Abstract
Mechanisms to increase plasma high-density lipoprotein (HDL) or to promote egress of cholesterol from cholesterol-loaded cells (e.g., foam cells from atherosclerotic lesions) remain an important target to regress heart disease. Reconstituted HDL (rHDL) serves as a valuable vehicle to promote cellular cholesterol efflux in vitro and in vivo. rHDL were prepared with wild type apolipoprotein (apo) A-I and the rare variant, apoA-I Milano (M), and each apolipoprotein was reconstituted with phosphatidylcholine (PC) or sphingomyelin (SM). The four distinct rHDL generated were incubated with CHO cells, J774 macrophages, and BHK cells in cellular cholesterol efflux assays. In each cell type, apoA-I(M) SM-rHDL promoted the greatest cholesterol efflux. In BHK cells, the cholesterol efflux capacities of all four distinct rHDL were greatly enhanced by increased expression of ABCG1. Efflux to PC-containing rHDL was stimulated by transfection of a nonfunctional ABCA1 mutant (W590S), suggesting that binding to ABCA1 represents a competing interaction. This interpretation was confirmed by binding experiments. The data show that cholesterol efflux activity is dependent upon the apoA-I protein employed, as well as the phospholipid constituent of the rHDL. Future studies designed to optimize the efflux capacity of therapeutic rHDL may improve the value of this emerging intervention strategy.
Keywords: apolipoprotein, ABCA1, ABCG1, reverse cholesterol transport, macrophage
Introduction
Apolipoprotein (apo) A-I, the predominant apolipoprotein found on high-density lipoprotein (HDL), plays a central role in reverse cholesterol transport (RCT), the pathway by which cholesterol is removed from peripheral tissues and returned to the liver for excretion from the body. Elevated HDL levels are associated with a decreased incidence of atherosclerosis and promotion of RCT by increasing apoA-I and lowering plasma and whole body cholesterol levels.
ApoA-I Milano (M) is a rare mutant form of this protein discovered in a population of heterozygous carriers living in a small town near Milan, Italy. These individuals display markedly reduced plasma HDL levels, a low prevalence of atherosclerosis, and increased lifespan compared with noncarriers (Franceschini et al. 1980). ApoA-I(M) differs from wild-type (WT) apoA-I by a single amino acid substitution (Arg173Cys). The mechanistic link between apoA-I(M) and athero-protection remains unclear, despite many in vitro and in vivo experiments examining this protein and its phenotypic effects. Improved cardiovascular outcomes were consistently seen in animals treated with apoA-I(M) in several arterial injury models (Chiesa et al. 2002; Li et al. 1999; Soma et al. 1995), including ischemia-reperfusion (Calabresi et al. 2003; Marchesi et al. 2004) and injury following coronary balloon angioplasty (Ameli et al. 1994).
In addition, human clinical trials have been conducted to investigate the efficacy of apoA-I(M)-phospholipid complex therapy. Using a primary efficacy measure of the change in percent atheroma volume, a placebo-controlled clinical trial found that patients receiving apoA-I(M)-rHDL had significant regression of coronary atherosclerosis compared with controls, as measured by intravenous ultrasound (Nissen et al. 2003). In a follow-up trial measuring regression of arterial atheroma volume and changes in lumen volume, Nicholls et al. (2006) reported that injection of rHDL containing apoA-I (M) produced significant but heterogenous regression of coronary atherosclerosis in regions containing high plaque burden.
Because the efficacy of this therapy is considered to be related to the ability of apoA-I(M) to efflux cholesterol, determining the optimal composition of apoA-I rHDL is critical to the potential success of this therapy. In an in vitro model of cholesterol efflux, apoA-I(M)-containing sera from human apoA-I(M) carriers and apoA-I(M) transgenic mice showed a similar ability to efflux cholesterol, compared with control sera, despite a nearly 70% lower apoA-I level in the apoA-I (M) transgenic mice (Franceschini et al. 1999). This led to the conclusion that apoA-I(M) has a significantly higher cholesterol efflux potential than WT apoA-I. Despite this, it has been shown that the overall amount of cholesterol efflux is not appreciably higher in an apoA-I(M) genetic background, because apoA-I(M) plasma HDL levels are much lower than in WT apoA-I in control mice. These data suggest that atheroprotection conferred by apoA-I(M) is not attributable to a net higher cellular lipid mobilization (Alexander et al. 2009b; Weibel et al. 2007), but rather the unique efflux promotion properties of apoA-I(M) rHDL. In the present study we compared WT apoA-I rHDL prepared with phosphatidylcholine (PC) and sphingomyelin (SM) against apoA-I(M) prepared with the same phospholipids. The results show that, in all cell culture models tested, apoA-I(M) SM rHDL are the most effective at cholesterol efflux.
Materials and methods
Recombinant apoA-I expression and isolation
WT apoA-I and apoA-I(M) were expressed in Escherichia coli and isolated as described earlier (Ryan et al. 2003). ApoA-I(M) was generated by site directed mutagenesis using the Quick-Change XL kit from Stratagene according to the manufacturer's instructions. Introduction of the desired mutation was verified by dideoxy automated DNA sequencing.
rHDL formation
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (PC) and egg sphingomyelin (SM) were purchased from Avanti Polar Lipids (Pelham, Ala., USA). The phospholipids (5 mg) were dissolved in chloroform/methanol (3:1, v/v) and dried under a stream of N2 gas as a thin film in a glass tube. Following dispersion of the lipid in 50 mmol/L sodium phosphate, pH 7.0, 150 mmol/L NaCl, 2 mg of apoA-I was added, and the sample bath-sonicated at 24 °C until clear. The solutions containing intact rHDL were then dialyzed for ~16 h in tris buffered saline (pH 7.4).
Cell culture
CHO and BHK cells were maintained in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S). J774 cells were maintained in RPMI Medium 1640 with 10% FBS and 1% P/S. Acetylated LDL was prepared as previously described (Kiss et al. 2005).
