Abstract
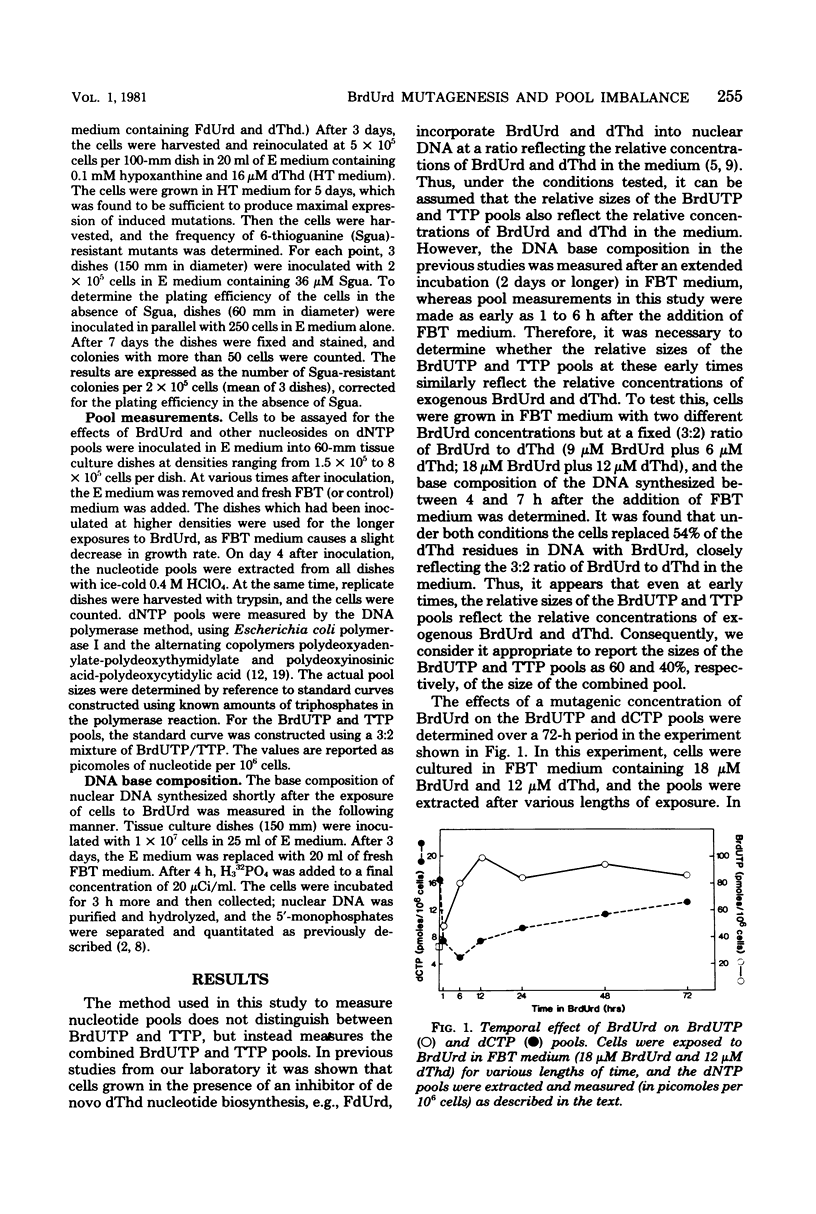
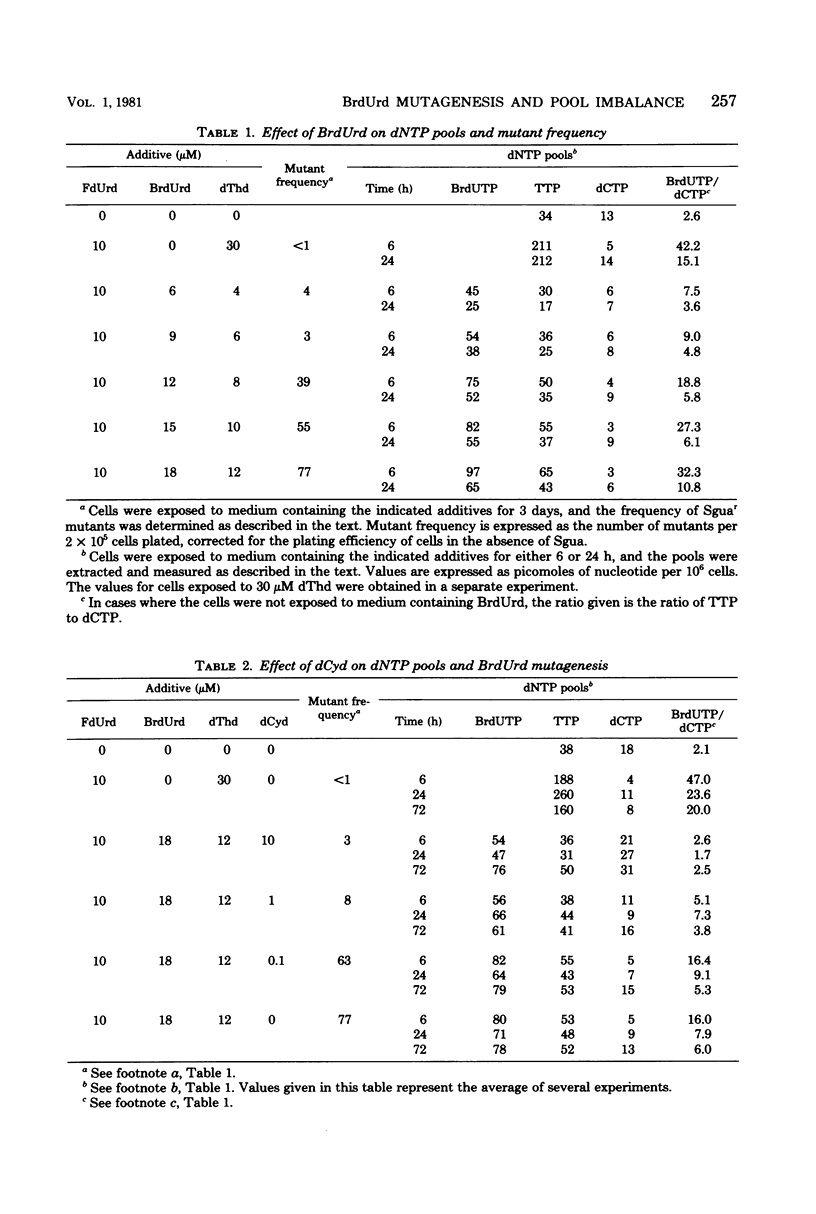
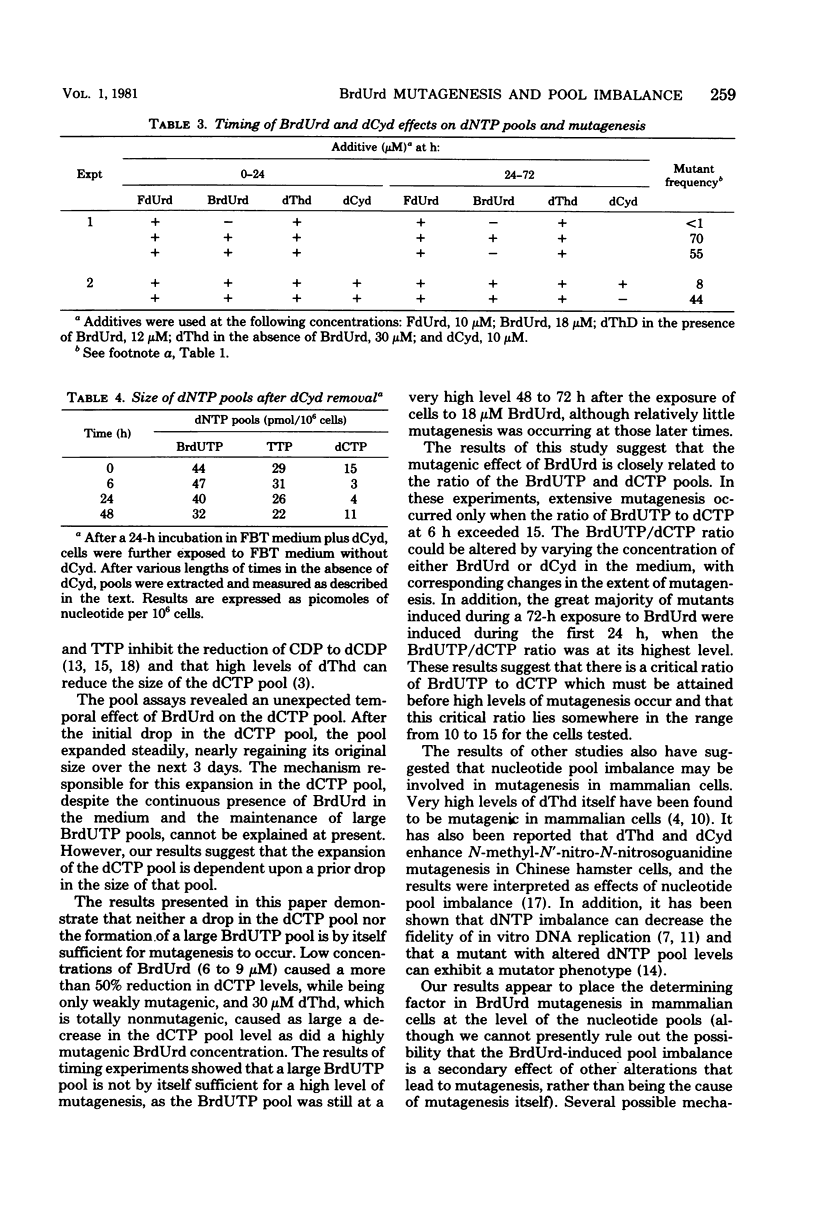
The relationship between bromodeoxyuridine (BrdUrd) mutagenesis in mammalian cells and the effects of BrdUrd on deoxyribonucleoside triphosphate pools was analyzed. It was found that the exposure of Syrian hamster melanoma cells to mutagenic concentrations of BrdUrd resulted in the formation of a large bromodeoxyuridine triphosphate (BrdUTP) pool, which remained at a high level for several days. In contrast, the size of the deoxycytidine triphosphate (dCTP) pool dropped rapidly after the addition of BrdUrd, reached a minimum at about 6 h, and then expanded gradually to nearly its original level over the next 3 days. The addition of lower concentrations of BrdUrd, which had less of a mutagenic effect, resulted in the formation of a smaller BrdUTP pool and a slightly smaller drop in the dCTP pool. When a high concentration of deoxycytidine was added at the same time as a normally mutagenic concentration of BrdUrd, the drop in the dCTP pool was prevented, as was BrdUrd mutagenesis. In all of these experiments, mutagenesis was related to the ratio of BrdUTP to dCTP in the cells. In addition, it was shown that mutagenesis occurred primarily during the first 24 h of BrdUrd exposure, when the BrdUTP/dCTP ratio was at its highest