Abstract
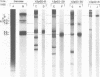
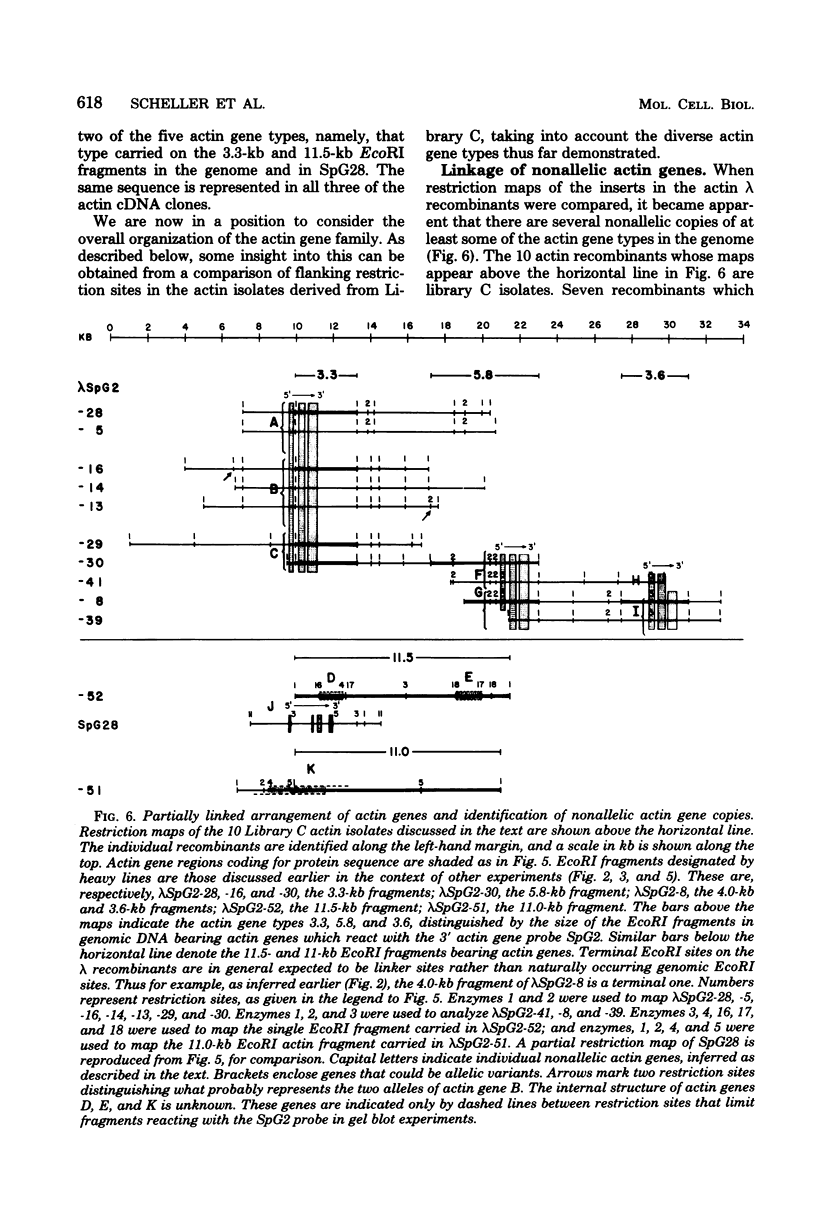
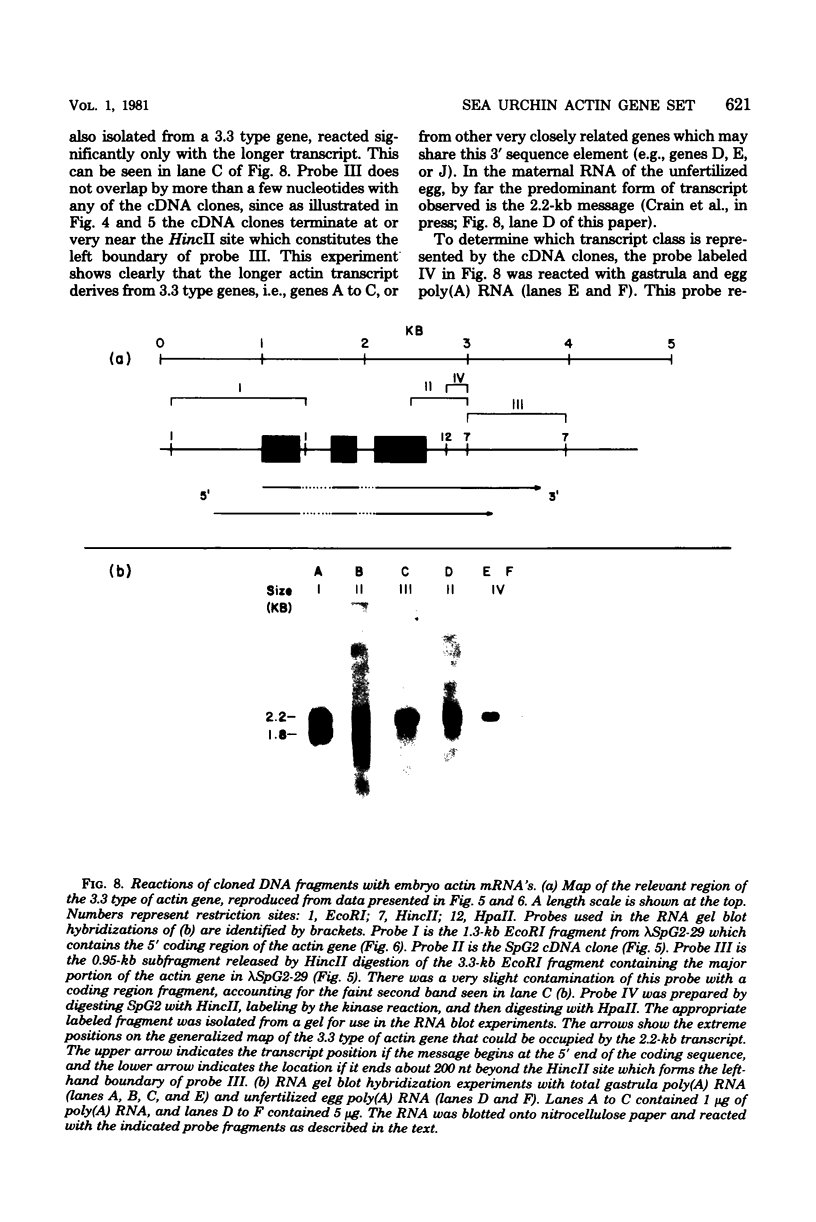
A set of at least 11 actin genes has been isolated from genomic recombinant deoxyribonucleic acid libraries of the sea urchin Strongylocentrotus purpuratus. Most of the isolates derive from a library which represents the genome of a single animal. There are at least five distinct types of sea urchin actin gene, some of which are represented by multiple copies in the genome. The actin gene types are distinguished by nonhomologous flanking sequences and intervening sequences, though the protein coding sequences appear in most cases to be quite similar. Eight of the 11 genes isolated have been recovered in lambda recombinants that contain two actin genes, linked at 5- to 9-kilobase distances. Restriction map overlaps suggest that the genome contains an array of at least three of these genes spaced over about 30 kilobases of deoxyribonucleic acid. In the linkage patterns observed, actin genes of diverse types were linked to each other. In early embryos, actin messenger ribonucleic acid (RNA) transcripts of 1.8 and 2.2 kilobases were found, and the longer of these transcripts was more prevalent in the maternal RNA of the egg. From RNA gel blot experiments, we conclude that the two transcripts derive from different actin gene types. Different repetitive sequences were located to either side of most of the actin genes, and in most observed cases the repeat sequences which were adjacent to actin genes of a given type were similar. The repeat sequences flanking the actin genes belonged to families which were transcribed, but those repeats in the neighborhood of the actin genes which have been investigated were not themselves represented in the stable RNAs of eggs or early embryos.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. M., Scheller R. H., Posakony J. W., McAllister L. B., Trabert S. G., Beall C., Britten R. J., Davidson E. H. Repetitive sequences of the sea urchin genome. Distribution of members of specific repetitive families. J Mol Biol. 1981 Jan 5;145(1):5–28. doi: 10.1016/0022-2836(81)90332-6. [DOI] [PubMed] [Google Scholar]
