Abstract
We have previously demonstrated that the mitochondria-derived cytoprotective peptide humanin (HN), when administered intratesticularly to rats, rescues germ cells from apoptosis secondary to testicular stress of hormonal deprivation induced by gonadotropin-releasing hormone antagonist (GnRH-A). To decipher the cellular mechanisms of HN action in the amelioration of GnRH-A induced germ cell apoptosis, adult male rats received the following treatments for 5 days: 1) daily intratesticular (IT) injections with saline (control); 2) a single subcutaneous injection of GnRH-A on day 1 and daily IT injection of saline; 3) daily IT injection of synthetic humanin (HN); and 4) GnRH-A injection on day 1 and daily IT injection of HN (GnRH-A+HN). HN alone had no effect on germ cell apoptosis. GnRH-A increased germ cell apoptosis and BAX in the testicular mitochondrial fractions. Synthetic HN decreased germ cell apoptosis induced by GnRH-A and BAX in the mitochondria. We deduced that the cytoprotective action of synthetic HN on GnRH-A induced germ cell apoptosis was mediated by attenuating p38 MAPK activity and increasing STAT3 phosphorylation. The effect of synthetic HN on the expression of endogenous rat HN in the testis was studied using rat HN specific antibody. GnRH-A treatment increased but concomitant treatment with synthetic HN reduced endogenous rat HN expression in both cytosolic and mitochondrial fractions in testis. Co-immunoprecipitation experiments demonstrated the increased rat HN was physically associated with BAX in the cytosolic testicular fractions after GnRH-A treatment. Double-immunofluorescence staining confirmed the co-localization of BAX and rat HN in the cytoplasm of Leydig cells and spermatocytes after GnRH-A treatment. We conclude that the cytoprotective effect of exogenously administered synthetic HN is mediated by interactions of endogenous rat HN with BAX in the cytoplasm preventing the entry of BAX to the mitochondria to govern the fate of germ cell survival or death during pro-apoptotic stress to the testis in rats.
INTRODUCTION
Germ cell apoptosis can be induced in a cell-specific manner by a variety of pro-apoptotic stressors, which include experimental male contraceptive approaches such as administration of gonadotropin-releasing hormone antagonist (GnRH-A), or testosterone (T) to suppress endogenous gonadotropins and thus intra-testicular T secretion (Ruwanpura et al, 2008; Sinha Hikim et al, 1993, 1995, 2003a). We found that preleptotene and pachytene spermatocytes and round spermatids at mid stages (VII–VIII) are the most susceptible germ cells to apoptosis after hormone deprivation in the rats (Sinha Hikim et al, 1993, 1995, 2003a; Vera Y et al, 2006). The findings were consistent across species in rodents (Lue et al, 2006), monkeys (Jia et al, 2007), and humans (Wang et al, 2007); demonstrating that germ cell apoptosis plays an important role in the organized regression of spermatogenesis after intratesticular T deprivation. In previous studies, we showed that the mitochondria-dependent intrinsic pathway serves as the indispensable signaling pathway for germ cell apoptosis across species after intratesticular hormonal deprivation (Green et al, 2000; Hengartner, 2000; Jia et al, 2007; Reed, 2000; Sinha Hikim et al, 2003a; Vera et al, 2004, 2006;). The activation of p38 mitogen-activated protein kinase (p38 MAPK) alters the BAX/BCL-2 rheostat in the mitochondria, allowing BAX to translocate to the mitochondria, thereby activating the caspase cascade and resulting in male germ cell apoptosis in response to hormonal deprivation (Jia et al, 2007; Vera et al, 2004).
Humanin (HN) is a mitochondria-derived, cytoprotective peptide expressed in neuronal tissue (Chiba et al, 2004; Hashimoto et al, 2001; Kariya et al, 2002, 2005; Matsuoka et al, 2004, 2009; Nishimoto et al, 2004; Xu et al, 2006, 2010), blood-derived cells (Wang et al, 2005), heart and blood vessels (Bachar et al, 2010; Jung and Van Nostrand, 2003; Muzumdar et al, 2010), pancreatic beta cells (Hoang et al, 2010), and testis (Lue et al, 2010; Moretti et al, 2010). We have demonstrated that exogenous HN mitigates GnRH-A or insulin-like growth factor binding protein-3 (IGFBP-3) induced male germ cell apoptosis in rats (Lue et al, 2010). However, the cellular mechanisms of HN action in the regulation of testicular germ cell homeostasis are not known and were examined in this study. The endogenous HN homologue in the rat, rat HN (also known as rattin), has 14 amino acids more than the human version and has similar biological effects (Caricasole et al, 2002). Rat HN can be immunologically distinguished from human version of HN secondary to the non-cross-reactivity of the anti-rattin antibody, in addition to the difference in molecular weight of the two peptides.
In this study, we first assessed BAX translocation from cytosol to mitochondria in experimental conditions of testicular stress to define the intracellular cytoprotective action of HN on germ cell apoptosis. We showed that HN exerted its cytoprotective action via activation of STAT-3 signaling while decreasing p38-MAP kinase, events upstream of apoptosis. We then examined the intracellular interaction of endogenous rat HN and BAX after induction of apoptosis by acute testicular stress induced by GnRH-A with or without concomitant exogenous synthetic HN administration.
MATERIALS AND METHODS
Animals and Experimental Protocols
Young adult 60-day-old male Sprague Dawley (SD) rats were purchased from Charles River Laboratories (Wilmington, MA) and housed in a standard animal facility under controlled temperature (22°C) and photoperiod of twelve hours of light and 12 hours of darkness with free access to food and water. The animal use protocol was reviewed and approved by the Institutional Animal Care and Use Committee of Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. Groups of three to four rats received the following treatment for five days: 1) Control (n=3): Daily intratesticular (IT) saline injection; 2) GnRH-A (n=3): GnRH-A (Acyline, 30 mg/kg body weight, obtained from the NICHD, Rockville, MD) subcutaneous injection on day 1 and daily IT saline injection; 3) HN (n=4): daily IT injection of 50 mcg HN (GeneMed Synthesis, Inc., San Antonio, TX); and 4) GnRH-A + HN (n=4): GnRH-A injection on day 1 and daily IT injection of 50 mcg HN. Rats were euthanized on day 6. Detailed experimental procedures were similar as published previously (Lue et al, 2010).
Tissue Preparation and Subcellular Fractionation
Both control and experimental animals were injected with heparin(130 IU/100g BW, IP) 15 min before a lethal injection of sodium pentobarbital (100 mg/kg BW, IP) to facilitate testicular perfusion using a whole-body perfusion technique (Lue et al, 1999). After perfusion with saline, one testis was removed and weighed. Portions of testicular parenchyma were snap frozen in liquid N2, and stored at −80°C for subcellular fractionation and Western blotting.
Mitochondrial and cytosolic fractions were prepared as described previously (Jia et al, 2007, 2009; Sinha Hikim et al 2003b; Vera et al, 2006). Saline-perfused testes were homogenized in HEPES buffer (0.25 M sucrose, 50 mM HEPES, 10 mM NaCl, 10 mM EDTA, 2 mM DTT) supplemented with protease inhibitors (Complete Protease Inhibitors; Roche, Basel, Switzerland). Mitochondrial and cytosolic fractions were isolated by repeated centrifugation at different speeds. The purity of the cytosolic and mitochondrial fractions was validated by Western blotting using antibodies to GAPDH (1:5000; Millipore, Billerica, MA) and cytochrome c oxidase subunit IV (1:500; Cell Signaling, Danvers, MA), respectively.
