Abstract
Corynebacterium diphtheriae utilizes hemin and hemoglobin (Hb) as iron sources during growth in iron-depleted environments, and recent studies have shown that the surface-exposed HtaA protein binds both hemin and Hb and also contributes to the utilization of hemin iron. Conserved (CR) domains within HtaA and in the associated hemin-binding protein, HtaB, are required for the ability to bind hemin and Hb. In this study, we identified and characterized two novel genetic loci in C. diphtheriae that encode factors that bind hemin and Hb. Both genetic systems contain two-gene operons that are transcriptionally regulated by DtxR and iron. The gene products of these operons are ChtA-ChtB and ChtC-CirA (previously DIP0522-DIP0523). The chtA and chtB genes are carried on a putative composite transposon associated with C. diphtheriae isolates that dominated the diphtheria outbreak in the former Soviet Union in the 1990s. ChtA and ChtC each contain a single N-terminal CR domain and exhibit significant sequence similarity to each other but only limited similarity with HtaA. The chtB and htaB gene products exhibited a high level of sequence similarity throughout their sequences, and both proteins contain a single CR domain. Whole-cell binding studies as well as protease analysis indicated that all four of the proteins encoded by these two operons are surface exposed, which is consistent with the presence of a transmembrane segment in their C-terminal regions. ChtA, ChtB, and ChtC are able to bind hemin and Hb, with ChtA showing the highest affinity. Site-directed mutagenesis showed that specific tyrosine residues within the ChtA CR domain were critical for hemin and Hb binding. Hemin iron utilization assays using various C. diphtheriae mutants indicate that deletion of the chtA-chtB region and the chtC gene has no affect on the ability of C. diphtheriae to use hemin or Hb as iron sources; however, a chtB htaB double mutant exhibits a significant decrease in hemin iron use, indicating a role in hemin transport for HtaB and ChtB.
INTRODUCTION
Corynebacterium diphtheriae is a Gram-positive bacterial pathogen and the etiological agent of the human respiratory disease diphtheria. Strains that harbor the βtox+ bacteriophage synthesize and secrete diphtheria toxin (DT), an iron-regulated exotoxin that is associated with severe human infection by this organism (1, 2). Virulence determinants in numerous bacterial pathogens are regulated by iron in manner similar to that of DT, where expression is repressed in high-iron environments and derepressed under iron-depleted conditions (3, 4). Iron-dependent transcriptional regulation of the tox gene, the structural gene for DT, is mediated by DtxR, an iron-binding regulatory factor that controls the expression of numerous genes in C. diphtheriae (5–8). Many iron-regulated factors in bacteria are involved in the transport and metabolism of iron, and certain bacterial pathogens encode factors that facilitate the utilization of host iron sources, including transferrin, lactoferrin, and various heme compounds such as hemoglobin (Hb) (9, 10).
Hemin transport systems were first described in Gram-negative bacteria (10, 11). In these organisms, hemin or Hb initially binds to outer membrane proteins, where hemin is extracted from Hb and subsequently mobilized into the periplasmic space. Once inside the periplasm, hemin-specific substrate-binding proteins bind to heme and facilitate the passage of the porphyrin through the membrane by means of heme-specific ABC transporters (11). The mechanism of heme transport through the cytoplasmic membrane is similar in Gram-negative and Gram-positive bacteria; however, the initial binding of heme or heme proteins at the surface of the bacteria is quite distinct between these organisms. In Gram-positive bacteria, such as Staphylococcus aureus, cell wall-anchored proteins designated iron-regulated surface determinants (Isd) are proposed to bind specifically to hemin or Hb at the bacterial surface, where hemin is moved through the cell wall by a cascade-like mechanism in which hemin is transferred between Isd proteins until it ultimately interacts with a hemin-specific ABC transporter at the membrane (12–14). Hemin binding by the Isd proteins in S. aureus and Bacillus anthracis and by the related Shr and Shp proteins in Streptococcus pyogenes occurs at a NEAT domain, a conserved region of approximately 125 amino acids (15–18). The Isd proteins in S. aureus and B. anthracis are covalently anchored directly to the cell wall by sortase enzymes (12, 19), whereas the Shr and Shp proteins appear to be tethered to the cytoplasmic membrane by means of a C-terminal membrane-spanning region (20). Additionally, the Shr protein binds to the extracellular matrix proteins fibronectin and laminin, which suggests that Shr may function as an adhesin (18).
C. diphtheriae encodes numerous iron transporters, including a high-affinity siderophore transport system encoded by the ciuABCDE genes (21) and a hemin iron utilization system encoded by hmuO (22) and the hmu genes (23–25). The hmuO gene encodes a heme oxygenase that is involved in the enzymatic degradation of intracellular heme with the subsequent release of the heme-associated iron (26). The hmu genetic cluster in C. diphtheriae encodes the HmuTUV ABC hemin transporter and the surface anchored hemin-binding proteins HtaA and HtaB (25). The hmu region is composed of three distinct operons, all of which are transcriptionally regulated by DtxR and iron. Deletion of hmuTUV, htaA, or the complete hmu region results in a reduced ability to use hemin and Hb as iron sources, which indicates a direct role for factors encoded by the hmu region in the transport of hemin (25). It was noted that deletion of the complete hmu genetic cluster failed to abolish the ability of C. diphtheriae to use hemin and Hb as iron sources, which indicates that additional systems are involved in the utilization of hemin iron (25).
The HtaA and HtaB proteins share sequence similarity over an approximately 150-amino-acid region (designated a CR domain) that is required for the hemin-binding properties of both proteins as well as the Hb-binding ability of HtaA (23, 25). In addition to the similarities in their CR domains, HtaA and HtaB both contain leader sequences indicating they are secreted; however, they also possess C-terminal transmembrane segments, which appear to be responsible for tethering these proteins to the cytoplasmic membrane. The 61-kDa HtaA protein contains two CR domains that bind hemin with similar affinities, and both domains bind Hb; the smaller hemin-binding HtaB protein (35.5 kDa) contains only a single CR domain and was not able to bind Hb using an in vitro enzyme-linked immunosorbent assay (ELISA) procedure (25). An alignment of CR domains among Corynebacterium species and related genera showed an overall sequence identity of approximately 25%; however, the sequence comparison revealed that two tyrosines and a single histidine residue were highly conserved among the CR domains. Tyrosine and histidine residues are often the critical ligands in coordinating the hemin-associated iron. Alanine substitutions of any three of these amino acids in the CR domains of HtaA results in a significantly reduced ability to bind hemin and Hb, with Tyr-49 and Tyr-361 in the CR1 and CR2 domains, respectively, resulting in the most severe defect in hemin and Hb binding (23). Consistent with the reduction in hemin binding, the Tyr-361 mutation also abolished the hemin uptake function of HtaA, underlining the critical importance of this residue for the hemin transport activity of this protein. The C. diphtheriae HtaA and HtaB CR domains show no significant sequence similarity to the hemin-binding NEAT domains described for S. aureus, B. anthracis, and S. pyogenes. However, as observed with the CR domains, tyrosine residues are also critical for hemin binding to the Isd NEAT domains (17).
In vitro studies showed that purified HtaA acquires hemin from Hb and that the holo form of HtaA (hemin-bound HtaA) can transfer hemin to HtaB (23). Based on this observation and on earlier findings, a model for hemin transport in C. diphtheriae was described. The model proposes that HtaA initially binds Hb at the bacterial surface, where the hemin is subsequently extracted from Hb and then mobilized to HtaB or directly to HmuT. HmuT is a surface-anchored, hemin-binding lipoprotein that facilitates the transfer of hemin through the cytoplasmic membrane via its cognate ABC transporter composed of HmuU and HmuV (25). Upon entry into the cytosol, it is proposed that heme is degraded by HmuO, resulting in the release of the heme-associated iron.
In this study, we identified and characterized additional hemin- and Hb-binding proteins in C. diphtheriae. The genes encoding these novel hemin-binding proteins, designated chtA, chtB, and chtC, are arranged in unlinked two-gene operons, chtA-chtB and cirA-chtC, in which transcription is iron and DtxR regulated. The chtA-chtB operon is flanked by inverted repeats of identical insertion sequence (IS) elements creating an apparent composite transposon. All four of the proteins are predicted to be surface exposed and anchored to the cytoplasmic membrane through a C-terminal transmembrane segment. ChtA and ChtC are high-molecular-weight proteins that contain a previously described CR domain in their N-terminal region that is critical for the hemin- and Hb-binding ability of these proteins.
MATERIALS AND METHODS
Bacterial strains and media.
The Escherichia coli and C. diphtheriae strains used in this study are listed in Table 1. Luria-Bertani (LB) medium was used for culturing of E. coli, and heart infusion broth (Difco, Detroit, MI) containing 0.2% Tween 80 (HIBTW) was used for routine growth of C. diphtheriae strains. Bacterial stocks were maintained in 20% glycerol at −80°C. Antibiotics were added to LB medium at 34 μg/ml for chloramphenicol, 50 μg/ml for kanamycin, 100 μg/ml for ampicillin, and 100 μg/ml for spectinomycin and to HIBTW for C. diphtheriae cultures at 2 μg/ml for chloramphenicol, 100 μg/ml for spectinomycin, and 50 μg/ml for kanamycin. HIBTW was made low iron by the addition of ethylenediamine di(o-hydroxyphenylacetic acid) (EDDA) at 12 μg/ml (unless indicated otherwise). Modified PGT (mPGT) is a semidefined low-iron medium that has been previously described (27). Antibiotics, EDDA, and Tween 80 were obtained from Sigma Chemical Co. (St. Louis, MO), and pure hemoglobin (human) was purchased from MP BioMedical (Solon, OH).