level. It appears that there is a critical ratio of BrdUTP to dCTP that must be attained for high levels of mutagenesis to occur and that the extent of mutagenesis is related to the ratio of the BrdUrd and dCTP pools.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold P. M. Mutagenic mechanism of 5-bromodeoxyuridine in Chinese hamster cells. Mutat Res. 1976 Sep;36(3):357–362. doi: 10.1016/0027-5107(76)90245-1. [DOI] [PubMed] [Google Scholar]
- Bick M. D., Davidson R. L. Total substitution of bromodeoxyuridine for thymidine in the DNA of a bromodeoxyuridine-dependent cell line. Proc Natl Acad Sci U S A. 1974 May;71(5):2082–2086. doi: 10.1073/pnas.71.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G., Reichard P. Effects of thymidine on deoxyribonucleoside triphosphate pools and deoxyribonucleic acid synthesis in Chinese hamster ovary cells. J Biol Chem. 1973 Jun 10;248(11):3904–3909. [PubMed] [Google Scholar]
- Bradley M. O., Sharkey N. A. Mutagenicity of thymidine to cultured Chinese hamster cells. Nature. 1978 Aug 10;274(5671):607–608. doi: 10.1038/274607a0. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R. Bromodeoxyuridine mutagenesis in mammalian cells is stimulated by thymidine and suppressed by deoxycytidine. Nature. 1978 Dec 14;276(5689):722–723. doi: 10.1038/276722a0. [DOI] [PubMed] [Google Scholar]