- Brosius J., Dull T. J., Noller H. F. Complete nucleotide sequence of a 23S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jan;77(1):201–204. doi: 10.1073/pnas.77.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn R. H., Lowry J. C., Kedes L. H. Histone genes of the sea urchin (S. purpuratus) cloned in E coli: order, polarity, and strandedness of the five histone-coding and spacer regions. Cell. 1976 Sep;9(1):147–161. doi: 10.1016/0092-8674(76)90060-x. [DOI] [PubMed] [Google Scholar]
- Costantini F. D., Britten R. J., Davidson E. H. Message sequences and short repetitive sequences are interspersed in sea urchin egg poly(A)+ RNAs. Nature. 1980 Sep 11;287(5778):111–117. doi: 10.1038/287111a0. [DOI] [PubMed] [Google Scholar]
- Costantini F. D., Scheller R. H., Britten R. J., Davidson E. H. Repetitive sequence transcripts in the mature sea urchin oocyte. Cell. 1978 Sep;15(1):173–187. doi: 10.1016/0092-8674(78)90093-4. [DOI] [PubMed] [Google Scholar]
- Davidson E. H., Hough B. R., Klein W. H., Britten R. J. Structural genes adjacent to interspersed repetitive DNA sequences. Cell. 1975 Mar;4(3):217–238. doi: 10.1016/0092-8674(75)90170-1. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Durica D. S., Schloss J. A., Crain W. R., Jr Organization of actin gene sequences in the sea urchin: molecular cloning of an intron-containing DNA sequence coding for a cytoplasmic actin. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5683–5687. doi: 10.1073/pnas.77.10.5683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firtel R. A., Timm R., Kimmel A. R., McKeown M. Unusual nucleotide sequences at the 5' end of actin genes in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6206–6210. doi: 10.1073/pnas.76.12.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Kindle K. L., Davidson N., Kindle K. L. The actin genes of Drosophila: a dispersed multigene family. Cell. 1980 Feb;19(2):365–378. doi: 10.1016/0092-8674(80)90511-5. [DOI] [PubMed] [Google Scholar]
- Infante A. A., Heilmann L. J. Distribution of messenger ribonucleic acid in polysomes and nonpolysomal particles of sea urchin embryos: translational control of actin synthesis. Biochemistry. 1981 Jan 6;20(1):1–8. doi: 10.1021/bi00504a001. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Coordinately expressed members of two chorion multi-gene families are clustered, alternating and divergently orientated. Nature. 1980 Apr 17;284(5757):635–638. doi: 10.1038/284635a0. [DOI] [PubMed] [Google Scholar]