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics or use | Reference or source |
|---|---|---|
| C. diphtheriae strains | ||
| 1737 | Wild type, Gravis biotype, Tox+ | 37 |
| 1737htaAΔ | htaA deletion mutant of 1737 | 25 |
| 1737htaBΔ | htaB deletion mutant of 1737 | 25 |
| 1737chtAΔ | chtA deletion mutant of 1737 | This study |
| 1737chtBΔ | chtB deletion mutant of 1737 | This study |
| 1737chtCΔ | chtC deletion mutant of 1737 | This study |
| 1737chtAΔ/chtBΔ | Deletion of chtA and chtB in 1737 | This study |
| 1737htaBΔ/chtBΔ | Deletion of htaB and chtB in 1737 | This study |
| 1737chtAΔ/chtCΔ/htaAΔ | Deletion of chtA, chtC, and htaA in 1737 | This study |
| 1737chtAΔ/chtCΔ | Deletion of chtA and chtC in 1737 | This study |
| C7(−) | Wild type, Tox− | 38 |
| C7dtxRΔ | C7 dtxR deletion mutant | 25 |
| E. coli strains | ||
| BL21(DE3) | Protein expression | Novagen |
| DH5α | Cloning strain | Invitrogen |
| S17-1 | RP4 mobilization functions | 32 |
| TOP10 | Cloning of PCR fragments | Invitrogen |
| XL1-Gold | Mutagenesis strain | Stratagene |
| Plasmids | ||
| pCR-Blunt-IITOPO | Cloning of PCR fragments, Knr | Invitrogen |
| pSPZ | Promoter probe, Spcr | 28 |
| pGEX-6P-1 | Expression vector (GST fusion), Ampr | GE Healthcare |
| pET24(a)+ | Expression vector, Knr | Millipore |
| pKN2.6Z | C. diphtheriae shuttle vector, Knr | 24 |
| pKhtaA | pKN2.6Z carrying the htaA gene | 25 |
Plasmid construction.
Plasmids used in this study are listed in Table 1. PCR-derived DNA fragments were initially cloned into the pCR-Blunt II-TOPO vector (Invitrogen), and 1737 genomic DNA was used as a template for PCR (unless otherwise indicated). The promoter probe vector pSPZ (28), which contains a promoterless lacZ gene and replicates at low copy in C. diphtheriae, was used for the construction of lacZ promoter fusions. PCR was used to amplify a 211-bp DNA fragment carrying the chtA promoter region, which included a 145-bp region upstream of the start codon for the chtA gene. The cirA promoter fusions, PO1 (269 bp) and PO2 (166 bp), have the same 3′ end within the cirA coding region but contain either 181 bp or 78 bp of sequence upstream of the cirA start codon, respectively. The PO3 construct (114 bp) maintains the identical 5′ end as the PO1 insert. All DNA fragments were cloned into the BamHI and SalI sites in pSPZ. The primers used for the construction of all plasmids and the approximate sizes of the amplified PCR products are provided in Table S1 in the supplemental material.
The vector pGEX-6P-1 (Amersham) was used for the expression of N-terminally glutathione S-transferase (GST)-tagged ChtA, ChtB, ChtC, and CirA. The coding regions for the chtA, chtB, chtC, and cirA genes, which lack the N-terminal leader sequence and C-terminal transmembrane and charged tails, were amplified by PCR and subsequently ligated into the EcoRI and XhoI sites of pGEX-6P-1.
Plasmid constructs that expressed the N-terminally Strep-tagged proteins ChtA, ChtA CR, ChtA C-terminal region, ChtB, and ChtC were constructed using the pET24a vector (Novagen). PCR-derived DNA fragments harboring the gene of interest were initially cloned into the pCR-Blunt II-TOPO vector and subsequently ligated into the NdeI and EcoRI sites of pET24a. The cloned genes carried deletions that removed the N-terminal secretion signals and the C-terminal transmembrane region.
DtxR purification.
DtxR was expressed and purified from E. coli DH5α/pDtxR-7 as described previously (29). Briefly, bacteria were recovered after centrifugation, and the cell pellet was resuspended in lysis buffer (42 mM NaH2PO4, 58 mMNa2HPO4, 50 mM NaCl, 5 mM MgCl2) containing protease inhibitors (Roche) and lysed with a French pressure cell using standard procedures. DtxR was purified by Ni-nitrilotriacetic acid (NTA) affinity chromatography (Qiagen). The cell lysate was incubated with Ni-NTA agarose at 4°C for 1 h and subsequently washed two times with 20 mM imidazole. DtxR was eluted with 250 mM imidazole and dialyzed in phosphate-buffered saline (PBS)–20% glycerol.
DNase I protection experiments.
The 269-bp insert in the pSPZ-PO1 construct was excised with BamHI/SphI and radiolabeled with [α-32P]dCTP at its 5′ end using the Klenow fragment as described previously (30). Various amounts of DtxR were added to the binding reaction mixture, which contained binding buffer (8.4 mM NaH2PO4, 11.6 mM Na2HPO4, 50 mM NaCl, 1 mM MgCl2, 200 μg bovine serum albumin [BSA], 2 μg salmon sperm DNA, 10% glycerol) with and without 150 μM Co2+, 5.7 μM 2-mercaptoethanol (Sigma), and approximately 60,000 cpm of radiolabeled DNA (0.034 pmol). After a 20-min incubation at room temperature, each sample was treated with DNase I (Roche) at room temperature. The digestion reactions were terminated with the addition of phenol-chloroform-isoamyl alcohol (25:24:1) (Invitrogen), and the mixtures were subsequently treated as previously described (31). Approximately 16,000 cpm of each sample was applied to a 5% polyacrylamide-urea sequencing gel. A sequencing ladder for the PO1 insert DNA was prepared by the Sanger method utilizing the USB-Sequenase version 2.0 DNA sequencing kit (USB Corporation, Cleveland, OH) as directed by the manufacturer. Reaction products were visualized by autoradiography.
Mutant construction.
A previously described allelic replacement technique (32) was used to construct nonpolar deletion mutations in the C. diphtheriae 1737 chtA, chtB, and chtC genes. Mutant construction utilized PCR to clone DNA fragments located upstream and downstream of the region targeted for deletion. Primers used for the construction of the mutants are listed in Table S1 in the supplemental material. PCR and Western blotting were used to confirm the mutations in all of the deletion mutants (not shown).
Site-directed mutagenesis.
Site-directed mutants were made using the QuikChange Lightning kit (Stratagene) according to the manufacturer's instructions. Briefly, 125 ng of each primer containing the targeted base change and 50 ng of plasmid template were used in the QuikChange reaction. Methylated template DNA was removed from the reaction by digestion with DpnI restriction endonuclease, and mutagenized DNA was recovered by transformation into XL1-Gold competent cells. The presence of the base changes was confirmed by sequence analysis. Plasmids used for site-directed mutagenesis were pET24a with the N-terminal Strep tag sequence containing the cloned chtA gene.
Protein expression and antibody production.
BL21(DE3) carrying the chtA, chtB, chtC, and cirA expression plasmids was grown in 100 ml of LB medium at 37°C to mid-log phase, at which time 1 to 0.5 mM isopropyl-β-d-thiogalactopyranoside (IPTG) was added and the cultures were allowed to grow for additional 2 to 3 h at 27 to 37°C before harvesting. The cultures were washed in 10 ml of 20 mM Tris-HCl (pH 7.5), and the pellet was either stored at −20°C until use or resuspended in lysis buffer for lysis with a French pressure cell or BugBuster (EMD Millipore). Cell debris was removed by centrifugation at 12,000 × g at 4°C, and the supernatant fraction containing the soluble protein was purified on Strep-Tactin columns (IBA or Neuromics) for Strep-tagged constructs. Soluble lysates containing the GST-tagged constructs were purified using GST-resin (GE Healthcare) by a batch method following the manufacturer's instructions. The purified GST-tagged ChtA, ChtB, ChtC, and CirA were used for the production of polyclonal antibodies in guinea pigs by standard methods (Cocalico Biologicals Inc.).
SDS-PAGE and Western blot analysis.
Proteins were separated by SDS-PAGE (33) and stained with Bio-Safe Coomassie blue (Bio-Rad) following the manufacturer's instructions. Western blot procedures were performed as described previously (25), and anti-ChtA, anti-ChtB, anti-ChtC, and anti-CirA antibodies were used at a 1:2,500 dilution. Antibodies raised against DtxR were use at a dilution of 1:5,000. Binding of the primary antibodies to immobilized proteins was detected with alkaline phosphatase-labeled secondary antibodies following established procedures (25).
Protein localization studies.
To determine whether proteins were secreted or cell associated, 1-ml cultures were grown to mid-log phase at 37°C in HIBTW medium containing 12 μg/ml EDDA (low-iron medium). Alternatively, bacteria were grown overnight in low-iron mPGT medium. Cultures were briefly centrifuged to pellet the cells, and the supernatant was recovered and passed through a 0.2-μm filter to remove bacteria. Proteins in the filtered supernatant were then precipitated with 10% trichloroacetic acid (TCA), washed with ethanol, dried, and then resuspended in an SDS gel loading buffer (33). Half of the sample was analyzed by SDS-PAGE and stained with Coomassie blue, and the remaining half was used for Western analysis. The resulting cell pellet was lysed and then resuspended in SDS loading buffer, and half the sample was analyzed by SDS-PAGE and half by Western blotting.