[3H]cholesterol labeling and cholesterol efflux assays
CHO and BHK cells were labeled with [3H]cholesterol by incubating with the respective culture media containing [3H] cholesterol (3 µCi/mL) incorporated into 5% FBS for 24 h. J774 macrophages were cholesterol-loaded and labeled at the same time by incubation with 25 μg/mL acetylated LDL (with [3H]cholesterol, 3 µCi/mL). Cells were washed with media plus 2 mg/mL BSA to remove unincorporated radioactivity, and cells underwent an equilibration step by incubating with media containing BSA overnight. After equilibration, cholesterol efflux studies were conducted by introducing 5 or 10 µg of specified rHDL complexes to the cells for 4 h. The media were collected after 4 h and the amount of [3H] cholesterol measured by liquid scintillation spectrometry (PerkinElmer). Cells were then treated with 0.5 mol/L NaOH overnight, and the amount of cellular [3H]cholesterol determined. Percent efflux was computed from the percentage [3H]cholesterol in the media over total [3H]cholesterol (media + cell). BHK cells overexpressing ABCG1, ABCA1, and a nonfunctional mutant, W590S ABCA1, were generated using mifepristone-inducible GeneSwitch system as previously described (Vaughan and Oram 2005, 2006). ABCG1 redistributes cell cholesterol to domains removable by HDL but not by lipid-depleted apolipoproteins (Vaughan and Oram 2006). ABCA1 and ABCG1 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL (Vaughan and Oram 2006). BHK cells were treated with 10 nmol/L mifepristone during the labeling and equilibration steps for a total of 48 h.
Binding studies
rHDL were radio-iodinated using iodobeads (Pierce Chemical Co., Rockford, Ill., USA) (Bailey et al. 2010b), and binding assays performed as previously described (Kiss et al. 2011). Specific activities were 159.7 counts per min/ng protein, 143.7, 141.6, and 142.9 counts per min/ng (WT-PC, WT-SM, Milano-PC, Milano-SM, respectively). Briefly, all media and cells were cooled to 4 °C prior to the experiment. Different concentrations of rHDL (1–100 μg) were added to BHK cells expressing the different transporters at 4 °C and incubated for 30 min. Cells were washed 3 times with PBS-BSA and 2 times with PBS. Then 0.1 mol/L NaOH was added to cells at room temperature to release bound rHDL. Radioactivity was determined in a gamma counter (PerkinElmer 1470), and the results were normalized by specific activity added and total cellular protein.
Analytical procedures
Protein concentrations were determined by the BCA assay (Pierce Chemical Co., Rockford, Ill., USA) using bovine serum albumin as a standard. Native-PAGE was performed on 4%–20% acrylamide gradient slab gels electrophoresed at 35 mA constant current. Gels were stained with Gel Code Blue (Pierce Chemical Co., Rockford, Ill., USA) according to manufacturer's instructions. BHK cell lysates were prepared and Western blotting of ABCA1, and ABCG1 was performed (GAPDH was used as a control, all antibodies from Santa Cruz) according to Bailey et al. (2010a).
Results
ApoA-I ·phospholipid rHDL
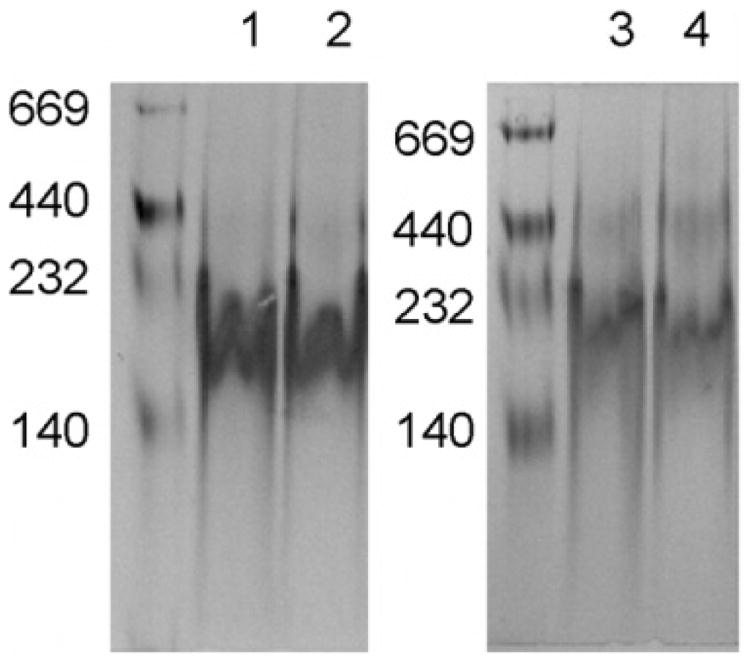
ApoA-I has been shown previously to interact with bilayer vesicles of phospholipid to form discrete, homogeneous rHDL that are organized as a disk-shaped bilayer, wherein two apoA-I molecules circumscribe the disk perimeter through interaction with phospholipid fatty acyl chains (Wu et al. 2007, 2009; Martin et al. 2006). In the present study, we employed two distinct bilayer forming phospholipids as substrates for rHDL formation. Whereas the phospholipids employed possess identical polar head groups, one is a glycerophospholipid, while the other is a sphingolipid. ApoA-I rHDL were characterized by native PAGE analysis (Fig. 1). The dimyristoyl molecular species of PC employed is advantageous for two reasons: (i) its phase transition temperature is 24 °C, promoting rHDL formation under standard laboratory conditions and (ii) the rHDL product is of uniform size. Thus, we compared rHDL generated with different apoA-I without the complication of multiple phospholipid species.
Fig. 1.
Similar sizes of rHDL with apoA-I(WT) and apoA-I(M). rHDL were made with PC with WT apoA-I (WT-PC; Lane 1) and apoA-I(M) (Milano-PC; Lane 2), or with SM with WT apoA-I (WT-SM; Lane 3) and apoA-I(M) (Milano-SM; Lane 4). These rHDL were applied to a NATIVE polyacrylamide gradient gel (4%–20%) for electrophoresis. Native molecular weight markers are shown.
Efflux studies
For each cell type (Chinese hamster ovary (CHO), J774 mouse macrophage, baby hamster kidney (BHK)), we utilized four different rHDL complexes that differ in their apoA-I and phospholipid components: WT apoA-I PC-rHDL (WT-PC rHDL), WT apoA-I SM-rHDL (WT-SM rHDL), apoA-I(M) PC-rHDL (Milano-PC rHDL), apoA-I(M) SM-rHDL (Milano-SM rHDL).