Measurements of Kinase Activation
Activation of p38 MAPK was measured using a specific assay kit (Cell Signaling Technology, Inc., Beverly, MA) as described previously (Vera et al, 2006). In brief, a monoclonal phospho-specific antibody to p38 MAPK (Thr180/Tyr182) was used to selectively immunoprecipitate active p38 MAPK from total testis lysates. The resulting immunoprecipitate was then incubated with activating transcription factor 2 (ATF2) fusion proteins in the presence of ATP and kinase buffer, which allows immunoprecipitated active p38 MAPK to phosphorylate ATF2. Phosphorylated ATF2 was assessed by Western blotting with phospho-ATF2 (Thr71) antibody (provided in the p38 MAPK activity assay kit, Cell Signaling Technology, Inc., Beverly, MA).
Western blotting Analysis
Western blotting was performed as described previously (Jia et al, 2007, 2009; Johnson et al, 2008; Vera et al, 2006). Endogenous rat HN was distinguished from exogenously administered synthetic HN because the anti-rattin antibody (Fitzgerald Industries Intl, Acton, MA) does not cross react with HN (data not shown). The predicted molecular weight of rat HN is 5 kDa compared with 2.7 kDa for human HN.
Proteins were denatured and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Invitrogen, Carlsbad, CA). After transferring, the immuno-blot PVDF membrane (Bio-Rad, Hercules, CA) was blocked for 1 h and then probed using anti-STAT3, pTyr705 STAT3, or pSer727 STAT3 antibody (Cell Signaling Technology, Inc., Beverly, MA); anti-rattin antibody (Fitzgerald Industries Intl, Acton, MA); anti-COX IV antibody (Cell Signaling Technology, Inc., Beverly, MA); anti-BAX and GAPDH antibody (Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C with constant shaking. After washing, the membrane was incubated with anti-mouse (Santa Cruz Biotechnology, Santa Cruz, CA) or anti-rabbit (Amersham Biosciences, Piscataway, NJ) IgG-HRP secondary antibody. All antibodies were diluted in blocking buffer. For immunodetection, membranes were incubated with enhanced chemiluminescence solutions per the manufacturer’s specifications (Amersham Biosciences, Piscataway, NJ), and exposed to Hyperfilm ECL (Denville Scientific Inc., Metuchen, NJ). Band density was determined using Bio-Rad Quantity One software (Hercules, CA). The numerical data from each sample was collected using the ratio of the band density of interest to that of loading control on each line of the immunoblots. The mean levels of the numerical data from samples from different treatment were compared by statistical analysis as described below. COX IV was used as loading control (normalization factor in density analysis) for BAX or rat HN expression in mitochondrial fraction and GAPDH was used as loading control (normalization factor in density analysis) for BAX expression in cytosolic fraction and STAT3 or rat HN expression in total lysate. ATF2 was used as loading control (normalization factor in density analysis) for pATF2 in p38MAPK activity assay.
Co-Immunoprecipitation
Co-immunoprecipitation (Co-IP) of rattin with anti-BAX antibody (Santa Cruz Biotechnology, Santa Cruz, CA) in the cytosolic and mitochondrial fractions was performed using the ExactaCruz™ F kit (Santa Cruz Biotechnology). Following incubation of the antibody against BAX with the immunoprecipitation matrix, the mitochondrial or cytosolic fractions in the matrix were pelleted by microcentrifugation at maximum speed for 30 seconds at 4°C and washed twice with 500 μl of PBS. After the final wash of the IP antibody-IP matrix complex, 1000 μg of cytosolic or mitochondrial fractions of testis lysate was added to the pelleted matrix and incubated at 4°C on a rotator overnight. The mixture was again pelleted by micro-centrifugation at maximum speed for 30 seconds at 4°C, washed three times with RIPA lysis buffer, and resuspended in 40 μl of reducing 2x Electrophoresis Sample Buffer (Santa Cruz Biotechnology, Santa Cruz, CA). After boiling samples for 3 minutes the supernatant was loaded onto a gel for electrophoresis. After incubation with anti-rattin antibody and secondary antibody, bands were visualized using the corresponding horseradish peroxidase (HRP) conjugated ExactaCruz™ F reagents and the enhanced chemiluminescence solutions per the manufacturer’s specifications (Amersham Biosciences, Piscataway, NJ).
Double Immunofluorescence Staining