A previously described procedure was used to determine the cellular location of cell-associated proteins (24) but with the following modifications. Briefly, 100-ml cultures of C. diphtheriae strain 1737 were grown overnight in mPGT medium with 0.5 μM FeSO4 (low-Fe conditions) at 37°C. Cells were pelleted, washed once with PBS, and resuspended in 5 ml of PBS, which was followed by lysozyme treatment and lysis of the bacteria using a French pressure cell. Cell debris was removed by centrifugation, and the soluble fraction, which contains both soluble and membrane proteins, was centrifuged at 100,000 × g to pellet the cytoplasmic membrane. The supernatant, which contains the soluble intracellular proteins, was recovered and stored at 4°C, while the membrane fraction was solubilized in 0.1% Triton X-100. The same procedure was followed for cells cultured in HIBTW medium containing EDDA (low-Fe conditions).
Hemoglobin iron utilization assays.
The hemoglobin utilization assay has been described previously (25). Briefly, Corynebacterium strains were grown overnight (20 to 22 h at 37°C) in HIBTW. The next day, overnight cultures were diluted with 1 ml of HIBTW and incubated for 1 h at 37°C. Then, 500 μl of the cultures was centrifuged and resuspended in 1 ml of mPGT medium with 1 μM FeSO4. Strains were grown for several hours at 37°C until log phase, at which time bacteria were inoculated at an optical density at 600 nm (OD600) of 0.03 into fresh mPGT medium that contained various supplements as indicated. After 20 to 22 h of growth at 37°C, the OD600 of the cultures was determined. All strains grew well in the presence of 1 μM FeSO4 (+Fe), and all strains were inhibited for growth under low-iron conditions (−Fe, 10 μM EDDA).
Identification of surface-exposed proteins in C. diphtheriae.
Surface exposure of proteins in C. diphtheriae was determined by two methods: by susceptibility of proteins to protease K treatment and by a whole-cell ELISA procedure. For the protease studies, C. diphtheriae was grown overnight (20 to 22 h at 37°C) in HIBTW, and the overnight culture was used to inoculate 1 ml of HIBTW containing 6 μg EDDA at an OD600 of 0.2. The EDDA culture was grown to log phase and then used to inoculate mPGT medium containing 0.5 μM FeSO4 (low-iron conditions) at an OD600 of 0.2, which was grown overnight at 37°C. The bacteria were harvested after centrifugation (3,000 rpm for 5 min), and the cells were resuspended in PBS, which was followed by addition of proteinase K (13 μg/ml) to the cell suspension. The reaction mixture was incubated for 30 min at room temperature, at which time the cells were pelleted and then washed three times with PBS. The bacteria were then treated with lysozyme, followed by the addition of 0.75% Sarkosyl to lyse the bacteria. Whole-cell protein preparations were boiled under reducing conditions prior to analysis by SDS-PAGE and Western blotting. Control samples were not treated with proteinase K. C. diphtheriae strain 1737htaAΔ/pKhtaA was used for these studies, since the expression of HtaA was easily monitored as a control to assess expression and detection of HtaA, a known surface-exposed protein.
Cell surface expression was also detected by ELISA as follows. C. diphtheriae 1737 wt and mutant strains were cultured as described above except that overnight inoculation was into mPGT medium containing either 0.5 μM FeSO4 (low-iron conditions) or 10 μM FeSO4 (high-iron conditions). After harvest and resuspension into PBS, the cells were adjusted to equivalent densities and then placed into wells of microtiter plates (96-well plates, polystyrene; Costar) for 20 to 22 h at 37°C. After the incubation, the plates were washed with PBS plus 0.05% Tween 20 (PBST), blocked for 1 h with 5% Blotto in PBST, and then incubated with the respective primary antibodies for 1 h. After incubation, an additional wash was done, and then the cells were incubated for 1 h with the appropriate alkaline phosphate-labeled secondary antibodies. All incubations were performed at 37°C. The plates were developed in the dark with 50 μl of p-nitrophenyl phosphate (pNPP) (Sigma) at room temperature. A final absorbance reading was taken when the low-iron wt sample reached an OD405 of approximately 1.
LacZ assays.
C. diphtheriae strains containing lacZ fusion constructs were grown overnight in HIBTW medium and then inoculated at an OD600 of 0.2 into fresh HIBTW medium in the presence of various supplements as indicated and grown to log phase. LacZ activity was determined on log-phase cultures as previously described (6). All results are the means from three independent experiments ± standard deviations.
Hemin binding analysis.
Purified GST-tagged and Strep-tagged fusion proteins were analyzed for their hemin-binding properties by UV-visible spectroscopy using a Beckman DU 640 spectrophotometer. Proteins at 2 μM in PBS buffer containing glycerol were assessed for their ability to bind hemin at various concentrations as indicated. Proteins were incubated in the presence of hemin for at least 15 min at room temperature before spectrophotometric analysis. UV-visible absorption scans were done using wavelengths between 280 and 600 nm. Absorbance spectra for all protein-hemin samples were zeroed against a reference cuvette that contained the respective hemin concentration in PBS buffer in the absence of protein.
Protein binding studies.
An ELISA method was used to assess the ability of various test proteins to bind immobilized Hb or to bind to various cell matrix proteins. Microtiter plates (96-well plates, polystyrene; Costar) were coated overnight at 37°C with 50 μl of 25-μg/ml Hb in PBS. After incubation with Hb, the plates were washed with PBS plus 0.05% Tween 20 (PBST) and blocked for 1 h with 5% Blotto in PBST, followed by incubation with the test protein at various protein concentrations (50 μl) for 1 h. After incubation, the plates were washed with PBST, and primary antibodies, anti-GST (1:1,000; GE Healthcare), or anti-Strep (1:1,000; Abcam Inc.) was then added to the plates for 1 h, followed by an additional wash and then a 1-h incubation with appropriate alkaline phosphate-labeled secondary antibodies. All incubations were performed at 37°C. The plates were developed in the dark with 50 μl of p-nitrophenyl phosphate (pNPP) (Sigma) at room temperature. A final absorbance reading was taken when the positive-control sample achieved an OD405 of approximately 1. The same procedure utilized to detect binding to Hb was used to detect binding to fibronectin, fibrinogen, transferrin, laminin, and collagen types I and IV, with the exception that plates for laminin and collagen were precoated (EMD Millipore) and the test proteins were used to coat the plates in the fibronectin and fibrinogen studies. The S. aureus positive-control proteins, ClfA and FnbB, were kindly provided by Rebecca Brady.
Computer analysis.
Amino acid sequence similarity searches were done using the BLAST program (34) at the National Center for Biotechnology Information and also using the BLAST server provided at the online site for the Sanger Institute (http://www.sanger.ac.uk/Projects/C_diphtheriae). The annotated genome sequence for C. diphtheriae strain NCTC13129 (35) is accessible in the EMBL/GenBank database under accession number BX248353. Multiple-sequence alignments were done using ClustalW2 at http://www.ebi.ac.uk/Tools/msa/clustalw2/.
RESULTS
Genetic analysis of the chtA-chtB and cirA-chtC operons.
In a previous study, we identified the chtA-chtB (chtAB) genetic region in a search for DtxR-binding sites (7). Transcription from a promoter upstream of chtA was shown to be repressed under high-iron conditions, and a putative DtxR-binding site overlapped the chtA promoter region. A closer examination of the chtAB region reveals that the two-gene operon is flanked by inverted repeats composed of identical putative IS elements, which show sequence similarity to IS150, an insertion sequence present in certain E. coli strains (Fig. 1A). The organization of this genetic region suggests that chtAB may be present on a composite transposon in C. diphtheriae strain NCTC13129. A pan-genomic analysis that reported the sequences of 13 C. diphtheriae strains (8) showed that six of these strains contained the chtAB genes; however, only NCTC13129, an isolate associated with the diphtheria outbreak in the former Soviet Union (FSU) in the 1990s, contained chtAB on a putative transposon (Table 2). We examined four additional isolates that were associated with the FSU diphtheria epidemic of the 1990s (36, 37) and found using PCR analysis that all of these strains contained chtAB genes that were flanked by IS elements in a manner identical to that observed with NCTC13129 (Table 2). It is striking that this closely related group of strains, which dominated the epidemic in the 1990s, has maintained this putative transposon that harbors the chtAB region. Two additional strains obtained from the FSU, 1751 and G4193 (37), which are separated geographically and temporally from the dominant clonal group associated with the 1990s outbreak, do not carry the chtAB genes (Table 2).
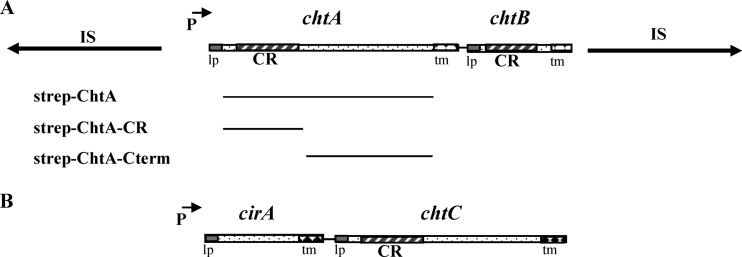
Fig 1.
(A) Genetic map of the chtA-chtB region. The chtA-chtB genes in NCTC13129 are present on a putative transposon that is composed of two identical and inverted insertion sequences (IS elements, indicated by lines with arrows). CR, conserved region; lp, leader peptide; tm, transmembrane region; P with arrow, DtxR and iron-regulated promoter. Below the genetic map are shown the regions of ChtA that were used to construct various Strep-tagged fusion proteins. (B) Genetic map of the cirA-chtC region in NCTC13129.
Table 2.
