Abstract
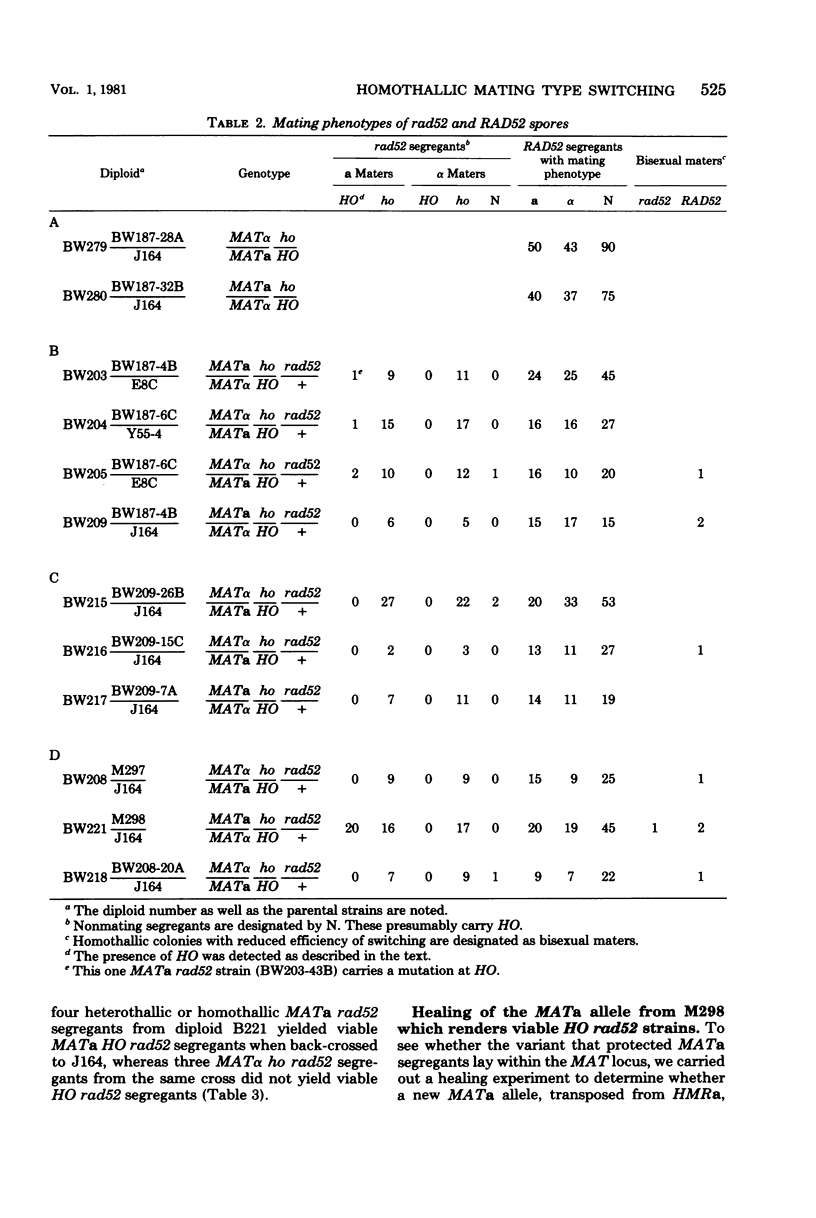
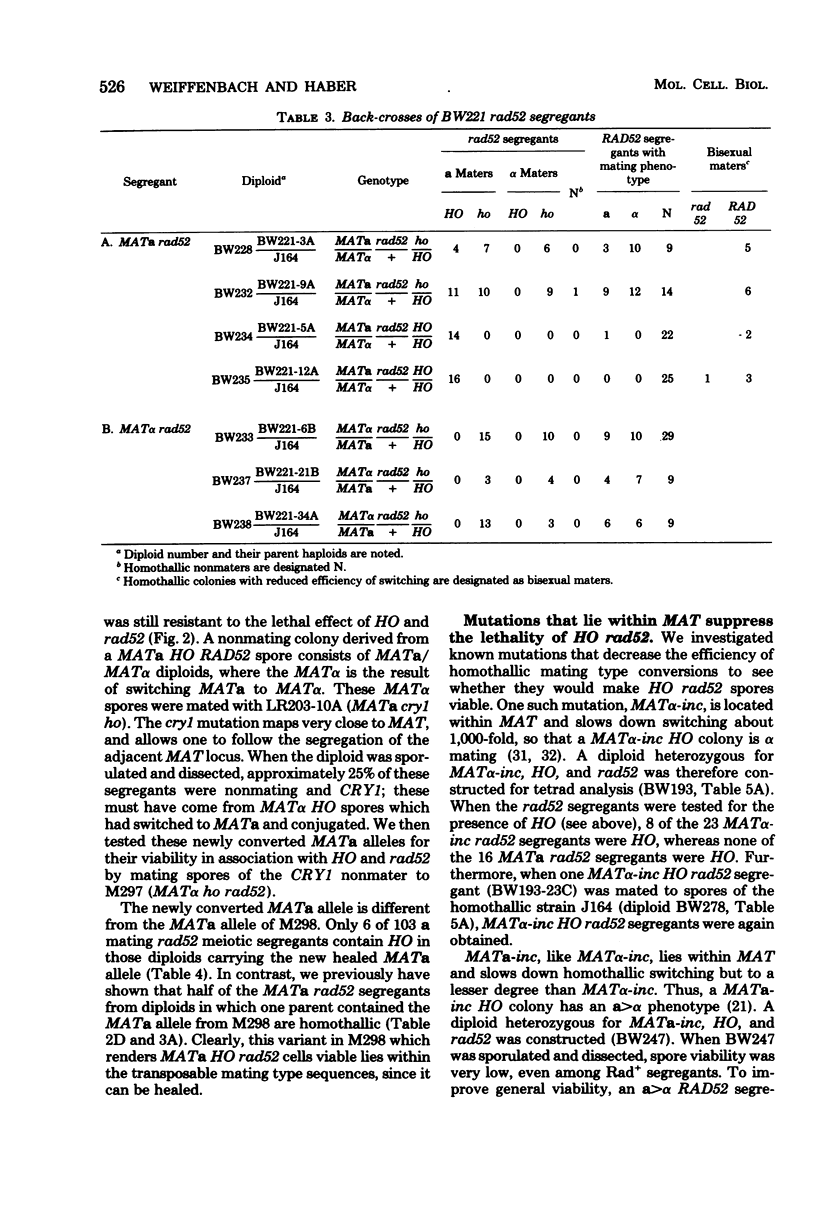
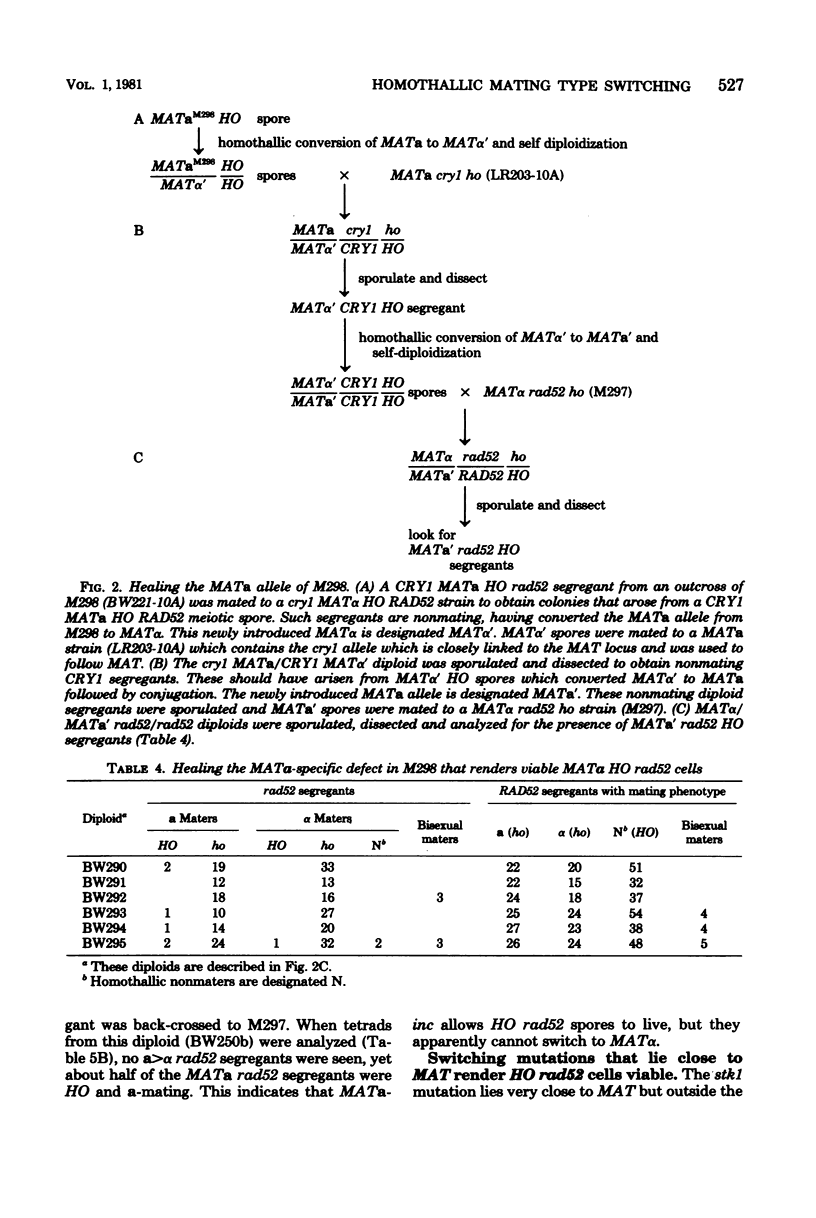
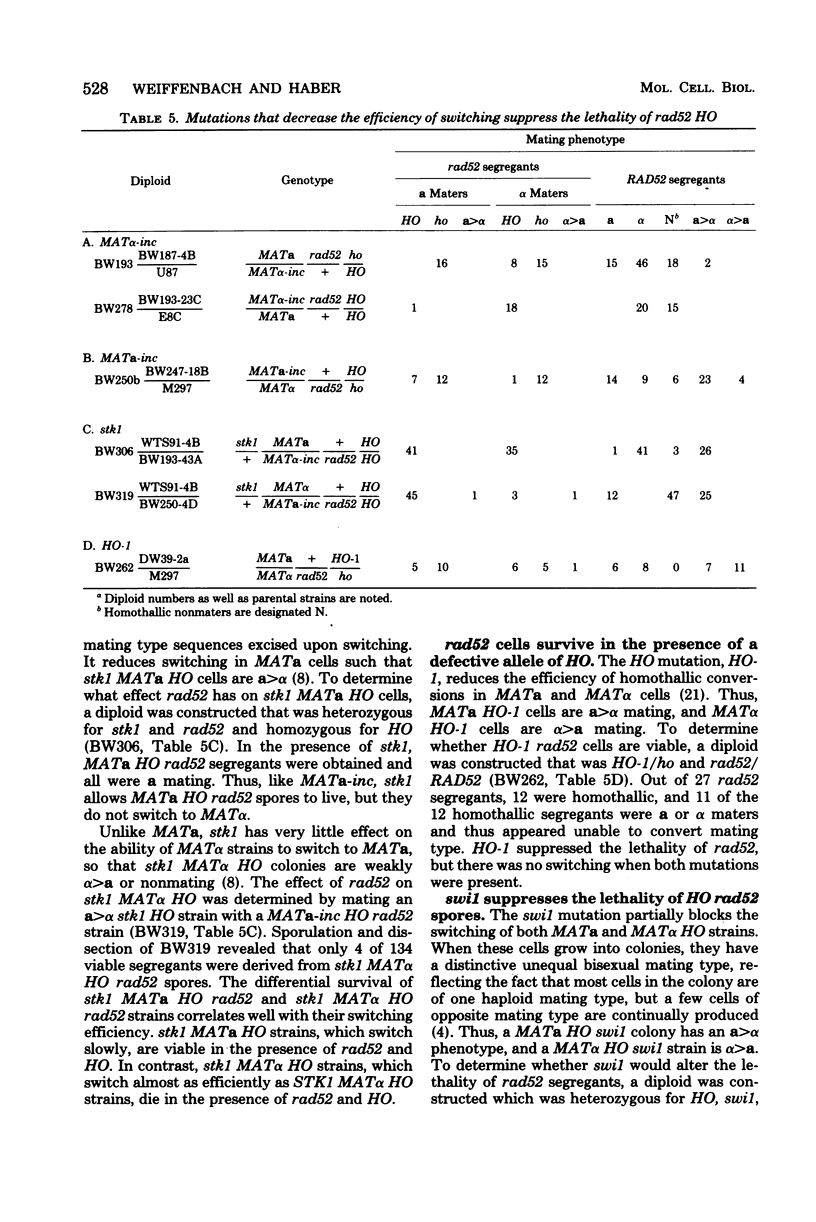
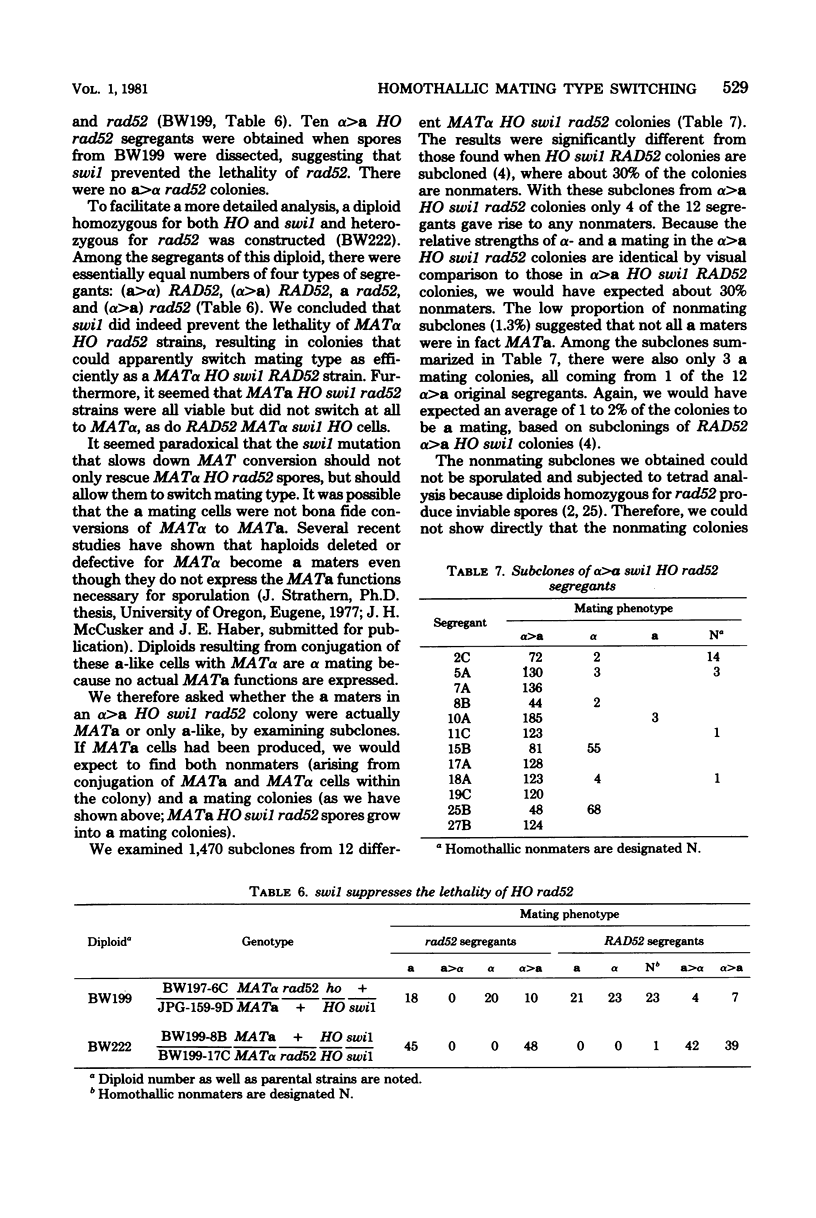
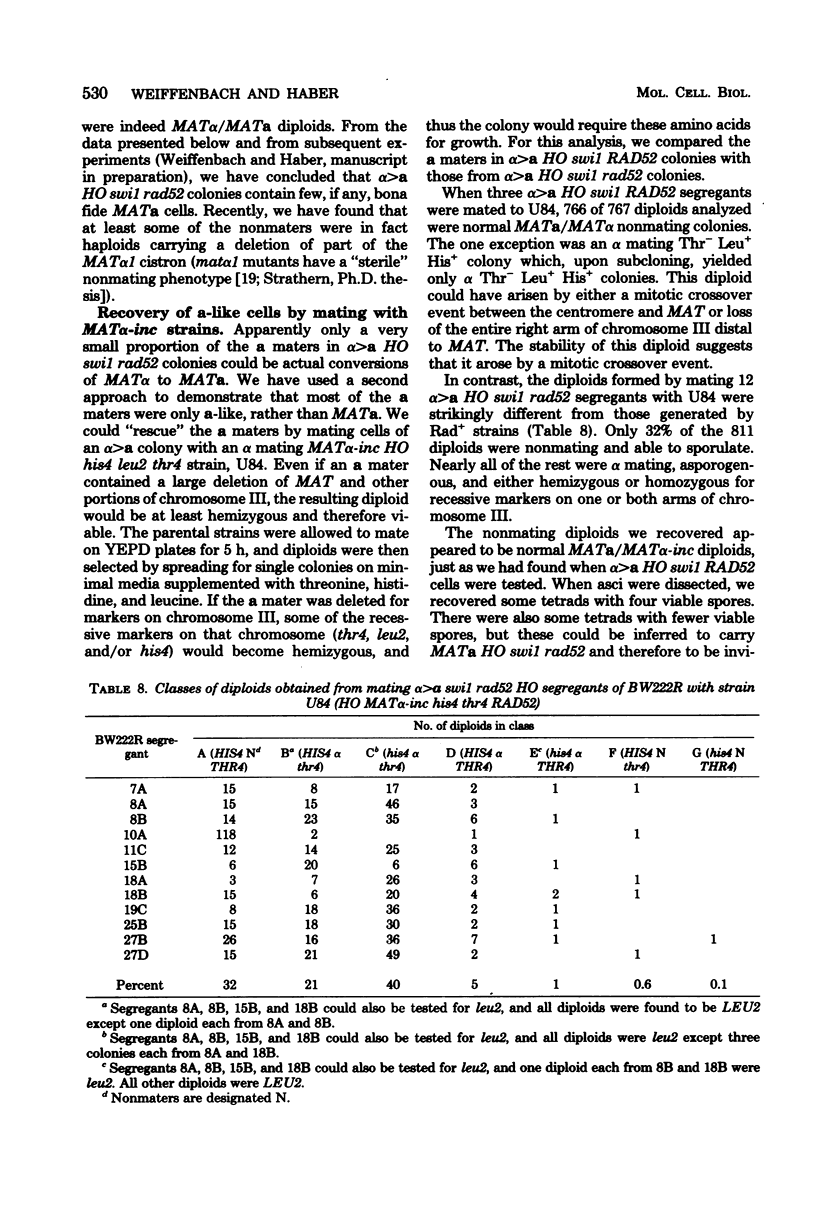
In homothallic cells of Saccharomyces cerevisiae, a or alpha mating type information at the mating type locus (MAT) is replaced by the transposition of the opposite mating type allele from HML alpha or HMRa. The rad52-1 mutation, which reduces mitotic and abolishes meiotic recombination, also affects homothallic switching (Malone and Esposito, Proc. Natl. Acad. Sci. U.S.A. 77:503-507, 1980). We have found that both HO rad52 MATa and HO rad52 MAT alpha cells die. This lethality is suppressed by mutations that substantially reduce but do not eliminate homothallic conversions. These mutations map at or near the MAT locus (MAT alpha inc, MATa-inc, MATa stk1) or are unlinked to MAT (HO-1 and swi1). These results suggest that the switching event itself is involved in the lethality. With the exception of swi1, HO rad52 strains carrying one of the above mutations cannot convert mating type at all. MAT alpha rad52 HO swi1 strains apparently can switch MAT alpha to