- Davidson R. L., Kaufman E. R., Dougherty C. P., Ouellette A. M., DiFolco C. M., Latt S. A. Induction of sister chromatid exchanges by BUdR is largely independent of the BUdR content of DNA. Nature. 1980 Mar 6;284(5751):74–76. doi: 10.1038/284074a0. [DOI] [PubMed] [Google Scholar]
- Fersht A. R. Fidelity of replication of phage phi X174 DNA by DNA polymerase III holoenzyme: spontaneous mutation by misincorporation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4946–4950. doi: 10.1073/pnas.76.10.4946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E. R., Davidson R. L. Bromodeoxyuridine mutagenesis in mammalian cells is stimulated by purine deoxyribonucleosides. Somatic Cell Genet. 1979 Sep;5(5):653–663. doi: 10.1007/BF01542701. [DOI] [PubMed] [Google Scholar]
- Kaufman E. R., Davidson R. L. Bromodeoxyuridine mutagenesis in mammalian cells: mutagenesis is independent of the amount of bromouracil in DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4982–4986. doi: 10.1073/pnas.75.10.4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman E. R., Davidson R. L. Novel phenotypes arising from selection of hamster melanoma cells for resistance to BUdR. Exp Cell Res. 1977 Jun;107(1):15–24. doi: 10.1016/0014-4827(77)90380-9. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Lindberg U., Skoog L. A method for the determination of dATP and dTTP in picomole amounts. Anal Biochem. 1970 Mar;34:152–160. doi: 10.1016/0003-2697(70)90096-5. [DOI] [PubMed] [Google Scholar]
