Abstract
The phase III AZA-001 study established that azacitidine significantly improves overall survival compared with conventional care regimens (hazard ratio 0.58 [95% confidence interval 0.43–0.77], P<0.001). This analysis was conducted to investigate the relationship between treatment response and overall survival. AZA-001 data were analyzed in a multivariate Cox regression analysis with response as a time-varying covariate. Response categories were “Overall Response” (defined as complete remission, partial remission, or any hematologic improvement) and “Stable Disease” (no complete or partial remission, hematologic improvement, or progression) or “Other” (e.g. disease progression). Achieving an Overall Response with azacitidine reduced risk of death by 95% compared with achieving an Overall Response with the conventional care regimens (hazard ratio 0.05 [95%CI: 0.01–0.43], P=0.006). Sensitivity analyses indicated that significantly improved overall survival remained manifest for patients with a hematologic improvement who had never achieved complete or partial remission (hazard ratio 0.19 [95%CI: 0.08–0.46], P<0.001). Stable Disease in both azacitidine-treated and conventional care-treated patients was also associated with a significantly reduced risk of death (hazard ratio 0.09, [95%CI: 0.06–0.15]; P<0.001). These results demonstrate azacitidine benefit on overall survival compared with conventional care regimens in patients with higher-risk myelodysplastic syndromes who achieve hematologic response but never attain complete or partial remission, in addition to the survival advantage conferred by achievement of complete or partial remission. This study was registered with clinicaltrials.gov (NCT00071799).
Introduction
Thirty percent of patients with myelodysplastic syndromes (MDS) present with higher-risk disease (International Prognostic Scoring System1 [IPSS] intermediate-2 or high) with an over 60% risk of progression to acute myelogenous leukemia (AML) and poor overall survival (OS).2,3 The primary goal of treatment for higher-risk MDS is prolonging OS by altering the natural disease course.4,5 Because treatment-related effects on OS may require years to measure, hematologic response has been the primary end point in most MDS clinical studies. Using treatment experience from AML, the general view has been that complete or partial remission (CR or PR) is a prerequisite for prolonged OS.6
The phase III randomized AZA-001 trial demonstrated that azacitidine (Vidaza®, Celgene Corporation, Summit, NJ, USA) significantly prolongs OS in higher-risk MDS compared with conventional care (CCR) (hazard ratio [HR] 0.58 [95%CI: 0.43–0.77], P<0.001).7 In the AZA-001 study, significantly more patients achieved hematologic improvement (HI)5 with azacitidine than with CCR (49% vs. 29%, respectively, P<0.0001). The paradigm that CR or PR is necessary to prolong OS is being challenged.4 This study was designed to assess whether CR or PR are obligate responses for improved OS with azacitidine and whether a best response of HI might be associated with improved OS as well. Analyses of OS by treatment response category can be misleading if an inappropriate statistical approach is used.9,10 To minimize potential biases, we used two approaches to assess the relationship between treatment response and OS in the AZA-001 study: a multivariate Cox regression analysis with response category as a time-varying covariate, and landmark analyses examining the response-survival relationship with response categorized at fixed time points.9–11
Design and Methods
AZA-001 (ClinicalTrials.gov Identifier:00071799) study design is reported in detail elsewhere.7
All patients provided written, informed consent before participating in the study. A multivariate Cox regression analysis with response as a time-varying covariate evaluated the relationship between hematologic response and OS. CR, PR, HI, stable disease (SD), and progression were defined by IWG MDS 2000 criteria.5 Stable Disease was defined as no evidence of progression without HI. Patient responses (CR and PR) were reviewed by an independent review committee (IRC) of international MDS experts blinded to treatment assignment, and an HI response was determined programmatically.
Response was classified in 3 categories: Overall Response (HI, PR, or CR), Stable Disease (without HI), and Other (e.g. disease progression). HI, PR, and CR were grouped in Overall Response because HI could be evaluated twice monthly while IRC-assessed PR or CR (requiring bone marrow samples) could be evaluated only every four months and we wanted to capture changes in response as soon as they occurred.