MATa. However, when we analyzed these a maters, we found that few, if any, of them were bona fide MATa cells. These a-like cells were instead either deleted for part of chromosome III distal to and including MAT or had lost the entire third chromosome. Approximately 30% of the time, an a-like cell could be repaired to a normal MATa genotype if the cell was mated to a RAD52 MAT alpha-inc strain. The effects of rad52 were also studied in mata/MAT alpha-inc rad52/rad52 ho/HO diploids. When this diploid attempted to switch mata to MATa, an unstable broken chromosome was generated in nearly every cell. These studies suggest that homothallic switching involves the formation of a double-stranded deoxyribonucleic acid break or a structure which is labile in rad52 cells and results in a broken chromosome. We propose that the production of a double-stranded deoxyribonucleic acid break is the lethal event in rad52 HO cells.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Game J. C., Mortimer R. K. A genetic study of x-ray sensitive mutants in yeast. Mutat Res. 1974 Sep;24(3):281–292. doi: 10.1016/0027-5107(74)90176-6. [DOI] [PubMed] [Google Scholar]
- Game J. C., Zamb T. J., Braun R. J., Resnick M., Roth R. M. The Role of Radiation (rad) Genes in Meiotic Recombination in Yeast. Genetics. 1980 Jan;94(1):51–68. doi: 10.1093/genetics/94.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAWTHORNE D. C. A DELETION IN YEAST AND ITS BEARING ON THE STRUCTURE OF THE MATING TYPE LOCUS. Genetics. 1963 Dec;48:1727–1729. doi: 10.1093/genetics/48.12.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Garvik B. A new gene affecting the efficiency of mating-type interconversions in homothallic strains of Saccharomyces cerevisiae. Genetics. 1977 Sep;87(1):33–50. doi: 10.1093/genetics/87.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., George J. P. A mutation that permits the expression of normally silent copies of mating-type information in Saccharomyces cerevisiae. Genetics. 1979 Sep;93(1):13–35. doi: 10.1093/genetics/93.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Mascioli D. W., Rogers D. T. Illegal transposition of mating-type genes in yeast. Cell. 1980 Jun;20(2):519–528. doi: 10.1016/0092-8674(80)90638-8. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Rogers D. T., McCusker J. H. Homothallic conversions of yeast mating-type genes occur by intrachromosomal recombination. Cell. 1980 Nov;22(1 Pt 1):277–289. doi: 10.1016/0092-8674(80)90175-0. [DOI] [PubMed] [Google Scholar]