Co-localization of BAX and rat HN in the testis was detected by confocal microscopy using double immunostaining as described (Johnson et al, 2008). In brief, after deparaffinization and rehydration, tissue sections were first incubated with the anti-BAX antibody (Santa Cruz Biotechnology, Santa Cruz, CA, mouse monoclonal, 1:100) and followed by FITC anti-mouse secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Then after incubation with an anti-rat HN antibody (Fitzgerald Industries Intl, Acton, MA, rabbit polyclonal, 1:100), the sections were probed with Alexa 633 anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA). For negative controls, sections were treated only with secondary antibody. Confocal imaging was performed using a Leica TCS-SP-MP confocal microscope equipped with a 488 nm argon laser for excitation of green fluorophores and a 633 nm helium-neon laser for excitation of red flurophores.
Statistical Analysis
Statistical analyses were performed using the SigmaStat 2.0 Program (Jandel Cooperation, San Rafael, CA). The Student-Newman-Keuls test after one-way repeated measures ANOVA was used to define statistical significance. Differences were considered significant if p<0.05.
RESULTS
Synthetic HN treatment attenuated GnRH-A induced apoptosis and BAX translocation from cytosol to mitochondria
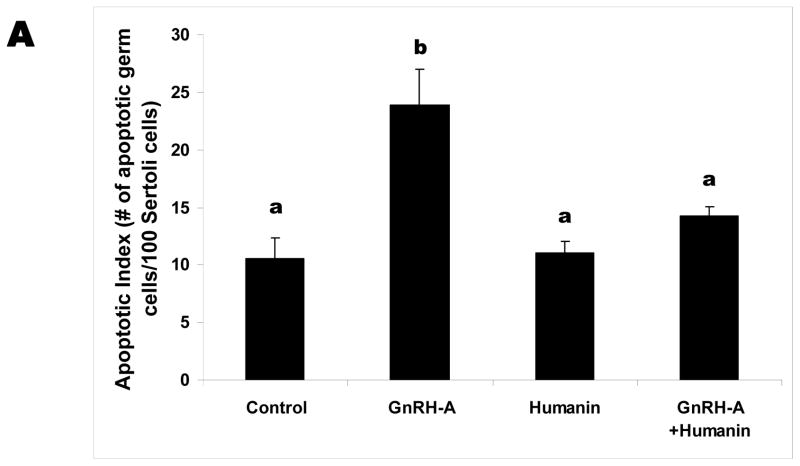
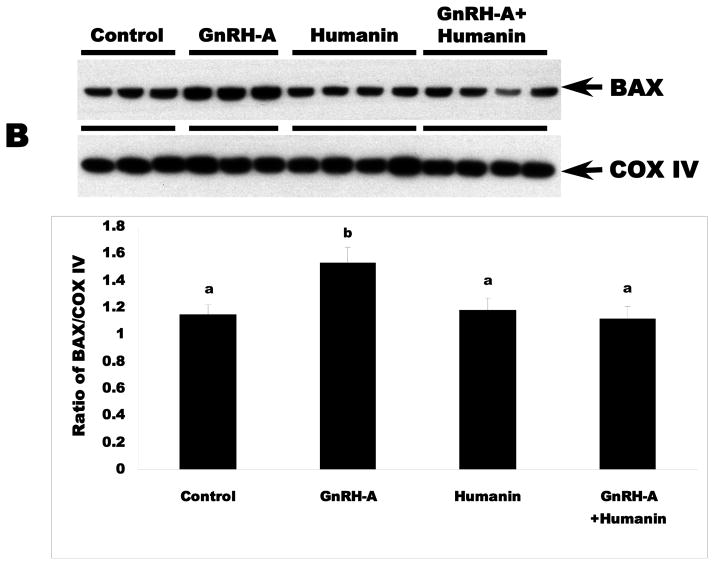
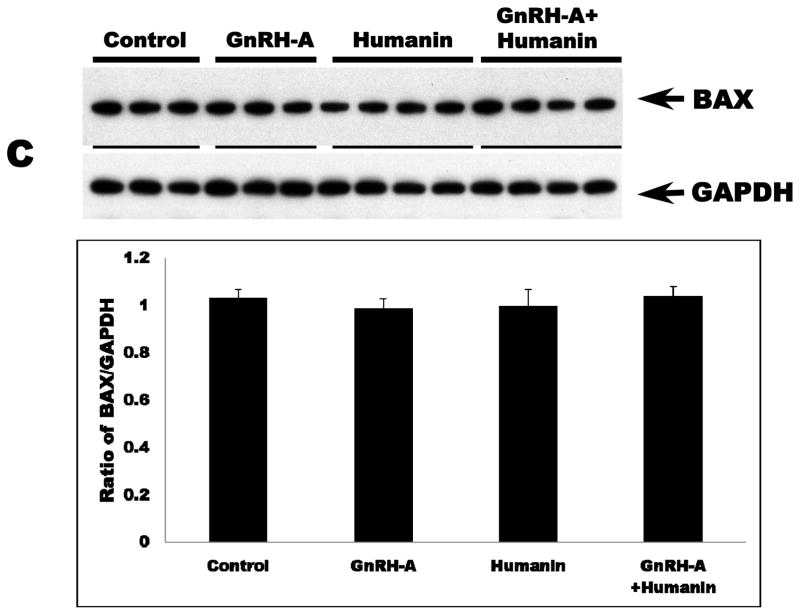
GnRH-A treatment increased germ cell apoptosis. Intratesticular administration of exogenous HN alone has no effect on the rate of germ cell apoptosis in the unstressed state, but ameliorated GnRH-A induced apoptosis (Fig. 1A). Consistent with our earlier study in male germ cells (Vera et al, 2006), GnRH-A induced apoptosis was not associated with changes in cytosolic BAX localization but was associated with increased BAX localization in the mitochondria (p<0.05) suggesting that the BAX translocation from cytosol to mitochondria is the key step in male germ cell apoptosis (Fig. 1B and C). Synthetic HN prevented GnRH-A induced BAX translocation to mitochondria to initiate the apoptosis cascade (Fig. 1B).
FIG. 1. Apoptosis induced by GnRH-A is associated with BAX translocation to the mitochondria and reversed by concomitant treatment with HN.
Germ cell apoptosis was quantified by TUNEL and expressed as apoptotic index (AI, number of apoptotic germ cells per 100 Sertoli cells)(A). The Western blots were used to assess the BAX expression in mitochondrial (B) and cytosolic (C) fractions in rat testis. A: GnRH-A treatment increased male germ cell apoptosis which is reduced by concomitant treatment with synthetic HN. Apoptosis is expressed as a number of apoptotic cells/Sertoli cells stained with TUNEL and counted manually. B: GnRH-A treatment increased BAX in the mitochondrial fractions. HN reversed the increased BAX observed with GnRH-A treatment. COX IV was used as loading control (normalization factor for density analysis in mitochondrial fractions). C: GnRH-A±HN treatment had no effect on BAX level in cytosolic fractions. GAPDH was used as loading control (normalization factor for density analysis). In each experiment, groups of 3 or 4 animals received treatment with vehicle (control, n=3), GnRH-A (n=3), HN (n=4), or GnRH-A+HN (n=4) as described in the methods. Values are means ± SD. Means with unlike superscripts are significantly (P<0.05) different.
Exogenous HN treatment increased STAT-3 phosphorylation but decreased p38 MAPK activity
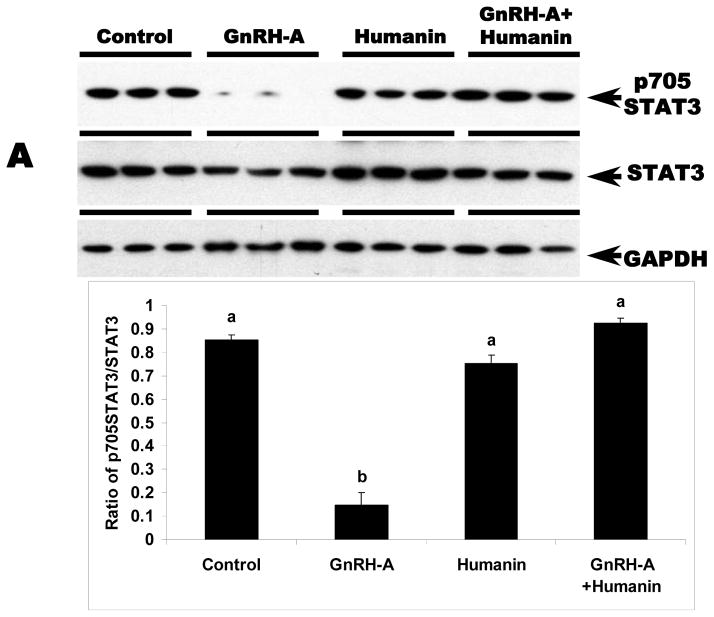
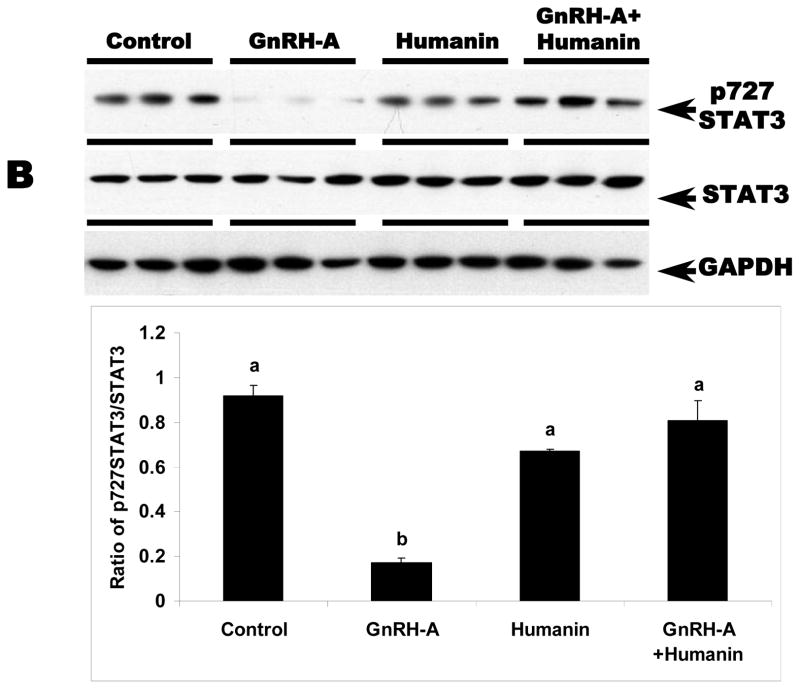
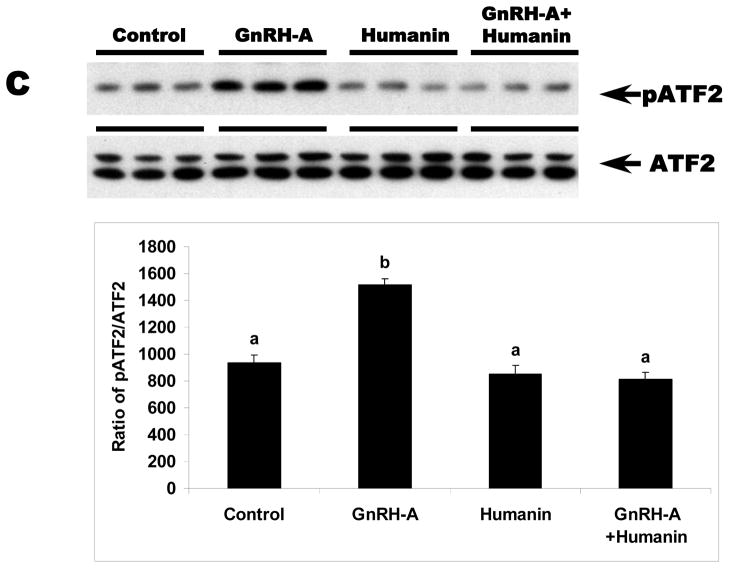
We examined whether HN exerts its cytoprotective action through STAT 3 phosphorylation pathway in germ cells similar to reports in neuronal cells (Chiba et al, 2009; Hashimoto et al, 2009; Matsuoka and Hashimoto, 2010). STAT3 is a transcriptional factor that is stimulated in response to cytokines and growth factors. STAT3 has two phosphorylation sites, Ser727 and Tyr705. Phosphorylation of these sites leads to activation of STAT3 (Schuringa et al, 2000). As expected, total STAT3 expression in the testis was not changed with any treatment (Fig. 2A and 2B). GnRH-A treatment suppressed both Ser727- and Tyr705-phosphorylated STAT3 in testes (p<0.05). Exogenous HN treatment restored STAT3 phosphorylation suppressed by GnRH-A in whole testis homogenates (p<0.05) (Fig 2A and 2B). Consistent with our earlier study that showed p38 MAPK is the key stress kinase that regulates male germ cell apoptosis, we demonstrated that GnRH-A increased p38 MAPK activity that was also attenuated by exogenous HN (p<0.05) (Fig. 2C).
