Abstract
Blind individuals show visual cortex activity during Braille reading. We examined whether such cross‐modal activations reflect processing somatosensory stimuli independent of language by identifying cortical activity during a one‐back vibrotactile matching task. Three groups (sighted, early‐onset, and late‐onset [>12 years] blind) detected whether paired vibrations (25 and 100 Hz), delivered to the right index finger, differed in frequency. Three successive paired vibrations, followed by a no‐stimulation interval, were presented in a long event‐related design. A fixed effects average z‐score analysis showed increased activity throughout the visuotopic visual cortex, where it was mostly restricted to foveal and parafoveal eccentricities. Early blind showed the most extensive distribution of activity. Late blind exhibited activity mostly in similar regions but with declining response magnitudes with age of blindness onset. Three sighted individuals had suprathreshold activity in V1 but negative responses elsewhere in visual cortex. Mixed effects ANOVA confirmed group distinctions in defined regions (V1, V3, V4v, V7, LOC, and MT). These results suggest cross‐modal adaptation to tactile stimulation in visual cortex independent of language processes. All groups showed increased activity in left primary (S1) and bilateral second somatosensory areas, but without response magnitude differences between groups throughout sensorimotor cortex. Early blind showed the greatest spatial extent of S1 activity. Blind participants had more extensive bilateral activity in anterior intraparietal sulcus and supramarginal gyrus. Extensive usage of touch in Braille reading may underlie observed S1 expansions in the reading finger representation. In addition, learned attentiveness to touch may explain similar expansion of parietal tactile attention regions. Hum. Brain Mapp. 23:210–228, 2004. © 2004 Wiley‐Liss, Inc.
Keywords: blindness, human, magnetic resonance imaging, visual cortex/*physiology
INTRODUCTION
Brain‐imaging studies indicate that most congenitally blind (EB) and many adventitiously blind (LB) people show activity in visual cortex to tactile and auditory stimuli and to spoken or Braille read words [for literature citations, see Burton2003; Sadato et al.,2002]. Engaging visual cortex in the absence of sight constitutes an important instance of cross‐modal neuroplasticity. These findings suggest that visual cortex adapts by acquiring a capacity to process tactile stimuli [Büchel et al.,1998; Burton et al.,2002a; Melzer et al.,2001; Sadato et al.,1996,1998,2002; Uhl et al.,1991]. However, the effects of tactile vs. language components involved in reading Braille were not separated in these studies. To avoid the problem of dissociating language processes from the tactile aspects of Braille, the present study examined visual cortex activity in blind people doing a totally non‐lexical task, a tactile vibration difference task. This task is also readily learned by sighted individuals, which is not the case for tactile Braille reading [Uhl et al.,1991].
Prior reports of visual cortex activity in blind people noted different activation patterns in striate and extrastriate cortex in early‐ and late‐onset blind Braille readers [Büchel et al.,1998; Burton et al.,2002a,2002b; Melzer et al.,2001; Sadato et al.,1996,2002], which raises a question of whether these differences related to early de‐afferentation. Thus, another aim of the present study was to determine whether activity present in striate and extrastriate visual cortex is comparable in EB vs. LB during performance of a tactile discrimination task.
This study also addressed an ongoing debate about whether blindness leads to adaptive plasticity in the somatosensory system similar to increased cortical representations of affected body regions after extensive use [Allard et al.,1991; Clark et al.,1988; Elbert et al.,1995; Jenkins et al.,1990; Karni et al.,1995; Recanzone et al.,1992a, 1992b, 1992c]. Braille literacy constitutes a good example of extensive and continuous use of the fingertips, especially for the predominant reading hand. Thus, it is possible that, as a consequence of neuroplasticity, blind Braille readers have altered representations for the reading hand in somatosensory and motor cortex. Such changes are theoretically likely in fluent Braille readers who have multiple years of practice in making the tactile discriminations used in reading Braille.
Changes in the somatosensory cortex of blind people have not been studied extensively [Sadato et al.,1996,1998,2002]. Supportive evidence for plasticity in sensorimotor cortex of Braille readers derives from transcranial magnetic stimulation (TMS) studies finding blockage of tactile stimulus detection from TMS to more scalp positions for the Braille reading finger [Pascual‐Leone and Torres,1993] and for expanded motor cortex maps for the first dorsal interosseous and the abductor digiti minimi of the reading hand [Pascual‐Leone et al.,1993]. Similarly, an enlarged hand representation in primary somatosensory cortex (S1) is suggested by finding SEPs distributed over a larger area from electrically stimulating the Braille reading finger [Pascual‐Leone and Torres,1993] and from neuromagnetic recordings of greater distance between the dipoles for stimulated fingers on the Braille reading hand [Sterr et al.,1998]. It is uncertain whether reorganization can occur at any age since Sterr and colleagues did not distinguish between EB vs. LB. There have been no fMRI analyses of somatosensory cortex in blind people that considered regional activity to controlled vibrotactile stimulation of the right index finger, which is the finger most extensively engaged by Braille readers [Millar,1997]. The present experiment aimed to determine whether activity in S1 or other somatosensory cortical areas, in response to passively applied and controlled vibrotactile stimuli, differs in magnitude, cortical location, or spatial extent in EB, LB, and sighted individuals. Using passively applied tactile stimulation, this study examined potential distinctions between groups without bias due to differences in visual or sensorimotor skills.
METHODS
Participants
Nine early blind (EB), nine late blind (LB), and eight normal sighted (NS) paid volunteers provided informed consent following guidelines approved by the Human Studies Committee of Washington University. Table I presents demographic characteristics of the blind participants and lists identification numbers, which were retained for blind people who volunteered in previous studies [Burton et al.,2002a,2002b,2003]. Except for a variety of ophthalmologic causes of blindness (Table I), all participants were free of neurological disease, had normal brain anatomy, and were age matched. All blind participants were Braille literate, read Braille for at least 1 hr/day, and had reading speeds that averaged >85 words per minute. On average, the EB read faster than the LB group, yet this difference was not significant (t = 0.37, df = 14, P = 0.7). Three EB and six LB participants had some light sensitivity, but none were currently able to read print nor could they navigate without aid. All LB learned to read print before the onset of blindness, which occurred at an average age of 21 years (SEM ± 3.96, range 7–41). All participants had scores >75% for right‐handedness on the Edinburgh inventory [Raczkowski et al.,1974] although three stated they were left handed. Except for two LB individuals, all blind participants preferred their right hands when asked to read Braille with one hand, but otherwise used both hands when reading (Table I): one for spatial guidance and the other for reading.
Table 1.
Demographic information*
| ID no. | Age | Sex | Preferred hand | Reading hand | wpm | Age of blindness onset | Light sensitivity | Years reading | Cause of blindness | % Correct |
|---|---|---|---|---|---|---|---|---|---|---|
| Early 1 | 54.0 | F | Right | Both | 145.4 | 0 | None | 40 | Retinopathy of prematurity | 100.0 |
| Early 7 | 72.0 | M | Right | Both | 40.4 | 5 | None | 65 | Cataracts | 100.0 |
| Early 9 | 48.0 | M | Right | Right | n/a | 0 | None | 49 | Retrolental fibroplasia | 100.0 |
| Early 10 | 37.0 | M | Right | Both | 71.2 | 0 | Slight | 28 | Retinopathy of prematurity | 94.7 |
| Early 11 | 27.0 | M | Right | Left | 58.7 | 0 | Some | 20 | Leber's congenital amaurosis | 100.0 |
| Early 12 | 26.0 | M | Right | Left | 60.2 | 0 | None | 22 | Retinitis pigmentosa | 100.0 |
| Early 13 | 70.0 | F | Right | Both | 88.7 | 0 | None | 60 | Eye infection | 100.0 |
| Early 14 | 58.0 | F | Right | Both | 137.0 | 0 | Light source | 58 | Genetic retinal pigmentation | 100.0 |
| Early 16 | 48.0 | F | Right | Both | 185.8 | 0 | None | 44 | Retinopathy of prematurity | 100.0 |
| Average | 48.3 | 97.3 | 0.9 | 42 | 99.4 | |||||
| SEM | 5.3 | 16.1 | 0.6 | 5 | 0.6 | |||||
| Late 1 | 40.0 | F | Right | Both | 69.0 | 6 | Yes | 40 | Rubella | 100.0 |
| Late 3 | 44.0 | M | Left | Left | 72.5 | 11 | None | 36 | Coates disease | 100.0 |
| Late 4 | 66.0 | M | Right | Left | 63.8 | 11 | None | 56 | Congenital glaucoma | 100.0 |
| Late 6 | 50.0 | F | Right | Both | 125.0 | 27 | None | 20 | Retinitis pigmentosa | 94.1 |
| Late 8 | 50.0 | F | Right | Both | 32.6 | 36 | Slight | 14 | Steven Johnson's Syndrome | 94.1 |
| Late 9 | 40.0 | M | Right | Right | n/b | 15 | Direct light | 22 | Retinitis pigmentosa | 100.0 |
| Late 11 | 47.0 | F | Left | Both | 182.3 | 20 | Slight | 41 | Retinopathy of prematurity | 94.7 |
| Late 13 | 61.0 | M | Right | Right | n/a | 41 | Slight | 13 | Diabetes/retinopathy | 88.2 |
| Late 14 | 18.0 | M | Right | Both | 71.7 | 18 | Some | 12 | Microcornea | 93.8 |
| Average | 46.2 | 88.1 | 17.9 | 28 | 96.1 | |||||
| SEM | 4.6 | 16.5 | 3.5 | 5 | 1.4 | |||||
| Sighted | 37.0 | M | Right | 76.5 | ||||||
| Sighted | 31.0 | M | Right | 100.0 | ||||||
| Sighted | 62.0 | M | Right | 100.0 | ||||||
| Sighted | 32.0 | M | Left | 100.0 | ||||||
| Sighted | 51.0 | F | Right | 82.4 | ||||||
| Sighted | 55.0 | M | Right | 100.0 | ||||||
| Sighted | 60.0 | M | Right | 100.0 | ||||||
| Sighted | 36.0 | M | Right | 100.0 | ||||||
| Average | 45.5 | 94.9 | ||||||||
| SEM | 4.5 | 3.4 |
n/a, Braille reading speed measurement not available; n/b, not Braille literate.
Vibrotactile Frequency Difference Task
The tactile discrimination task involved judging whether paired sinusoidal vibrations that were applied to the right index fingertip matched or differed in frequency (one‐back same/different judgment). Each sinusoidal vibration lasted 750 ms and the interval between vibrations in a pair was 300 ms. A vibration pair and a following 1.5‐s interval for participant responses were considered a trial. Each pair of tactile stimuli occurred during a single imaging frame of 2.84 s (Fig. 1). An equal number of vibrations at 25 and 100 Hz were presented, and each was equated for subjective intensity with displacement amplitudes of 130 and 35 μm, respectively [Sinclair and Burton,1996]. A large difference in vibration frequencies made it easy for participants to learn the task with just a few practice trials prior to scanning. Separate activation of different mechanoreceptors was unlikely because stimulus amplitudes, though adjusted for subjective intensity, were still of sufficient magnitude that the stimuli did not selectively engage different receptor types.1 Matched and unmatched vibration pairs were, respectively, defined as non‐target and target trials. The time for three successive trials and a blank interval of ∼14 s without stimulation were subsequently analyzed as one 8‐frame epoch (Fig. 1,0–22.72 s) that was subsequently modeled as 3 frames ON and 5 frames OFF. Each fMRI run contained 20 epochs of which 3 randomized epochs (15%) contained one or two target trials. The average incidence of target trials was ∼7% for four successive image runs (17/240 trials). All epochs that had at least one target trial were analyzed as target epochs. The non‐target epochs contained only non‐target trials.