CHO cells
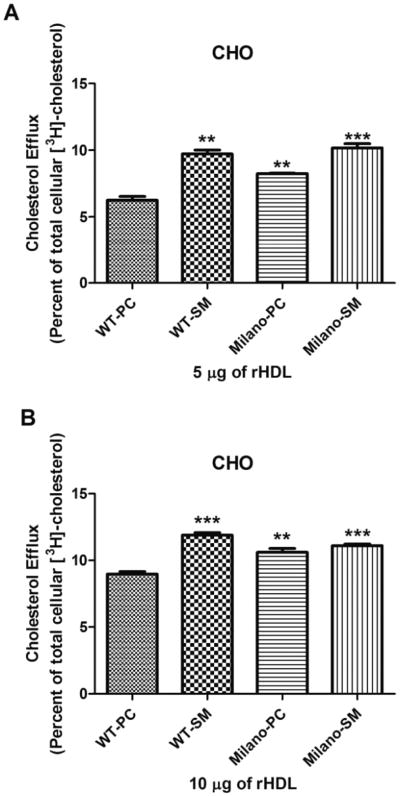
CHO cells are a standard cell type commonly used due to their ease of manipulation and transfection. CHO cells express ABCA1 and ABCG1 to mediate cholesterol efflux, although at low levels (Gelissen et al. 2006; Sankaranarayanan et al. 2009). rHDL prepared with WT apoA-I and SM were more effective than rHDL prepared with WT apoA-I and PC (Fig. 2). When apoA-I(M) was studied, SM containing rHDL showed enhanced efflux compared with PC containing rHDL. These experiments were performed at rHDL concentrations of 5 μg (Fig. 2, panel A) and 10 μg (Fig. 2, panel B), which represent the linear range of concentrations that promote efflux (below saturation levels) to maximize the differences between rHDL complexes. Results were similar for 5 μg and 10 μg, except that the results for 10 μg had a higher relative specific cholesterol efflux, and the differences decreased because the amount of efflux approached saturation (maximal amount of cholesterol that the rHDL can efflux). The results demonstrate that in PC-containing rHDL, apoA-I(M) is more efficient than WT apoA-I, and that SM is more efficient than PC in both WT apoA-I and apoA-I(M)-containing rHDL.
Fig. 2.
Optimizing efflux in CHO cells. Efflux assays were performed with the four rHDL (5 μg in panel A; 10 μg in panel B) in cholesterol loaded CHO cells. Results are expressed as the amount of [3H]cholesterol found in the medium as a percent of the total radioactivity (medium + cell radioactivity). Results shown are the average (± SD) of three experiments performed in quadruplicate. Milano-SM is the most effective rHDL. In comparison with WT-PC, significance is shown; ** p ≤ 0.01, *** p ≤ 0.001.
J774 macrophages
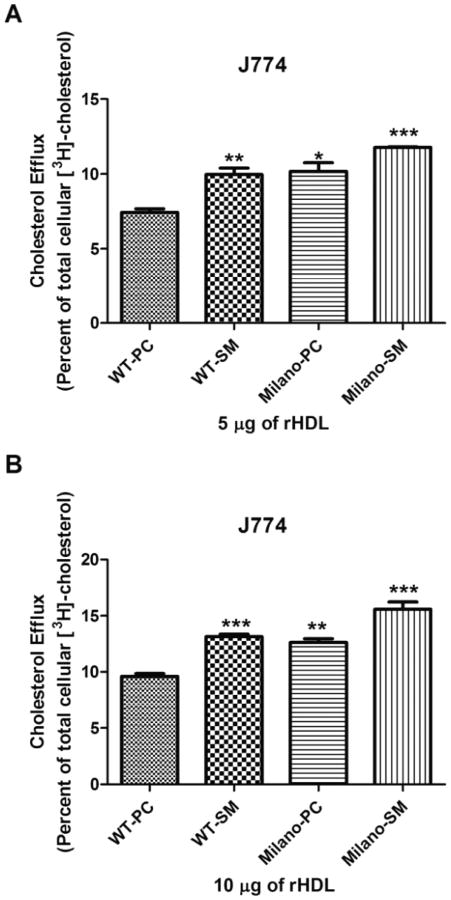
J774 macrophages are a murine cell line commonly used for efflux studies because they express robust levels of ABCA1 and ABCG1 (Khovidhunkit et al. 2003). In addition, because these macrophages express scavenger receptor class A, they can be readily loaded with cholesterol to mimic foam cells in atherosclerotic lesions. In this cell type, WT-SM rHDL were more effective than WT-PC rHDL (Fig. 3). Similarly, Milano-SM rHDL were more efficient than Milano-PC. Interestingly, Milano-PC rHDL and Milano-SM rHDL were more efficient than WT-PC rHDL and WT-SM rHDL, respectively. As with CHO cells, these experiments were performed at rHDL concentrations of 5 mg (Fig. 3, panel A) and 10 μg (Fig. 3, panel B), which represent the linear range of concentrations that promote efflux (below saturation levels) to maximize the differences between rHDL complexes. Results were very similar for 5 mg and 10 mg, with no apparent saturation due to the high efflux capacity of J774 macrophages. The results demonstrate that apoA-I (M) is more efficient than WT apoA-I, and SM is more efficient than PC, such that in combination, Milano-SM rHDL promote significantly greater cholesterol efflux than any other combination rHDL.
Fig. 3.
Optimizing efflux in J774 cells. Efflux assays were performed with the four rHDL (5 μg panel A; 10 mg panel B) in cholesterol loaded J774 macrophages. Results are expressed as the amount of [3H]-cholesterol found in the medium as a percent of the total radioactivity (medium + cell radioactivity). Results shown are the average (± SD) of three experiments performed in quadruplicate. Milano is better than WT, SM is better than PC and together there is an additive effect to make Milano-SM the most effective rHDL. In comparison to WT-PC, significance is shown; * p ≤0.05, ** p ≤0.01, *** p ≤0.001.