FIG. 2. HN stimulated STAT3 phosphorylation and decreased p38 MAP kinase activation in rat testis.
Western blots for STAT3 phosphorylation and p38 MAPK activation in the rat testis total homogenates. A and B: GnRH-A treatment suppressed phosphorylation of STAT3 at both Tyr705 (A, top lane) and Ser727 (B, top lane). HN treatment restored both forms of phosphorylated STAT3. GAPDH was used as loading control (A, bottom lane and B, bottom lane), but STAT3 was used as normalization factor in density analysis (A, middle lane and B, middle lane). C: GnRH-A treatment induced activation of p38 MAPK which is reflected by phosphorylation of ATF2 in this assay. HN combined with GnRH-A treatment suppressed the increased p38 MAPK activation. ATF2 was used as sample control (normalization factor in density analysis). Values are means ± SD. Means with unlike superscripts are significantly (P<0.05) different.
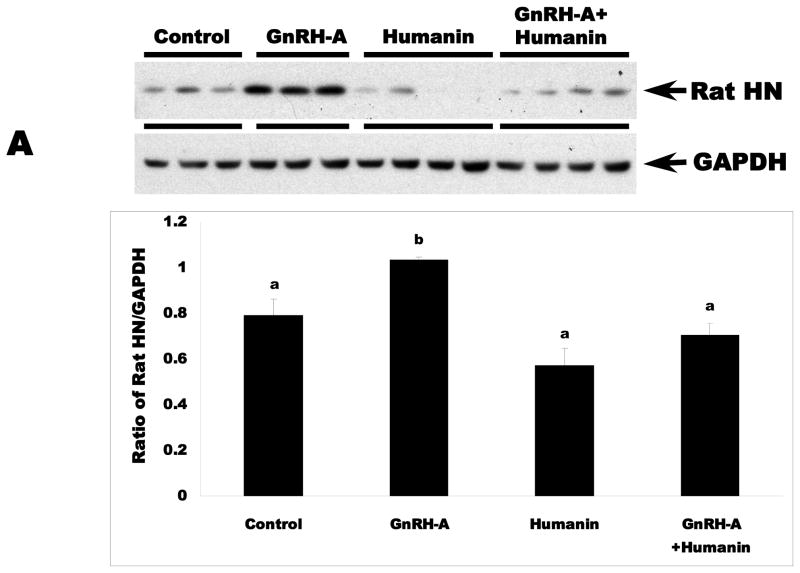
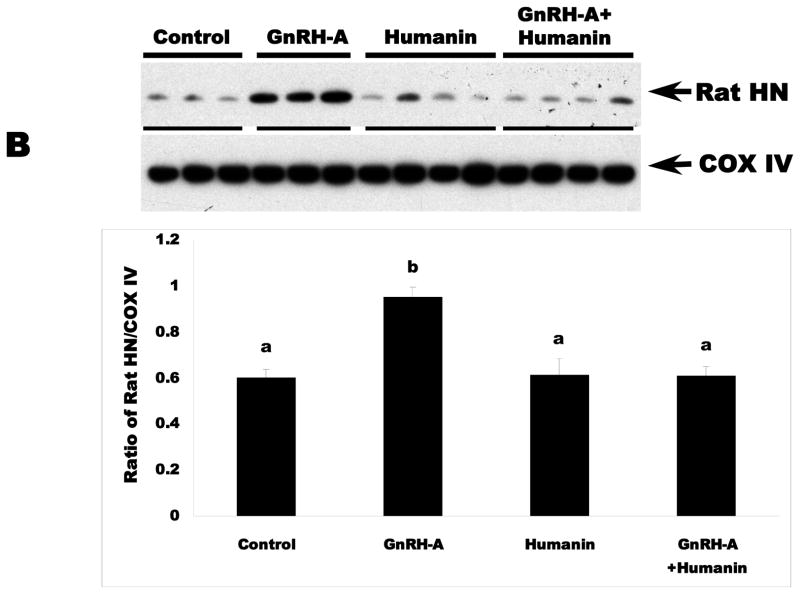
Endogenous rat HN protein expression is related to germ cell apoptosis in rat testis
To determine the role of endogenous rat HN in male germ cell apoptosis, we examined the expression of endogenous rat HN in fractionated testicular cytosol or mitochondria from control and treated rats by immunoblotting. Similar to unfractionated testicular homogenates (data not shown), GnRH-A treatment increased rat HN expression in the cytosolic (Fig. 2A) and mitochondrial fractions (Fig. 2B) as compared to controls (p<0.05). Intratesticular administration of HN suppressed endogenous rat HN expression to the control levels in the cytosolic and mitochondrial fractions of rats treated with GnRH-A. Thus, germ cell survival and death is associated with the modulation of endogenous rat HN expression in rat testis.
Rat HN associated with BAX was retained in the cytoplasm and undetectable in the mitochondria during germ cell apoptosis
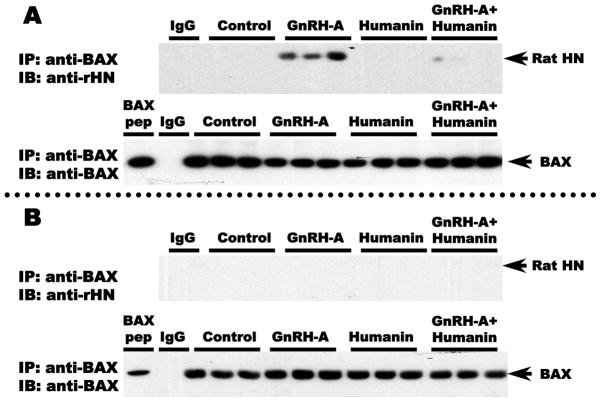
To determine the contribution of the interaction between endogenous rat HN and BAX to the cytoprotective effects of HN on germ cell apoptosis, we examined the association of these two peptides in the cytosolic and mitochondrial fractions of rat testicular extracts. Co-immunoprecipitation experiments with anti-BAX antibody showed that there was no detectable interaction between rat HN and BAX in both cytosolic and mitochondrial fractions in control testes. However, we observed that a rat HN:BAX complex was detectable in the cytosolic fraction in response to GnRH-A treatment that was associated with increased germ cell apoptosis (Fig. 3A). In contrast, rat HN:BAX complexes were not detected in the mitochondrial fractions of any testis sample (Fig. 3B), thus indicating that BAX associated with endogenous rat HN was retained in the cytosolic fractions and not present in any of the mitochondrial fractions.