- Haber J. E., Savage W. T., Raposa S. M., Weiffenbach B., Rowe L. B. Mutations preventing transpositions of yeast mating type alleles. Proc Natl Acad Sci U S A. 1980 May;77(5):2824–2828. doi: 10.1073/pnas.77.5.2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Weiffenbach B., Rogers D. T., McCusker J., Rowe L. B. Chromosomal rearrangements accompanying yeast mating-type switching: evidence for a gene-conversion model. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):991–1002. doi: 10.1101/sqb.1981.045.01.115. [DOI] [PubMed] [Google Scholar]
- Harashima S., Nogi Y., Oshima Y. The genetic system controlling homothallism in Saccharomyces yeasts. Genetics. 1974 Aug;77(4):639–650. doi: 10.1093/genetics/77.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Herskowitz I. Interconversion of Yeast Mating Types II. Restoration of Mating Ability to Sterile Mutants in Homothallic and Heterothallic Strains. Genetics. 1977 Mar;85(3):373–393. doi: 10.1093/genetics/85.3.373b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J., Strathern J. N., Klar A. J. Transposable mating type genes in Saccharomyces cerevisiae. Nature. 1979 Nov 29;282(5738):478–473. doi: 10.1038/282478a0. [DOI] [PubMed] [Google Scholar]