BHK cells
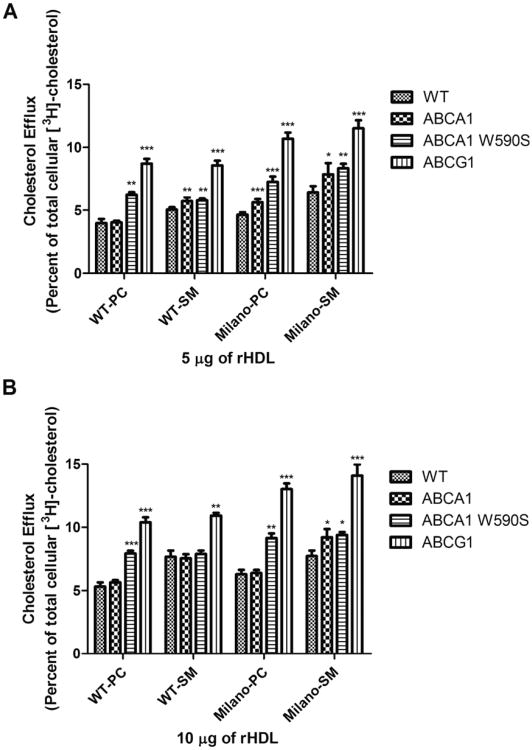
To assess the relative contribution of ABCA1 and ABCG1 to the overall efflux potential of a given rHDL, we employed BHK cells with inducible expression of ABCA1 and ABCG1 (Supplementary data Figs. 1 and 2).1 From the literature, it is anticipated that rHDL will preferentially interact with ABCG1 (Yvan-Charvet et al. 2010; Kennedy et al. 2005). Thus, as a negative control, we included BHK cells that in-ducibly express a nonfunctional mutant ABCA1 (W590S). In control BHK cells, WT-SM rHDL were more effective than WT-PC rHDL (Fig. 4; Supplementary data Fig. 3).1 Similarly, Milano-SM rHDL were more effective than Milano-PC rHDL. Upon expression of ABCA1, no additional efflux was observed with WT-PC rHDL, suggesting that ABCA1 is not predominantly involved in efflux to rHDL, as previously shown (Yvan-Charvet et al. 2010; Kennedy et al. 2005; Wang et al. 2004). Interestingly, expression of the nonfunctional mutant ABCA1 resulted in enhanced efflux to WT-PC rHDL. We speculate that ABCA1 and ABCG1 compete for binding to rHDL, but that efflux productive binding only occurs with ABCG1. Because rHDL does not bind nonfunctional ABCA1, a greater amount of rHDL is available for productive interaction with ABCG1. Consistent with the hypothesis that rHDL productively interacts with ABCG1, the highest efflux to WT-PC rHDL was observed upon ABCG1 expression. The Milano-PC rHDL efflux profile mirrored these results, except at a higher basal level of cholesterol efflux. Results obtained with SM rHDLs were somewhat different, however. WT-SM rHDL showed enhanced efflux compared with WT-PC rHDL. Upon expression of ABCA1, a significant relative enhancement of efflux was observed by WT-SM rHDL (at 5 μg rHDL), unlike results obtained with WT-PC rHDL. This enhanced efflux was not affected by expression of the nonfunctional ABCA1, suggesting that WT-SM rHDL interacts poorly with ABCA1. As expected, upon expression of ABCG1, the highest level of efflux was observed. The results were very similar for Milano-SM rHDL, except that the basal level of efflux was increased. Thus, apoA-I(M) is more efficient than WT apoA-I, and SM is more efficient than PC, such that in combination, Milano-SM rHDL are significantly more effective than any other combination of rHDL (Fig. 4; Supplementary data Fig. 3). Importantly, it appears that the presence of PC in rHDL confers enhanced binding capability to ABCA1, which in this case is a competing dead end reaction. Conversely, SM reduced the interaction of rHDL with ABCA1 and further promoted ABCG1-mediated efflux. The data show that differences in cholesterol efflux capacity are dependent upon the cell type, the apoA-I protein employed, as well as the lipid constituent of the rHDL.
Fig. 4.
Optimizing efflux in BHK cells. Efflux assays were performed with the four rHDL (5 μg panel A; 10 μg panel B) in the different cholesterol loaded BHK cells: BHK cells with no expressed protein (WT); BHK cells expressing ABCA1 (ABCA1); BHK cells expressing a dominant negative mutant of ABCA1 (ABCA1 W590S); and BHK cells expressing ABCG1 (ABCG1). Results are expressed as the amount of [3H]-cholesterol found in the medium as a percent of the total radioactivity (medium + cell radioactivity). Results shown are the average (± SD) of three experiments performed in quadruplicate. Expression of ABCG1 greatly increases the capacity of rHDL to promote net cholesterol efflux. In comparison to WT BHK cells in each set, significance is shown; * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001.
rHDL binding
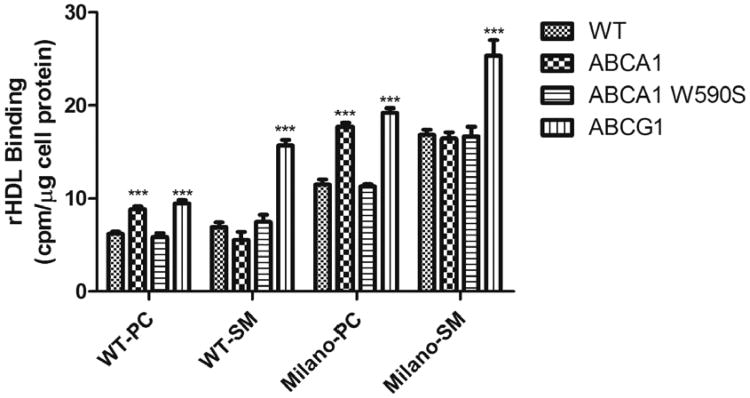
The observation that BHK cells expressing nonfunctional ABCA1 display increased efflux, compared with ABCA1-expressing cells when using PC containing rHDL, led us to speculate that WT-PC rHDL and Milano-PC rHDL may bind ABCA1, but not nonfunctional ABCA1, as a competing interaction. To confirm these observations, binding studies were performed. Radiolabeled rHDL were allowed to interact with BHK cells expressing different transporters at 4 °C, and then bound radioactivity was measured. Firstly, rHDL containing apoA-I(M) bound 2–3-fold more avidly to BHK cells than WT apoA-I rHDL (Fig. 5; Supplementary data Fig. 4). Secondly, for WT-PC rHDL, a demonstrable increase in binding to ABCA1 and ABCG1-expressing cells was observed, with no increase in binding to cells expressing the nonfunctional W590S ABCA1, compared with control BHK cells. This pattern was also observed for Milano-PC rHDL, except a higher level of binding was observed. These results show the WT-PC rHDL and Milano-PC rHDL bind to ABCA1-expressing cells, and this result may explain the decreased efflux for PC containing rHDL in these cells. Thirdly, for WT-SM rHDL, we observed a moderate decrease in binding to ABCA1-expressing cells, a moderate increase in binding to ABCA1 W590S-expressing cells, and a sizeable and significant increase in binding to ABCG1-expressing cells. This pattern was also observed for Milano-SM rHDL, except at a higher level of binding. These results show that SM-containing rHDL bind more poorly to ABCA1-expressing cells, and much more strongly to ABCG1-expressing cells. These binding results correlate well with the efflux results and explain why SM-containing rHDL may be a better efflux substrate.
Fig. 5.
rHDL binding to BHK cells. Binding assays were performed with the four radiolabeled rHDL (10 μg apoA-I protein) on BHK cells with no expressed protein (WT), BHK cells expressing ABCA1 (ABCA1), BHK cells expressing a nonfunctional mutant ABCA1 (ABCA1 W590S), and BHK cells expressing ABCG1 (ABCG1). Results are expressed as the mean cpm/mg cell protein ±SD, normalized to added specific activity. Each experimental condition was performed in triplicate, and the results presented here are the average of two experiments. In comparison with WT BHK cells in each set, significance is shown; *** p ≤ 0.001.