FIG. 3. Endogenous rat HN was increased by GnRH-A, which was attenuated by concomitant exogenous HN treatment in cytosolic (A) and mitochondrial fractions (B) in rat testis.
Western blots for rat HN in cytosolic and mitochondrial fractions in rat testis. In both cytosolic (A, Upper panel) and mitochondrial (B, lower panel) testicular fractions, endogenous rat HN was low in the control group and increased after treatment with GnRH-A. Exogenous treatment with synthetic HN suppressed the GnRH-A induced increase in endogenous rat HN. GAPDH and COX IV were used as loading controls (used for normalization of data analysis) in cytosolic and mitochondrial fractions, respectively. Values are means ± SD. Means with unlike superscripts are significantly (P<0.05) different.
Co-localization of rat HN and BAX was detected in Leydig cell and spermatocytes after GnRH-A treatment
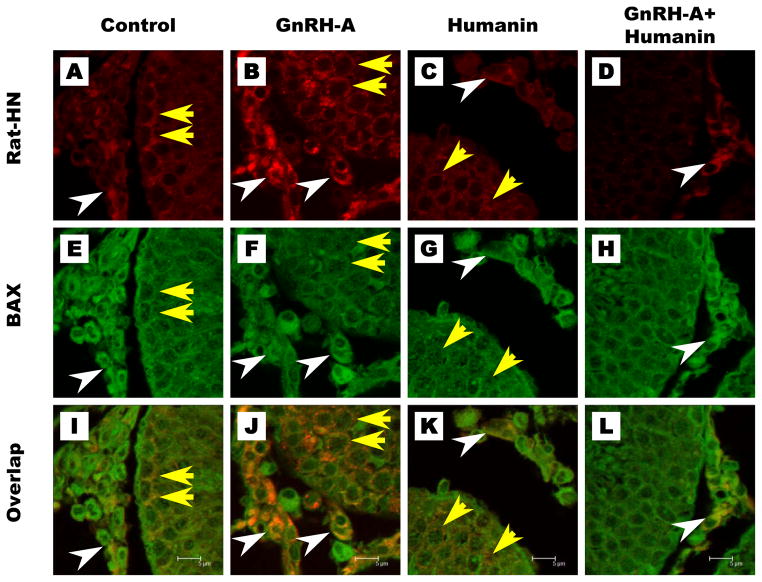
To demonstrate the interaction between endogenous rat HN and BAX in the rat, we used double immunofluorescence staining with rat HN and BAX antibodies on the testis sections examined using confocal microscopy. The confocal images showed that endogenous rat HN and Bax were localized in spermatocytes and Leydig cells of control testes (Fig. 4A, E, I). HN treatment alone did not change their expression (Fig. 4C, G, K). GnRH-A treatment markedly increased rat HN and Bax expression and colocalization in spermatocytes and Leydig cells (Fig. 4B, F, J), which was neutralized by co-treatment with HN (Fig. 4D, H, L). These data supported that interaction between BAX and endogenous rat HN increased in both Leydig cells and germ cells after pro-apoptotic stress in rat.
FIG. 4. Endogenous rat HN interacts with BAX in cytosolic but not mitochondrial fractions of rat testis.
Anti-BAX antibody or IgG (negative control for the immunoprecipitation, IP: IgG) was used to immunoprecipitate rat HN (IP: anti-BAX). Western blots were used to detect endogenous rat HN expression after anti-BAX IP (IB: anti-rat HN). Endogenous rat HN expression was not detected after IP with rabbit IgG in GnRH-A treated testis tissue (IgG, right lane). After anti-BAX IP, endogenous rat HN was detected in testis cytosolic fractions after treatment with GnRH-A but not in the control or synthetic HN treated groups; synthetic HN reduced the GnRH-A induced rat HN expression in the cytosol (A, upper panel). After anti-BAX IP, endogenous rat HN was undetectable in the mitochondrial fraction in the control and all treatment groups (B, lower panel). Endogenous BAX (IB: Anti-BAX) was detected in both testicular cytosolic (A, upper panel) and mitochondrial fractions (B, lower panel) after IP with anti-BAX antibody (IP: Anti-BAX).
DISCUSSION
We have previously demonstrated that HN, a broad-spectrum cytoprotective factor (Bachar et al, 2010; Chiba et al, 2004; Hashimoto et al, 2001; Hoang et al, 2010; Jung and Van Nostrand, 2003; Kariya et al, 2002, 2005; Lue et al, 2010; Matsuoka et al, 2004, 2009; Moretti et al, 2010; Muzumdar et al, 2010; Nishimoto et al, 2004; Wang et al, 2005; Xu et al, 2006, 2010), prevents GnRH-A induced male germ cell apoptosis in rats (Lue et al, 2010). HN is a peptide that inhibits Alzheimer Disease (AD)-related and non-AD-related neuronal cell death (Chiba et al, 2004, 2005; Hashimoto et al, 2001; Kariya et al, 2002, 2005; Matsuoka et al, 2004, 2009; Nishimoto et al, 2004; Xu et al, 2006, 2010). HN also ameliorates cognitive impairment in AD-relevant mouse models (Chiba et al, 2005, 2009; Krejcova et al, 2004; Kunesová et al, 2008; Mamiya et al, 2001; Miao et al, 2008; Tajima et al, 2005; Yamada et al, 2008). HN is a secreted protein that acts to inhibit AD-related death in vitro by binding to receptors on the cell membrane (Hashimoto et al, 2001). Hashimoto et al. described a novel HN receptor on the cell membrane that mediates neuroprotective activity. This trimeric receptor belongs to the IL-6 receptor family (with three components: CNTFR, IL-27 receptor WSX1, and gp130) and signals via STAT3 activation (Hashimoto et al, 2009; Matsuoka and Hashimoto, 2010).
BAX, a soluble pro-apoptotic member of the BCL-2 protein family, locates diffusely throughout the cytosol in healthy cells (Wolter et al. 1997). Translocation of BAX from cytosol to mitochondria is a key step to promote cell apoptosis (Wolter et al. 1997; Gross et al, 1998; Hsu and Youle, 1997). Our prior publications have shown that the mitochondria-dependent BAX/BCL-2 pathway is the main initiator for germ cell apoptosis cascade in the testis (Vera et al, 2006; Jia et al, 2007, 2009). The intracellular interaction of HN to inhibit apoptosis is mediated through binding to pro-apoptotic proteins of the BCL-2 family with a BH3 domain such as BAX, BIMEL, and BID. Prior studies (Guo, et al., 2003) have demonstrated that HN prevents the translocation of BAX from cytosol to mitochondria and suppresses cytochrome c release in vitro. HN also binds to BIMEL, a BH3 domain only protein, to prevent apoptosis (Luciano, et al., 2005). HN binds and nullifies BID activity by blocking its activation of BAX and BAK to prevent apoptosis (Zhai, et al., 2005). In this study, we provide in vivo evidence showing exogenous HN reversed the GnRH-A induced increases in BAX in mitochondrial fractions of the testis. This confirms our previous finding that BAX, through the mitochondria-dependent pathway, is the key pathway of HN cytoprotection in male germ cell apoptosis in the testis (Vera et al, 2006). One of the reasons why we did not find a decrease in the levels of BAX in cytosol is because abundant BAX protein is present in the cytosolic reservoir compared with the translocated BAX protein in mitochondrial fractions. So the decrease in cytosolic BAX level was too small compared to the vast pool of cytosolic BAX to be detected. Previous published work from our group studied GnRH-A induced germ cell apoptosis and showed consistently that there was an increase in mitochondrial BAX but no change of cytosolic BAX level in the GnRH-Antagonist treated compared with control animals (Hikim, et al., 2003, Vera, et al., 2006). It has been also been shown that conformational change of BAX induced by pro-apoptotic stimuli is the more important step occurring in the cytosol of cells undergoing apoptosis (Dewson et al., 2003). Thus as shown in prior studies from our group, it is not surprising to see BAX translocation to the mitochondria without observable changes in cytosol detected by Western blot.