Figure 1.
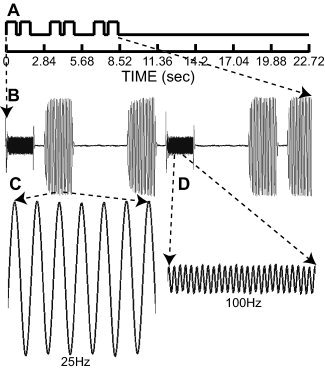
Task paradigm and records of tactile vibration stimuli. A: Single analysis epochs consisted of three successive pairs of tactile vibrations between 0 and 8.52 s (frames 1–3) followed by ∼14 s (8.52 to 22.72 s or frames 4–8) with no stimulation. Each vibration lasted 750 ms and the interval between pairs was 300 ms. This was followed by 1.5 s for participant responses. Each vibration pair occurred within, but was not synchronized with, the beginning of an image frame. B: Paired vibrations were matched (25–25 Hz or 100–100 Hz) or unmatched frequencies (25 and 100 Hz). Matched pairs were non‐targets and required no overt responses; unmatched pairs were targets that participants noted by briefly elevating their left index finger. The illustrated epoch shows two target trials followed by one non‐target trial and was analyzed as a target epoch. C,D: Expanded views of displacement signals for 25‐ and 100‐Hz vibrations recorded during a scan session. Amplitudes of the two frequencies were adjusted to create equal subjective intensities.
Participants were instructed to signal detection of a target trial by elevating their left index finger. Non‐target trials required no overt movements. Finger movement was visually monitored and tallied for accuracy. Participants were informed about the low frequency of target trials and were instructed to remain vigilant throughout a run.
Parametrically controlled vibrotactile stimuli were delivered using an air‐cooled, Ling Dynamic Systems vibrator [Sinclair and Burton,1996] that was made MR‐compatible by copper and mμ‐metal shielding, and by shielding all cables. The mμ‐metal shielding and placement of the device driver several meters from the scanner isolated the driver's ferromagnetic core from the MR magnetic field. This distance was achieved through a 12‐foot by ½ inch titanium rod that attached to the drive shaft of the vibrator. This rod ended in a 3.5‐mm‐diameter plastic probe tip that touched the fingertip. Alignment and stabilization of the drive rod were achieved by floating it on concentric, frictionless air bearings at 100 psi. An external titanium cylinder housed the drive rod and air bearings. The vibrator was capable of delivering 10–200‐μm sinusoidal excursions at frequencies of 5–200 Hz. Prior calibrations determined settings for selected vibratory amplitudes that were under computer control during the experiments. An optical transducer continuously monitored displacement of the drive rod to ∼5 μm resolution, and these signals were recorded (Fig. 1). MRI experiments with phantoms demonstrated that the vibrator did not introduce any noise into structural or functional data.
An arch attached to the scanner bed held in place the distal end of the vibrator and a hand rest. The hand rest aligned the participant's extended index finger to meet the probe tip. A collar attached to the arch securely fastened to the external titanium cylinder of the vibrator. A platform with a central hole (5.5 mm diameter) was mounted on the end of the vibrator. Participants rested their fingertip over this hole rather than pushing directly against the probe tip, which lay flush with the platform surface, leaving a 1‐mm annulus.
MRI Acquisition and Reconstruction
Images were acquired on a Siemens 3 Tesla Allegra scanner using a standard bird cage head coil. Functional images were collected with a single‐shot, gradient echo, Siemens echo‐planar (EPI) sequence (TR = 2,840 ms, TE = 30 ms, 90° flip angle) and 32 contiguous 4‐mm horizontal slices with in‐plane resolution of 4 × 4 mm. Functional images were acquired parallel to the AC–PC plane. Functional slice prescriptions were automatically computed on the basis of an unsupervised procedure that registered a coarse (2 × 2 × 2 mm, TR = 722 ms, TE = 3.93 ms, flip angle = 8°, TI = 380 ms) MP‐RAGE sagittal scan [Mugler and Brookeman,1990] to an atlas representative target image. Structural images included a T2‐weighted SE horizontal scan (1.33 × 1.33 × 3 mm, TR = 8,430 ms, TE = 98 ms) and a high resolution (1 × 1 × 1.25 mm) T1‐weighted sagittal MP‐RAGE (TR = 2,100 ms, TE = 3.93 ms, flip angle = 7°, TI = 1,000 ms).
Functional data were passed through a sequence of unsupervised steps to minimize artifact: (1) compensation for asynchronous slice acquisition; (2) removal of systematic odd vs. even slice intensity differences due to imperfect slice excitation profiles that result from contiguous, interleaved slice acquisition; and (3) realignment to compensate for head motion within and across runs using difference image variance minimization [Friston et al.,1995a; Snyder1996]. A single resampling using fast 3‐D cubic spline interpolation produced results very similar to those obtained by sinc interpolation [Hajnal et al.,1995].
Our atlas template was produced by mutual co‐registration (12 parameter affine warp) of MP‐RAGE images from 12 normal, young adult individuals. This average conforms to the Talairach system [Talairach and Tournoux,1988] as defined by the SN procedure [Lancaster et al.,1995]. Atlas transformation of functional (EPI) data was achieved by computing a sequence of affine transforms as follows: EPI → T2W → MP‐RAGE → atlas representative target. T2W was a conventional T2 weighted image, the inclusion of which helped to minimize systematic EPI → MP‐RAGE misregistration caused by EPI distortion and susceptibility artifacts [Ojemann et al.,1997]. Cross‐modal registration was accomplished using an in‐house variant of the method of Andersson et al. [1995]. This procedure required no editing of extracranial structures and performed with precision comparable to or better than AIR [Woods et al.,1993]. Algebraic composition of transforms (matrix multiplication) generated the functional EPI → atlas transform. Reslicing the functional data (or any intermediate image) in register with the atlas then involved only one interpolation. All statistical analyses were conducted in an atlas space of 2 mm3 and after spatial smoothing (4 mm FWHM).
Statistical Analyses
Blood oxygen level dependent (BOLD) responses were analyzed in four stages. First, fixed effects linear methods [Friston et al.,1995b; Zarahn et al.,1997] were used to compute t‐statistic maps of significant activity for each epoch type (target and non‐target) and each participant. This analysis assumed a delayed gamma function model [Boynton et al.,1996] that was convolved with the epoch time course shown in Figure 1 (3 frames ON, 0–8.52 s; 5 frames OFF, 8.52–22.72 s) to compute the expected hemodynamic response function (HRF). The latter was cross‐correlated with the estimated time course of BOLD responses per voxel. The cross‐correlation magnitudes represented the extent that the estimated response time courses followed an assumed HRF. The residuals from this fit were used to obtain t‐statistics per voxel. The t‐statistic maps for each individual were converted to equally probable z‐scores that were thresholded on the basis of Monte Carlo simulations [Forman et al.,1995] at a multiple‐comparisons corrected false‐detection rate of P = 0.05 (z = 4) over at least 6 contiguous, face‐connected voxels. Inspection of these maps established the pattern of activity in individual participants (e.g., Fig. 4).
Figure 4.
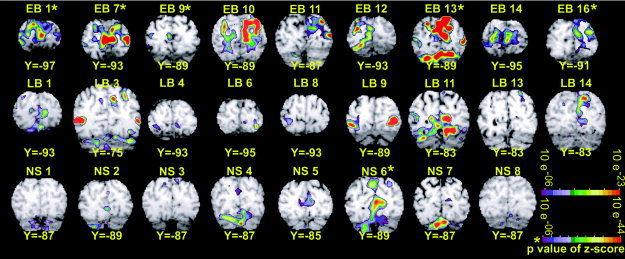
Individual participant z‐score maps showing activity in posterior occipital cortex. Coronal sections (in Talairach atlas space) [Talairach and Tournoux,1988] for each participant were selected to show the maximum activity in or near V1. Participant labels cross‐reference to demographic characteristics listed in Table I (early blind, EB; late blind, LB; sighted, NS).
Next, a fixed‐effects analysis strategy was used to generate average z‐score maps for each of three groups (EB, LB, and NS) by epoch type (target and non‐target). The averages, corrected by the square root of sample size, were based on unthresholded z‐score maps for individuals [Bosch,2000]. These maps were projected onto smoothed and flattened representations of a canonical cortical surface [Van Essen,2002a,b] to facilitate evaluation of regional activity elicited by each epoch type and for comparison of response topography in blind vs. sighted people (Fig. 2).
Figure 2.
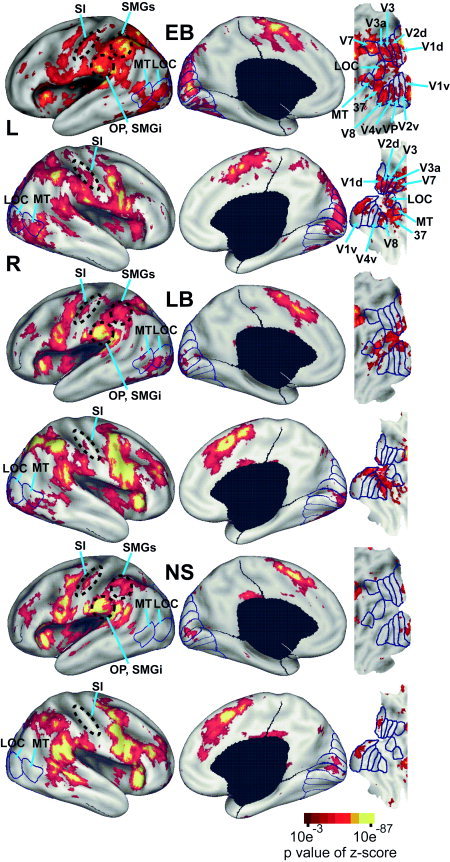
Average z‐score maps for positive BOLD responses by group (early blind, EB; late blind, LB; sighted, NS) for non‐target epochs are shown overlying inflated canonical hemispheres and flat maps of visual cortex (description and methods for creating flat maps can be found in Drury et al. [1996], Van Essen et al. [1998,2001], and Van Essen [2004]. Borders and labeling of visual areas are from prior identifications in sighted people [Hadjikhani et al.,1998; Tootell et al.,1996,1997,1998; Van Essen,2002a,2004]. LOC: lateral occipital complex; MT: medial temporal area; OP/SMGi: parietal operculum/ inferior supramarginal gyrus; S1: primary somatosensory area; SMGs: superior supramarginal gyrus; V1d, V1v: dorsal and ventral primary visual areas; V2d, V2v: dorsal and ventral second visual areas; V3, V3A: third visual areas; V4v: ventral fourth visual area; VP: ventral posterior visual area; V7: seventh visual area; V8: eighth visual area.