Discussion
ApoA-I(M) represents an interesting scientific paradox: the mutation destabilizes apoA-I leading to lower plasma HDL levels, but is correlated with higher lipid efflux and athero-protection, a trait usually associated with high levels of HDL. Biophysical studies of apoA-I(M) protein indicate that it is less stable than WT apoA-I. While mature apoA-I(M) is more susceptible to guanidine-HCl induced denaturation, the α-helical content is the same as WT apoA-I (Zhu et al. 2005). When complexed with 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine, apoA-I(M) forms two distinct 12.5 and 7.8 nm diameter particles and displays significantly higher protease sensitivity than WT apoA-I (Calabresi et al. 2001). The N-terminus of the protein is largely spared from limited proteolysis, while the C-terminus is more sensitive than WT apoA-I, displaying six sites in the central and carboxy-terminal portions of the protein (Calabresi et al. 2001). An additional characterization of several amino acid substitutions at position 173 showed that three specific mutations destabilized the lipid-free protein to a greater extent than that observed with WT apoA-I (R173K > R173S > R173C) (Alexander et al. 2009a). Furthermore, compared with WT apoA-I, apoA-I(M) has a lower affinity for lipids, while R173S apoA-I displays intermediate affinity. Mice expressing R173S apoA-I also display an intermediate HDL and cholesterol lowering phenotype compared with WT apoA-I or apoA-I(M). From these findings, it was suggested that replacement of Arg173 by Cys contributes to the altered functional properties of apoA-I(M). Based on an intra α-helical salt bridge observed between Arg173 and Glu169 in the X-ray crystal structure of WT apoA-I, these authors also suggest that disruption of this salt bridge, by the combined loss of the positively charged arginine and repulsion due to the cysteine substitution, causes apoA-I(M) to be significantly destabilized, altering its normal function. It is plausible that apoA-I molecules that are less stable as a monomer or form less homo-oligomers may have altered properties (Weers et al. 2011). However, in apoA-I(M), these changes manifest altered self-association properties (Bankston and Carta 2010), LCAT activation (Calabresi et al. 1997), efflux properties (Favari et al. 2007; Bielicki et al. 1997; Weibel et al. 2007), stability in plasma (Perez-Mendez et al. 2000), and effects on RCT (Alexander et al. 2009b). On the other hand, introduction of cysteine at position 173 has been proposed to induce intermolecular disulfide bond formation with either another apoA-I(M) or apoA-II (Wang et al. 2001), which may confer beneficial properties. Despite the mounting evidence that the Cys173 in apoA-I(M) destabilizes the protein, it remains largely unclear exactly how much of the apoA-I(M) phenotype is attributable to “reorganized” interactions or to some other unidentified property. Further studies are required to elucidate the mechanism by which apoA-I(M) imparts its atheroprotective effects. Regardless of the mechanism, use of apoA-I(M) rHDL has proven to be a successful strategy to reduce atherosclerotic burden in both animal models and humans (Ibanez et al. 2008; Nicholls et al. 2006; Nissen et al. 2003; Parolini et al. 2008; Shah et al. 2001; Wang et al. 2006; Zhao and Brown 2008).
The experiments presented here provide new insight regarding optimization of apoA-I(M) rHDL for therapeutic use. It is evident that addition of SM in lieu of PC increases the capacity of rHDL to promote cellular lipid efflux. The use of SM in rHDL has been previously studied (Marmillot et al. 2007; Rye et al. 1996) and shown to have profound effects. Rye et al. (1996) demonstrated that the presence of SM on rHDL altered the conformation of apoA-I on the rHDL surface, inhibited LCAT activity (because SM cannot be an acyl group donor in the LCAT reaction), but did not affect CETP-mediated transfers, all deemed as bad for use in promoting cholesterol efflux and reverse cholesterol transport. Interestingly, we now know that apoA-I(M) also has an altered structural conformation and impaired LCAT activation ability, so it is conceivable the effect of SM is partially mitigated. Marmillot et al. (2007) demonstrated that SM-containing rHDL gave rise to enhanced efflux in a RCT model (Marmillot et al. 2007). To our knowledge, the present study is the first to combine the use of apoA-I(M) and SM, and we would predict their effects to be additive.
For these experiments, we utilized CHO, J774, and BHK cells. It could be argued that CHO cells represent a poor model for efflux and are nonphysiological. However, CHO cells demonstrate efflux properties similar to fibroblasts and are commonly used for efflux and other measures. J774 macrophages represent an excellent model of macrophages, the most physiologically relevant cell type. We did in fact find that apoA-I(M) and SM had a significantly increased effect on cholesterol efflux. To elucidate the role of ABCA1 and ABCG1 in efflux, we took advantage of an inducible promoter system in BHK cells. Results with these cells clearly show that most efflux is attributable to ABCG1, and that interaction with ABCA1 represents a competing interaction that is largely nonproductive with rHDL as substrate. In the presence of a nonfunctional ABCA1, total cholesterol efflux was enhanced. Although we did not perform such an experiment, we would expect that co-expression of nonfunctional ABCA1 and functional ABCG1 would result in the highest measurable cholesterol efflux. Interestingly, we only observed this competing effect of ABCA1 with PC-containing rHDL and not SM-containing rHDL, and our binding results confirm these observations. The results demonstrate that SM-containing rHDL do not bind ABCA1 as well as PC-containing rHDL.
Use of rHDL as a substrate for efflux is very different than apolipoprotein-induced, efflux-dependent HDL biogenesis. The net result might be the same (i.e., net mobilization of cholesterol from peripheral cells promoting the RCT pathway), but the mechanisms are different. HDL biogenesis involved interaction of lipid-free or poorly lipidated apoA-I to ABCA1, acquisition of phospholipid, and free cholesterol and formation of a discoidal HDL particle. In the other case, addition of rHDL obviates the necessity of interacting with ABCA1, and the rHDL can directly interact with ABCG1 to promote net bulk cholesterol efflux. However, we observed that rHDL made with PC can interact with ABCA1, and this represents a competing interaction that results in less overall efflux (Figs. 4 and 5). However, rHDL with SM does not interact with ABCA1 (compare the efflux and binding for each rHDL with ABCA1 and the nonfunctional ABCA1 W590S). Moreover, SM and cholesterol have a high affinity for each other, and this physical property likely contributes to the heightened efflux. For these reasons, SM serves as the best acceptor of cholesterol. Furthermore, we find that cells expressing ABCG1 have the highest rate of efflux to rHDL. In the literature, the role of ABCG1 has been controversial in relation to ABCA1 and its effect on cholesterol efflux and atherosclerosis (Tarling et al. 2010; Wiersma et al. 2009; Burgess et al. 2008; Out et al. 2008; Yvan-Charvet et al. 2007; Wang et al. 2007; Ranalletta et al. 2006; Out et al. 2006). For example, Mukhamedova et al. (2008) reported experiments demonstrating optimization of cholesterol efflux to lipid-free apoA-I and HDL and the relatively ineffectual role of ABCG1 in promoting cholesterol efflux in RAW 264.7 macrophages (Mukhamedova et al. 2008). On the other hand, there may be a SM specific efflux from ABCG1 and a PC specific efflux from ABCA1 (Kobayashi et al. 2006; Sano et al. 2007; Matsuo et al. 2011), which could suggest a specific interaction of ABCG1 with SM. If SM rHDL can selectively target ABCG1 and avoid a competing nonproductive interaction with ABCA1, it should be possible to enhance cholesterol efflux. To validate this concept, it will be important to assess the effect of SM rHDL on macrophage cholesterol storage and atherosclerosis development/regression in an animal or human model.