Analysis of the CR-containing genes chtAB, cirA-chtC, and htaA in C. diphtheriae strains
| Straina | Origin/characteristics | chtAB-Tnb | cirA-chtCc | htaAd | Reference |
|---|---|---|---|---|---|
| NCTC13129* | FSU/epidemic clone | + | + | + | 35 |
| 1716 | FSU/epidemic clone | + | + | + | 37 |
| 1718 | FSU/epidemic clone | + | + | + | 37 |
| 1737 | FSU/epidemic clone | + | + | + | 37 |
| 1897 | FSU/epidemic clone | + | + | + | 37 |
| 1751 | FSU | − | − | ? | 37 |
| G4193 | FSU | − | − | ? | 37 |
| C7* | USA/lab strain | − | − | − | 38 |
| PW8* | USA/vaccine strain | − | − | − | 40 |
| CDCE8392* | CDC | − | − | − | 8 |
| 31A* | Brazil/Tox+ | − | − | − | 8 |
| 241* | Brazil/Tox− | ± | + | + | 8 |
| VA01* | Brazil/Tox− | ± | + | + | 8 |
| HC01* | Brazil/endocarditis | ± | + | + | 8 |
| HC02* | Brazil/endocarditis | − | + | + | 8 |
| HC03* | Brazil/endocarditis | ± | + | + | 8 |
| HC04* | Brazil/endocarditis | ± | + | + | 8 |
| INCA402* | Brazil/pneumonia | − | − | − | 8 |
| BH8* | Brazil | − | − | − | 8 |
Asterisks identify strains analyzed in the pan-genomic study described in reference 8.
+, chtAB present on transposon; ±, chtAB present but not associated with a transposon; −, chtAB absent.
+, cirA-chtC genes present; −, genes absent.
+, htaA wild-type copy; −, htaA contains point mutations, functional status unknown (HtaA protein not detected in C7); ?, htaA status unknown.
Sequence analysis of ChtA (83.9 kDa) and ChtB (32.6 kDa) indicates that both proteins are predicted to contain an N-terminal leader peptide, which suggests that they are secreted, but they also possess a C-terminal transmembrane region that may anchor the proteins to the cytoplasmic membrane (Fig. 1A). Both ChtA and ChtB contain a single CR domain, a region associated with hemin and Hb binding in the C. diphtheriae HtaA and HtaB proteins (25). ChtA shares sequence similarity with HtaA only over the approximately 150-amino-acid region of the CR domain (35% similarity), while ChtB shares 44% identity and 63% similarity with HtaB over the full length of the protein (not shown).
The cirA-chtC region was identified based on sequence similarity between ChtC and ChtA. Analysis of the predicted products of cirA and chtC shows that both CirA (37 kDa) and ChtC (74.3 kDa) are structurally similar to ChtA and ChtB; both proteins contain putative N-terminal secretion signals and C-terminal transmembrane regions (Fig. 1B). However, CirA lacks a putative CR domain, while ChtC contains a single putative CR domain in its N-terminal region. ChtC shares approximately 30% amino acid sequence identity and 47% similarity with ChtA throughout the length of the protein, and the two proteins have 44% identity and 61% similarity between their N-terminal CR domains. CirA shows no significant sequence similarity to any proteins of known function. The cirA-chtC genes are present in seven of 13 strains examined in the pan-genomic analysis, including NCTC13129 (Table 2). PCR analysis showed that the cirA-chtC genes are also present in the four additional isolates associated with the outbreak in the FSU described above but are lacking in the two strains from the FSU that are not associated with the diphtheria epidemic (Table 2). No IS elements or phage genes are present in sequences flanking the cirA-chtC region. The chtAB and cirA-chtC operons are not closely linked on the NCTC13129 genome: the chtA start codon is at position 1545506, while cirA is at position 493763.
It was also noted from the pan-genomic analysis that the well-studied C7 strain (38) lacked both the chtAB and cirA-chtC regions and also contained a frameshift mutation in htaA, which is predicted to result in premature termination of HtaA. The truncated C7 HtaA protein, if synthesized, would likely be secreted, since the membrane-anchoring motif is deleted. It was further observed that the C7 htaB gene contains a 166-bp deletion at its 3′ end, which is predicted to remove the C-terminal transmembrane region. Western blot analyses that examined proteins in whole-cell lysates and culture supernatants from strain C7 failed to identify any protein bands that reacted with the anti-HtaA or anti-HtaB polyclonal antibodies, which indicates that strain C7 does not produce HtaA or HtaB or any truncated products derived from these proteins (data not shown). The C7 strain contains a functional hemin-specific ABC transporter encoded by the hmuTUV genes (25), and since strains with mutations in the C7 hmuTUV genes show only a partial defect in the ability to use hemin and Hb as iron sources, the C7 strain likely contains additional mechanisms for utilizing hemin iron that have yet to be identified. The pan-genomic analysis also revealed that several C. diphtheriae strains, in addition to C7, contain mutations in the htaA gene, indicating that the presence of point mutations in htaA is not unique to the C7 strain (Table 2).
Transcription of the chtAB and cirA-chtC regions is regulated by iron and DtxR.
Proteins associated with hemin iron utilization are frequently regulated by iron and/or hemin. Since ChtA, ChtB, and ChtC contain a region that shares sequence similarity to the hemin-binding CR domain in HtaA, we sought to determine if the promoters upstream of the chtAB and cirA-chtC operons are controlled by iron, heme or possibly other metals. DNA fragments containing the regions upstream of chtA, chtB, cirA, and chtC were placed into the promoter probe vector pSPZ and examined for transcriptional activity in wild-type strains C7 and 1737 and in a dtxR deletion mutant of C. diphtheriae C7. A dtxR deletion mutant of 1737 is unstable and could not be used to examine gene expression (M. P. Schmitt, unpublished observation). Similar LacZ activity was observed for wild-type C7 and 1737 in all expression constructs. Transcription analysis of a region that extends 145 bp upstream of the start of chtA, which includes the putative DtxR-binding site, exhibited promoter activity in the wild-type strain that was strongly repressed under high-iron conditions (+Fe) as measured in β-galactosidase assays (−Fe, 246.5 ± 7.5 U; +Fe, 8.8 ± 1.2 U), which is consistent with previous findings (7). The iron-dependent repression of chtA was alleviated in a dtxR mutant (−Fe, 216 ± 24.6 U; +Fe, 285 ± 12.5 U). The presence of Hb or the divalent metals Mn and Zn did not have a significant effect on transcription (data not shown). No promoter activity was detected in the approximately 200-bp intergenic region upstream of chtB.
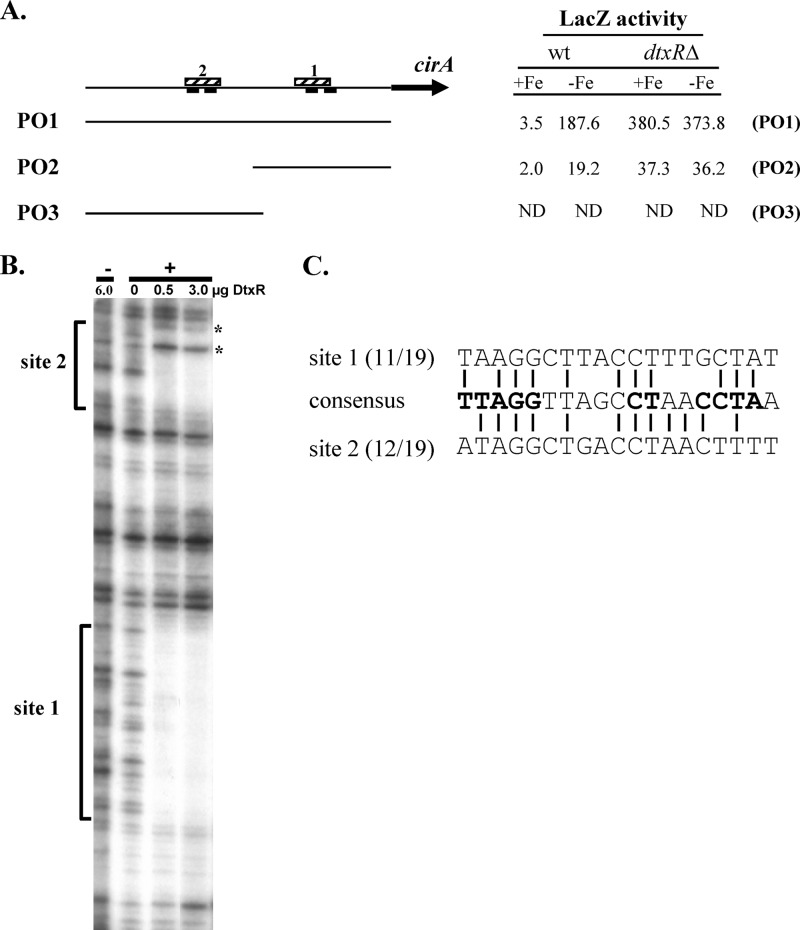
The region upstream of cirA does not contain a sequence that shows high similarity with the 19-bp consensus DtxR-binding site (7). Despite this observation, transcriptional analysis of this region from the cirA-lacZ promoter fusion construct pSPZ-PO1 (PO1) showed strong iron- and DtxR-dependent regulation (Fig. 2A). In an attempt to determine the location of the DtxR-binding site and the associated iron-regulated promoter upstream of cirA, a deletion of the 5′ region of the DNA fragment harboring the cirA promoter was constructed and analyzed. This deletion construct, designated pSPZ-PO2 (PO2), removed two putative −10 promoter elements but preserved two additional downstream regions that may serve as −10 promoter sequences (Fig. 2A). Expression from the PO2 construct showed greatly reduced transcriptional activity but maintained full iron- and DtxR-dependent repression, suggesting that a DtxR-binding site was located on the smaller fragment present in the PO2 construct. To more precisely define the location of the DtxR-binding site, we performed DNase I footprinting using purified DtxR and the PO1 DNA insert in the presence and absence of a divalent metal, which is required for the DNA-binding activity of DtxR. Surprisingly, the results showed that DtxR protected two noncontiguous 30-bp regions separated by approximately 60 bp (Fig. 2A and B). Regions showing moderate similarity to DtxR-binding sites were identified in each of the protected sequences. The site within the PO2 fragment (site 1) shared 11/19 bp with the consensus DtxR-binding site and included 9 of the 11 most conserved residues within the consensus binding sequence (Fig. 2C). Two possible −10 elements were located within this protected region, and the DtxR-binding site overlapped both putative promoter elements (Fig. 2A). The second DtxR-binding site (site 2) was present only on the larger PO1 insert and matched 12/19 bp of the consensus sequence (Fig. 2A and C). To determine if promoter activity was present on this upstream region that contained site 2, an additional fusion construct was generated and designated pSPZ-PO3 (PO3). This fragment had the identical 5′ sequence as PO1, and its 3′ terminus overlapped the 5′ region of PO2 (Fig. 2A). No promoter activity was detected from the fragment in PO3 in either of the C. diphtheriae strains tested (Fig. 2A). Moreover, no promoter activity was detected from the PO3 fragment when examined in the opposite orientation in the pSPZ expression vector (not shown). The reason for the reduced promoter activity on PO2 relative to that detected from PO1 has not been determined, but clearly sequences upstream of the PO2 insert are required for optimal transcription.