The cytoprotective effect of HN has been reported to be mediated by phosphorylation of STAT3 (Chiba et al, 2009; Hashimoto et al, 2005, 2009). We showed that STAT3 phosphorylation (at both Ser727 and Tyr705) decreased after GnRH-A treatment leading to germ cell death, while HN restored both phosphorylated forms of STAT3 and rescued cells from apoptosis. We also reported previously that p38 MAPK is the main signaling pathway for germ cell apoptosis in the testis (Vera et al, 2006; Jia et al, 2009). Exogenous HN suppressed GnRH-A induced p38 MAPK activation but not ERK, confirming our previous finding that p38 MAPK is the key pathway of HN rescue action in male germ cell apoptosis in the testis. Our data supports that the cytoprotective effect of HN in male germ cell appeared to be mediated by suppressing p38 MAPK and increasing STAT3 signaling, events upstream of BAX translocation to the mitochondria to induce the apoptosis cascade. This suggests that the action of HN on rescuing germ cell apoptosis may result from HN binding to the cell membrane CNTFR/WSX1/gp130 receptors activating STAT3 pathway (Hashimoto et al, 2009; Matsuoka and Hashimoto, 2010).
We then investigated whether endogenous rat HN responds to inducers of germ cell death and to exogenous HN. We found increases in endogenous rat HN protein levels in the cytosol and mitochondria of the testes after GnRH-A treatment. This increase in rat HN within the testis was attenuated by exogenous administration of HN, concomitant with the amelioration of germ cell apoptosis induced by GnRH-A as previously reported (Lue et al, 2010). The mechanisms by which exogenous HN decreased the GnRH-A induced rise in endogenous rat HN is unclear. One possible explanation is that synthetic HN acts through the STAT-3 or p38 MAPK pathways to decrease GnRH-A induced apoptosis thereby decreasing the need for an increase in endogenous rat HN to bind to BAX. We have demonstrated in prior experiments that synthetic HN binds to BAX in vitro (Jia, et al., 2010). If synthetic HN can gain access to the cytosol to bind to BAX to prevent BAX from translocation to the mitochondria, endogenous HN will not be necessary to counteract the stress induced by hormonal deprivation caused by GnRH-A treatment. This might result in a decrease in endogenous HN. Our present data suggest that, as a response to germ cell stress induced by pro-apoptotic factors, endogenous HN is increased in an attempt to protect the cells from the exogenous insult.
With co-immunoprecipitation studies, we showed that BAX-associated rat HN was increased by GnRH-A treatment probably due to a compensatory effort to protect germ cells and Leydig cells against agents causing testicular stress. Alternatively, our results could be attributed solely to an increase in endogenous HN expression after GNRH-A treatment. Some BCL-2 family members (such as BCL-2) constitutively localize to the mitochondrial membrane whereas others such as BAX translocate from cytoplasm to mitochondria to activate the intrinsic apoptosis cascade (Cory and Adams, 2002; Danial and Korsmeyer 2004). Furthermore, insertion of BAX into mitochondrial membranes has been shown to play an essential role in releasing cytochrome c from the mitochondrial membrane space to the cytoplasm in various cell systems including the testis to initiate apoptosis (Eskes et al, 1998; Shimizu et al, 1999; Sinha Hikim et al, 2005). Data reported in this study show that rat HN bound to BAX was co-localized mainly in the cytoplasm of Leydig cells and germ cells. Importantly, HN:BAX complexes are sequestered in the cytoplasm but not in the mitochondria of testis homogenates when germ cell apoptosis is induced by GnRH-A. This suggests that the interaction between rat HN and BAX may prevent targeting of BAX to the mitochondria to initiate cell death. In this study we have validated and expanded a previous observation that HN binds BAX in vitro (Zhai et al, 2005; Luciano et al, 2005) to prevent apoptosis of neuronal cells in culture. We have shown for the first time that when BAX was associated with rat HN, HN:BAX complex is not translocated to the mitochondria in vivo in the testis. Combined with our published data that IGFBP-3 binds both HN (Ikonen et al, 2003) and BAX (Jia et al, 2010), we conclude that HN, BAX, and IGFBP-3 modulate each other’s actions to determine germ cell survival or apoptosis. Although the molecular mechanisms of the cytoprotective actions of HN have been demonstrated in somatic cells, the exact mechanism of HN action in germ cells is still being unraveled. This may include both the binding of HN to cell surface receptors to activate the signaling pathway via STAT-3 as well as intracellular interaction of HN with the BAX-BCL2 family of proteins.
In summary, we have demonstrated that exogenous HN exerts its cytoprotective effect to prevent testicular stress induced by GnRH-A by the suppression of HN:BAX complexes translocation from cytosol to mitochondria. Importantly, we have shown that endogenous HN is induced in stressed cells undergoing apoptosis. The endogenous HN homologue, rat HN, restricts BAX translocation to the mitochondria from the cytoplasm. This cytoplasmic sequestration may also play a role in the cytoprotective effect of HN in male germ cell apoptosis. HN may therefore be important in the regulation of germ cell homeostasis and could be targeted for the treatment of prevention of male fertility, development of male contraception, as well as testicular diseases such as germ cell tumors.
FIG. 5. Co-localization of endogenous rat HN and BAX in rat testis.
Rat HN expression (red) was low in control and HN groups (Fig. 5A, C), increased in germ cells (yellow arrows) and Leydig cells (white arrows) with GnRH-A treatment (Fig. 5B) which was reduced by concomitant treatment with HN(Fig. 5D). BAX (green) was detected in both Leydig cells and germ cells in all treatment groups (Fig. 5E–5H). Confocal microscopy showed co-localization of rat HN and BAX (orange) both in Leydig cells and germ cells mainly after GnRH-A treatment (Fig. 5J), which was decreased to control level (Fig. 5I) after GnRH-A+HN co-treatment (Fig. 5L).
Acknowledgments
We thank the UCLA Clinical and Translational Science Institute UCLA CTSI Grant UL1TR000124 for providing Confocal Microscopy Technology Core support for this study.
