Abstract
Background and Purpose
Blood–brain barrier disruption and consequent vasogenic edema formation codetermine the clinical course of intracerebral hemorrhage (ICH). This study examined the effect of PHA-543613, a novel α7 nicotinic acetylcholine receptor agonist, on blood–brain barrier preservation after ICH.
Methods
Male CD-1 mice, subjected to intrastriatal blood infusion, received PHA-543613 alone or in combination with α7 nicotinic acetylcholine receptor antagonist methyllycaconitine or phosphatidylinositol 3-kinase inhibitor wortmannin.
Results
PHA-543613 alone, but not in combination with methyllycaconitine or wortmannin, inhibited glycogen synthase kinase-3β, thus, stabilizing β-catenin and tight junction proteins, which was paralleled by improved blood–brain barrier stability and ameliorated neurofunctional deficits in ICH animals.
Conclusions
PHA-543613 preserved blood–brain barrier integrity after ICH, possibly through phosphatidylinositol 3-kinase-Akt–induced inhibition of glycogen synthase kinase-3β and β-catenin stabilization.
Keywords: α7 nicotinic acetylcholine receptor, blood–brain barrier, intracerebral hemorrhage, mice, PHA-543613
Vasogenic brain edema develops after intracerebral hemorrhage (ICH) as a consequence of increased blood–brain barrier (BBB) permeability.1 The hematoma-induced disintegration of endothelial tight junctions facilitates capillary leakage, and the mass effect of extravasated fluid contributes to the patient’s delayed neurological deterioration.2 Tight junction proteins, such as claudins, occludin, and junctional adhesion molecule-1, span the membrane of 2 adjacent endothelial cells, thus, forming the initial barrier between blood and brain.1 Moreover, decreased expression of these trans-membrane proteins indicates reduced barrier integrity and increased paracellular permeability.3,4
Previous studies demonstrated the regulatory role of β-catenin in claudin-3 and claudin-5 gene expression, both being essential BBB constituents.3,5 Unphosphorylated, cytosolic β-catenin translocates into the nucleus, and, by binding to the T cell factor/lymphoid enhancer factor, it promotes claudin-3 transcription.3 Contrary to this barrier-maintaining effect, β-catenin has also been reported to form a complex with the Forkhead box O1 transcription factor, which subsequently represses claudin-5 transcription.5
After hemorrhagic stroke, cytotoxic events activate the ubiquitously expressed glycogen synthase kinase-3β (GSK-3β),6 which phosphorylates serine and threonine residues of β-catenin, initiating its ubiquitin-dependent proteasomal degradation.7 However, pharmacological stimulation of the α7 nicotinic acetylcholine receptor (α7nAChR) activates the phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, and Akt has been shown to inhibit GSK-3β, thereby stabilizing β-catenin.6
Our earlier work demonstrated that selective α7nAChR stimulation reduced brain edema in rodents subjected to experimental hemorrhagic stroke,6,8 but the mechanism remains poorly understood. Because functional α7nAChRs are located in cerebral microvascular endothelial cells,9 we hypothesized that PHA-543613, a selective α7nAChR agonist, would stabilize β-catenin through PI3K-Akt–induced inhibition of GSK-3β, therefore, increasing claudin-3 expression and improving BBB functional integrity after experimental ICH in mice.
Material and Methods
Animal Surgery and Experimental Groups
All procedures were approved by the Institutional Animal Care and Use Committee. One hundred eleven male CD-1 mice (35–45 g; Charles River, Wilmington, MA) were subjected to either ICH or sham operation. A stereotactically guided, autologous whole blood double injection model was used to mimic right-sided intrastriatal bleeds.10 Details are available in the online-only Data Supplement. ICH animals received intraperitoneal injections of α7nAChR agonist PHA-543613 (12 mg/kg; 1 hour post-ICH), PHA-543613+α7nAChR antagonist methyllycaconitine (MLA; 6 mg/kg; intraperitoneally at 45 minutes post-ICH), PHA-543613+PI3K inhibitor wortmannin (15 μL/kg; intravenously at 30 minutes post-ICH), or MLA and wortmannin alone. Control animals received vehicle administrations. All drugs were purchased from Sigma-Aldrich (St. Louis, MO), processed, and administered according to protocols reported previously.6,8
Assessment of Sensorimotor Deficits
The modified Garcia neuroscore and the forelimb placing test were used to assess sensorimotor deficits in mice at 24 and 72 hours after surgery, as previously reported.6 Details are available in the online-only Data Supplement.