Fig 2.
The cirA promoter is regulated by iron and DtxR. (A) Map of the region upstream of the cirA gene, showing putative −10 elements (solid bars below line) and DtxR-binding sites 1 and 2 (hatched bars above line). DNA sequences carried by the various promoter fusions PO1, PO2, and PO3 are shown. LacZ activities from the various promoter fusions in wt C. diphtheriae C7 and a dtxRΔ mutant from strains grown in high (+Fe)- or low (−Fe)-iron medium are shown. ND, none detected. (B) DNase I protection at the cirA promoter region. DtxR protects two distinct regions upstream of cirA, indicated by brackets as site 1 and site 2. Asterisks indicate hypercleavable sites. +, presence of divalent metal Co2+; −, absence of divalent metal Co2+. (C) Alignment of the two DtxR-binding sites upstream of cirA with the 19-bp consensus DtxR-binding site. The most highly conserved bases in the consensus sequence are in bold.
No promoter activity was detected in the approximately 100-bp intergenic region upstream of chtC, which suggests that cirA and chtC are expressed as a single transcriptional unit (not shown).
Expression and localization of ChtAB and CirA-ChtC.
To analyze the products of chtA, chtB, cirA, and chtC, each of the genes was expressed in E. coli as an N-terminal GST fusion, and the purified recombinant proteins were used to generate polyclonal antibodies. Western blot analysis revealed that expression of these four proteins in wild-type C. diphtheriae strain 1737 is strongly iron regulated, which is consistent with the findings from the transcription studies and strongly suggests that each of the operons is expressed as a single transcriptional unit. (Fig. 3).
Fig 3.
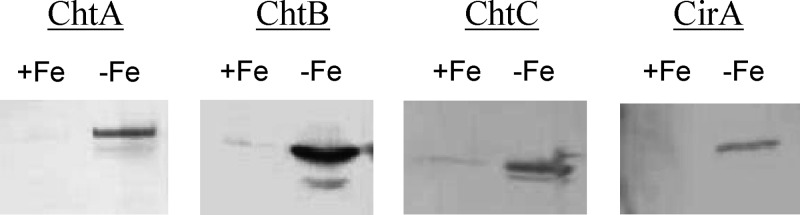
Expression of ChtA, ChtB, ChtC, and CirA is repressed by iron in the C. diphtheriae wild-type strain 1737. Bacteria were grown in high- or low-iron HIBTW medium. Proteins from either whole cells (ChtC and CirA) or supernatant fractions (ChtA and ChtB were located predominately in the supernatant in HIBTW medium) were detected by Western blotting using polyclonal antiserum specific to the proteins indicated. Samples were loaded using equivalent protein levels. +Fe, high-iron medium; −Fe, iron-depleted medium.
Protein localization studies showed that ChtA, ChtB, ChtC, and CirA were predominately associated with the cytoplasmic membrane when the cells were grown in mPGT medium (Fig. 4), which suggests that all of these proteins are anchored to the membrane. However, we observed that full-length ChtA and ChtB proteins were largely secreted when culture was in nutrient-rich HIBTW medium (Fig. 3); it was previously noted that HtaA was also found predominately in the culture supernatant after growth in HIBTW medium (25). The reason for the secretion of these proteins in nutrient-rich medium is not known. DtxR, an intracellular protein, was used as a control in the localization studies and was found exclusively in the cytosolic fraction (reference 25 and data not shown).
Fig 4.
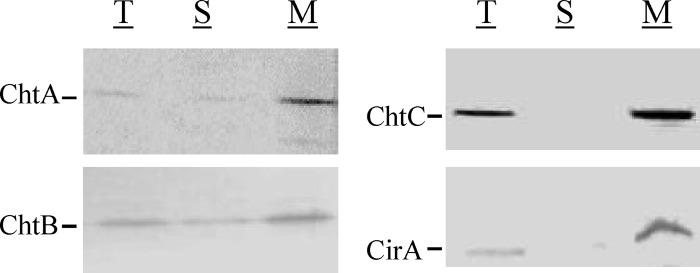
Protein localization studies show that ChtA, ChtB, ChtC, and CirA are associated predominately with the membrane fraction in C. diphtheriae 1737. Cultures were grown in mPGT medium under low-Fe (0.5 μM) conditions and bacteria were fractionated as described in Materials and Methods. Polyclonal antiserum specific to the proteins indicated was used for detection of total cellular proteins (T), soluble cytosolic proteins (S), and membrane-associated proteins (M). Samples were loaded using equivalent protein levels.
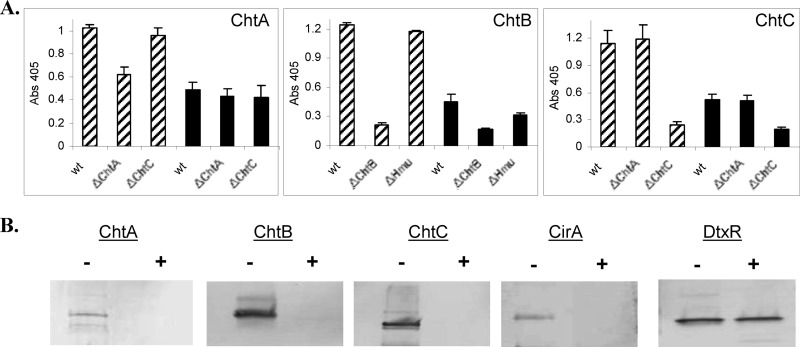
ChtA, ChtB, CirA, and ChtC are surface exposed.
To determine if proteins were present on the surface of C. diphtheriae, we employed a whole-cell ELISA, in which bacteria were grown in either high- or low-iron medium and subsequently bound to microtiter plates. Proteins on the surface of the fixed bacteria were detected by specific antiserum as described in Materials and Methods. As shown in Fig. 5A, significantly higher levels of ChtA, ChtB, and ChtC were detected under iron-depleted conditions than in high-iron medium in the wild-type strain. Similarly, higher levels of the targeted proteins were detected in the wild-type strain than in the mutant strain when the bacteria were cultured under low-iron conditions (an irrelevant mutant strain was also examined as a control, and it showed levels similar to those in the wild type). The high background in the chtA deletion mutant (ΔchtA) may be due to cross-reactivity with ChtC (Fig. 5A, left panel). These findings were further validated in studies in which intact bacteria were treated with proteinase K to assess the susceptibility of surface proteins to protease digestion. It is predicted that surface-exposed proteins would be degraded after exposure to protease. As shown in Fig. 5B, ChtA, ChtB, ChtC, and CirA were all degraded after exposure of whole cells to proteinase K. DtxR, an intracellular protein used as a control, was resistant to protease treatment as expected. DtxR could not be used as a control in the ELISA study due to high nonspecific background activity (not shown). CirA was examined only in the proteinase K study.
Fig 5.
ChtA, ChtB, ChtC and CirA are surface exposed in C. diphtheriae. (A) A whole-cell ELISA was used to detect surface proteins in strain 1737. C. diphtheriae strains were grown in mPGT medium under either high-iron (solid bars) or low-iron (hatched bars) conditions. Equivalent amounts of cells were fixed to the plates, and antiserum specific to the protein of interest was used to detect surface proteins. Values represent the means from three independent experiments (± standard deviations [SD]). (B) Proteinase K was used to detect the presence of surface proteins in C. diphtheriae. See Materials and Methods for experimental details. Bacteria were grown in low-iron mPGT medium, and intact bacteria were incubated in the presence (+) or absence (−) of proteinase K. Proteins from whole cells were examined by Western blotting using the relevant antiserum.
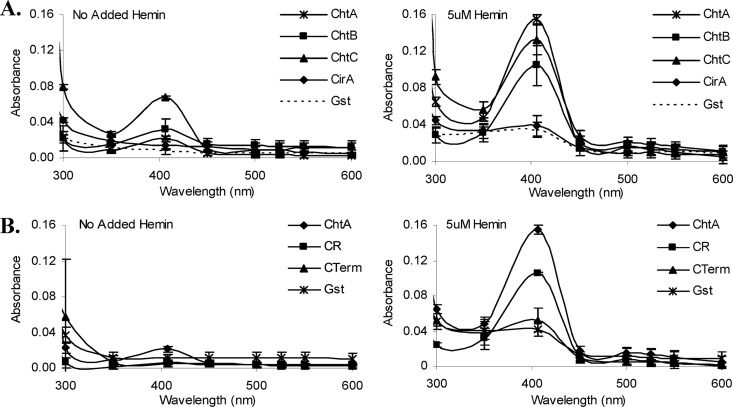
ChtA, ChtB, and ChtC are hemin-binding proteins.
