Abstract
The phylogenetic affiliation and physiological characteristics (e.g., Ks and maximum specific growth rate [μmax]) of an anaerobic ammonium oxidation (anammox) bacterium, “Candidatus Scalindua sp.,” enriched from the marine sediment of Hiroshima Bay, Japan, were investigated. “Candidatus Scalindua sp.” exhibits higher affinity for nitrite and a lower growth rate and yield than the known anammox species.
TEXT
Anaerobic ammonium oxidation (anammox) is a microbial process in which ammonium is oxidized to nitrogen gas with nitrite as the electron acceptor under anoxic conditions (1–3). At least five candidate genera have been tentatively proposed in this taxon (4). The “Candidatus Scalindua” group is primarily found in marine environments (5–10). Previous studies indicate that “Candidatus Scalindua” contains taxonomically diverse members, while only a few members of the “Candidatus Scalindua” group have been successfully grown in enrichment cultures so far (11–15). Physiological characteristics of anammox bacteria affiliated to “Candidatus Scalindua” have been demonstrated only partially (11, 12, 16, 17) compared with the freshwater anammox bacteria (18–23). In this study, the phylogenetic affiliation and physiological characteristics of an anammox bacterium previously enriched from marine sediments of Hiroshima Bay, Japan, were determined. Anaerobic batch experiments were performed to determine the following physiological parameters: (i) growth temperature, pH, and salinity ranges, (ii) inhibition by ammonium and nitrite, (iii) half-saturation constants (Ks) for nitrite and ammonium, (iv) accumulation and consumption of hydrazine after the addition of hydroxylamine, and (v) biomass yield. The maximum specific growth rate (μmax) and the ultrastructure of the anammox bacterium were also determined.
Biomass samples were obtained from a 7-liter membrane bioreactor (MBR) inoculated with anammox biofilms (13, 14) to obtain free-living anammox bacterial cells related to “Candidatus Scalindua.” Cells in the MBR were collected for fluorescence in situ hybridization (FISH) and phylogenetic analyses, transmission electron microscopy (TEM), and anaerobic batch experiments. The detailed procedures are described in the supplemental material.
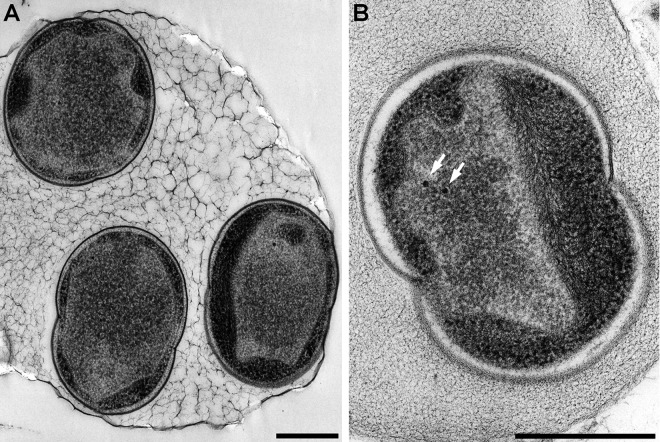
A single species of “Candidatus Scalindua sp.” was successfully enriched in free-living cells (see Fig. S1 in the supplemental material) using the MBR in this study, where the anammox bacteria that hybridized with the Sca1129b probe accounted for 88.8% ± 3.1% of all the bacteria. The partial sequences of the 16S rRNA gene from 83 clones were grouped into a single operational taxonomic unit (OTU) based on 97% sequence identity. Four nearly full-length 16S rRNA gene sequences from the OTU (99.7% identity among the four sequences) shared 96.9% to 97.0% identity with the sequence of “Candidatus Scalindua wagneri” (Fig. 1; see also Table S2 in the supplemental material). Such low sequence similarity suggests that the members of “Candidatus Scalindua sp.” are affiliated with a different anammox species in the “Candidatus Scalindua” lineage. The cellular structure of “Candidatus Scalindua sp.” has shown three separate compartments that include electron-dense particles and no pilus-like appendages (Fig. 2), as reported by van Niftrik et al. (24, 25).
Fig 1.