Tissue Processing and Analysis
Mice were euthanized at 24 and 72 hours after surgery to evaluate brain water content (brain edema) via the wet weight/dry weight method, as previously described.10 Standard protocols were used to conduct Evans blue and Western blot assays at 24 hours after surgery.6,10 Details are available in the online-only Data Supplement.
Statistical Analysis
Data are expressed as mean±SEM. Behavior data were analyzed with Kruskal–Wallis 1-way ANOVA on ranks, followed by the Student–Newman–Keuls method. All other data were analyzed by 1-way ANOVA, followed by the Tukey test. A P value of <0.05 was considered statistically significant.
Results
PHA-543613 Attenuated Sensorimotor Deficits and Brain Edema After Experimental ICH
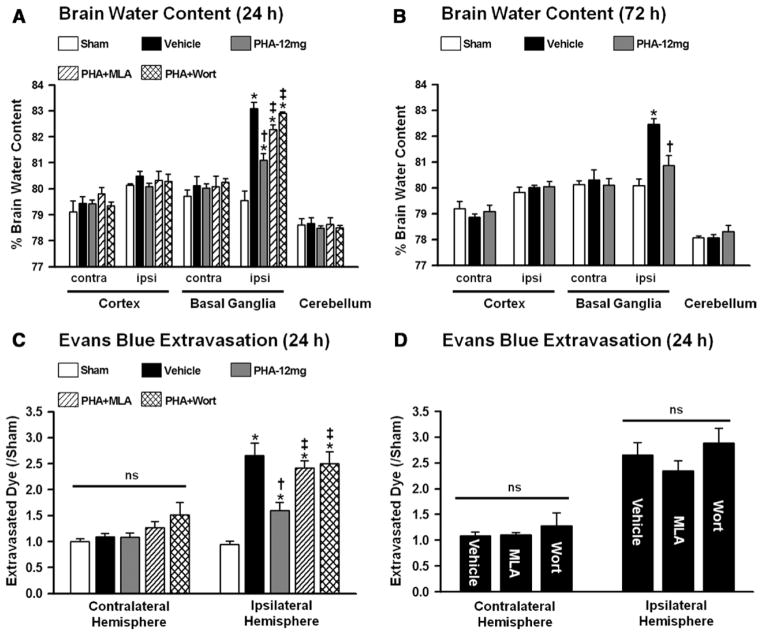
Sensorimotor deficits and brain water content were evaluated in mice at 24 and 72 hours after surgery (n=6 per group). Mice subjected to ICH showed significant sensorimotor deficits compared with sham-operated animals (P<0.05; Figure 1A and 1B). However, PHA-543613 treatment (PHA-12 mg) improved neuroscore as well as forelimb placing performance at 24 and 72 hours after surgery (P<0.05, compared with vehicle). Mice receiving PHA-543613 in combination with α7nAChR antagonist MLA (PHA+MLA) or PI3K inhibitor wortmannin (PHA+Wort) failed to demonstrate improved sensorimotor function in both behavioral tests (P>0.05, compared with vehicle). Furthermore, PHA-543613 significantly reduced the water content within the ipsilateral basal ganglia at 24 and 72 hours after ICH induction (P<0.05, compared with vehicle; Figure 2A and 2B). Mice receiving PHA-543613 in combination with MLA (PHA+MLA) or wortmannin (PHA+Wort), however, failed to demonstrate reduced brain edema after ICH (P>0.05, compared with vehicle).