- Kaback D. B., Angerer L. M., Davidson N. Improved methods for the formation and stabilization of R-loops. Nucleic Acids Res. 1979 Jun 11;6(7):2499–2317. doi: 10.1093/nar/6.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindle K. L., Firtel R. A. Identification and analysis of Dictyostelium actin genes, a family of moderately repeated genes. Cell. 1978 Nov;15(3):763–778. doi: 10.1016/0092-8674(78)90262-3. [DOI] [PubMed] [Google Scholar]
- Konkel D. A., Maizel J. V., Jr, Leder P. The evolution and sequence comparison of two recently diverged mouse chromosomal beta--globin genes. Cell. 1979 Nov;18(3):865–873. doi: 10.1016/0092-8674(79)90138-7. [DOI] [PubMed] [Google Scholar]
- Lacy E., Hardison R. C., Quon D., Maniatis T. The linkage arrangement of four rabbit beta-like globin genes. Cell. 1979 Dec;18(4):1273–1283. doi: 10.1016/0092-8674(79)90238-1. [DOI] [PubMed] [Google Scholar]
- Lasky L. A., Lev Z., Xin J. H., Britten R. J., Davidson E. H. Messenger RNA prevalence in sea urchin embryos measured with cloned cDNAs. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5317–5321. doi: 10.1073/pnas.77.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Hansen J. N., Konkel D., Leder A., Nishioka Y., Talkington C. Mouse globin system: a functional and evolutionary analysis. Science. 1980 Sep 19;209(4463):1336–1342. doi: 10.1126/science.7414319. [DOI] [PubMed] [Google Scholar]
- Lee A. S., Thomas T. L., Lev Z., Britten R. J., Davidson E. H. Four sizes of transcript produced by a single sea urchin gene expressed in early embryos. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3259–3263. doi: 10.1073/pnas.77.6.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev Z., Thomas T. L., Lee A. S., Angerer R. C., Britten R. J., Davidson E. H. Developmental expression of two cloned sequences coding for rare sea urchin embryo messages. Dev Biol. 1980 May;76(2):322–340. doi: 10.1016/0012-1606(80)90382-6. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown M., Taylor W. C., Kindle K. L., Firtel R. A., Bender W., Davidson N. Multiple, heterogeneous actin genes in Dictyostelium. Cell. 1978 Nov;15(3):789–800. doi: 10.1016/0092-8674(78)90264-7. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Tilghman S. M., Ovitt C., Fornwald J., Largen M. T. Structure and developmental expression of the chick alpha-actin gene. Nucleic Acids Res. 1980 Nov 11;8(21):4989–5005. doi: 10.1093/nar/8.21.4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal A., Garapin A., Cami B., Perrin F., Mandel J. L., LeMeur M., Brégégègre F., Gannon F., LePennec J. P., Chambon P. The ovalbumin gene region: common features in the organisation of three genes expressed in chicken oviduct under hormonal control. Nature. 1979 May 10;279(5709):125–132. doi: 10.1038/279125a0. [DOI] [PubMed] [Google Scholar]
- Scheller R. H., Costantini F. D., Kozlowski M. R., Britten R. J., Davidson E. H. Specific representation of cloned repetitive DNA sequences in sea urchin RNAs. Cell. 1978 Sep;15(1):189–203. doi: 10.1016/0092-8674(78)90094-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Tobin S. L., Zulauf E., Sánchez F., Craig E. A., McCarthy B. J. Multiple actin-related sequences in the Drosophila melanogaster genome. Cell. 1980 Jan;19(1):121–131. doi: 10.1016/0092-8674(80)90393-1. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. Actin amino-acid sequences. Comparison of actins from calf thymus, bovine brain, and SV40-transformed mouse 3T3 cells with rabbit skeletal muscle actin. Eur J Biochem. 1978 Oct 16;90(3):451–462. doi: 10.1111/j.1432-1033.1978.tb12624.x. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. At least six different actins are expressed in a higher mammal: an analysis based on the amino acid sequence of the amino-terminal tryptic peptide. J Mol Biol. 1978 Dec 25;126(4):783–802. doi: 10.1016/0022-2836(78)90020-7. [DOI] [PubMed] [Google Scholar]
- Wahli W., Dawid I. B., Wyler T., Weber R., Ryffel G. U. Comparative analysis of the structural organization of two closely related vitellogenin genes in X. laevis. Cell. 1980 May;20(1):107–117. doi: 10.1016/0092-8674(80)90239-1. [DOI] [PubMed] [Google Scholar]
- Zain S., Sambrook J., Roberts R. J., Keller W., Fried M., Dunn A. R. Nucleotide sequence analysis of the leader segments in a cloned copy of adenovirus 2 fiber mRNA. Cell. 1979 Apr;16(4):851–861. doi: 10.1016/0092-8674(79)90100-4. [DOI] [PubMed] [Google Scholar]