The second analysis of group‐level differences utilized a repeated measures, mixed effects ANOVA, treating participants as a random factor and time (8 image frames), epoch type, and group as fixed factors. This analysis computed main effects of time, epoch type, group, and the associated interactions. The statistical maps indicated the distribution of voxels with significant F‐ratios. The dependent variable of percent change in MR signal per voxel was calculated using estimates from the GLM obtained in each participant. F‐ratios for each factor in the ANOVA models were converted to z‐scores whose degrees of freedom were adjusted for covariance (sphericity correction), thresholded at P = 0.05 for a z‐score of 4 over 12 contiguous and face‐connected voxels, and multiple comparison corrected on the basis of Monte Carlo simulations on random noise patterns [similar to the method described by Forman et al.,1995]. The z‐score map for the main effect of time identified regions in which BOLD responses were significantly modulated by the experimental paradigm independent of any a priori assumptions of an expected HRF. The time‐by‐group interaction identified regions in which the response profiles varied between the groups (see Fig. 5). The time‐by‐epoch‐type interaction identified regions in which the response profile changed depending on whether the epoch included any target trials (see Fig. 8).
Figure 5.
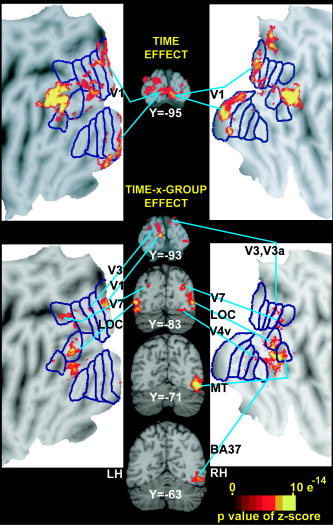
Selected coronal sections (in Talairach atlas space) [Talairach and Tournoux,1988] and flat maps through visual cortex regions identified from the effects of time (top) and time‐by‐group (bottom) factors in a voxel‐based ANOVA. See Figure 2 for abbreviations and citations that explain flat maps. LH, left hemisphere; RH, right hemisphere.
Figure 8.
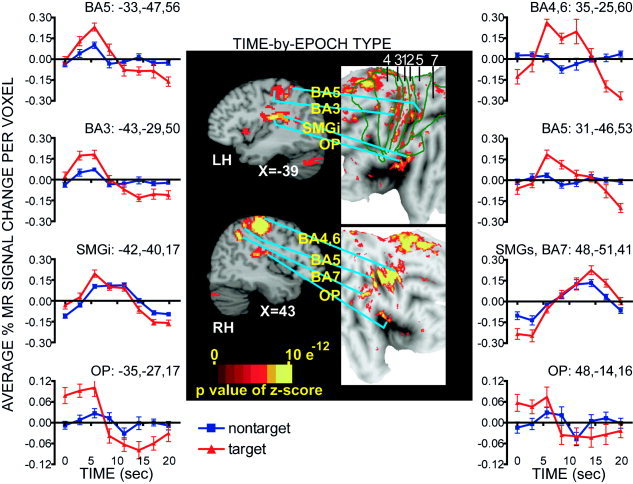
Selected sagittal sections (in Talairach atlas space) [Talairach and Tournoux,1988] and flat maps through sensorimotor cortex regions identified from the effect of the time‐by‐epoch‐type factor in a voxel based ANOVA. Brodmann area (BA) borders for BA 4, 3, 1, 2, 5, and 7 taken from a canonical brain [Van Essen et al.,1998; Van Essen,2002a,b]. Regional time courses extracted for each epoch type are plotted for regions where cross‐correlation response magnitudes differed significantly between target and non‐target epochs. Plots from the left hemisphere (LH) regions are shown on the left and those from the right hemisphere (RH) regions are shown on the right in the vertical order that the regions are labeled on the brain images. OP: parietal operculum;SMGi, SMGs: inferior and superior parts of supramarginal gyrus.
To explore group‐dependent differences with greater power than afforded by voxel‐wise group analysis, objectively defined regions of interest (ROI) were determined as follows. The z‐score maps for the time, time‐by‐group, and time‐by‐epoch factors from the ANOVA were separately submitted to a peak (local extremum) search algorithm that identified multiple loci of maximal z‐scores (threshold z = 4). Loci separated by less than 13.5 mm were consolidated (by center of mass calculation) to eliminate excess subdivisions due to noise in spatially extended responses. Spherical (6.75 mm radius) ROIs were centered on the identified peaks whose coordinates are listed in Tables II and III. These tables also note Brodmann areas (BA) from the Talairach atlas that encompassed the peaks [Talairach and Tournoux,1988]. Regional normalized response time courses were extracted from each participant and were averaged for each group by epoch type (see Fig. 6) and across groups for each epoch type (see Fig. 8).
Table II.
Regional t‐test analyses of groups*
| X,Y,Z | Region | EB vs NS | LB vs NS | EB vs LB | ||||
|---|---|---|---|---|---|---|---|---|
| t | P | t | P | t | P | |||
| Time effect | ||||||||
| Target epochs | ||||||||
| Left Hem (LH) | −3,−94,−2 | LBCS, V1 | 1.09 | 0.2941 | 1.32 | 0.2066 | 2.14 | 0.0492 |
| Right Hem (RH) | 20,−94,−4 | LBCS, V1 | 1.10 | 0.2899 | 0.21 | 0.8365 | 0.50 | 0.6250 |
| Non‐target epochs | ||||||||
| LH | −3,−94,−2 | LBCS, V1 | 1.83 | 0.0886 | 0.49 | 0.6326 | 2.10 | 0.0529 |
| RH | 20,−94,−4 | LBCS, V1 | 1.51 | 0.1533 | 1.06 | 0.3059 | 0.11 | 0.9139 |
| Time‐by‐group effect | ||||||||
| Target epochs | ||||||||
| LH | −3,−93,4 | UBCS, V1 | 2.72 | 0.0166 | 0.20 | 0.8442 | 2.99 | 0.0092 |
| −5,−94,13 | Cun, V3 | 3.26 | 0.0057 | 0.89 | 0.3875 | 2.50 | 0.0245 | |
| −20,−88,19 | MOG, V7 | 3.32 | 0.0051 | 3.25 | 0.0054 | 1.67 | 0.1156 | |
| −40,−82,−6 | IOG, LOC | 5.28 | 0.0001 | 4.20 | 0.0008 | 1.90 | 0.0768 | |
| −33,−74,−16 | ITG/FG, BA37 | 5.21 | 0.0001 | 6.41 | 0.0000 | 0.79 | 0.4418 | |
| RH | 14,−90,25 | SOG, V3 | 4.33 | 0.0007 | 1.57 | 0.1373 | 2.51 | 0.0240 |
| 29,−80,−14 | FG, V4v | 3.80 | 0.0020 | 1.96 | 0.0688 | 2.00 | 0.0639 | |
| 25,−87,17 | MOG, V7 | 3.27 | 0.0056 | 2.85 | 0.0122 | 1.65 | 0.1197 | |
| 38,−83,5 | IOG, LOC | 3.12 | 0.0075 | 2.59 | 0.0205 | 1.80 | 0.0920 | |
| 46,−72,−7 | ITG, MT | 6.78 | 0.0000 | 5.04 | 0.0001 | 1.51 | 0.1518 | |
| 43,−59,−13 | ITG/FG, BA37 | 4.19 | 0.0009 | 3.46 | 0.0035 | 1.93 | 0.0727 | |
| 53,−6,37 | PCG, BA3,1 | 3.10 | 0.0078 | 2.84 | 0.0124 | 1.04 | 0.3148 | |
| 33,21,3 | Insula | 4.15 | 0.0010 | 0.36 | 0.7239 | 4.65 | 0.0003 | |
| Non‐target epochs | ||||||||
| LH | −3,−93,4 | UBCS, V1 | 2.68 | 0.0179 | 0.69 | 0.5007 | 2.61 | 0.0197 |
| −5,−94,13 | Cun, V3 | 3.31 | 0.0052 | 0.64 | 0.5318 | 3.12 | 0.0070 | |
| −20,−88,19 | MOG, V7 | 2.71 | 0.0169 | 1.84 | 0.0856 | 1.74 | 0.1023 | |
| −40,−82,−6 | IOG, LOC | 2.86 | 0.0126 | 3.54 | 0.0030 | 1.13 | 0.2762 | |
| −33,−74,−16 | ITG,BA37 | 1.90 | 0.0782 | 3.93 | 0.0013 | 0.31 | 0.7608 | |
| RH | 14,−90,25 | SOG, V3 | 2.82 | 0.0136 | 0.55 | 0.5904 | 2.80 | 0.0135 |
| 29,−80,−14 | FG, V4v | 3.10 | 0.0078 | 2.69 | 0.0168 | 0.60 | 0.5575 | |
| 25,−87,17 | MOG, V7 | 2.72 | 0.0166 | 1.38 | 0.1878 | 3.29 | 0.0050 | |
| 38,−83,5 | IOG, LOC | 2.88 | 0.0121 | 1.34 | 0.2002 | 4.67 | 0.0003 | |
| 46,−72,−7 | ITG, MT | 5.70 | 0.0001 | 2.98 | 0.0093 | 2.87 | 0.0117 | |
| 43,−59,−13 | ITG/FG, BA37 | 3.72 | 0.0023 | 4.13 | 0.0009 | 1.91 | 0.0754 | |
| 53,−6,37 | PCG, BA3,1 | 2.89 | 0.0119 | 2.78 | 0.0140 | 0.40 | 0.6948 | |
| 33,21,3 | Insula | 3.79 | 0.0020 | 0.16 | 0.8750 | 4.10 | 0.0010 | |
BA, Brodmann Area; Cun, cuneus; FG, fusiform g.; IOG, inferior occipital g.; ITG, inferior temporal g.; LOC, lateral occipital complex; LBCS, lower bank calcarine s.; MOG, middle occipital g.; PCG, postcentral g.; SOG, superior occipital g.; UBCS, upper bank calcarine s.
Table III.