Figure 1.

A, Modified Garcia neuroscore. B, Forelimb placing test. Values are expressed as mean±SEM. *P<0.05 compared with sham, †P<0.05 compared with vehicle, ‡P<0.05 compared with PHA-12 mg. N=6 per group.
Figure 2.
A, Brain water content at 24 and (B) 72 hours. C and D, Evans blue extravasation at 24 hours. Values are expressed as mean±SEM; *P≤0.05 compared with sham, †P≤0.05 compared with vehicle, ‡P≤0.05 compared with PHA-12 mg. N=5 to 6 per group. Contra indicates contralateral; ipsi, ipsilateral; MLA, methyllycaconitine; and ns, not significant.
PHA-543613 Preserved BBB Integrity After Experimental ICH
Functional barrier properties were evaluated via Evans blue assays, conducted at 24 hours after surgery (n=5–6 per group). Significantly more extravasated dye was measured in the ipsilateral hemispheres of mice subjected to ICH (P<0.05, compared with sham; Figure 2C). PHA-543613 preserved BBB integrity after ICH, which was associated with significantly reduced dye extravasation in treated animals (P<0.05, compared with vehicle). Mice receiving PHA-543613 in combination with MLA (PHA+MLA) or wortmannin (PHA+Wort) failed to demonstrate reduced dye extravasation into the ipsilateral brain hemisphere after ICH (P≥0.05, compared with vehicle). When given alone, MLA and wortmannin did not increase the BBB rupture to a greater extent than vehicle administration (P≥0.05; Figure 2D). The amount of extravasated dye within the contralateral hemisphere was similar between all groups (P≥0.05; Figure 2C and 2D).
PHA-543613 Reduced GSK-3β Activation, Thereby Stabilizing β-Catenin and Tight Junction Proteins
Western blot analyses of the ipsilateral brain hemisphere were conducted at 24 hours after surgery (n=5 per group). Changes in protein expression of phosphorylated and, therefore, activated GSK-3β (p–GSK-3β, Tyr216) was quantified as a ratio to total GSK-3β (Figure 3A). GSK-3 phosphorylation was significantly increased in the ipsilateral brain hemisphere of mice subjected to ICH (P<0.05, compared with sham). However, PHA-543613 treatment significantly reduced the p–GSK-3β/GSK-3β ratio (P<0.05, compared with vehicle), which was reversed by MLA and wortmannin (P<0.05, compared with PHA-12 mg). β-catenin phosphorylation enhances its degradation. Increased phosphorylated β-catenin (p–β-catenin; Ser33/37 and Thr41) levels were found in vehicle animal brains (P<0.05, compared with sham; Figure 3B). PHA-543613 significantly reduced the p–β-catenin/β-catenin ratio (P<0.05, compared with vehicle), which was tendentially abolished by MLA (P=0.05) and reversed by wort-mannin (P<0.05). Experimental ICH reduced claudin-3 and claudin-5 levels (P<0.05, compared with sham; Figure 3C and 3D), but PHA-543613 treatment increased their expression significantly (P<0.05, compared with vehicle). Mice in the PHA+MLA group presented significantly reduced claudin-3 levels (P<0.05) and tended to reduce claudin-5 levels (P=0.09) when compared with vehicle animals. Wortmannin reversed the initial increase of claudin-3 and claudin-5 by PHA-543613 (P<0.05).
Figure 3.
Western blot analysis of (A) p–GSK-3β/GSK-3β, (B) p–β-catenin/β-catenin, (C) claudin-3, and (D) claudin-5. Values are expressed as mean±SEM, normalized to sham. *P<0.05 compared with sham, †P<0.05 compared with vehicle, ‡P<0.05 compared with PHA-12 mg. N=5 per group. GSK-3β indicates glycogen synthase kinase-3β; and MLA, methyllycaconitine.