Response was evaluated as a time-varying covariate, with patients’ response classification changing each time clinical status changed. Thus, a patient starting study with Stable Disease, achieving HI at Day 84, PR at Day 195, and sustaining PR until Day 581 would enter the model as Stable Disease for Days 0–83, HI for Days 84–194, and PR for Days 195–581.
OS was evaluated using a Cox proportional hazards model stratified by FAB and IPSS, including treatment as a factor. Time-varying covariates of Overall Response and Stable Disease, and terms for Overall Response-by-treatment and Stable Disease-by-treatment, were added to the model. HR and associated 95% confidence intervals (CI) are reported from this model; HR and P value were adjusted for presence of all factors. Sensitivity analyses evaluated the relationship between HI without an investigator-assessed response of CR or PR and OS. (IRC-adjudicated CR+PR rates at any time were small; because investigator-reported CR+PR rates were higher, they increased the probability of detecting whether CR and PR were driving the OS benefit.) The sensitivity analysis evaluated OS in patients with a PR or CR during the study, and in those with an HI as best response (i.e. without an investigator-reported PR or CR on-study). A similar method was used to assess the relationship between OS and Stable Disease. Landmark (“snapshot”) analyses were performed to corroborate the Cox regression analysis and to avoid biases inherent in classifying patients by best response during study. Landmark analyses evaluated the relationship between OS and response at three, six, and nine months. Median OS and 2-year OS rates were estimated using Kaplan-Meier methods. HRs and 95% CIs were from a Cox proportional hazards model and the effect of treatment on OS within response groups was evaluated using a 2-sided log rank test. Exploratory logistical regression analyses investigated differences in baseline clinical characteristics between: 1) patients in the azacitidine and CCR groups with Stable Disease as best response at six months; and 2) patients (in both treatment arms) who achieved an Overall Response and patients who maintained Stable Disease over the 9-month period.