ChtA, ChtB, ChtC, and CirA were examined for their ability to bind hemin using UV-visible spectroscopy. Recombinant proteins purified from E. coli were incubated in the presence of either 5 μM hemin or no added hemin, and the spectrum was analyzed (Fig. 6A). A peak absorbance at 406 nm in the presence of 5 μM hemin was detected for ChtA, ChtC, and ChtB, which is consistent with hemin binding to protein; no significant absorbance peak was observed with CirA or GST, which was used as a negative control (Fig. 6A). The lack of a putative CR domain in CirA is consistent with its inability to bind hemin. Hemin binding by ChtA is associated with the CR domain, since a Strep-tagged fusion protein carrying the N-terminal CR region was able to bind hemin, while a fusion protein harboring the remaining C-terminal portion of the protein failed to bind hemin (Fig. 6B and Fig. 1A).
Fig 6.
(A) ChtA, ChtB, and ChtC are hemin-binding proteins. UV-visible spectroscopy was used to examine the hemin-binding properties of purified GST-CirA, Strep-ChtA, Strep-ChtB, and Strep-ChtC. Proteins at 2 μM were incubated for 15 min in the presence of 5 μM hemin or with no added hemin prior to spectral analysis. Similar results were observed for GST-tagged ChtA, ChtB, and ChtC (data not shown). GST was included as a negative control. Values are the means from three independent experiments (± SD). (B) The CR domain of ChtA binds hemin. UV-visible spectroscopy was used to examine the hemin-binding properties of the CR domain (CR) of ChtA and the C-terminal region (CTerm) of ChtA using Strep-tagged fusion proteins harboring these specific regions. The same procedures described for panel A above were used for these studies.
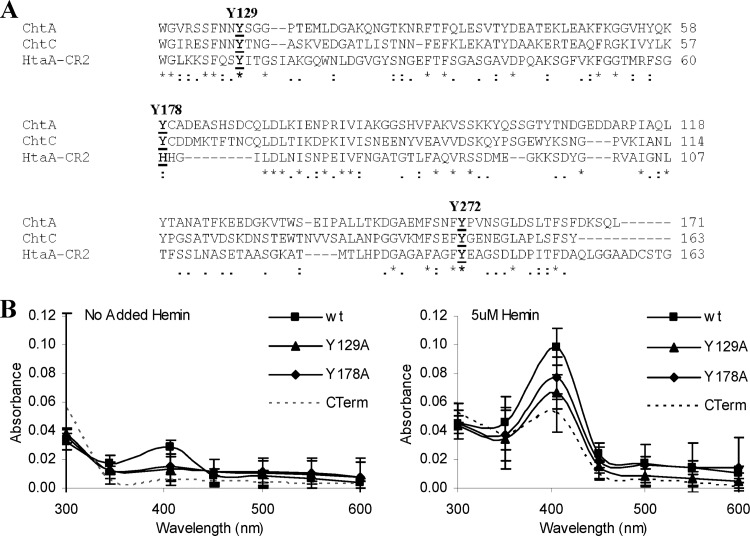
An amino acid alignment of the CR domains between ChtA, ChtC, and HtaA showed that the two highly conserved tyrosine residues previously found in the HtaA CR domains were also conserved in the CR domain from ChtA and ChtC (Y129 and Y272); however, a conserved histidine residue in the HtaA CR domain, which was shown to be critical for hemin and Hb binding (23), aligns with a tyrosine residue (Y178) in both ChtA and ChtC (Fig. 7A). A comparison of the CR domains between ChtC and ChtA reveals numerous conserved residues that are not shared with the HtaA CR domain, suggesting that additional residues in the ChtC and ChtA CR domains may be important for hemin binding or other, yet-unidentified functions for this region. The ChtB CR domain showed high sequence similarity to the CR domain of HtaB and maintains the conserved tyrosine and histidine residues previously described in the HtaB and HtaA CR domains (not shown) (23).
Fig 7.
(A) Sequence alignment of CR domains from the C. diphtheriae ChtA, ChtC, and HtaA proteins. Conserved tyrosine residues are indicated in bold and above the sequences. Asterisks indicate identity; colons and periods indicate sequence similarity. (B) Conserved tyrosine residues in the CR domain of ChtA are critical for optimal hemin binding. UV-visible spectroscopy was used to assess the hemin binding capability of proteins with various tyrosine-to-alanine substitutions in the CR domain of Strep-tagged ChtA. Proteins at 2 μM were incubated for 15 min in the presence of 5 μM hemin or with no added hemin prior to analysis. Values are the means from three independent experiments (± SD). The difference in peak absorbance (406 nm) between the wt and the Y129A mutant is significant at a P value of <0.01, and the difference in peak absorbance between wt and the Y178A, Y178H, and Y272A mutants is significant at a P value of <0.05.
Site-directed mutagenesis was used to change the three conserved tyrosine residues in ChtA (Y129, Y178, and Y272) to alanine residues (Fig. 7A). Because of the high similarity between the CR domains of ChtA and ChtC, only the CR domain of ChtA was examined in the site-directed mutagenesis studies. The ChtA proteins containing these amino substitutions were purified and analyzed for their ability to bind hemin. All three alanine substitutions exhibited significantly reduced hemin binding relative to that of the wild-type protein, with the Y129A substitution resulting in the most significant decrease in absorbance at 406 nm (Fig. 7B, and data not shown for Y272A). Substitution of a histidine at residue Y178 (Y178H) in ChtA showed a decrease in absorbance in the presence of hemin that was not significantly different from that observed with the alanine substitution, Y178A (not shown). In the HtaA and HtaB proteins, a histidine is present in the position equivalent to Y178, and alanine substitutions for this conserved histidine in HtaA resulted in a sharp decrease in absorbance in the presence of hemin, indicating that this histidine residue is critical for hemin binding by HtaA (23). The findings here with ChtA indicate that a histidine cannot replace the tyrosine at Y178 and maintain optimal hemin binding, which suggests that there may be differences in sequence specificity in the hemin-binding site between HtaA and ChtA. All of the alanine and histidine substitution variants exhibited stability similar to that of the wild-type ChtA protein (not shown).
Hemoglobin binding studies.
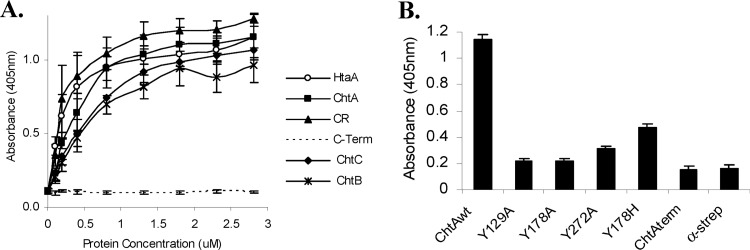
Since certain proteins that are able to bind hemin also have the capability to bind Hb, we tested ChtA, ChtC, and ChtB for their ability to bind Hb using an ELISA procedure (23). As shown in Fig. 8A, the ChtA CR domain showed an ability to bind Hb that was similar to that of HtaA (Kd [dissociation constant] values of 0.24 μM and 0.28 μM, respectively), while the full-length ChtA protein exhibited a slightly reduced binding affinity (Kd = 0.41 μM). ChtA showed a higher affinity for Hb binding than either ChtC (Kd = 0.52 μM) or ChtB (Kd = 0.51 μM). The ChtA C-terminal region and CirA failed to bind Hb (Fig. 8A and B, and data not shown for CirA). Purified ChtA containing alanine substitutions in the conserved tyrosine resides in the CR domain showed significantly reduced ability to bind Hb, with the substitutions in Y129 and Y178 exhibiting the sharpest decrease in Hb binding (Fig. 8B).
Fig 8.
ChtA, ChtB, and ChtC are hemoglobin-binding proteins. (A) Hb binding by Strep-tagged ChtA, ChtA-CR (CR), ChtA-CTerm (C-Term), ChtC, and ChtB was assessed at various protein concentrations as indicated using an ELISA. The Hb-binding protein HtaA was used as a positive control. Values represent the means from three independent experiments (±SD). Binding was detected using anti-Strep antibodies. (B) The effect of amino acid substitutions in ChtA on Hb binding. Hb binding by the various Strep constructs (200 nM) was assessed as for panel A. Values represent the means from three independent experiments (±SD). Binding was detected using anti-Strep antibodies. α-strep, negative-control wells coated only with Hb.
Utilization of Hb as an iron source by the chtAB and cirA-chtC genes.