Regional t‐test analyses of epoch types (Non Target–Target)*
| Time‐by‐epoch type effect | ||||
|---|---|---|---|---|
| X,Y,Z | Region | t | P | |
| LH | ||||
| Ft | −16,21,38 | SFS, BA8 | 4.80 | 0.0001 |
| −39,10,03 | Insula | −5.69 | 0.0000 | |
| −35,07,38 | MFG, BA8 | 2.89 | 0.0082 | |
| −05,07,37 | CG, BA32 | −4.27 | 0.0003 | |
| −08,−18,62 | SMA, BA6 | −2.76 | 0.0112 | |
| Pt | −43,−29,50 | PCG, BA3 | −3.35 | 0.0028 |
| −17,−34,65 | PCG, BA3,1 | −2.41 | 0.0242 | |
| −56,−18,37 | PCG, BA1 | −3.65 | 0.0013 | |
| −31,−37,44 | PCG, BA2 | −2.35 | 0.0189 | |
| −35,−27,17 | Parietal Oper, OP | −5.15 | 0.0000 | |
| −42,−40,17 | SMGi, BA40 | −2.89 | 0.0039 | |
| −18,−48,64 | SMGs, BA7 | 2.11 | 0.0463 | |
| −14,−29,47 | CG, BA31 | −2.78 | 0.0055 | |
| RH | ||||
| Ft | 03,−14,54 | MeFG, SMA | −7.85 | 0.0000 |
| 05,−02,46 | CG, BA31 | −9.69 | 0.0000 | |
| 23,−16,61 | PrCG, BA4,6 | −4.63 | 0.0001 | |
| 35,−25,60 | PrCG, BA4 | −4.34 | 0.0002 | |
| Pt | 51,−18,44 | PCG, BA3,1 | −2.44 | 0.0228 |
| 48,−14,16 | Parietal Oper, OP | −2.51 | 0.0196 | |
| 57,−45,25 | SMGi, BA40 | −5.19 | 0.0000 | |
| 31,−46,53 | SPL, BA5 | −5.46 | 0.0000 | |
| 51,−31,39 | SMGs, BA7 | −2.68 | 0.0133 | |
| 48,−51,41 | SMGs, BA7 | −3.76 | 0.0010 | |
| 03,−38,57 | PreCuneus, BA5 | 2.49 | 0.0205 | |
| 14,−44,16 | CG, BA31 | 3.92 | 0.0007 | |
| 01,−61,63 | PreCuneus, BA7 | −3.90 | 0.0007 | |
| 00,−72,53 | PreCuneus, BA7 | −5.31 | 0.0000 | |
CG: cingulate g.; Ft, frontal cortex; IFG: inferior frontal g., frontal cortex; LH: left hemisphere; MeFG: medial frontal g.; Pt: parietal cortex; MFG: middle frontal g.; PCG: postcentral g.; PrCG: precentral g.; RH: right hemisphere; SFS: superior frontal s.; SPL: superior parietal lobule; SMG: supramarginal g.
Figure 6.
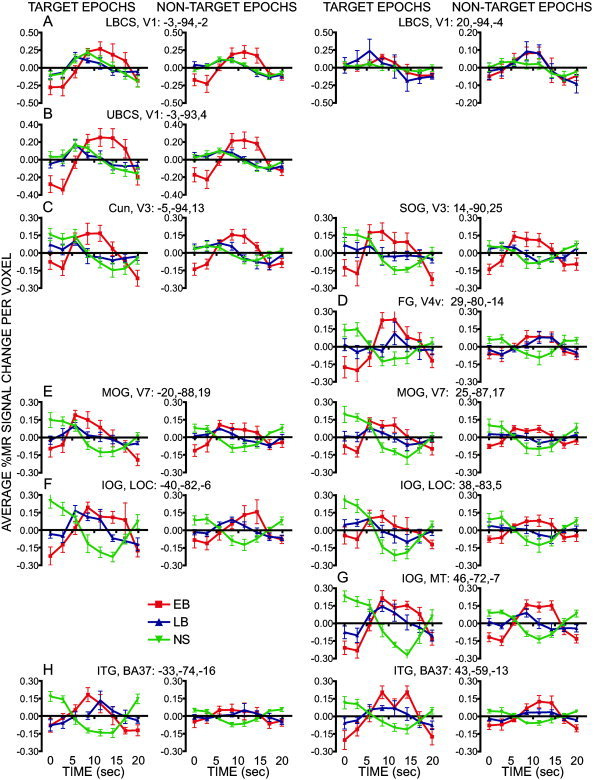
Regional time courses extracted for each epoch type and group are plotted for regions of interest identified from the effects of the time (A) or time‐by‐group (B–H) in a voxel based ANOVA. Data at each time point show group mean and S.E.M. (early blind, EB; late blind, LB; sighted, NS). Plots from left hemisphere regions are shown in the left two columns and those from right hemisphere regions are shown in the right two columns. Each region is identified by its cortical location, visual area, and atlas coordinate location for the regional center of mass. The plots are arranged to follow the top‐to‐bottom and left‐right order of regions shown on the coronal sections in Figure 5. Cun: cuneus gyrus; FG: fusiform g; IOG: inferior occipital g; LG: lingual g; MOG: middle occipital g; SOG: superior occipital g.
Group differences in response magnitudes were assessed with unpaired t‐tests. A model‐free assessment of regional response magnitude was used for pair‐wise comparisons between the three groups (EB vs. NS, LB vs. NS, and EB vs. LB). This approach was needed because response magnitudes calculated from cross‐correlation with a single HRF model were not comparable given the varied time courses observed among the groups (see Fig. 6). Participant regional response magnitudes were computed for each epoch type as the difference between the average of responses in frames 4–6 (8.52–17.04 s), which followed the end of tactile stimulation, minus the average baseline signals in frames 1 (0–2.84 s) and 8 (19.88–22.72 s).
Task differences in response magnitudes were assessed using random effects paired t‐tests. Regional cross‐correlation magnitudes [based on a fixed delay model, Boynton et al.,1996] were computed for each epoch type (target and non‐target). This computation of response magnitude per epoch type was appropriate because response time courses were more similar within a region for target and non‐target epochs (see Fig. 8).
RESULTS
Task Performance
Participants were >75% accurate in the detection of target trials (Table I). Accuracy was not significantly different between blind and sighted groups and was unaffected by age of blindness onset.
Overview of Activated Cortex Based on Fixed Effects Average Z‐score Maps
Parietal cortex
Average z‐score maps from non‐target epochs (Fig. 2) show that activity increased posterior to the central sulcus along the middle of the postcentral gyrus (Fig. 2, S1). EB compared to LB and NS had a greater spatial extent of postcentral activity in S1 (Fig. 2). All groups showed predominantly overlapping response regions in the parietal operculum and inferior supramarginal gyrus (Fig. 2, OP/SMGi)2 and along the anterior intraparietal sulcal cortex and superior supramarginal gyrus (Fig. 2, SMGs). However, in the superior parietal lobule the trend for differential activity was EB>LB>NS in left SMGs and bilaterally in cortex medial to the anterior half of the intraparietal sulcus (Fig. 2).
All groups showed activity in S1 and OP, contralateral (left hemisphere) to the stimulated finger with similar magnitude positive BOLD responses during non‐target epochs (Fig. 3B). A 16‐mm diameter S1 region, located on the crown of the postcentral gyrus in presumptive BA 1 (Fig. 3A), was centered on the spatial extent of S1 activity identified in Figure 2 for the NS participants. The boundaries of this S1 region extended anterior into the posterior bank of the central sulcus where it presumably included BA 3 cortex. A 16‐mm‐diameter left OP region was centered on the most superficial upper bank portion of the lateral sulcus, which encompassed the average activity noted across the parietal operculum (Fig. 2). The exact mirror coordinates were selected for a right OP region, where response magnitudes were similar and strongly correlated (n = 208, r = 0.72, P < 0.0001) with those in the left OP (Fig. 3C). The average % MR signal changes per voxel in these S1 and OP regions were unaffected by the age of blindness onset, as shown by the horizontal slopes in Figure 3D (S1: m = −0.001, r = 0.07; OP, left: m = −0.001, r = 0.05; OP, right: m = −0.006, r = 0.27).
Figure 3.
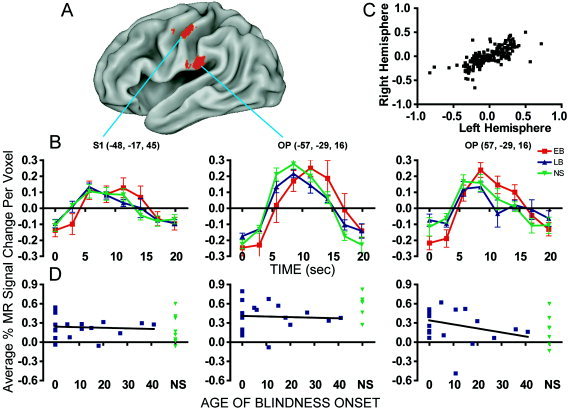
BOLD responses during non‐target epochs in somatosensory cortex. A: Locations of analyzed S1 and OP regions in the left hemisphere are illustrated on a partially inflated canonical brain. B: Response time courses for each group (early blind, EB; late blind, LB; and sighted, NS) in S1 and bilaterally in OP. Talairach coordinates of the regional centers‐of‐mass are listed in parentheses. C: Left and right hemisphere time course cross‐correlation. Each point is one of the measurements in the time course. The axes are in average % MR signal change per voxel. D: Response magnitudes in somatosensory regions at different ages of blindness onset. Linear regression lines were fit to data from blind participants only. Results from NS are shown on the extreme right of each graph.
Frontal cortex
Anterior inferior frontal operculum and medial superior frontal gyrus showed relatively similar activity distributions in all groups (Fig. 2). In the left prefrontal cortex there was a trend of NS>LB>EB.
Occipital‐temporal cortex
A flattened rendering of this cortex (Fig. 2, right column) is shown with the distribution of activity overlaid by likely borders of visuotopic visual areas previously identified in sighted people [Hadjikhani et al.,1998; Tootell et al.,1996,1997,1998; Van Essen2002a,2004]. These flat maps are artificially split along the calcarine sulcus and show upper bank regions more superior. Within each visual region, these maps plot more posterior cortical locations closer to the split.
Activity extended from the occipital pole to adjacent occipital‐temporal and occipital‐parietal cortex in EB. The activity affected the upper and lower banks of the calcarine sulcus, lingual gyrus, cuneus, fusiform gyrus, occipital gyri, and inferior temporal gyrus, which in reference to defined visual areas in sighted people involved V1 to MT (Fig. 2). On the right, activity was especially concentrated closer to the calcarine sulcus split, which indicates a more posterior cortical location (Fig. 2). Individual z‐score maps from all EB, although variable, confirm the posterior location of suprathreshold activity in V1 (Fig. 4).
In LB, bilateral activity was restricted to posterior occipital and adjacent occipital‐temporal cortex where it involved extreme posterior domains of both banks of the calcarine sulcus, especially on the left, and thus affected V1‐V4v regions representing foveal and parafoveal representations as defined in sighted people [Tootell et al.,1995,1997,1998] (Fig. 2). Individual z‐score maps from only three LB participants had statistically significant responses in the V1 region (Fig. 4, LB1, 11, and 14). Other LB participants may have contributed to the V1 pattern shown in the average z‐score maps, but the activity in these individuals was not statistically significant on its own.