Results
Demographic and disease characteristics of the 358 patients (azacitidine n=179, CCR n=179) included in AZA-001 and patient disposition are reported elsewhere.7 Median follow up was 21.1 months. Patients received a median of 9 azacitidine treatment cycles (range 1–39), 4.5 LDAC cycles (1–15; n=49), one intensive chemotherapy cycle (1–3; n=25), or supportive care for a median 6.2 months (range 0.2–25.8; n=105).7
Survival in patients with Overall Response
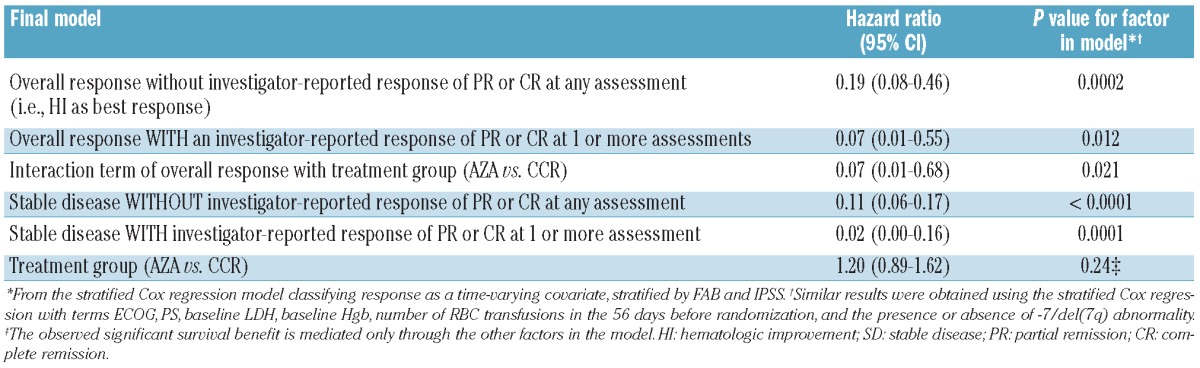
The multivariate Cox proportional hazard model using response as a time-varying covariate indicated that achieving Overall Response (HI, PR, or CR) with either azacitidine or CCR was associated with an 84% reduced risk of death (HR 0.16, 95%CI: 0.07–0.37; P<0.0001) compared with no Overall Response (Table 1). However, the response-by-treatment interaction analysis showed that achieving Overall Response with azacitidine was associated with a 95% reduced risk of death compared with achieving Overall Response with CCR (HR 0.05, 95%CI: 0.01–0.43; P=0.006). In the multivariate model, treatment group alone as a factor in the model was not significant (P=0.26), indicating that the observed significant survival benefit with azacitidine compared with CCR is mediated through the other factors in the model (i.e. achievement of Overall Response). Sensitivity analysis demonstrated that achievement of HI without investigator-reported response of CR or PR at any time during the study remained associated with improved OS (Table 2). (Investigator-reported response of CR or PR at any time was achieved by 51 azacitidine-treated patients [29%] and 21 CCR patients [12%].7) The treatment effect of azacitidine versus CCR for improved OS remained significant in this model (HR 0.07, 95%CI: 0.01–0.68; P=0.021).
Table 1.
Overall survival: multivariate Cox regression analysis with response as a time-varying covariate.
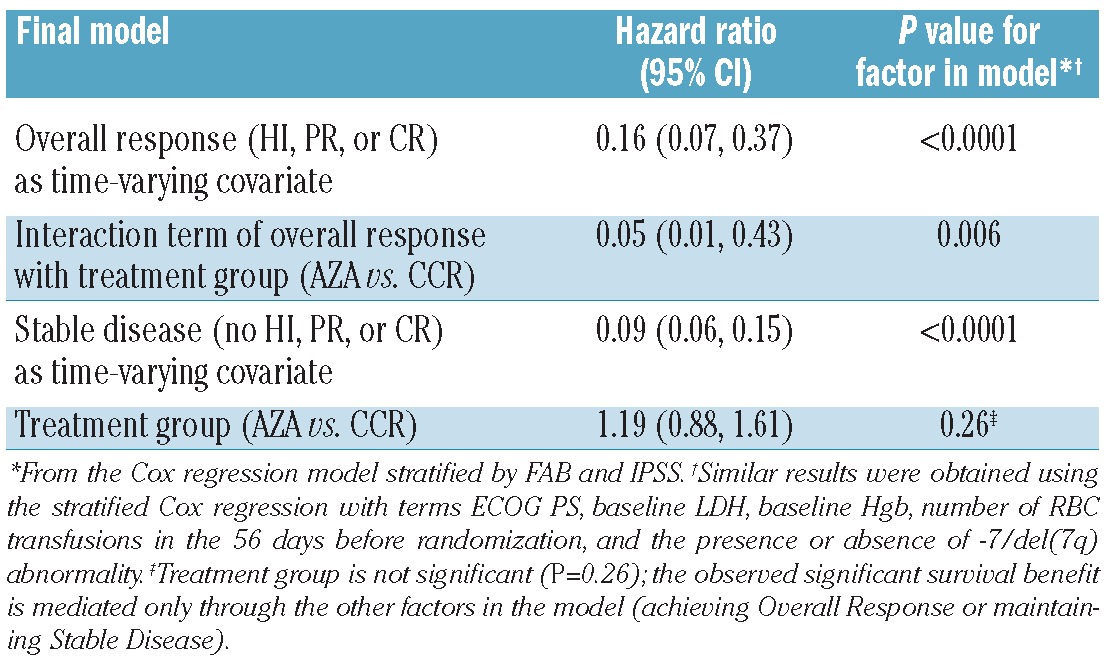
Table 2.
Sensitivity analyses of investigator-reported best responses of CR or PR versus best responses without CR or PR.