References
- Bachar AR, Scheffer L, Schroeder AS, Nakamura HK, Cobb LJ, Oh YK, Lerman LO, Pagano RE, Cohen P, Lerman A. Humanin is expressed in human vascular walls and has a cytoprotective effect against oxidized LDL-induced oxidative stress. Cardiovasc Res. 2010;88:360–366. doi: 10.1093/cvr/cvq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caricasole A, Bruno V, Cappuccio I, Melchiorri D, Copani A, Nicoletti F. A novel rat gene encoding a Humanin-like peptide endowed with broad neuroprotective activity. The FASEB Journal. 2002;16:1331–1333. doi: 10.1096/fj.02-0018fje. [DOI] [PubMed] [Google Scholar]
- Chiba T, Hashimoto Y, Tajima H, Yamada M, Kato R, Niikura T. Neuroprotective effect of activity-dependent neurotrophic factor against toxicity from familial amyotrophic lateral sclerosis-linked mutant SOD1 in vitro and in vivo. J Neurosci Res. 2004;78:542–552. doi: 10.1002/jnr.20305. [DOI] [PubMed] [Google Scholar]
- Chiba T, Yamada M, Hashimoto Y, Sato M, Sasabe J, Kita Y, Terashita K, Aiso S, Nishimoto I, Matsuoka M. Development of a femtomolar-acting Humanin derivative named colivelin by attaching activity-dependent neurotrophic factor to its N terminus: characterization of colivelin-mediated neuroprotection against Alzheimer’s disease-relevant insults in vitro and in vivo. J Neurosci. 2005;25:10252–10261. doi: 10.1523/JNEUROSCI.3348-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba T, Yamada M, Sasabe J, Terashita K, Shimoda M, Matsuoka M, Aiso S. Amyloid-beta causes memory impairment by disturbing the JAK2/STAT3 axis in hippocampal neurons. Mol Psychiatry. 2009;14:206–222. doi: 10.1038/mp.2008.105. [DOI] [PubMed] [Google Scholar]
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Dewson G, Snowden RT, Almond JB, Dyer MJ, Cohen GM. Conformational change and mitochondrial translocation of Bax accompany proteasome inhibitor-induced apoptosis of chronic lymphocytic leukemic cells. Oncogene. 2003;22:2643–2654. doi: 10.1038/sj.onc.1206326. [DOI] [PubMed] [Google Scholar]
- Eskes R, Antonsson B, Osen-Sand A, Montessuit S, Richter C, Sadoul R, Mazzei G, Nichols A, Martinou JC. Bax-induced cytochrome C release from mitochondria is independent of the permeability transition pore but highly dependent on Mg2+ ions. J Cell Biol. 1998;143:217–24. doi: 10.1083/jcb.143.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: paper wraps stone blunts scissors. Cell. 2000;102:1–4. doi: 10.1016/s0092-8674(00)00003-9. [DOI] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo B, Zhai D, Cabezas E, Welsh K, Nouraini S, Satterthwait AC, Reed JC. Humanin peptide suppresses apoptosis by interfering with Bax activation. Nature. 2003;423:456–461. doi: 10.1038/nature01627. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Kurita M, Aiso S, Nishimoto I, Matsuoka M. Humanin inhibits neuronal cell death by interacting with a cytokine receptor complex or complexes involving CNTF receptor α/WSX-1/gp130. Mol Biol Cell. 2009;20:2864–2873. doi: 10.1091/mbc.E09-02-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Niikura T, Tajima H, YasukawaT SH, Ito Y, Kita Y, Kawasumi M, Kouyama K, Doyu M, Sobue G, Koide T, Tsuji S, Lang J, Kurokawa K, Nishimoto I. A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer’s disease genes and Aβ. Proc Natl Acad Sci USA. 2001;98:6336–6341. doi: 10.1073/pnas.101133498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Suzuki H, Aiso S, Niikura T, Nishimoto I, Matsuoka M. Involvement of tyrosine kinases and STAT3 in Humanin-mediated neuroprotection. Life Sci. 2005;77:3092–3104. doi: 10.1016/j.lfs.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hoang PT, Park P, Cobb LJ, Paharkova-Vatchkova V, Hakimi M, Cohen P. The neurosurvival factor humanin inhibits beta-cell apoptosis via signal transducer and activator of transcription 3 activation and delays and ameliorates diabetes in nonobese diabetic mice. Metabolism. 2010;59:343–349. doi: 10.1016/j.metabol.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen M, Liu B, Hashimoto Y, Ma L, Lee KW, Niikura T, Nishimoto I, Cohen P. Interaction between the Alzheimer’s survival peptide Humanin and insulin-like growth factor-binding protein 3 regulates cell survival and apoptosis. Proc Natl Acad Sci USA. 2003;100:13042–13047. doi: 10.1073/pnas.2135111100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Castellanos J, Wang C, Sinha-Hikim I, Lue Y, Swerdloff RS, Sinha-Hikim AP. Mitogen-Activated Protein Kinase Signaling in Male Germ Cell Apoptosis in the Rat: Biol. Reprod. 2009;80:771–780. doi: 10.1095/biolreprod.108.072843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lee KW, Swerdloff R, Hwang D, Cobb LJ, Sinha Hikim A, Lue YH, Cohen P, Wang C. Interaction of insulin-like growth factor-binding protein-3 and BAX in mitochondria promotes male germ cell apoptosis. J Biol Chem. 2010;285:1726–1732. doi: 10.1074/jbc.M109.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Sinha Hikim AP, Swerdloff RS, Lue YH, Vera Y, Zhang XS, Hu ZY, Li YC, Liu YX, Wang C. Signaling pathways for germ cell death in adult Cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod. 2007;77:83–92. doi: 10.1095/biolreprod.106.058594. [DOI] [PubMed] [Google Scholar]
- Johnson C, Jia Y, Wang C, Lue YH, Swerdloff RS, Zhang XS, Hu ZY, Li YC, Liu YX, Hikim AP. Role of caspase 2 in apoptotic signaling in primate and murine germ cells. Biol Reprod. 2008;79:806–814. doi: 10.1095/biolreprod.108.068833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SS, Van Nostrand WE. Humanin rescues human cerebrovascular smooth muscle cells from Abeta-induced toxicity. J Neurochem. 2003;84:266–272. doi: 10.1046/j.1471-4159.2003.01524.x. [DOI] [PubMed] [Google Scholar]
- Kariya S, Hirano M, Nagai Y, Furiya Y, Fujikake N, Toda T. Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches. J Mol Neurosci. 2005;25:165–169. doi: 10.1385/JMN:25:2:165. [DOI] [PubMed] [Google Scholar]
- Kariya S, Takahashi N, Ooba N, Kawahara M, Nakayama H, Ueno S. Humanin inhibits cell death of serum-deprived PC12h cells. Neuro Report. 2002;13:903–907. doi: 10.1097/00001756-200205070-00034. [DOI] [PubMed] [Google Scholar]
- Krejcova G, Patocka J, Slaninova J. Effect of humanin analogues on experimentally induced impairment of spatial memory in rats. J Pept Sci. 2004;10:636–639. doi: 10.1002/psc.569. [DOI] [PubMed] [Google Scholar]
- Kunesová G, Hlavácek J, Patocka J, Evangelou A, Zikos C, Benaki D, Paravatou-Petsotas M, Pelecanou M, Livaniou E, Slaninova J. The multiple T-maze in vivo testing of the neuroprotective effect of humanin analogues. Peptides. 2008;29:1982–1987. doi: 10.1016/j.peptides.2008.06.019. [DOI] [PubMed] [Google Scholar]
- Luciano F, Zhai D, Zhu X, Bailly-Maitre B, Ricci JE, Satterthwait AC, Reed JC. Cytoprotective peptide humanin binds and inhibits proapoptotic Bcl-2/Bax family protein BimEL. J Biol Chem. 2005;280:15825–15835. doi: 10.1074/jbc.M413062200. [DOI] [PubMed] [Google Scholar]