In NS, minimal occipital cortex activity was largely restricted to the occipital cortex for V1 where it was centered some distance from the extreme posterior pole. This portion of the calcarine sulcal cortex includes the parafoveal parts of V1 (Fig. 2). Despite the apparent consistent location of V1 responses to vibrotactile stimuli in NS, only three NS participants showed statistically significant positive BOLD responses in V1 (Fig. 4, NS2, NS5, and NS6). Statistically non‐significant activity in individual z‐score maps may have contributed to the distribution shown in the average NS z‐score map.
Mixed Effects Group Analysis of Group Differences
Regions identified from the mixed effects ANOVA analysis did not involve any assumptions about the shape or magnitude of hemodynamic responses. Results from the time (BOLD response over the course of an 8 frame epoch) and time‐by‐group effects indicate, respectively, cortical areas with significant BOLD modulations irrespective of blindness and those areas uniquely activated in the different groups. Most of the identified regions had positive BOLD responses in blind participants and were located in areas normally associated with vision in sighted individuals. The fixed effects group average z‐score maps showed activity distributed more widely than the regions identified with the ANOVA. However, this difference arises because the ANOVA is less affected by large contributions from a few individuals and shows regions generally utilized in the vibrotactile task. The importance of the ANOVA results is that they allow high confidence in generalizing the involvement of the identified regions.
Areas along and near the banks of the calcarine sulcus, V1, V2
The map based on the time effect revealed significant modulation over the course of an epoch along the calcarine sulcus (Fig. 5, Y = −95) and adjoining lingual gyrus and cuneus. Bilateral activity was identified along both banks of the calcarine sulcus but the peaks were centered on the lower bank (LBCS) in posterior occipital cortex within this portion of the time effect map. Visualization of these voxels on a flat map, overlaid with borders for the visuotopic visual areas defined in sighted people (Van Essen,2004], shows the modulated activity involved V1, especially on the right. Separate V2 regions in the lingual gyrus and cuneus, however, were not objectively definable given proximity to the V1 responses and the parameter settings used in our peak search. These time effects in the ANOVA are consistent with all groups showing similar positive BOLD responses bilaterally in LBCS, V1 regions (Fig. 6, row A). There was a trend of larger responses in EB vs. LB on the left during both epochs (Table II, LBCS, V1, P = 0.049 and 0.052).
The time‐by‐group analysis identified another calcarine sulcal region with activation that differed between the groups. The peak for this region was on the left upper bank (UBCS) away from the posterior occipital pole (Fig. 5, Y = −93). The flat map illustrates that this region was confined to V1 (Fig. 5, left flat map) and did not involve the neighboring cuneus or V2d region, which indicates that only the UBCS, V1 region had differing responses between the groups. The time course plots from the UBCS, V1 region show that the major source of the between group variance was significantly larger positive BOLD responses in EB compared to LB and NS and predominantly late negative responses in LB and NS (Fig. 6, row B; Table II: UBCS, V1).
Cuneus and superior occipital gyrus, V3‐V3a
The time effect map (Fig. 5, Y = −95) and the time‐by‐group effect map (Fig. 5, Y = −93) showed similar distributions of significant voxels in the cuneus (Cun) and adjoining superior occipital gyrus (SOG). The flat map shows that these regions involved visuotopic visual areas V3‐V3a (Fig. 5), which is consistent with the distributions shown for EB and LB but not NS in the average z‐score maps (Fig. 2). Time courses for the bilateral Cun and SOG regions identified from the time‐by‐group effect show large sustained positive BOLD responses in EB compared to earlier shallow positive followed by delayed negative BOLD responses in LB and NS groups for target and non‐target epochs (Fig. 6, row C; Table II: Cun, V3 and SOG, V3‐V3a).
V1 vs. V3
A similar pattern of group differences is seen throughout the V1 and V3 territory for both epochs. This especially includes a sustained late positive BOLD response in EB. The group differences, however, are slightly less marked in LBCS compared to UBCS and V3 (Fig. 6, Table II), such that the LBCS appears in the time effect map (Fig. 5) and UBCS and V3 appear in the time‐by‐group effect map (Fig. 5).
Fusiform gyrus, V4v
The right fusiform gyrus (FG) contained significant voxels in the time‐by‐group effect map (Fig. 5, Y = −83), which the flat map shows were located within the probable borders of visual area V4v. Positive BOLD responses during non‐target epochs in EB and LB, and during target epochs in EB, differed significantly from the negative BOLD responses in NS (Fig. 6, row D; Table II, FG, V4v).
Middle occipital gyrus, V7
Bilaterally, the middle occipital gyrus (MOG) contained significant voxels in the time‐by‐group effect map (Fig. 5, Y = −83) that fell within the probable boundaries set for the V7 visual area [Van Essen,2004]. EB showed consistently larger positive BOLD responses compared to LB (Fig. 6, row E). EB responses differed significantly from negative BOLD responses in NS for both epoch types (Table II, V7, MOG). Smaller LB positive responses also differed significantly from NS responses during target epochs (Table II). On the right, during non‐target epochs, however, LB responses were slightly negative (Fig. 6, row E) and differed significantly from EB responses (Table II).
Inferior occipital gyrus, LOC
Bilaterally the inferior occipital gyrus (IOG) contained significant voxels in the time‐by‐group effect map (Fig. 5, Y = −83) that fell within the probable borders of LOC identified in sighted people [Tootell et al.,1996]. Other studies extend the boundaries of LOC anterior and ventral [i.e., Amedi et al.,2002; Van Essen,2004], which encompass the presence of widespread activity throughout IOG in EB and LB, but not NS (Fig. 2). EB had relatively similar positive BOLD responses bilaterally for both epoch types that were significantly larger than the negative BOLD responses in NS (Table II, IOG, LOC). Responses in LB resembled those for EB only on the left (Fig. 6, row F) where they similarly differed significantly from the NS responses (Table II).
Posterior inferior temporal sulcus and gyrus, MT
The right posterior inferior temporal gyral/sulcal region (ITG) contained significant voxels in the map for the time‐by‐group effect (Fig. 5, Y = −71). The flat maps include this posterior portion of ITG/ITS within the probable borders of the MT/V5 visual area in sighted people [Tootell et al.,1995,1996; Tootell et al.,1995]. Both groups of blind people showed similar positive BOLD responses in MT/V5 (Fig. 6, row G) during both epoch types that differed significantly from the negative BOLD responses in NS (Table II, IOG, MT). On non‐target epochs, the EB responses were larger than those in LB.
Anterior inferior temporal and fusiform gyri, BA 37
The map for the time‐by‐group effect showed a cluster of significant voxels further anterior that were centered in the inferior temporal gyrus (Fig. 5, Y = −63); the cluster extended medially into the fusiform gyrus (ITG/FG). This distribution, which lay outside probable visuotopic visual areas defined in the flat maps, is labeled BA37 in the Talairach atlas [Talairach and Tournoux,1988]. EB and LB had significantly larger positive responses during target epochs than the negative responses recorded in NS bilaterally within this ITG/FG region (Fig. 6, row H; Table II, ITG/FG, BA37). During non‐target epochs, EB and LB again differed significantly from NS in the right hemisphere (Table II).
Summary
Across most of the visual cortex, activity in EB always showed a positive peak at a mid‐time interval. In contrast, NS activity tended to do the reverse. These distinctions are least evident in V1 and more evident in more distant extrastriate regions. LB activity mimicked NS activity in V1, but increasingly approximated EB activity in more distant extrastriate regions.
In general, activity distributions observed with fixed (Fig. 2) and mixed (Fig. 5) effect analyses provided complementary results. However, discrepancies were greatest where a group's temporal response profile departed from the HRF used in the z‐score analyses. The random effect correction used in the ANOVA discounted distortions introduced into the average z‐score map by response magnitudes in a few participants. Furthermore, z‐score maps indicate response direction while the ANOVA time factor shows the presence of response modulation.
Angular gyrus, BA 39
An extended cluster of significant voxels was centered in the angular gyrus in the time effect map (Fig. 5). This distribution, which lay anterior to the visuotopic area borders illustrated in the flat maps, is labeled BA39 in the Talairach atlas [Talairach and Tournoux,1988]. All groups showed similar negative BOLD responses in this region.
Age of blindness onset
Average MR signal changes per voxel in visual cortex decreased with the age of blindness onset. Within the probable borders of V1, V3, V4v, MT, and LOC, for example, activity during both epoch types declined for the LB group who had lost sight after age 12 (Fig. 7). However, some LB individuals showed suprathreshold activity (Fig. 4). None of the linear regressions were significantly different from a zero slope nor do they differ between regions. This likely reflected the small degrees of freedom and mostly single data points for ages >0.
Figure 7.
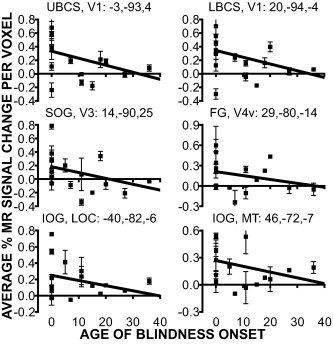
Magnitude of BOLD responses as a function of age of blindness onset in defined regions. Title for each graph identifies the anatomical location, visual area, and atlas coordinates for the centers of mass from the ANOVA effects (see Table II). Each point shows single participant mean and S.E.M. for target and non‐target epoch types.
Mixed Effects Group Analysis of Epoch Type Differences in Parietal and Frontal Cortex
The following emphasizes a contrast between responses across groups to the target and nontarget epoch types because, with one exception: there were no group differences in frontal and parietal regions. Only a right postcentral gyral region, identified in the time‐by‐group effect map (Table II, PCG, BA3, 1), showed significantly larger responses in EB and LB vs. NS for both epoch types.
Parietal cortex
All groups had significantly larger positive BOLD responses in all somatosensory cortex regions during target epochs (Fig. 8, Table III, negative t‐values when comparing non‐target to target epochs). Left hemisphere regions, contralateral to the stimulated finger, included subdivisions of the postcentral gyrus that spanned from the posterior bank of the central sulcus to the anterior bank of the postcentral sulcus (BA 3, 1, and 2). In addition, a large swath of activity occupied the parietal operculum and adjoining SMGi. On the right, the regions included the postcentral gyrus (BA3,1), parietal operculum, and adjoining SMGi (Table III). Response time courses differed between epoch types especially in OP bilaterally. As shown in Figure 8, the positive aspect of the responses in OP during target epochs peaked within ∼5 sec; this was followed by a pronounced negativity with a peak at ∼15 s. There was only a small positive BOLD response during non‐target trials (Fig. 8, OP graphs). Additional right hemisphere activity was noted in the cortex medial to the anterior tip of the intraparietal sulcus (BA 5) and several regions within SMGs and precuneus (BA7). The peak of the positive response in SMGs was especially delayed to ∼15 s.
Frontal cortex
Right hemisphere motor areas in the precentral gyrus (BA 4 and 6), bilaterally in medial frontal cortex (supplementary motor area, BA6) and adjacent cingulate cortex (BA32) had significantly larger positive BOLD responses during target epochs (Fig. 8, Table III).