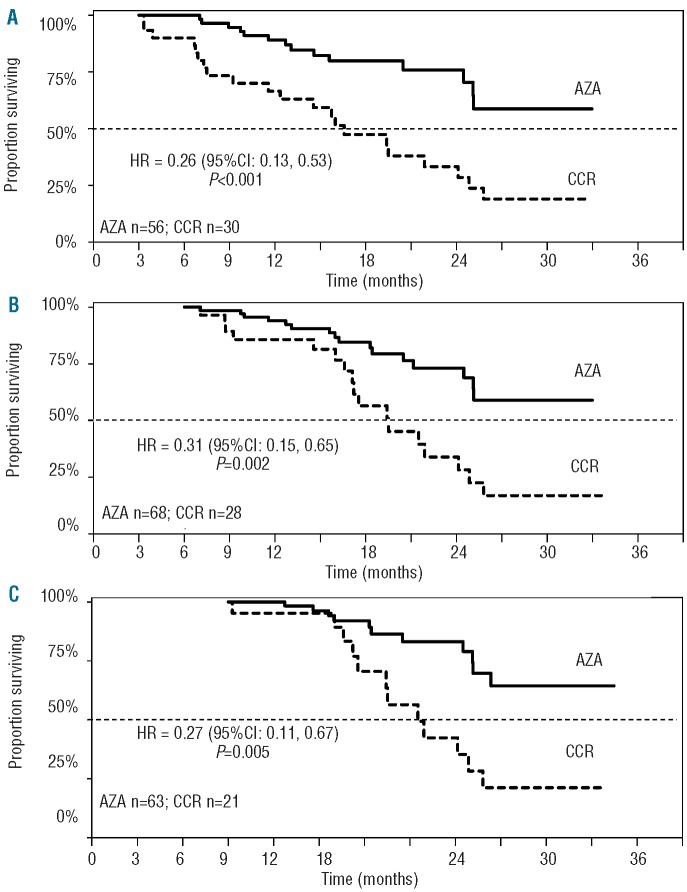
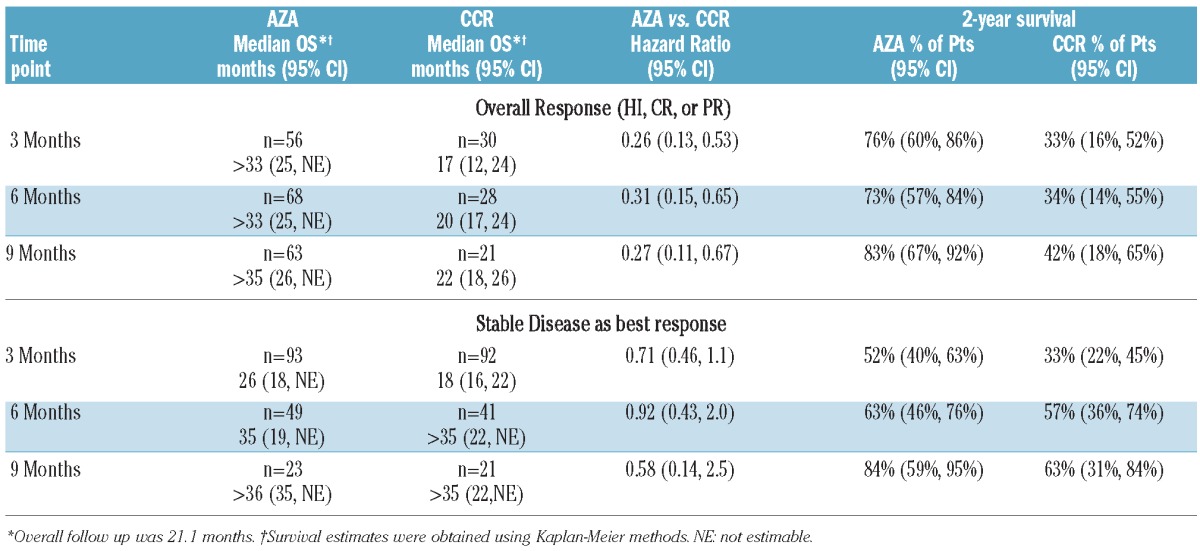
Landmark analyses supported results of the multivariate analysis (Figure 1). Median OS within the Overall Response group was not reached in patients receiving azacitidine at any landmark, while median OS in the CCR group ranged from 16.6 to 21.5 months. HRs for OS with azacitidine versus CCR ranged from 0.26 to 0.31. Estimated OS rates at two years in patients who achieved Overall Response were approximately 2-fold higher in the azacitidine group at each landmark than in the CCR group (73–83% vs. 33–42%, respectively) (Table 3).