Conclusion
The effect of SM-containing rHDL in vivo is currently unknown. The in vitro results presented here, however, suggest that Milano-SM rHDL enhance cholesterol efflux by ~20% compared with Milano-PC rHDL, thereby providing evidence that SM-containing rHDL represent a significant advantage to in vivo therapies.
Supplementary Material
Acknowledgments
We would like to thank Cyril Martin and Anne Smith and for expert technical help. This work was supported by CIHR (MOP-89972), the Heart and Stroke Foundation of Québec, and NIH (HL 64159).
Abbreviations
- apoA-I
apolipoprotein A-I
- ABC
ATP-binding cassette
- dimyristoyl phosphatidylcholine
PC
- HDL
high density lipoprotein
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- rHDL
reconstituted HDL
- RCT
reverse cholesterol transport
- SM
sphingomyelin
- WT
wild-type
Footnotes
Supplementary data are available with the article through the journal Web site at http://nrcresearchpress.com/doi/suppl/10.1139/o2012-015.
Contributor Information
Cheng-I J. Ma, Cardiovascular Research Laboratories, Department of Medicine, Royal Victoria Hospital, 687 Pine Avenue West, Montreal, QC H3A 1A1, Canada
Jennifer A. Beckstead, Center for Prevention of Obesity, Cardiovascular Disease and Diabetes, Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr. Way, Oakland CA 94609, USA
Airlia Thompson, Center for Prevention of Obesity, Cardiovascular Disease and Diabetes, Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr. Way, Oakland CA 94609, USA.
Anouar Hafiane, Cardiovascular Research Laboratories, Department of Medicine, Royal Victoria Hospital, 687 Pine Avenue West, Montreal, QC H3A 1A1, Canada.
Rui Hao Leo Wang, Cardiovascular Research Laboratories, Department of Medicine, Royal Victoria Hospital, 687 Pine Avenue West, Montreal, QC H3A 1A1, Canada.
Robert O. Ryan, Center for Prevention of Obesity, Cardiovascular Disease and Diabetes, Children's Hospital Oakland Research Institute, 5700 Martin Luther King Jr. Way, Oakland CA 94609, USA
Robert S. Kiss, Cardiovascular Research Laboratories, Department of Medicine, Royal Victoria Hospital, 687 Pine Avenue West, Montreal, QC H3A 1A1, Canada
References
- Alexander ET, Tanaka M, Kono M, Saito H, Rader DJ, Phillips MC. Structural and functional consequences of the Milano mutation (R173C) in human apolipoprotein A-I. J Lipid Res. 2009a;50(7):1409–1419. doi: 10.1194/jlr.M800578-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander ET, Weibel GL, Joshi MR, Vedhachalam C, de la Llera-Moya M, Rothblat GH, et al. Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler Thromb Vasc Biol. 2009b;29(10):1496–1501. doi: 10.1161/ATVBAHA.109.191379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli S, Hultgardh-Nilsson A, Cercek B, Shah PK, Forrester JS, Ageland H, Nilsson J. Recombinant apolipoprotein A-I Milano reduces intimal thickening after balloon injury in hypercholesterolemic rabbits. Circulation. 1994;90(4):1935–1941. doi: 10.1161/01.CIR.90.4.1935. [DOI] [PubMed] [Google Scholar]
- Bailey D, Jahagirdar R, Gordon A, Hafiane A, Campbell S, Chatur S et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J Am Coll Cardiol. 2010a;55(23):2580–2589. doi: 10.1016/j.jacc.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Bailey D, Ruel I, Hafiane A, Cochrane H, Iatan I, Jauhiainen M et al. Analysis of lipid transfer activity between model nascent HDL particles and plasma lipoproteins: implications for current concepts of nascent HDL maturation and genesis. J Lipid Res. 2010b;51(4):785–797. doi: 10.1194/jlr.M001875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankston TE, Carta G. Apolipoprotein A-I(Milano) anion exchange chromatography: Self association and adsorption equilibrium. Biotechnol J. 2010;5(10):1028–1039. doi: 10.1002/biot.201000221. [DOI] [PubMed] [Google Scholar]
- Bielicki JK, McCall MR, Stoltzfus LJ, Ravandi A, Kuksis A, Rubin EM, Forte TM. Evidence that apolipoprotein A-IMilano has reduced capacity, compared with wild-type apolipoprotein A-I, to recruit membrane cholesterol. Arterioscler Thromb Vasc Biol. 1997;17(9):1637–1643. doi: 10.1161/01.ATV.17.9.1637. [DOI] [PubMed] [Google Scholar]
- Burgess B, Naus K, Chan J, Hirsch-Reinshagen V, Tansley G, Matzke L et al. Overexpression of human ABCG1 does not affect atherosclerosis in fat-fed ApoE-deficient mice. Arter-ioscler Thromb Vasc Biol. 2008;28(10):1731–1737. doi: 10.1161/ATVBAHA.108.168542. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Franceschini G, Burkybile A, Jonas A. Activation of lecithin cholesterol acyltransferase by a disulfide-linked apolipoprotein A-I dimer. Biochem Biophys Res Commun. 1997;232(2):345–349. doi: 10.1006/bbrc.1997.6286. [DOI] [PubMed] [Google Scholar]
- Calabresi L, Tedeschi G, Treu C, Ronchi S, Galbiati D, Airoldi S et al. Limited proteolysis of a disulfide-linked apoA-I dimer in reconstituted HDL. J Lipid Res. 2001;42(6):935–942. [PubMed] [Google Scholar]
- Calabresi L, Rossoni G, Gomaraschi M, Sisto F, Berti F, Franceschini G. High-density lipoproteins protect isolated rat hearts from ischemia-reperfusion injury by reducing cardiac tumor necrosis factor-alpha content and enhancing prostaglandin release. Circ Res. 2003;92(3):330–337. doi: 10.1161/01.RES.0000054201.60308.1A. [DOI] [PubMed] [Google Scholar]
- Chiesa G, Monteggia E, Marchesi M, Lorenzon P, Laucello M, Lorusso V et al. Recombinant apolipoprotein A-I(Milano) infusion into rabbit carotid artery rapidly removes lipid from fatty streaks. Circ Res. 2002;90(9):974–980. doi: 10.1161/01.RES.0000018422.31717.EE. [DOI] [PubMed] [Google Scholar]