Left superior and medial portions of SMGs (BA7), superior frontal sulcus and middle frontal gyrus (BA8), and right medial parietal cortex in the precuneus (BA5) and posterior cingulate (BA31) showed significantly more negative BOLD responses during target epochs (Table III, positive t‐values).
DISCUSSION
Visual Cortex Activity to Vibrotactile Stimulation
A relatively simple vibrotactile discrimination task activated striate and extrastriate parts of visual cortex in blind people. Early blind showed the greatest involvement of visual cortex, but a few LB participants had some visual cortex activity that included instances of V1 responses. Prior findings that visual cortex in blind people exhibits striking responsiveness during language tasks may be a consequence of adaptations to process non‐visual information. This interpretation is consistent with demonstrations of cross‐modal visual cortex (including V1) activity in visually deprived animals where language is not a factor [Hyvärinen et al.,1981; Kahn and Krubitzer,2002; Newton et al.,2002; Rauschecker,1995].
Many studies show that tactile stimulation, including that associated with Braille reading, activates visual cortex in blind people [Amedi et al.,2003; Büchel et al.,1998; Burton et al.,2002a; Gizewski et al.,2003; Melzer et al.,2001; Sadato et al.,1996,1998,2002; Uhl et al.,1991,1993]. However, several issues confound interpretation of these earlier findings because few previous studies utilized a strictly passive tactile discrimination task. First, explicit language effects were present in studies where participants read Braille words [Amedi et al.,2003; Burton et al.,2002a; Gizewski et al.,2003; Melzer et al.,2001; Sadato et al.,1996,1998; Uhl et al.,1991,1993]. Language processes also might have been engaged in studies that used Braille letter patterns [Büchel et al.,1998; Sadato et al.,2002] since a proficient Braille reader could first identify the Braille letter as an aid to the tactile discrimination task. This strategy might explain the higher percentage of correct trials in a Braille pattern match/no‐match task that was reported for early blind participants, who are normally better readers (EB 80.7% vs. 57.8% for LB and 59.2% for NS) [Sadato et al.,2002]. Second, studies that used non‐Braille tactile stimulation, mostly as controls [Gizewski et al.,2003; Sadato et al.,1996,1998; Uhl et al.,1991,1993], included haptic behavior for stimulus delivery, which intertwined the effects of hand movement with tactile stimulation [Gizewski et al.,2003; Sadato et al.,2002], or used uncontrolled manual application of the stimuli [Uhl et al.,1991,1993].
The present study avoided a language confound by using single frequency sinusoidal vibrations that were readily compared without naming the stimuli. The mixed effects ANOVA of time‐by‐group or time‐by‐epoch‐type effects found no indication that activity in the language areas [Bookheimer,2002] differed among the groups or epoch types, which suggests that any possible verbal tagging of the stimuli was identical among the groups. In addition, haptic behavior was avoided by passively stimulating the same fingertip location in all participants with controlled and consistently specified vibrations. Possible movement of the stimulated finger was also minimized by passively constraining the hand and fingertip and by supporting the arm on cushions. However, a task requiring some discrimination behavior was potentially of critical importance to revealing cross‐modal adaptations in visual cortex. Merely stimulating a cutaneous nerve electrically does not appear to activate visual cortex in blind people [Gizewski et al.,2003].
There was no long‐term memory component to the one‐back vibrotactile matching task, and participants needed only a few training trials to learn the task just minutes before scanning. However, short‐term (300 ms) sensory memory was needed for the matching task. Thus, neither long‐term verbal memory [Amedi et al.,2003] nor higher level language processes [Burton,2003; Burton et al.,2003] adequately account for group differences in visual cortex activity to tactile stimulation.
Cross‐modal adaptation vs. an existing feature
A parsimonious interpretation of the present findings is that somatosensory activation of visual cortex is a necessary adaptation to blindness at least in Braille readers. Others have suggested these changes constitute a competition between modalities that are coupled with anatomical networks designed for single modalities and are expressions of an existing metamodal capability [Pascual‐Leone and Hamilton,2001]. A corollary notion is that the sensory systems are not separate entities, but are capable of being influenced by different modalities depending on the task. For example, unimodal visual activation of extrastriate visual areas in the fusiform and lingual gyri can be affected by a touched hand on the same side as the visual stimulus [Macaluso and Driver,2001; Macaluso et al.,2002].
Recent studies with sighted people strengthen the suggestion that tactile processing in visual cortex derives from an existing somatosensory processing capability rather than a dramatic alteration induced by blindness. The principal findings were that visual cortex excitability increased after 60 min of no vision [Boroojerdi et al.,2000] and responded to tactile stimulation after subjects wore blindfolds for 5 days [Pascual‐Leone and Hamilton,2001]. Sighted people also show increased tactile acuity following exposure to short‐term visual deprivation (90 min) [Facchini and Aglioti2003]; (5 days) [Kauffman et al.,2002]. The heightened tactile acuity obtained in sighted participants resembles that reported in congenitally blind people [Goldreich and Kanics,2003; Stevens et al.,1996; Van Boven et al.,2000].
An existing metamodal capacity in visual cortex predicts that tactile stimulation ought to activate similar visual cortical areas despite differences in age of blindness onset (ABO). However, despite evidence for some visual cortex activity in all late blind individuals, early blind showed responses in more visual cortical areas. This suggests that ABO inversely affects visual cortex plasticity [Cohen et al.,1999]. In addition, early blind are more adaptive than late blind because they showed significantly greater response magnitudes in visual cortex regions defined from the time‐by‐group effect (i.e., V1, V3, V7, LOC, and MT), and the magnitude of the responses in these regions declined in late blind who lost all sight after age 12 years. These results suggest greater adaptive changes in early blind rather than an existing metamodal capacity revealed by any blindness. Except for three sighted people showing activity in an isolated part of V1, no other visual areas showed positive responses in the sighted group, although these individuals wore blindfolds for >1 hr during a scanning session. This indicates that a visual cortex response to tactile stimulation in blindness is not necessarily an existing property that reveals a metamodal capacity. Thus, cross‐modal responses in blind people constitute adaptations that have a critical period of susceptibility [Cohen et al.,1999] as these changes are more likely to be expressed at younger ABO and are especially prominent in congenital or early blind people.
Distinctions between visual areas
Comparisons between the current average z‐score maps and those noted previously [Burton et al.,2002a,2002b,2003] show several similarities and one notable difference in the organization and extent of activated visual cortex. As in these previous studies, the early blind group had the most extensive bilateral activation in areas V1‐MT. In contrast to prior findings, the average z‐score maps in the early blind showed activity throughout the foveal and adjacent parafoveal eccentricities of retinotopic components of the visual areas. The average z‐score maps in late blind showed activity that was even more confined to the foveal eccentricities. This contrasts with little or no activity in comparable foveal eccentricities in our prior studies, all of which involved a language task [Burton et al.,2002a,2002b,2003]. A speculative interpretation of this difference is that in the present study participants focused attention on tactile stimulation of a single fingertip as opposed to language. Consistent with this notion of task‐based distinctions in visual cortex processing, we observed declining response magnitudes in all visual cortical areas at different ages of blindness onset, which contrasts with relatively constant V1 response magnitudes across all blind people, irrespective of ABO for semantic and phonological language tasks [Burton et al.,2003].
An interpretation of visual cortical areas activated by a tactile task is to view the group responses to touch as uniform and holistic rather than as activity in specific visuotopic entities defined in sighted people. This suggestion gains support from finding overall similarities between the temporal profiles of BOLD responses between regions. Some of this comparability possibly derives from overlapping contributions of activity between neighboring regions, given limitations in intersubject averaging. However, the distance between the UBCS and several extrastriate regions (e.g., LOC in IOG) partially allays this caution. Nevertheless, task modulation of responses in the upper bank of the calcarine sulcus predicts the time course of responses in the cuneus or inferior occipital gyrus (i.e., in the left hemisphere V1 vs. V3 and V1 vs. LOC).
It remains unknown whether the activity in these different regions indicates distinct processes. However, differences in activated portions of visual cortex observed in various studies (the current study vs. Burton et al.,2002a,2002b,2003] suggest selective processing appropriate to task demands, which also implicates the functional utility of this activity in blind people. Thus, parts of visual cortex essential for somatosensory processing differ from those engaged during semantic language tasks in blind people. Differences in the spatial extent of visual cortex activated in early compared to late blind people also implies possible distinctions in the way that visual cortex contributes to task performance in these two groups. Clarifying the nature of these processes will require further study.
Visual stimulus attributes that engage these regions in sighted people have few parallels in blindness. For example, activity in or adjacent to the calcarine sulcus, though clearly involving V1, do not point toward processing shape details of a tactile stimulus, for which V1 is highly selective for visual stimulation in sighted people. Similarly, although tactile motion activates MT in sighted people [Hagen et al.,2002], there was no movement across the skin in the vibrotactile stimulation that activated MT in blind but not sighted people in the present study. However, we used canonical anatomical boundaries to reference the active regions in blind people to the visual areas identified in sighted people as an implied interpretation that these regions have selective, and as yet undefined, functions in blind people.
Visual areas, like the lateral occipital complex (LOC), that show responsiveness to somatosensory stimuli in sighted people, might provide similar functions even with blindness. For example, portions of LOC show activity during somatosensory macro‐object recognition tasks in sighted people [Amedi et al.,2002; James et al.,2002; Stoesz et al.,2003]. But these findings do not discount a likely cross‐modal interpretation of LOC activity in blind people because in the present study the non‐spatial vibrotactile discrimination task did not evoke LOC responses in sighted people. This is consistent with a previous report of no LOC activity for a discrimination task that involved micro‐spatial tactile features [Stoesz et al.,2003]. Visual imagery of objects or spatial locations also activates comparable parieto‐occipital regions in sighted and blind people [Arno et al.,2001; Vanlierde et al.,2003]. Tactile‐visual cross‐modal shape‐matching activates a related posterior portion of parietal‐occipital cortex in sighted people [Saito et al.,2003]. Some sighted participants who had V1 responses in the present study might have used visual imagery to “reconstruct the local geometry” [Kosslyn et al.,2001] of the vibrator on the finger. However, in the present study the dominant effect in most visual areas in sighted people was a delayed and sustained negative BOLD response, which normally suggests suppressed activity [Drevets et al.,1995; Gusnard and Raichle2001; Haxby et al.,1994]. This further emphasizes that the positive BOLD responses in these same visual areas in blind people represent a mechanism adapted to tactile discriminations rather than visual imagery of the tactile stimulation.
Somatosensory Cortex Activity
Primary somatosensory cortex, S1
An extensive literature shows expansions in primary somatosensory cortex (S1) of the representation of used fingers [Buonomano and Merzenich,1998; Elbert et al.,1995; Jenkins et al.,1990; Merzenich and Jenkins1993; Ramachandran and Hirstein1998; Recanzone et al.,1992c]. Because Braille literacy relies on extensive and continuous finger usage, Braille proficient blind people might have an expanded S1 region contralateral to their Braille reading hand. Only the early blind group showed an expanded zone of left hemisphere S1 activity. These individuals generally read faster, were daily Braille readers, and mostly read with their right hand. Larger representations of sensorimotor regions have been reported previously in the distribution of surface‐evoked potentials [Pascual‐Leone and Torres,1993] and in neuromagnetic recordings of a greater distance between the dipoles for stimulated fingers on the Braille reading hand [Sterr et al.,1998].