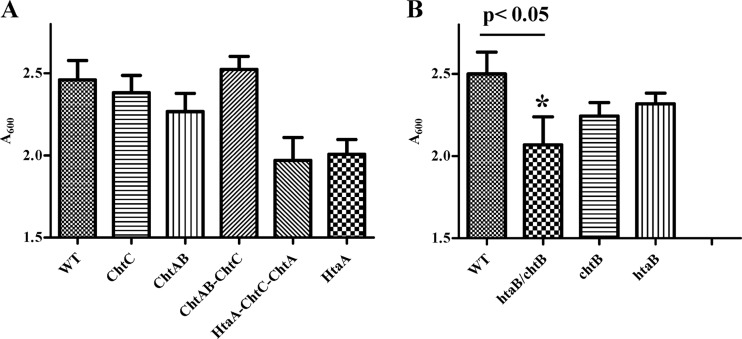
Since proteins that are associated with hemin and Hb binding are often involved in hemin transport and the subsequent use of heme iron, we constructed defined mutations in the C. diphtheriae chtA, chtB, and chtC genes in strain 1737 to determine if any of these genes function in the use of hemin or Hb as an iron source. Analysis of C. diphtheriae mutants that carried deletions in the chtAB operon or chtC or of a triple mutant deleted for chtAB and chtC revealed that these mutants had growth profiles that were not significantly different from that of the wild-type strain when Hb was the sole iron source (Fig. 9A). This finding suggests that these gene products are not involved in the uptake and use of hemin iron. It was previously reported that C. diphtheriae 1737 deleted for htaA had a significant reduction in growth relative to the wild type when hemin or Hb served as the sole iron source. Since HtaA shares similar structural and hemin-binding properties with ChtA and ChtC, it is possible that any defect in hemin iron utilization associated with mutations in chtA and chtC may be complemented or masked by the wild-type copy of htaA. To test for this possibility, we constructed a triple mutant of the 1737 strain in which htaA, chtA, and chtC are deleted from the chromosome. This strain had an Hb iron utilization phenotype that was not significantly different from that of the htaA single mutant, suggesting that the chtA and chtC genes are not involved in Hb iron use in the assay used in this study (Fig. 9A). Hemin was also used as the sole iron source for these studies and showed results similar to those for Hb (data not shown).
Fig 9.
Hb iron utilization assays. Nonpolar deletion mutants were constructed in C. diphtheriae 1737 and assessed for their ability to use Hb as the sole iron source for growth in low-iron mPGT medium. Cultures were grown overnight at 37°C, and cell density was measured by A600. Results are the means from three independent experiments ± standard deviations. The growth difference between the wt and the chtB-htaB double mutant (*) was significant at a P value of <0.05.
In an earlier report, we showed that mutations in htaB had no effect on growth when Hb was the only iron source (25). In this report, we show that HtaB has significant sequence similarity to ChtB, suggesting that these two proteins may have similar functions. If the proteins do share similar activities, this may explain why htaB or chtB single deletion mutants have no detectable phenotype in Hb iron utilization assays, since one of these proteins would be functional in an htaB or chtB single mutant. To assess this observation, we constructed a chtB-htaB double mutant and tested this strain in Hb iron utilization assays. The double mutant showed a significant reduction in growth relative to that of the wild-type strain when Hb was the sole iron source (Fig. 9B) (P < 0.05). Neither of the single mutants exhibited growth characteristics on Hb that were significantly different from those of the wild type, although both mutants showed trends of reduced growth relative to that of the wild-type strain (Fig. 9B). This finding suggests that HtaB and ChtB have similar functions in heme iron utilization. Since these proteins are both surface-exposed hemin-binding proteins, these current results suggest that HtaB and ChtB are involved in the uptake of hemin at the cell surface, and it seems likely that they may be able to substitute for one another in this transport function.
ChtA, ChtC, and HtaA are unable to bind extracellular matrix proteins.
Previous studies in bacterial pathogens show that surface proteins involved in metal or hemin transport are involved in mediating adhesion to extracellular matrix proteins or plasma proteins (18, 39). Because of the surface localization of ChtA, ChtC, and HtaA, we sought to determine whether these large surface-anchored proteins in C. diphtheriae may recognize cellular matrix molecules or plasma proteins. We employed an ELISA procedure, similar to that described above for the Hb binding studies, to assess the ability of ChtA, ChtC, and HtaA to bind to fibronectin and fibrinogen. None of the three proteins showed significant binding to these eukaryotic proteins, while positive-control proteins showed strong binding (see Fig. S1 in the supplemental material). ChtA, ChtC, and HtaA also exhibited no significant binding to transferrin, laminin, and collagen types I and IV (not shown).
DISCUSSION
A recent pan-genomic study reporting the sequences of 13 C. diphtheriae isolates revealed that a large diversity exists among the genomes of these various C. diphtheriae strains (8). This high degree of variability is found in many different genetic processes, including systems involved in the acquisition of iron and in the binding and utilization of hemin and Hb as essential iron sources. We and others showed that two iron-regulated ABC-type iron transport operons, irp1 and irp2, previously identified in C. diphtheriae C7, were absent in strain NCTC13129, the first C. diphtheriae isolate to have its genome sequence determined (35). Similarly, certain iron acquisition systems found in NCTC13129 were not detected in the C7 strain (7). These findings, and others from the pan-genomic analysis, show that different virulent isolates of C. diphtheriae appear to utilize or at least encode different iron acquisitions systems. It remains to be determined which of these various iron transporters are functional and whether they contribute to the virulence or survival of these C. diphtheriae strains.
In this study, we examined a pair of genetic loci composed of two-gene operons that are designated chtA-chtB and cirA-chtC. While these genes are not conserved in all the C. diphtheriae strains presented in Table 2, there appears to be a strong association between these genes: 18 of the 19 strains examined either carry both operons or lack both genetic regions. Interestingly, the transposon-associated chtAB system is found in a closely related group of strains that dominated the diphtheria outbreak in the former Soviet Union (FSU) in the 1990s (37). Although the chtAB genes are present in other isolates, these strains are not associated with a transposable element (Table 2). While it is possible that the chtAB genes were acquired by the FSU isolates by means of horizontal transfer, perhaps a more interesting question is whether these genes provided any advantage to this closely related group of C. diphtheriae strains that grew to dominate the FSU epidemic in the 1990s. High-molecular-weight surface proteins such as ChtA and ChtC that have hemin- and Hb-binding capabilities may have provided a survival advantage to certain C. diphtheriae strains by means of various mechanisms, including enhanced iron acquisition or stronger adhesion to epithelial cell surfaces. The function of these proteins in the epidemic strains is an issue that remains to be determined and may be difficult to resolve in the absence of relevant animal models for this disease.
Both the chtAB and cirA-chtC systems are transcriptionally regulated in an iron-dependent manner by the global regulatory factor DtxR. Western blot analysis showed that all four proteins are similarly regulated by iron, and since promoter activity was detected only upstream of the first gene in each operon, this strongly suggests that the two operons are regulated by a single iron-regulated promoter. We used DNase I footprinting to identify two DtxR-binding sites upstream of cirA. Neither of these binding sites was identified in the pan-genomic study in which the authors performed genome-wide searches for DtxR-binding sites in 13 C. diphtheriae strains (8). While both sites showed a relatively poor match to the consensus DtxR-binding site, they both displayed a very strong and distinct footprint in the binding study, suggesting that both sites are functional. While the genetic and expression studies indicate that site 1 is important for controlling a promoter involved in cirA transcription, it is unclear what role, if any, site 2 has in gene regulation. No promoter activity in either direction was detected from the fusion construct that carried only site 2. It is possible that as-yet-unidentified environmental stimuli and/or regulatory activators may be required for transcriptional activation from a putative DtxR-regulated promoter that overlaps binding site 2.
The ChtA, ChtB, ChtC, and CirA gene products were all found to be membrane associated and exposed on the surface, and with the exception of CirA, which lacked a CR domain, all of the proteins bound hemin and Hb. The binding of hemin and Hb occurred through the CR domain in ChtA, and it appears likely that this motif is required for hemin and Hb binding in ChtC and ChtB because of the sequence similarities between these regions. Both ChtA and ChtC exhibit only limited homology with HtaA, and this similarity is confined to the CR domains. We previously reported that deletion of htaA resulted in a reduced ability to use hemin and Hb as iron sources. In this study, we showed that various strains deleted for chtA or chtC, either as single deletions or in various combinations, were unaffected in their ability to use hemin and Hb as iron sources, suggesting that ChtA and ChtC have no role in hemin iron use when hemin or Hb serves as the sole iron sources.
It was previously proposed that the surface-exposed HtaB may function as a component in a hemin relay mechanism in which it initially receives hemin from HtaA and then transfers hemin to HmuT, the substrate-binding protein associated with the hemin-specific ABC transporter. However, it was shown previously that a C. diphtheriae htaB mutant was not defective in the use of Hb as an iron source (25). In this study, we show that a chtB mutation in combination with the deletion of htaB results in a significant reduction in the use of hemin and Hb as iron sources: the strain with the chtB mutation alone did not exhibit a significant reduction in hemin iron use compared to the wild type. The ChtB and HtaB proteins share a high level of sequence similarity, and it appears likely that these proteins can substitute for each other in the transport of hemin.
While ChtB appears to complement the activity of HtaB, the findings from this report suggest that ChtA and ChtC have a function distinct from that of HtaA, despite similarities in size, structure, cellular location, and the ability to bind hemin and Hb. The CR domains of ChtC and ChtA show a higher degree of similarity to each other than they do to the CR domains in HtaA, which may reflect a difference in binding specificity or even ligand specificity between these CR domains. Another notable difference between these proteins is the large, 450-amino-acid C-terminal region in ChtA and ChtC that has no known function and no homology to any described structural motifs as determined by Pfam analysis. These differences between ChtA/ChtC and HtaA may indicate that the primary physiological function of ChtA and ChtC may be as receptors for as-yet-unidentified iron- or heme-binding proteins or as adhesins to cell surface matrix proteins that were not specifically examined in this study. Our laboratory has recently shown that ChtA and ChtC are not involved in the utilization of heme iron from either Hb-Hp complexes or the heme-bound forms of human serum albumin or hemopexin (Schmitt, unpublished data). Additional studies will be needed to identify the physiological function of these novel iron-regulated hemin-binding proteins in C. diphtheriae.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the intramural research program at the Center for Biologics Evaluation and Research, Food and Drug Administration.
We thank Diana Oram and Scott Stibitz for helpful comments on the manuscript and Rebecca Brady for providing the recombinant proteins ClfA and FbnB.