- Favari E, Gomaraschi M, Zanotti I, Bernini F, Lee-Rueckert M, Kovanen PT et al. A unique protease-sensitive high density lipoprotein particle containing the apolipoprotein A-I (Milano) dimer effectively promotes ATP-binding Cassette A1-mediated cell cholesterol efflux. J Biol Chem. 2007;282(8):5125–5132. doi: 10.1074/jbc.M609336200. [DOI] [PubMed] [Google Scholar]
- Franceschini G, Sirtori CR, Capurso A, II, Weisgraber KH, Mahley RW. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980;66(5):892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschini G, Calabresi L, Chiesa G, Parolini C, Sirtori CR, Canavesi M, Bernini F. Increased cholesterol efflux potential of sera from ApoA-IMilano carriers and transgenic mice. Arterioscler Thromb Vasc Biol. 1999;19(5):1257–1262. doi: 10.1161/01.ATV.19.5.1257. [DOI] [PubMed] [Google Scholar]
- Gelissen IC, Harris M, Rye KA, Quinn C, Brown AJ, Kockx M et al. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler Thromb Vasc Biol. 2006;26(3):534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- Ibanez B, Vilahur G, Cimmino G, Speidl WS, Pinero A, Choi BG et al. Rapid change in plaque size, composition, and molecular footprint after recombinant apolipoprotein A-I Milano (ETC-216) administration: magnetic resonance imaging study in an experimental model of atherosclerosis. J Am Coll Cardiol. 2008;51(11):1104–1109. doi: 10.1016/j.jacc.2007.09.071. [DOI] [PubMed] [Google Scholar]
- Kennedy MA, Barrera GC, Nakamura K, Baldan A, Tarr P, Fishbein MC et al. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1(2):121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Endotoxin down-regulates ABCG5 and ABCG8 in mouse liver and ABCA1 and ABCG1 in J774 murine macrophages: differential role of LXR. J Lipid Res. 2003;44(9):1728–1736. doi: 10.1194/jlr.M300100-JLR200. [DOI] [PubMed] [Google Scholar]
- Kiss RS, Maric J, Marcel YL. Lipid efflux in human and mouse macrophagic cells: evidence for differential regulation of phospholipid and cholesterol efflux. J Lipid Res. 2005;46(9):1877–1887. doi: 10.1194/jlr.M400482-JLR200. [DOI] [PubMed] [Google Scholar]
- Kiss RS, You Z, Genest J, Jr, Behm DJ, Giaid A. Urotensin II differentially regulates macrophage and hepatic cholesterol homeostasis. Peptides. 2011;32(5):956–963. doi: 10.1016/j.peptides.2011.02.016. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Takanezawa Y, Hirata T, Shimizu Y, Misasa K, Kioka N, et al. Efflux of sphingomyelin, cholesterol, and phosphatidylcholine by ABCG1. J Lipid Res. 2006;47(8):1791–1802. doi: 10.1194/jlr.M500546-JLR200. [DOI] [PubMed] [Google Scholar]
- Li D, Weng S, Yang B, Zander DS, Saldeen T, Nichols WW et al. Inhibition of arterial thrombus formation by ApoA1 Milano. Arterioscler Thromb Vasc Biol. 1999;19(2):378–383. doi: 10.1161/01.ATV.19.2.378. [DOI] [PubMed] [Google Scholar]
- Marchesi M, Booth EA, Davis T, Bisgaier CL, Lucchesi BR. Apolipoprotein A-IMilano and 1-palmitoyl-2-oleoyl phosphatidylcholine complex (ETC-216) protects the in vivo rabbit heart from regional ischemia-reperfusion injury. J Pharmacol Exp Ther. 2004;311(3):1023–1031. doi: 10.1124/jpet.104.070789. [DOI] [PubMed] [Google Scholar]
- Marmillot P, Patel S, Lakshman MR. Reverse cholesterol transport is regulated by varying fatty acyl chain saturation and sphingomyelin content in reconstituted high-density lipoproteins. Metabolism. 2007;56(2):251–259. doi: 10.1016/j.metabol.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin DD, Budamagunta MS, Ryan RO, Voss JC, Oda MN. Apolipoprotein A-I assumes a “looped belt” conformation on reconstituted high density lipoprotein. J Biol Chem. 2006;281(29):20418–20426. doi: 10.1074/jbc.M602077200. [DOI] [PubMed] [Google Scholar]
- Matsuo M, Campenot RB, Vance DE, Ueda K, Vance JE. Involvement of low-density lipoprotein receptor-related protein and ABCG1 in stimulation of axonal extension by apoE-containing lipoproteins. Biochim Biophys Acta. 2011;1811(1):31–38. doi: 10.1016/j.bbalip.2010.10.004. [DOI] [PubMed] [Google Scholar]
- Mukhamedova N, Escher G, D'Souza W, Tchoua U, Grant A, Krozowski Z et al. Enhancing apolipoprotein A-I-dependent cholesterol efflux elevates cholesterol export from macrophages in vivo. J Lipid Res. 2008;49(11):2312–2322. doi: 10.1194/jlr.M800095-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls SJ, Tuzcu EM, Sipahi I, Schoenhagen P, Crowe T, Kapadia S, Nissen SE. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J Am Coll Cardiol. 2006;47(5):992–997. doi: 10.1016/j.jacc.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M et al. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003;290(17):2292–2300. doi: 10.1001/jama.290.17.2292. [DOI] [PubMed] [Google Scholar]
- Out R, Hoekstra M, Hildebrand RB, Kruit JK, Meurs I, Li Z et al. Macrophage ABCG1 deletion disrupts lipid home-ostasis in alveolar macrophages and moderately influences atherosclerotic lesion development in LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26(10):2295–2300. doi: 10.1161/01.ATV.0000237629.29842.4c. [DOI] [PubMed] [Google Scholar]
- Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol. 2008;28(2):258–264. doi: 10.1161/ATVBAHA.107.156935. [DOI] [PubMed] [Google Scholar]
- Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L et al. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51(11):1098–1103. doi: 10.1016/j.jacc.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Perez-Mendez O, Bruckert E, Franceschini G, Duhal N, Lacroix B, Bonte JP et al. Metabolism of apolipoproteins AI and AII in subjects carrying similar apoAI mutations, apoAI Milano and apoAI Paris. Atherosclerosis. 2000;148(2):317–325. doi: 10.1016/S0021-9150(99)00279-8. [DOI] [PubMed] [Google Scholar]
- Ranalletta M, Wang N, Han S, Yvan-Charvet L, Welch C, Tall AR. Decreased atherosclerosis in low-density lipoprotein receptor knockout mice transplanted with Abcg1–/– bone marrow. Arterioscler Thromb Vasc Biol. 2006;26(10):2308–2315. doi: 10.1161/01.ATV.0000242275.92915.43. [DOI] [PubMed] [Google Scholar]