Figure 1.
Median survival in patients with overall response (HI, PR, or CR) at 3-month (A), 6-month (B), and 9-month (C) landmark measures.
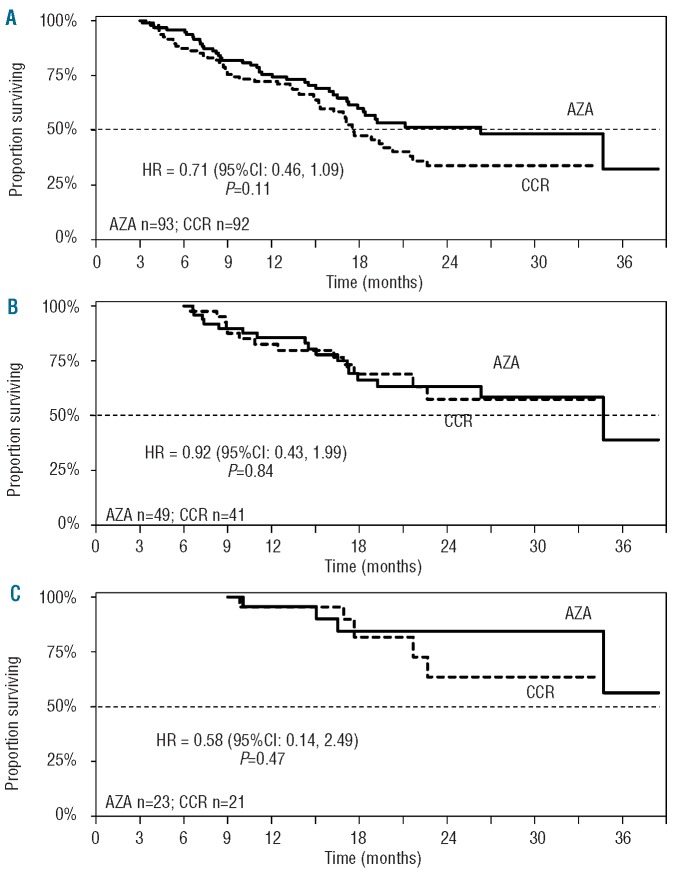
Table 3.
Kaplan-Meier estimates of median OS and OS at 2 years in patients who achieved an overall response and in patients with stable disease as a best response.
At three months, 56 azacitidine patients and 30 CCR patients had achieved an Overall Response (Figure 3). Proportionately more patients in the azacitidine group retained an Overall Response at six months (48 of 56, 86%) than did patients receiving CCR (15 of 30, 50%). Of patients with an Overall Response at six months, 54 of 68 patients (79%) in the azacitidine group and 21 of 28 (75%) in the CCR group retained an Overall Response at nine months.
Figure 3.