Discussion
The present study aimed to explain how α7nAChR stimulation minimized cerebral edema formation in mice subjected to ICH. Consistent with our previous work,6 PHA-543613 administration reduced total perihematomal brain edema, which we evaluated via the wet weight/dry weight method. Because this technique does not distinguish between vasogenic and cytotoxic brain edema,10 we incorporated Evans blue assays to determine the functional integrity of the BBB. As expected, PHA-543613 reduced BBB permeability after ICH, which was associated with less albumin-bound dye extravasation into the affected brain hemisphere. Therefore, we propose that PHA-543613 reduced brain edema formation after ICH, at least to some extent, by preserving BBB functional integrity.
Although primarily expressed by neurons and microglia, functional α7nAChRs have been located in BBB-comprising microvascular endothelial cells.9 In addition to their function as ligand-gated ion channels, α7nAChR stimulation activates PI3K-Akt signaling through an unidentified mechanism.11 Moreover, Akt has been reported to antagonize hemorrhage-induced GSK-3β activation,6 resulting in dephosphorylation of GSK-3β substrates, including β-catenin.7 In accordance with this theory, we found quantitatively more phosphorylated GSK-3β and β-catenin in the brains of ICH animals, but PHA-543613 administration reversed these hemorrhage-evoked molecular consequences.
Unphosphorylated β-catenin associates with sequence-specific DNA-binding factors of the T cell factor/lymphoid enhancer factor family, thus, promoting the transcription of claudin-3,3 which we found highly expressed in PHA-543613–treated ICH mouse brains. Contrary to this barrier-maintaining effect, β-catenin, when in complex with Forkhead box O1, represses endothelial-specific claudin-5 expression.5 Surprisingly, the present study found that PHA-543613–induced β-catenin stabilization was associated with increased claudin-5 expression. This discrepancy may be attributed to PI3K-Akt–induced Forkhead box O1 inhibition, as previously reported.5 Consistent with our findings, pharmacological β-catenin stabilization increased claudin-5 expression in a rat model of focal cerebral ischemia.12
The observed functional and morphological changes, evoked by PHA-543613, were ameliorated or reversed by coadministration of MLA or wortmannin with the treatment. MLA is a competitive inhibitor of α7nAChR; and wortmannin irreversibly inhibits PI3K, thus preventing Akt activation.8 Yet, both interventions failed to induce BBB disruption, brain edema, and neurofunctional deficits significantly after experimental ICH in mice, when administered alone.6 Based on these findings, we suggest that functional α7nAChRs and PI3K are required for PHA-543613–induced BBB protection.
Claudin-3 and claudin-5 are transmembrane proteins essential for maintaining the diffusion barrier provided by tight junctions.3,13 The selective loss of claudin-3 in cerebral microvascular endothelial cells has been linked to pathological conditions, including autoimmune encephalomyelitis and human glioblastoma multiforme.13 Additionally, claudin-5 knockout mice presented increased BBB permeability and died shortly after birth.4 Therefore, prospective treatments that effectively increase claudin-3 and claudin-5 expressions may ameliorate BBB breakdown in various central nervous system diseases.
The present study has several limitations. First, we did not implement the collagenase-induced ICH mouse model. Collagenases are proteolytic enzymes that may impair the BBB to a greater extent than blood toxicity alone.14 In fact, Hijioka et al15 observed that α7nAChR stimulation failed to significantly reduce brain edema in a collagenase-induced ICH model. Furthermore, pathophysiological mechanisms of edema formation may differ between these models. Second, β-catenin is a structural adaptor protein of endothelial adherens junctions and may, therefore, directly generate BBB stabilization.7 Third, anti-inflammatory11 and antiapoptotic6,8 properties of α7nAChR agonism were not evaluated in this study.