- Ryan RO, Forte TM, Oda MN. Optimized bacterial expression of human apolipoprotein A-I. Protein Expr Purif. 2003;27(1):98–103. doi: 10.1016/S1046-5928(02)00568-5. [DOI] [PubMed] [Google Scholar]
- Rye KA, Hime NJ, Barter PJ. The influence of sphingomyelin on the structure and function of reconstituted high density lipoproteins. J Biol Chem. 1996;271(8):4243–4250. doi: 10.1074/jbc.271.8.42430. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan S, Oram JF, Asztalos BF, Vaughan AM, Lund-Katz S, Adorni MP et al. Effects of acceptor composition and mechanism of ABCG1-mediated cellular free cholesterol efflux. J Lipid Res. 2009;50(2):275–284. doi: 10.1194/jlr.M800362-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano O, Kobayashi A, Nagao K, Kumagai K, Kioka N, Hanada K et al. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J Lipid Res. 2007;48(11):2377–2384. doi: 10.1194/jlr.M700139-JLR200. [DOI] [PubMed] [Google Scholar]
- Shah PK, Yano J, Reyes O, Chyu KY, Kaul S, Bisgaier CL et al. High-dose recombinant apolipoprotein A-I(milano) mobilizes tissue cholesterol and rapidly reduces plaque lipid and macrophage content in apolipoprotein e-deficient mice. Potential implications for acute plaque stabilization. Circulation. 2001;103(25):3047–3050. doi: 10.1161/hc2501.092494. [DOI] [PubMed] [Google Scholar]
- Soma MR, Donetti E, Parolini C, Sirtori CR, Fumagalli R, Franceschini G. Recombinant apolipoprotein A-IMilano dimer inhibits carotid intimal thickening induced by perivascular manipulation in rabbits. Circ Res. 1995;76(3):405–411. doi: 10.1161/01.RES.76.3.405. [DOI] [PubMed] [Google Scholar]
- Tarling EJ, Bojanic DD, Tangirala RK, Wang X, Lovgren-Sandblom A, Lusis AJ et al. Impaired development of atherosclerosis in Abcg1–/– Apoe–/– mice: identification of specific oxysterols that both accumulate in Abcg1–/– Apoe–/– tissues and induce apoptosis. Arterioscler Thromb Vasc Biol. 2010;30(6):1174–1180. doi: 10.1161/ATVBAHA.110.205617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J Biol Chem. 2005;280(34):30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- Vaughan AM, Oram JF. ABCA1 and ABCG1 or ABCG4 act sequentially to remove cellular cholesterol and generate cholesterol-rich HDL. J Lipid Res. 2006;47(11):2433–2443. doi: 10.1194/jlr.M600218-JLR200. [DOI] [PubMed] [Google Scholar]
- Wang WQ, Moses AS, Francis GA. Cholesterol mobilization by free and lipid-bound apoAI(Milano) and apoAI (Milano)-apoAII heterodimers. Biochemistry. 2001;40(12):3666–3673. doi: 10.1021/bi002141j. [DOI] [PubMed] [Google Scholar]
- Wang N, Lan D, Chen W, Matsuura F, Tall AR. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA. 2004;101(26):9774–9779. doi: 10.1073/pnas.0403506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Sharifi BG, Pan T, Song L, Yukht A, Shah PK. Bone marrow transplantation shows superior atheroprotec-tive effects of gene therapy with apolipoprotein A-I Milano compared with wild-type apolipoprotein A-I in hyperlipidemic mice. J Am Coll Cardiol. 2006;48(7):1459–1468. doi: 10.1016/j.jacc.2006.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH et al. Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117(8):2216–2224. doi: 10.1172/JCI32057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weers PM, Patel AB, Wan LC, Guigard E, Kay CM, Hafiane A et al. Novel N-terminal mutation of human apolipoprotein A-I reduces self-association and impairs LCAT activation. J Lipid Res. 2011;52(1):35–44. doi: 10.1194/jlr.M007500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weibel GL, Alexander ET, Joshi MR, Rader DJ, Lund-Katz S, Phillips MC, Rothblat GH. Wild-type ApoA-I and the Milano variant have similar abilities to stimulate cellular lipid mobilization and efflux. Arterioscler Thromb Vasc Biol. 2007;27(9):2022–2029. doi: 10.1161/ATVBAHA.107.148403. [DOI] [PubMed] [Google Scholar]
- Wiersma H, Nijstad N, de Boer JF, Out R, Hogewerf W, Van Berkel TJ, et al. Lack of Abcg1 results in decreased plasma HDL cholesterol levels and increased biliary cholesterol secretion in mice fed a high cholesterol diet. Atherosclerosis. 2009;206(1):141–147. doi: 10.1016/j.atherosclerosis.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Wu Z, Wagner MA, Zheng L, Parks JS, Shy JM, III, Smith JD et al. The refined structure of nascent HDL reveals a key functional domain for particle maturation and dysfunction. Nat Struct Mol Biol. 2007;14(9):861–868. doi: 10.1038/nsmb1284. [DOI] [PubMed] [Google Scholar]
- Wu Z, Gogonea V, Lee X, Wagner MA, Li XM, Huang Y et al. Double superhelix model of high density lipoprotein. J Biol Chem. 2009;284(52):36605–36619. doi: 10.1074/jbc.M109.039537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R et al. Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice. J Clin Invest. 2007;117(12):3900–3908. doi: 10.1172/JCI33372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Wang N, Tall AR. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler Thromb Vasc Biol. 2010;30(2):139–143. doi: 10.1161/ATVBAHA.108.179283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao XQ, Brown BG. ApoA-I(Milano)/phospholipid complex: clinical implications of dose-response studies in rabbit atherosclerosis with intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008;51(11):1110–1111. doi: 10.1016/j.jacc.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Zhu X, Wu G, Zeng W, Xue H, Chen B. Cysteine mutants of human apolipoprotein A-I: a study of secondary structural and functional properties. J Lipid Res. 2005;46(6):1303–1311. doi: 10.1194/jlr.M400401-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.