- Kassir Y., Simchen G. Regulation of mating and meiosis in yeast by the mating-type region. Genetics. 1976 Feb;82(2):187–206. doi: 10.1093/genetics/82.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S. Activation of mating type genes by transposition in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4539–4543. doi: 10.1073/pnas.76.9.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., Fogel S., Radin D. N. Switching of a mating-type a mutant allele in budding yeast Saccharomyces cerevisiae. Genetics. 1979 Jul;92(3):759–776. doi: 10.1093/genetics/92.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J. Mating-Type Functions for Meiosis and Sporulation in Yeast Act through Cytoplasm. Genetics. 1980 Mar;94(3):597–605. doi: 10.1093/genetics/94.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar A. J., McIndoo J., Strathern J. N., Hicks J. B. Evidence for a physical interaction between the transposed and the substituted sequences during mating type gene transposition in yeast. Cell. 1980 Nov;22(1 Pt 1):291–298. doi: 10.1016/0092-8674(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Mackay V., Manney T. R. Mutations affecting sexual conjugation and related processes in Saccharomyces cerevisiae. I. Isolation and phenotypic characterization of nonmating mutants. Genetics. 1974 Feb;76(2):255–271. doi: 10.1093/genetics/76.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascioli D. W., Haber J. E. A CIS-Acting Mutation within the MATa Locus of SACCHAROMYCES CEREVISIAE That Prevents Efficient Homothallic Mating-Type Switching. Genetics. 1980 Feb;94(2):341–360. doi: 10.1093/genetics/94.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima Y., Takano I. Mating types in Saccharomyces: their convertibility and homothallism. Genetics. 1971 Mar;67(3):327–335. doi: 10.1093/genetics/67.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Isolation and characterization of MMS-sensitive mutants of Saccharomyces cerevisiae. Genetics. 1977 May;86(1):33–55. doi: 10.1093/genetics/86.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Blair L. C., Herskowitz I. Healing of mat mutations and control of mating type interconversion by the mating type locus in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3425–3429. doi: 10.1073/pnas.76.7.3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Newlon C. S., Herskowitz I., Hicks J. B. Isolation of a circular