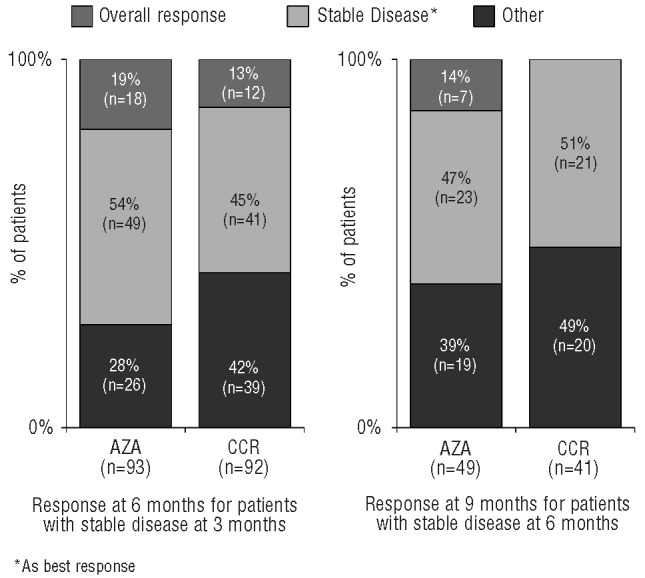
Change in response with continued treatment in patients with stable disease as best response.
Survival in patients with Stable Disease
In the multivariate analysis, Stable Disease (without HI) was associated with a 91% reduction in risk of death (HR 0.09, 95%CI: 0.06–0.15; P<0.0001) throughout the course of the study (Table 1). The significant association between Stable Disease and improved OS was not treatment-specific, occurring with both azacitidine and CCR treatment. Sensitivity analysis showed Stable Disease was associated with significantly improved OS even when no investigator-reported CR or PR was achieved during the study (Table 2). Similar results were obtained when the covariates ECOG PS, LDH, and Hgb at baseline, number RBC transfusions before randomization, and presence/absence of −7/del(7q) abnormality were included in the model (data not shown).
Results of landmark analyses were consistent with the multivariate analysis: there was no statistically significant difference in median OS between azacitidine and CCR treatment groups for patients with Stable Disease as the best response at three, six, or nine months (Figure 2, Table 3). Estimated 2-year OS rates in patients with Stable Disease as best response at three months were 52% and 33% for azacitidine and CCR patients, respectively, and rose to 84% and 63%, respectively, for patients with Stable Disease as their best response at month 9 (Table 3).
Figure 2.
Median survival in patients with stable disease as best response at 3-month (A), 6-month (B), and 9-month (C) landmark measures.
Of 93 azacitidine patients and 92 CCR patients with Stable Disease as their best response at three months, approximately half (azacitidine 53%, CCR 45%) maintained Stable Disease at six months (Figure 3). Eighteen azacitidine patients (19%) and 12 CCR patients (13%) with Stable Disease at three months achieved an Overall Response at six months, and 7 azacitidine patients (14%) and no CCR patient with Stable Disease at six months achieved an Overall Response by nine months.
Univariate logistical regression analysis showed significant differences in some hematologic indices at baseline between the subgroups of patients in the azacitidine (n=49) and CCR (n=41) treatment arms with Stable Disease as their best response at six months (Online Supplementary Table S1). In patients with available baseline cytogenetic data, a significantly higher proportion of those receiving azacitidine had abnormal (vs. normal) cytogenetics: 52% vs. 29% of CCR patients, P=0.038 (data not shown), and azacitidine patients had more frequent baseline cytopenias. In addition, patients receiving azacitidine had lower median platelet counts (68 vs. 107 x 109/L with CCR; P=0.045) and 10% were platelet transfusion-dependent, compared with none of the CCR patients. These imbalances were not present at baseline in the overall randomized cohorts in AZA-001.7 Two baseline characteristics remained significant in the multivariate model for OS: number of cytopenias (P=0.007) and presence of abnormal cytogenetics (P=0.016). Logistical regression analyses to determine whether patients who achieved an Overall Response at some point during the 9-month period had different clinical characteristics at baseline than patients who maintained Stable Disease as their best response included 106 azacitidine patients (Overall Response, n=83; Stable Disease, n=23) and 64 CCR patients (Overall Response, n=43; Stable Disease, n=21). In both treatment arms, a significantly higher proportion of patients who were RBC transfusion-dependent at baseline achieved an Overall Response rather than maintaining Stable Disease when compared with patients who were transfusion-independent at baseline. Logistical regression analyses to determine whether patients who achieved an Overall Response at some point during the 9-month period had different clinical characteristics at baseline than patients who maintained Stable Disease as their best response included 106 azacitidine patients (Overall Response, n=83; Stable Disease, n=23) and 64 CCR patients (Overall Response, n=43; Stable Disease, n=21). In both treatment arms, a significantly higher proportion of patients who were RBC transfusion-dependent at baseline achieved an Overall Response rather than maintaining Stable Disease when compared with patients who were transfusion-independent at baseline.