- Lue Y, Swerdloff R, Liu Q, Mehta H, Hikim AS, Lee KW, Jia Y, Hwang D, Cobb LJ, Cohen P, Wang C. Opposing roles of insulin-like growth factor binding protein 3 and humanin in the regulation of testicular germ cell apoptosis. Endocrinology. 2010;151:350–357. doi: 10.1210/en.2009-0577. [DOI] [PubMed] [Google Scholar]
- Lue Y, Wang C, Liu YX, Hikim AP, Zhang XS, Ng CM, Hu ZY, Li YC, Leung A, Swerdloff RS. Transient testicular warming enhances the suppressive effect of testosterone on spermatogenesis in adult cynomolgus monkeys (Macaca fascicularis) J Clin Endocrinol Metab. 2006;91:539–545. doi: 10.1210/jc.2005-1808. [DOI] [PubMed] [Google Scholar]
- Lue YH, Sinha Hikim AP, Swerdloff RS, Im P, Taing KS, Bui T, Leung A, Wang C. Single exposure to heat induces stage-specific germ cell apoptosis in rats: role of intratesticular testosterone (T) on stage specificity. Endocrinology. 1999;140:1709–1717. doi: 10.1210/endo.140.4.6629. [DOI] [PubMed] [Google Scholar]
- Mamiya T, Ukai M. [Gly(14)]-Humanin improved the learning and memory impairment induced by scopolamine in vivo. Br J Pharmacol. 2001;134:1597–1599. doi: 10.1038/sj.bjp.0704429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Hashimoto Y, Aiso S, Nishimoto I. Humanin and colivelin: neuronal-death-suppressing peptides for Alzheimer’s disease and amyotrophic lateral sclerosis. CNS Drug Rev. 2004;12:113–122. doi: 10.1111/j.1527-3458.2006.00113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka M, Hashimoto Y. Humanin and the receptors for humanin. Mol Neurobiol. 2010;41:22–28. doi: 10.1007/s12035-009-8090-z. [DOI] [PubMed] [Google Scholar]
- Matsuoka M. Humanin: a defender against Alzheimer’s disease? Recent Pat CNS Drug Discov. 2009;4:37–42. doi: 10.2174/157488909787002609. [DOI] [PubMed] [Google Scholar]
- Miao J, Zhang W, Yin R, Liu R, Su C, Lei G, Li Z. S14G HN ameliorates Abeta25–35-induced behavioral deficits by reducing neuroinflammatory responses and apoptosis in mice. Neuropeptides. 2008;42:557–567. doi: 10.1016/j.npep.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Moretti E, Giannerini V, Rossini L, Matsuoka M, Trabalzini L, Collodel G. Immunolocalization of humanin in human sperm and testis. Fertil Steril. 2010;94(7):2888–2890. doi: 10.1016/j.fertnstert.2010.04.075. [DOI] [PubMed] [Google Scholar]
- Muzumdar RH, Huffman DM, Calvert JW, Jha S, Weinberg Y, Cui L, Nemkal A, Atzmon G, Klein L, Gundewar S, Ji SY, Lavu M, Predmore BL, Lefer DJ. Acute humanin therapy attenuates myocardial ischemia and reperfusion injury in mice. Arterioscler Thromb Vasc Biol. 2010;30:1940–1948. doi: 10.1161/ATVBAHA.110.205997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I, Matsuoka M, Niikura T. Unravelling the role of Humanin. Trends Mol Med. 2004;10:102–105. doi: 10.1016/j.molmed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Reed JC. Mechanisms of apoptosis. Am J Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruwanpura SM, McLachlan RI, Stanton PG, Loveland KL, Meachem SJ. Pathways involved in testicular germ cell apoptosis in immature rats after FSH suppression. J Endocrinol. 2008;197:35–43. doi: 10.1677/JOE-07-0637. [DOI] [PubMed] [Google Scholar]
- Schuringa JJ, Wierenga ATJ, Kruijer W, Vellenga E. Constitutive Stat3, Tyr705, and Ser727 phosphorylation in acute myeloid leukemia cells caused by the autocrine secretion of interleukin-6. Blood. 2000;95:3765–3770. [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Rajavashisth TB, Sinha HI, Lue Y, Bonavera JJ, Leung A, Wang C, Swerdloff RS. Significance of apoptosis in the temporal and stage specific loss of germ cells in the adult rat after gonadotropin deprivation. Biol Reprod. 2005;57:1193–1201. doi: 10.1095/biolreprod57.5.1193. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Lue Y, Diaz-Romero M, Yen PH, Wang C, Swerdloff RS. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003a;85:175–182. doi: 10.1016/s0960-0760(03)00193-6. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Lue Y, Yamamoto CM, Vera Y, Rodriguez S, Yen PH, Soeng K, Wang C, Swerdloff RS. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology. 2003b;144:3167–3175. doi: 10.1210/en.2003-0175. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Swerdloff RS. Temporal and stage-specific changes in spermatogenesis of rat after gonadotropin deprivation by a potent gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1993;133:2161–2170. doi: 10.1210/endo.133.5.8404667. [DOI] [PubMed] [Google Scholar]
- Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Involvement of apoptosis in the induction of germ cell degeneration in adult rats after gonadotropin-releasing hormone antagonist treatment. Endocrinology. 1995;136:2770–2775. doi: 10.1210/endo.136.6.7750502. [DOI] [PubMed] [Google Scholar]
- Tajima H, Kawasumi M, Chiba T, Yamada M, Yamashita K, Nawa M, Kita Y, Kouyama K, Aiso S, Matsuoka M, Niikura T, Nishimoto I. A humanin derivative, S14G-HN, prevents amyloid-beta-induced memory impairment in mice. J Neurosci Res. 2005;79:714–723. doi: 10.1002/jnr.20391. [DOI] [PubMed] [Google Scholar]
- Vera Y, Diaz-Romero M, Rodriguez S, Lue Y, Wang C, Swerdloff RS, Sinha Hikim AP. Mitochondria-dependent pathway is involved in heat-induced male germ cell death: Lessons from mutant mice. Biol Reprod. 2004;70:1534–1540. doi: 10.1095/biolreprod.103.024661. [DOI] [PubMed] [Google Scholar]
- Vera Y, Erkkila K, Wang C, Nunez C, Kyttanen S, Lue Y, Dunkel L, Swerdloff RS, Sinha Hikim AP. Involvement of p38 mitogen-activated protein kinase and inducible nitric oxide synthase in apoptotic signaling of murine and human male germ cells after hormone deprivation. Mol Endocrinol. 2006;20:1597–1609. doi: 10.1210/me.2005-0395. [DOI] [PubMed] [Google Scholar]
- Wang C, Cui YG, Wang XH, Jia Y, Sinha HA, Lue YH, Tong JS, Qian LX, Sha JH, Zhou ZM, Hull L, Leung A, Swerdloff RS. Transient Scrotal Hyperthermia and Levonorgestrel Enhance Testosterone-Induced Spermatogenesis Suppression in Men through Increased Germ Cell Apoptosis. J Clin Endocrinol Metab. 2007;92:3292–3304. doi: 10.1210/jc.2007-0367. [DOI] [PubMed] [Google Scholar]
- Wang D, Li H, Yuan H, et al. Humanin delays apoptosis in K562 cells by downregulation of P38 MAP kinase. Apoptosis. 2005;10:963–971. doi: 10.1007/s10495-005-1191-x. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Chua CC, Gao J, Hamdy RC, Chua BH. Humanin is a novel neuroprotective agent against stroke. Stroke. 2006;37:2613–2619. doi: 10.1161/01.STR.0000242772.94277.1f. [DOI] [PubMed] [Google Scholar]
- Xu X, Chua KW, Chua CC, et al. Synergistic protective effects of humanin and necrostatin-1 on hypoxia and ischemia/reperfusion injury. Brain Res. 2010;1355:189–94. doi: 10.1016/j.brainres.2010.07.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Chiba T, Sasabe J, Terashita K, Aiso S, Matsuoka M. Nasal colivelin treatment ameliorates memory impairment related to Alzheimer’s disease. Neuropsychopharmaco. 2008;33:2020–2032. doi: 10.1038/sj.npp.1301591. [DOI] [PubMed] [Google Scholar]
- Zhai D, Luciano F, Zhu X, Guo B, Satterthwait AC, Reed JC. Humanin binds and nullifies Bid activity by blocking its activation of Bax and Bak. J Biol Chem. 2005;280:15815–15824. doi: 10.1074/jbc.M411902200. [DOI] [PubMed] [Google Scholar]