derivative of yeast chromosome III: implications for the mechanism of mating type interconversion. Cell. 1979 Oct;18(2):309–319. doi: 10.1016/0092-8674(79)90050-3. [DOI] [PubMed] [Google Scholar]
- Strathern J. N., Spatola E., McGill C., Hicks J. B. Structure and organization of transposable mating type cassettes in Saccharomyces yeasts. Proc Natl Acad Sci U S A. 1980 May;77(5):2839–2843. doi: 10.1073/pnas.77.5.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano I., Arima K. Evidence of the Insensitivity of the alpha-inc Allele to the Function of the Homothallic Genes in Saccharomyces Yeasts. Genetics. 1979 Feb;91(2):245–254. doi: 10.1093/genetics/91.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano I., Kusumi T., Oshima Y. An alpha mating-type allele insensitive to the mutagenic action of the homothallic gene system in Saccharomyces diastaticus. Mol Gen Genet. 1973 Oct 16;126(1):19–28. doi: 10.1007/BF00333478. [DOI] [PubMed] [Google Scholar]
- Takano I., Oshima Y. Mutational nature of an allele-specific conversion of the mating type by the homothallic gene HO alpha in Saccharomyces. Genetics. 1970 Jul;65(3):421–427. doi: 10.1093/genetics/65.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]