Discussion
Results of these analyses show that achieving Overall Response (HI, CR, or PR) significantly improves OS in patients with higher-risk MDS, with a strong treatment effect favoring azacitidine over CCR. Azacitidine-treated patients who achieved Overall Response had a 95% reduced risk of death compared with CCR-treated patients who achieved Overall Response. Sensitivity analyses supported the significant OS benefit of Overall Response in patients with HI (i.e. improvement of cytopenias) as best response, without ever achieving CR or PR. Importantly, sensitivity analyses supported the significant treatment benefit of azacitidine on OS in patients with HI as a best response (93% reduced risk of death with azacitidine vs. CCR). The significant response-by-treatment interaction in patients with Overall Response favoring azacitidine suggests that hematologic responses with azacitidine are qualitatively different from those with CCR. In addition, of all patients who maintained Stable Disease as best response at six months, those who received azacitidine had worse pre-treatment clinical characteristics (increased abnormal cytogenetics, more trilineage cytopenias) than those who received CCR, suggesting sicker patients were able to maintain SD with azacitidine.
There are no definitive guidelines regarding when to stop azacitidine treatment in patients who maintain Stable Disease. Azacitidine, a DNA methyltransferase inhibitor, requires multiple cycles to modulate clonal hematopoietic abnormalities.15,16 This multivariate Cox regression analysis suggests Stable Disease without HI may have OS benefits in patients treated with azacitidine or CCR. Our results also showed that with continued treatment, about one-third of azacitidine-treated patients with Stable Disease at three months will achieve Overall Response by nine months; however, this should be interpreted cautiously, since this is a retrospective analysis and by month 9 patient numbers were quite small. Recent MDS treatment guidelines recommend a minimum of 6 azacitidine treatment cycles in patients with higher-risk MDS with or without an IWG response, and maintenance therapy for as long as patients continue to benefit.14,17,18 Further study is needed to clarify our findings. Whether to continue azacitidine treatment after six cycles in patients with SD remains uncertain, but may be clarified in a prospective, randomized discontinuation trial. The physician’s decision to continue azacitidine treatment in patients with stable disease, is best made in collaboration with the patient, taking tolerance, toxicity, and treatment-related quality of life into consideration.
Assessing the relationship between response and OS is subject to known biases, e.g. patients with longer OS have more time to attain better responses. To minimize biases, a Cox regression analysis with response as a time-varying covariate supported by landmark analyses is recommended when assessing OS-response relationships.9–11 These two different analytic approaches reached similar conclusions in this analysis. Nevertheless, there are acknowledged limitations to analyses of OS by response in general10 and in MDS in particular. The possibility that response is a marker of intrinsically good prognosis rather than treatment effect cannot be excluded.10 In MDS, rates of disease progression vary. In some cases, Stable Disease may reflect variability in disease course rather than a therapeutic effect. In addition, the multivariate analysis is limited in that it accounts for patient status at each assessment, regardless of previous status, e.g. Stable Disease after CR is treated the same as Stable Disease without prior response. This limitation is addressed somewhat by results of the landmark analyses which provide OS estimates only for patients alive and on-study at each landmark with response categorized at that landmark, and by results of the sensitivity analysis showing that presence or absence of investigator-reported CR or PR did not influence the significant improvement in OS in patients with HI or Stable Disease as their best treatment response.