S1 expansions only in early blind might reflect different Braille reading styles from the late blind. Millar [1997] has shown that proficient Braille readers attend to a temporally dynamic texture stimulus based on the dot‐gap density differences across Braille cells and words. Fluent readers also use top‐down lexical and linguistic information to direct Braille reading. Less fluent readers, who often are late blind, apparently rely more on the shape of individual Braille cells and utilize phonemic and phonological clues to extract meaning from a text [Millar,1997]. These distinguishable reading styles could reflect different levels of tactile information usage. Although there was no statistical difference in the reading speeds of our two groups, we did not rigorously assess their ability to extract meaning from the test text. Our slowest readers were late blind, and average images that included activity from these individuals might have diminished average S1 spatial extent in this group.
In contrast, S1 response magnitudes were identical between the groups for each of the epoch types, and did not change with age of blindness onset. A prior report from a PET study showed larger regional cerebral blood flows in sensorimotor cortex contralateral to the preferred Braille reading finger in early blind [Sadato et al.,1998]. Generally, greater rCBF in PET would coincide with greater response magnitude in fMRI. Task differences and group differences in performance might explain the absence of correspondence between PET and fMRI measurements. In the PET study, early blind showed larger responses to a discrimination vs. non‐discrimination task [Sadato et al.,1998]. But only the early blind group correctly discriminated between the Braille patterns, possibly because their Braille fluency allowed them to identify the letters. The other two groups had chance performance on the discrimination task, and thus the cognitive effects of tactile stimulation and responses were equivalent between discrimination and non‐discrimination tasks. In the present study discrimination was required for all epochs. There was also no imbalance in performance accuracy across groups, which suggests an explanation for finding no group differences in S1 response magnitudes for each epoch type. However, the average S1 response magnitude across groups to the target was greater than that to the non‐target epoch. This difference possibly reflected the addition of left finger elevations to signal detection of matched vibration frequencies on target trials.
Differences in performing the left finger elevations also might explain significant group distinctions observed in the right S1 region. The early blind especially tended to elevate their entire hand and wrist when responding. In contrast, nearly all late blind and all sighted participants made discrete movements isolated to the left index finger.
Second somatosensory cortex, S2
Two regions were defined in the S2 area from the time‐by‐epoch‐type effect. One occupied the parietal operculum (OP) where it overlapped previously identified S2 [Burton,2001; Burton et al.,1993; Ledberg et al.,1995]. The other was posterior and juxtaposed in the inferior part of the supramarginal gyrus (SMGi), a region possibly analogous to monkey area 7b [Burton,2001; Eidelberg and Galaburda,1984]. There were no group differences in spatial extent or response magnitude bilaterally in either region. The OP region had brief, short latency positive BOLD responses during non‐target epochs and additionally showed sustained late negativity during target epochs. Exclusively positive BOLD responses in SMGi peaked later and were more sustained than the OP responses for both epoch types. Target epochs evoked larger responses possibly because of added effort to elevate the left index finger during target trials. In contrast, Sadato and colleagues reported decreased rCBF [Sadato et al.,1998] or negative BOLD responses [Sadato et al.,2002] in a parietal opercular S2 region in early blind, which they contrasted with positive activity in late blind and sighted people. We did not confirm this effect even in larger bilateral regions that were centered on the parietal operculum as they did. Responses within these larger regions were highly correlated and showed no significant changes in response magnitudes with age of blindness onset or between sighted and any blind group.
Reports of substantial S2 activity during sensory guided motor tasks [Binkofski et al.,1999a,b] suggest an alternative interpretation of the findings from Sadato and colleagues [Sadato et al.,2002]. In the present study, delayed negative S2 responses occurred during the target epochs when left finger extensions were required. The negative responses mirrored predictable strong positive responses in the right motor cortex and area 5 that possibly contributed to the movements. The absence of OP negative responses during non‐target epochs followed the absence of required movements. The studies of Sadato and colleagues involved complicated motor responses because the match/no‐match tactile discriminations drove selected button pushes with the non‐stimulated hand. Only automatic alternating button pushes were required on non‐discrimination trials, thus requiring no initial guidance from sensory information. However, because only early blind individuals correctly performed the tactile discrimination task in the study by Sadato and colleagues, they may also have been the only ones whose motor response selections were specifically guided and hence appropriately suppressed sensory information.
We observed later negativity only in the smaller opercular S2 region defined from the time‐by‐epoch‐type effect. This suggests that only a component of opercular S2 might show motor suppression of sensory input. The SMGi area identified from the time‐by‐epoch‐type effect had positive BOLD responses to the vibration stimuli in both epochs. The larger opercular S2 region, which included part of the SMGi area and was similar to the volume used by Sadato and colleagues, also showed no negative BOLD responses.
Parietal tactile attention regions
The rostral extent of the intraparietal sulcus and adjoining superior part of the supramarginal gyrus (SMGs) showed activity reflecting tactile attention [reviewed in Burton et al.,1999; Burton and Sinclair,2000]. In the present study, a greater spatial extent of right SMGs was noted, which is consistent with clinical findings that right parietal strokes lead to greater tactile neglect [Critchley,1953]. Activity in this cortex possibly reflected the sustained vigilance needed to detect infrequent target trials. Blind participants showed larger SMGs spatial extent bilaterally. This difference suggests that blind people might have used more neural resources due to learned skills in attending to tactile stimuli.
Acknowledgements
We are indebted to Patti Schonlau for recruiting blind people for this study, Dr. A. Snyder for image reconstruction and region identification routines, Dr. M. McAvoy for statistical analysis of scanner images, and John Kreitler for design and construction of the tactile stimulator.
Footnotes
Regional activity distributions did not differ in contrasts between trials with only 25‐ and 100‐Hz vibrations.
Region labeled OP includes the second somatosensory areas (S2). However, a distinct S2 is not distinguishable in the current images given the continuity of activity in SMGi.
REFERENCES
- Allard T, Clark SA, Jenkins WM, Merzenich MM (1991): Reorganization of somatosensory area 3b representations in adult owl monkeys after digital syndactyly. J Neurophysiol 66: 1048–1058. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, Hendler T, Malach R, Zohary E (2002): Convergence of visual and tactile shape processing in the human lateral occipital complex. Cereb Cortex 12: 1202–12. [DOI] [PubMed] [Google Scholar]
- Amedi A, Raz N, Pianka P, Malach R, Zohary E (2003): Early 'visual' cortex activation correlates with superior verbal memory performance in the blind. Nat Neurosci 6: 758–66. [DOI] [PubMed] [Google Scholar]
- Andersson JL, Sundin A, Valind S (1995): A method for coregistration of PET and MR brain images. J Nucl Med 36: 1307–1315. [PubMed] [Google Scholar]
- Arno P, De Volder AG, Vanlierde A, Wanet‐Defalque MC, Streel E, Robert A, Sanabria‐Bohorquez S, Veraart C (2001): Occipital activation by pattern recognition in the early blind using auditory substitution for vision. Neuroimage 13: 632–645. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz RJ, Rizzolatti G, Freund H (1999a): A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur J Neurosci 11: 3276–3286. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Stephan KM, Rizzolatti G, Seitz RJ, Freund HJ (1999b): A parieto‐premotor network for object manipulation: evidence from neuroimaging. Exp Brain Res 128: 210–213. [DOI] [PubMed] [Google Scholar]
- Bookheimer S (2002): Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci 25: 151–88. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Bushara KO, Corwell B, Immisch I, Battaglia F, Muellbacher W, Cohen LG (2000): Enhanced excitability of the human visual cortex induced by short‐term light deprivation. Cereb Cortex 10: 529–534. [DOI] [PubMed] [Google Scholar]
- Bosch V (2000): Statistical analysis of multi‐subject fMRI data: assessment of focal activations. J Magn Reson Imag 11: 61–64. [DOI] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GH, Heeger DJ (1996): Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci 16: 4207–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büchel C, Price C, Frackowiak RS, Friston K (1998): Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain 121: 409–419. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM (1998): Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186. [DOI] [PubMed] [Google Scholar]
- Burton H (2001): Brain imaging studies of human somatosensory cortical regions: results from brain imnaging studies in humans In: Nelson RJ, editor. The somatosensory system: deciphering the brain's own body image. Boca Raton: CRC Press; p 27–72. [Google Scholar]
- Burton H (2003): Visual cortex activity in early and late blind people. J Neurosci 23: 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ (2000): Attending to and remembering tactile stimuli: a review of brain imaging data and single neuron responses. J Clin Neurophysiol 17: 575–591. [DOI] [PubMed] [Google Scholar]
- Burton H, Videen TO, Raichle ME (1993): Tactile‐vibration‐activated foci in insular and parietal‐opercular cortex studied with positron emission tomography: mapping the second somatosensory area in humans. Somatosens Mot Res 10: 297–308. [DOI] [PubMed] [Google Scholar]
- Burton H, Abend NS, MacLeod A‐MK, Sinclair RJ, Snyder AZ, Raichle ME (1999): Tactile attention tasks enhance activation in somatosensory regions of parietal cortex: a positron emission tomography study. Cerebral Cortex 9: 662–674. [DOI] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Conturo TE, Akbudak E, Ollinger JM, Raichle ME (2002a): Adaptive changes in early and late blind: a fMRI study of Braille reading. J Neurophysiol 87: 589–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, Diamond J, Raichle ME (2002b): Adaptive changes in early and late blind: a fMRI study of verb generation to heard nouns. J Neurophysiol 88: 3359–3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Diamond JB, McDermott KB (2003): Dissociating cortical regions activated by semantic and phonological tasks to heard words: a fMRI study in blind and sighted individuals. J Neurophysiol 90: 1965–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SA, Allard T, Jenkins WM, Merzenich MM (1988): Receptive fields in the body‐surface map in adult cortex defined by temporally correlated inputs. Nature 332: 444–445. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Weeks RA, Sadato N, Celnik P, Ishii K, Hallett M (1999): Period of susceptibility for cross‐modal plasticity in the blind. Ann Neurol 45: 451–460. [DOI] [PubMed] [Google Scholar]
- Critchley M (1953): The parietal lobe. London: Edward Arnold. [Google Scholar]