- MOORE G. E. IN VITRO CULTURES OF A PIGMENTED HAMSTER MELANOMA CELL LINE. Exp Cell Res. 1964 Nov;36:422–423. doi: 10.1016/0014-4827(64)90224-1. [DOI] [PubMed] [Google Scholar]
- Meuth M., Green H. Induction of a deoxycytidineless state in cultured mammalian cells by bromodeoxyuridine. Cell. 1974 Jun;2(2):109–112. doi: 10.1016/0092-8674(74)90099-3. [DOI] [PubMed] [Google Scholar]
- Meuth M., L'Heureux-Huard N., Trudel M. Characterization of a mutator gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6505–6509. doi: 10.1073/pnas.76.12.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore E. C., Hurlbert R. B. Regulation of mammalian deoxyribonucleotide biosynthesis by nucleotides as activators and inhibitors. J Biol Chem. 1966 Oct 25;241(20):4802–4809. [PubMed] [Google Scholar]
- Peterson A. R., Landolph J. R., Peterson H., Heidelberger C. Mutagenesis of Chinese hamster cells is facilitated by thymidine and deoxycytidine. Nature. 1978 Nov 30;276(5687):508–510. doi: 10.1038/276508a0. [DOI] [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Skoog L. An enzymatic method for the determination of dCTP and dGTP in picomole amounts. Eur J Biochem. 1970 Dec;17(2):202–208. doi: 10.1111/j.1432-1033.1970.tb01154.x. [DOI] [PubMed] [Google Scholar]