These are the first results from a large, phase III controlled trial to clearly demonstrate that a response of HI with azacitidine is associated with significantly prolonged OS compared with CCR in patients with higher-risk MDS.
Acknowledgments
The authors would like to thank Neil Malone (Celgene Corporation) and Sheila Truten (MC2, Wynnewood, PA, USA) who provided editorial assistance during manuscript development.
Footnotes
Funding
This work was supported by Celgene Corporation, Summit, NJ, USA.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997. (6);89:2079–88 [PubMed] [Google Scholar]
- 2.Fukumoto JS, Greenberg PL. Management of patients with higher risk myelodysplastic syndromes. Crit Rev Oncol Hematol. 2005;56:(2)179–92 [DOI] [PubMed] [Google Scholar]
- 3.Gore SD, Hermes-DeSantis ER. Enhancing survival outcomes in the management of patients with higher-risk myelodysplastic syndromes. Cancer Control. 2009;16 Suppl:2–10 [DOI] [PubMed] [Google Scholar]
- 4.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25 [DOI] [PubMed] [Google Scholar]
- 5.Cheson BD, Bennett JM, Kantarjian H, Pinto A, Schiffer CA, Nimer SD, et al. Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood. 2000; 96(12):3671–4 [PubMed] [Google Scholar]
- 6.Freireich EJ, Gehan EA, Sulman D, Boggs DR, Frei E., III The effect of chemotherapy on acute leukemia in the human. J Chronic Dis. 1961;14:593–608 [DOI] [PubMed] [Google Scholar]
- 7.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol. 2009;10(3):223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.List A, Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Gore SD, Bennett JM, et al. Azacitidine (AZA) extends overall survival in higher-risk myelodysplastic syndromes (MDS) without necessity for complete remission. J Clin Oncol. 2008;26(155):7006 [Google Scholar]
- 9.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response and other comparisons of time-to-event by outcome variables. J Clin Oncol. 2008;26(24): 3913–5 [DOI] [PubMed] [Google Scholar]
- 10.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol 1983;1(11):710–9 [DOI] [PubMed] [Google Scholar]
- 11.Simon R, Makuch RW. A non-parametric graphical representation of the relationship between survival and the occurrence of an event: application to responder versus non-repsonder bias. Stat Med. 1984;3(1):35–44 [DOI] [PubMed] [Google Scholar]
- 12.Akaike H. A new look at the statistical model identification. IEEEE Trans Automat Contr. 1974;19(6):716–23 [Google Scholar]
- 13.Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20(10):2429–40 [DOI] [PubMed] [Google Scholar]
- 14.Vidaza® [printed page leaflet]. Summit, NJ: Celgene Corporation; 2011 [Google Scholar]
- 15.Christman JK, Mendelsohn N, Herzog D, Schneiderman N. Effect of 5-azacytidine on differentiation and DNA methylation in human promyelocytic leukemia cells (HL-60). Cancer Res. 1983;43(2):763–9 [PubMed] [Google Scholar]
- 16.Razin A, Cedar H. DNA methylation and gene expression. Microbiol Rev. 1991;55(3): 451–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santini V, Alessandrino PE, Angelucci E, Barosi G, Billio A, Di Maio M, et al. Clinical management of myelodysplastic syndromes: update of SIE, SIES, GITMO practice guidelines. Leuk Res. 2010;34(12):1576–88 [DOI] [PubMed] [Google Scholar]
- 18.Fenaux P, Bowen D, Gattermann N, Hellström-Lindberg E, Hofmann WK, Pfeilstöcker M, et al. Practical use of azacitidine in higher-risk myelodysplastic syndromes: an expert panel opinion. Leuk Res. 2010;34(11):1410–6 [DOI] [PubMed] [Google Scholar]