- Drevets WC, Burton H, Videen TO, Snyder AZ, Simpson JR, Jr. , Raichle ME (1995): Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 373: 249–252. [DOI] [PubMed] [Google Scholar]
- Drury HA, Van Essen DC, Anderson CH, Lee CW, Coogan TA, Lewis JW (1996): Computerized mappings of the cerebral cortex. A multiresolution flattening method and a surface‐based coordinate system. J Cogn Neurosci 8: 1–28. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Galaburda AM (1984): Inferior parietal lobule: divergent architectonic asymmetries in the human brain. Arch Neurol 41: 843–852. [DOI] [PubMed] [Google Scholar]
- Elbert T, Pantev C, Wienbruch C, Rockstroh B, Taub E (1995): Increased cortical representation of the fingers of the left hand in string players. Science 270: 305–307. [DOI] [PubMed] [Google Scholar]
- Facchini S, Aglioti SM (2003): Short term light deprivation increases tactile spatial acuity in humans. Neurology 60: 1998–1999. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC (1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Friston K, Ashburner J, Poline J, Frith C, Heather J, Frackowiak R (1995a): Spatial registration and normalization of images. Hum Brain Mapping 2: 165–189. [Google Scholar]
- Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R (1995b): Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapping 2: 189–210. [Google Scholar]
- Gizewski ER, Gasser T, de Greiff A, Boehm A, Forsting M (2003): Cross‐modal plasticity for sensory and motor activation patterns in blind subjects. Neuroimage 19: 968–975. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM (2003): Tactile acuity is enhanced in blindness. J Neurosci 23: 3439–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME (2001): Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci 2: 685–694. [DOI] [PubMed] [Google Scholar]
- Hadjikhani N, Liu AK, Dale AM, Cavanagh P, Tootell RB (1998): Retinotopy and color sensitivity in human visual cortical area V8. Nat Neurosci 1: 235–241. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, McGlone F, Essick G, Dancer C, Pardo JV (2002): Tactile motion activates the human middle temporal/V5 (MT/V5) complex. Eur J Neurosci 16: 957–964. [DOI] [PubMed] [Google Scholar]
- Hajnal JV, Saeed N, Soar EJ, Oatridge A, Young IR, Bydder GM (1995): A registration and interpolation procedure for subvoxel matching of serially acquired MR images. J Comput Assist Tomogr 19: 289–296. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL (1994): The functional organization of human extrastriate cortex: a PET‐rCBF study of selective attention to faces and locations. J Neurosci 14: 6336–6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvärinen J, Carlson S, Hyvarinen L (1981): Early visual deprivation alters modality of neuronal responses in area 19 of monkey cortex. Neurosci Lett 26: 239–243. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA (2002): Haptic study of three‐dimensional objects activates extrastriate visual areas. Neuropsychologia 40: 1706–1714. [DOI] [PubMed] [Google Scholar]
- Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic‐Robles E (1990): Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol 63: 82–104. [DOI] [PubMed] [Google Scholar]
- Kahn DM, Krubitzer L (2002): Massive cross‐modal cortical plasticity and the emergence of a new cortical area in developmentally blind mammals. Proc Natl Acad Sci USA 99: 11429–11434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG (1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Kauffman T, Theoret H, Pascual‐Leone A (2002): Braille character discrimination in blindfolded human subjects. Neuroreport 13: 571–574. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Ganis G, Thompson WL (2001): Neural foundations of imagery. Nat Rev Neurosci 2: 635–642. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT (1995): A modality‐independent approach to spatial normalization of tomographic images of the human brain. Hum Brain Mapping 3: 209–223. [Google Scholar]
- Ledberg A, O'Sullivan BT, Kinomura S, Roland PE (1995): Somatosensory activations of the parietal operculum of man. A PET study. Eur J Neurosci 7: 1934–1941. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Driver J (2001): Spatial attention and crossmodal interactions between vision and touch. Neuropsychologia 39: 1304–1316. [DOI] [PubMed] [Google Scholar]
- Macaluso E, Frith CD, Driver J (2002): Crossmodal spatial influences of touch on extrastriate visual areas take current gaze direction into account. Neuron 34: 647–658. [DOI] [PubMed] [Google Scholar]
- Melzer P, Morgan VL, Pickens DR, Price RR, Wall RS, Ebner FF (2001): Cortical activation during Braille reading is influenced by early visual experience in subjects with severe visual disability: a correlational fMRI study. Hum Brain Mapp 14: 186–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Jenkins WM (1993): Reorganization of cortical representations of the hand following alterations of skin inputs induced by nerve injury, skin island transfers, and experience. J Hand Ther 6: 89–104. [DOI] [PubMed] [Google Scholar]
- Millar S 1997. Reading by touch. London: Routledge; 337 p. [Google Scholar]
- Mugler JPd, Brookeman JR (1990): Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE). Magn Reson Med 15: 152–157. [DOI] [PubMed] [Google Scholar]
- Newton JR, Sikes RW, Skavenski AA (2002): Cross‐modal plasticity after monocular enucleation of the adult rabbit. Exp Brain Res 144: 423–429. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE (1997): Anatomic localization and quantitative analysis of gradient refocused echo‐planar fMRI susceptibility artifacts. Neuroimage 6: 156–167. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Hamilton R (2001): The metamodal organization of the brain. Prog Brain Res 134: 427–445. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Torres F (1993): Plasticity of the sensorimotor cortex representation of the reading finger in Braille readers. Brain 116: 39–52. [DOI] [PubMed] [Google Scholar]
- Pascual‐Leone A, Cammarota A, Wassermann EM, Brasil‐Neto JP, Cohen LG, Hallett M (1993): Modulation of motor cortical outputs to the reading hand of braille readers. Ann Neurol 34: 33–37. [DOI] [PubMed] [Google Scholar]
- Raczkowski D, Kalat JW, Nebes R (1974): Reliability and validity of some handedness questionnaire items. Neuropsychologia 12: 43–47. [DOI] [PubMed] [Google Scholar]
- Ramachandran VS, Hirstein W (1998): The perception of phantom limbs. The D. O. Hebb lecture. Brain 121: 1603–1630. [DOI] [PubMed] [Google Scholar]
- Rauschecker JP (1995): Compensatory plasticity and sensory substitution in the cerebral cortex. Trends Neurosci 18: 36–43. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Jenkins WM, Hradek GT, Merzenich MM (1992a): Progressive improvement in discriminative abilities in adult owl monkeys performing a tactile frequency discrimination task.. J Neurophysiol 67: 1015–1030. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM (1992b): Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. J Neurophysiol 67: 1057–1070. [DOI] [PubMed] [Google Scholar]
- Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR (1992c): Topographic reorganization of the hand representation in cortical area 3b of owl monkeys trained in a frequency‐discrimination task. J Neurophysiol 67: 1031–1056. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual‐Leone A, Grafman J, Ibanez V, Deiber MP, Dold G, Hallett M (1996): Activation of the primary visual cortex by Braille reading in blind subjects. Nature 380: 526–528. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual‐Leone A, Grafman J, Deiber MP, Ibanez V, Hallett M (1998): Neural networks for Braille reading by the blind. Brain 121: 1213–1229. [DOI] [PubMed] [Google Scholar]
- Sadato N, Okada T, Honda M, Yonekura Y (2002): Critical period for cross‐modal plasticity in blind humans: a functional MRI study. Neuroimage 16: 389–400. [DOI] [PubMed] [Google Scholar]
- Saito DN, Okada T, Morita Y, Yonekura Y, Sadato N (2003): Tactile‐visual cross‐modal shape matching: a functional MRI study. Brain Res Cogn Brain Res 17: 14–25. [DOI] [PubMed] [Google Scholar]
- Sinclair RJ, Burton H (1996): Discrimination of vibrotactile frequencies in a delayed pair comparison task. Percept Psychophys 58: 680–692. [DOI] [PubMed] [Google Scholar]
- Snyder AZ 1996. Difference image vs. ratio image error function forms in PET‐PET realignment In: Bailey D, Jones T, editors. Quantification of brain function using PET. San Diego: Academic Press; p 131–137. [Google Scholar]
- Sterr A, Muller MM, Elbert T, Rockstroh B, Pantev C, Taub E (1998): Perceptual correlates of changes in cortical representation of fingers in blind multifinger Braille readers. J Neurosci 18: 4417–4423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J, Foulke E, Patterson M (1996): Tactile acuity, aging, and braille reading in long‐term blindness. J Exp Psych: Applied 2: 91–106. [Google Scholar]
- Stoesz MR, Zhang M, Weisser VD, Prather SC, Mao H, Sathian K (2003): Neural networks active during tactile form perception: common and differential activity during macrospatial and microspatial tasks. Int J Psychophysiol 50: 41–49. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P 1988. Coplanar stereotaxic atlas of the human brain. New York: Thieme Medical; 122 p. [Google Scholar]
- Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW (1995): Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci 15: 3215–3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Dale AM, Sereno MI, Malach R (1996): New images from human visual cortex. Trends Neurosci 19: 481–489. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM (1997): Functional analysis of V3A and related areas in human visual cortex. J Neurosci 17: 7060–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tootell RB, Hadjikhani NK, Vanduffel W, Liu AK, Mendola JD, Sereno MI, Dale AM (1998): Functional analysis of primary visual cortex (V1) in humans. Proc Natl Acad Sci USA 95: 811–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl F, Franzen P, Lindinger G, Lang W, Deecke L (1991): On the functionality of the visually deprived occipital cortex in early blind persons. Neuroscience Letters 124: 256–259. [DOI] [PubMed] [Google Scholar]
- Uhl F, Franzen P, Podreka I, Steiner M, Deecke L (1993): Increased regional cerebral blood flow in inferior occipital cortex and cerebellum of early blind humans. Neurosci Lett 150: 162–164. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Hamilton RH, Kauffman T, Keenan JP, Pascual‐Leone A (2000): Tactile spatial resolution in blind braille readers. Neurology 54: 2230–2236. [DOI] [PubMed] [Google Scholar]
- Van Essen DC (2002a): Organization of visual areas in Macaque and human cerebral cortex In: Chalupa L, Werner J, editors. The visual neurosciences. Boston: MIT Press. [Google Scholar]
- Van Essen DC (2002b): Windows on the brain. The emerging role of atlases and databases in neuroscience. Curr Op Neurobiol 12: 574–579. [DOI] [PubMed] [Google Scholar]
- Van Essen DC (2004): Organization of visual areas in Macaque and human cerebral cortex In: Chalupa L, Werner JS, editors. The Visual Neurosciences: MIT Press; p 507–521. [Google Scholar]
- Van Essen D, Drury H, Joshi S, Miller M (1998): Functional and structural mapping of human cerebral cortex: solutions are in the surfaces. Proc Natl Acad Sci USA 95: 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Drury HA, Dickson J, Harwell J, Hanlon D, Anderson CH (2001): An integrated software suite for surface‐based analyses of cerebral cortex. J Am Med Inform Assoc 8: 443–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlierde A, De Volder AG, Wanet‐Defalque MC, Veraart C (2003): Occipito‐parietal cortex activation during visuo‐spatial imagery in early blind humans. Neuroimage 19: 698–709. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR (1993): MRI‐PET registration with automated algorithm. J Comput Assist Tomogr 17: 536–546. [DOI] [PubMed] [Google Scholar]
- Zarahn E, Aguirre G, D'Esposito M (1997): A trial‐based experimental design for fMRI. Neuroimage 6: 122–138. [DOI] [PubMed] [Google Scholar]