Footnotes
Published ahead of print 12 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00244-13.
REFERENCES
- 1. Collier RJ. 2001. Understanding the mode of action of diphtheria toxin: a perspective on progress during the 20th century. Toxicon 39:1793–1803 [DOI] [PubMed] [Google Scholar]
- 2. Barksdale L. 1970. Corynebacterium diphtheriae and its relatives. Bacteriol. Rev. 34:378–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cornelis P, Andrews SC. 2010. Iron uptake and homeostasis in microorganisms. Caister Academic Press, Norfolk, United Kingdom [Google Scholar]
- 4. Crosa JH, Mey AR, Payne SM. 2004. Iron transport in bacteria. ASM Press, Washington DC [Google Scholar]
- 5. Boyd J, Oza MN, Murphy JR. 1990. Molecular cloning and DNA sequence analysis of a diphtheria tox iron-dependent regulatory element (dtxR) from Corynebacterium diphtheriae. Proc. Natl. Acad. Sci. U. S. A. 87:5968–5972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schmitt MP, Holmes RK. 1991. Iron-dependent regulation of diphtheria toxin and siderophore expression by the cloned Corynebacterium diphtheriae repressor gene dtxR in C. diphtheriae C7 strains. Infect. Immun. 59:1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kunkle CA, Schmitt MP. 2003. Analysis of the Corynebacterium diphtheriae DtxR regulon: identification of a putative siderophore synthesis and transport system that is similar to the Yersinia high-pathogenicity island-encoded yersiniabactin synthesis and uptake system. J. Bacteriol. 185:6826–6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Trost E, Blom J, de Castro Soares S, Huang IH, Al-Dilaimi A, Schroder J, Jaenicke S, Dorella FA, Rocha FS, Miyoshi A, Azevedo V, Schneider MP, Silva A, Camello TC, Sabbadini PS, Santos CS, Santos LS, Hirata R, Jr, Mattos-Guaraldi AL, Efstratiou A, Schmitt MP, Ton-That H, Tauch A. 2012. Pangenomic study of Corynebacterium diphtheriae that provides insights into the genomic diversity of pathogenic isolates from cases of classical diphtheria, endocarditis, and pneumonia. J. Bacteriol. 194:3199–3215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammer ND, Skaar EP. 2011. Molecular mechanisms of Staphylococcus aureus iron acquisition. Annu. Rev. Microbiol. 65:129–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wyckoff EE, Mey AR, Payne SM. 2007. Iron acquisition in Vibrio cholerae. Biometals 20:405–416 [DOI] [PubMed] [Google Scholar]
- 11. Stojiljkovic I, Hantke K. 1994. Transport of haemin across the cytoplasmic membrane through a haemin-specific periplasmic binding-protein-dependent transport system in Yersinia enterocolitica. Mol. Microbiol. 13:719–732 [DOI] [PubMed] [Google Scholar]
- 12. Mazmanian SK, Skaar EP, Gaspar AH, Humayun M, Gornicki P, Jelenska J, Joachmiak A, Missiakas DM, Schneewind O. 2003. Passage of heme-iron across the envelope of Staphylococcus aureus. Science 299:906–909 [DOI] [PubMed] [Google Scholar]
- 13. Nobles CL, Maresso AW. 2011. The theft of host heme by Gram-positive pathogenic bacteria. Metallomics 3:788–796 [DOI] [PubMed] [Google Scholar]
- 14. Torres VJ, Pishchany G, Humayun M, Schneewind O, Skaar EP. 2006. Staphylococcus aureus IsdB is a hemoglobin receptor required for heme iron utilization. J. Bacteriol. 188:8421–8429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andrade MA, Ciccarelli FD, Perez-Iratxeta C, Bork P. 2002. NEAT: a domain duplicated in genes near the components of a putative Fe3+ siderophore transporter from Gram-positive pathogenic bacteria. Genome Biol. 3:RESEARCH0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grigg JC, Vermeiren CL, Heinrichs DE, Murphy ME. 2007. Haem recognition by a Staphylococcus aureus NEAT domain. Mol. Microbiol. 63:139–149 [DOI] [PubMed] [Google Scholar]
- 17. Pilpa RM, Robson SA, Villareal VA, Wong ML, Phillips M, Clubb RT. 2009. Functionally distinct NEAT (NEAr Transporter) domains within the Staphylococcus aureus IsdH/HarA protein extract heme from methemoglobin. J. Biol. Chem. 284:1166–1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ouattara M, Cunha EB, Li X, Huang YS, Dixon D, Eichenbaum Z. 2010. Shr of group A streptococcus is a new type of composite NEAT protein involved in sequestering haem from methaemoglobin. Mol. Microbiol. 78:739–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anzaldi LL, Skaar EP. 2010. Overcoming the heme paradox: heme toxicity and tolerance in bacterial pathogens. Infect. Immun. 78:4977–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fisher M, Huang YS, Li X, McIver KS, Toukoki C, Eichenbaum Z. 2008. Shr is a broad-spectrum surface receptor that contributes to adherence and virulence in group A streptococcus. Infect. Immun. 76:5006–5015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kunkle CA, Schmitt MP. 2005. Analysis of a DtxR-regulated iron transport and siderophore biosynthesis gene cluster in Corynebacterium diphtheriae. J. Bacteriol. 187:422–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schmitt MP. 1997. Utilization of host iron sources by Corynebacterium diphtheriae: identification of a gene whose product is homologous to eukaryotic heme oxygenases and is required for acquisition of iron from heme and hemoglobin. J. Bacteriol. 179:838–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Allen CE, Schmitt MP. 2011. Novel hemin binding domains in the Corynebacterium diphtheriae HtaA protein interact with hemoglobin and are critical for heme iron utilization by HtaA. J. Bacteriol. 193:5374–5385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Drazek ES, Hammack CA, Schmitt MP. 2000. Corynebacterium diphtheriae genes required for acquisition of iron from haemin and haemoglobin are homologous to ABC haemin transporters. Mol. Microbiol. 36:68–84 [DOI] [PubMed] [Google Scholar]
- 25. Allen CE, Schmitt MP. 2009. HtaA is an iron-regulated hemin binding protein involved in the utilization of heme iron in Corynebacterium diphtheriae. J. Bacteriol. 191:2638–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wilks A, Schmitt MP. 1998. Expression and characterization of a heme oxygenase (Hmu O) from Corynebacterium diphtheriae. Iron acquisition requires oxidative cleavage of the heme macrocycle. J. Biol. Chem. 273:837–841 [DOI] [PubMed] [Google Scholar]
- 27. Tai SP, Krafft AE, Nootheti P, Holmes RK. 1990. Coordinate regulation of siderophore and diphtheria toxin production by iron in Corynebacterium diphtheriae. Microb. Pathog. 9:267–273 [DOI] [PubMed] [Google Scholar]
- 28. Oram DM, Jacobson AD, Holmes RK. 2006. Transcription of the contiguous sigB, dtxR, and galE genes in Corynebacterium diphtheriae: evidence for multiple transcripts and regulation by environmental factors. J. Bacteriol. 188:2959–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schmitt MP, Holmes RK. 1993. Analysis of diphtheria toxin repressor-operator interactions and characterization of a mutant repressor with decreased binding activity for divalent metals. Mol. Microbiol. 9:173–181 [DOI] [PubMed] [Google Scholar]
- 30. Schmitt MP, Holmes RK. 1994. Cloning, sequence, and footprint analysis of two promoter/operators from Corynebacterium diphtheriae that are regulated by the diphtheria toxin repressor (DtxR) and iron. J. Bacteriol. 176:1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Burgos JM, Schmitt MP. 2012. The ChrA response regulator in Corynebacterium diphtheriae controls hemin-regulated gene expression through binding to the hmuO and hrtAB promoter regions. J. Bacteriol. 194:1717–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ton-That H, Schneewind O. 2003. Assembly of pili on the surface of Corynebacterium diphtheriae. Mol. Microbiol. 50:1429–1438 [DOI] [PubMed] [Google Scholar]
- 33. Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685 [DOI] [PubMed] [Google Scholar]
- 34. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410 [DOI] [PubMed] [Google Scholar]
- 35. Cerdeno-Tarraga AM, Efstratiou A, Dover LG, Holden MT, Pallen M, Bentley SD, Besra GS, Churcher C, James KD, De Zoysa A, Chillingworth T, Cronin A, Dowd L, Feltwell T, Hamlin N, Holroyd S, Jagels K, Moule S, Quail MA, Rabbinowitsch E, Rutherford KM, Thomson NR, Unwin L, Whitehead S, Barrell BG, Parkhill J. 2003. The complete genome sequence and analysis of Corynebacterium diphtheriae NCTC13129. Nucleic Acids Res. 31:6516–6523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vitek CR, Wharton M. 1998. Diphtheria in the former Soviet Union: reemergence of a pandemic disease. Emerg. Infect. Dis. 4:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Popovic T, Kombarova SY, Reeves MW, Nakao H, Mazurova IK, Wharton M, Wachsmuth IK, Wenger JD. 1996. Molecular epidemiology of diphtheria in Russia, 1985-1994. J. Infect. Dis. 174:1064–1072 [DOI] [PubMed] [Google Scholar]
- 38. Freeman VJ. 1951. Studies on the virulence of bacteriophage-infected strains of Corynebacterium diphtheriae. J. Bacteriol. 61:675–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clarke SR, Wiltshire MD, Foster SJ. 2004. IsdA of Staphylococcus aureus is a broad spectrum, iron-regulated adhesin. Mol. Microbiol. 51:1509–1519 [DOI] [PubMed] [Google Scholar]
- 40. Park WH, Williams AW. 1896. The production of diphtheria toxin. J. Exp. Med. 1:164–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.