In summary, α7nAChR agonist PHA-543613 reversed ICH-induced brain edema, possibly by stabilizing β-catenin and, therefore, increasing claudin-3 and claudin-5 expressions. These molecular changes were paralleled by preserved BBB integrity and improved sensorimotor deficits in ICH animals. However, α7nAChR antagonist MLA and PI3K inhibitor wortmannin reversed the treatment effects. Based on these findings, we conclude that α7nAChR-induced activation of the PI3K-Akt pathway stabilized β-catenin through GSK-3β inhibition, thereby increasing subsequent claudin-3 and claudin-5 expression.
Acknowledgments
Sources of Funding
This research was supported by the National Institutes of Health grant RO1 NS053407 to Dr Zhang.
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.111.000427/-/DC1.
Disclosures
None.
References
- 1.Keep RF, Xiang J, Ennis SR, Andjelkovic A, Hua Y, Xi G, et al. Blood-brain barrier function in intracerebral hemorrhage. Acta Neurochir Suppl. 2008;105:73–77. doi: 10.1007/978-3-211-09469-3_15. [DOI] [PubMed] [Google Scholar]
- 2.Gebel JM, Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, et al. Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke. 2002;33:2636–2641. doi: 10.1161/01.str.0000035283.34109.ea. [DOI] [PubMed] [Google Scholar]
- 3.Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taddei A, Giampietro C, Conti A, Orsenigo F, Breviario F, Pirazzoli V, et al. Endothelial adherens junctions control tight junctions by VE-cadherin-mediated upregulation of claudin-5. Nat Cell Biol. 2008;10:923–934. doi: 10.1038/ncb1752. [DOI] [PubMed] [Google Scholar]
- 6.Krafft PR, Altay O, Rolland WB, Duris K, Lekic T, Tang J, et al. α7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3β inhibition in a mouse model of intracerebral hemorrhage. Stroke. 2012;43:844–850. doi: 10.1161/STROKEAHA.111.639989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aberle H, Bauer A, Stappert J, Kispert A, Kemler R. Beta-catenin is a target for the ubiquitin-proteasome pathway. EMBO J. 1997;16:3797–3804. doi: 10.1093/emboj/16.13.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. α 7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011;42:3530–3536. doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moccia F, Frost C, Berra-Romani R, Tanzi F, Adams DJ. Expression and function of neuronal nicotinic ACh receptors in rat microvascular endothelial cells. Am J Physiol Heart Circ Physiol. 2004;286:H486–H491. doi: 10.1152/ajpheart.00620.2003. [DOI] [PubMed] [Google Scholar]
- 10.Ma Q, Huang B, Khatibi N, Rolland W, II, Suzuki H, Zhang JH, et al. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Ann Neurol. 2011;70:920–931. doi: 10.1002/ana.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing Y, Zhang X, Zhao K, Cui L, Wang L, Dong L, et al. Beneficial effects of sulindac in focal cerebral ischemia: a positive role in Wnt/β-catenin pathway. Brain Res. 2012;1482:71–80. doi: 10.1016/j.brainres.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 13.Wolburg H, Wolburg-Buchholz K, Kraus J, Rascher-Eggstein G, Liebner S, Hamm S, et al. Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol. 2003;105:586–592. doi: 10.1007/s00401-003-0688-z. [DOI] [PubMed] [Google Scholar]
- 14.MacLellan CL, Silasi G, Poon CC, Edmundson CL, Buist R, Peeling J, et al. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J Cereb Blood Flow Metab. 2008;28:516–525. doi: 10.1038/sj.jcbfm.9600548. [DOI] [PubMed] [Google Scholar]
- 15.Hijioka M, Matsushita H, Ishibashi H, Hisatsune A, Isohama Y, Katsuki H. α7 Nicotinic acetylcholine receptor agonist attenuates neuropathological changes associated with intracerebral hemorrhage in mice. Neuroscience. 2012;222:10–19. doi: 10.1016/j.neuroscience.2012.07.024. [DOI] [PubMed] [Google Scholar]