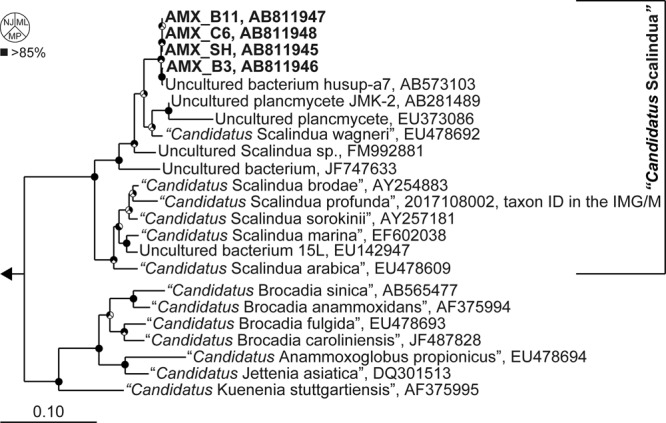
Maximum-likelihood tree based on 16S rRNA gene sequences. The GenBank/EMBL/DDBJ accession numbers are indicated. The scale bar represents the number of nucleotide changes per sequence position. Pie charts at the nodes represent the confidence of the branch topology results, and bootstrap values (1,000 resamplings) greater than 85% are filled in black (the neighbor-joining method, NJ, for the upper-left sector, the maximum-likelihood method, ML, for the upper-right sector, or the maximum-parsimony method, MP, for the bottom sector). The designation of “Ca. Scalindua” is indicated on the right. The Planctomyces brasiliensis sequence (CP002546) served as an outgroup to root the tree. ID, identification; IMG/M, Integrated Microbial Genomes with Microbiome Samples.
Fig 2.
Transmission electron micrographs of “Candidatus Scalindua sp.” enriched in the MBR, showing the cells which were about to be divided. All cells in panels A and B were divided into three separate compartments by individual membranes: the paryphoplasm, the riboplasm, and the anammoxosome compartments. In panel A, all cells were occupied by the voluminous anammoxosome. In panel B, the white arrows indicate condensed and electron-dense particles. Scale bars, 500 nm.
Table 1 summarizes the physiological characteristics of “Candidatus Scalindua sp.” and other anammox bacteria (10, 12, 18–23, 26). The optimal temperature and pH ranges of “Candidatus Scalindua sp.” (10 to 30°C and pH 6.0 to 8.5, respectively) (Fig. 3A and B) were lower than those of other anammox species (i.e., 15 to 45°C and pH 6.5 to 9.0, respectively) (10, 12, 19, 23, 26). Anammox activities of “Candidatus Scalindua sp.” were observed under conditions of 0.8% to 4.0% salinity, whereas no activity was detected in the absence of salinity. This outcome indicates that “Candidatus Scalindua sp.” is a halophilic bacterium. “Candidatus Scalindua sp.” accumulated hydrazine after the spike addition of hydroxylamine (see Fig. S2 in the supplemental material), which is a phenomenon commonly observed in the known anammox bacteria (12, 18, 21, 23).
Table 1.
Physiological characteristics of “Candidatus Scalindua sp.” and four anammox bacteria identified elsewhere: “Candidatus Brocadia sinica,” “Candidatus Brocadia anammoxidans,” “Candidatus Kuenenia stuttgartiensis,” and “Candidatus Scalindua profunda”
| Parameter | Value(s) for: |
||||
|---|---|---|---|---|---|
| “Ca. Scalindua sp.” | “Ca. Brocadia sinica” | “Ca. Brocadia anammoxidans” | “Ca. Kuenenia stuttgartiensis” | “Ca. Scalindua profunda” | |
| Growth temp (°C) | 10–30 | 25–45 | 20–43 | 25–37 | 15–45 |
| Growth pH | 6.0–8.5 | 7.0–8.8 | 6.7–8.3 | 6.5–9.0 | 7.4 |
| Growth salinity (%) or level of salinity (mmol) | 1.5–4.0 | <3 | Not determined | 200 mmol (chloride) | 3.3 |
| Biomass yield (mmol C [mmol N]−1) | 0.030 | 0.063 | 0.07 | Not determined | Not determined |
| Ks for ammonium (μM) | 3 | 28 ± 4 | <5 | Not determined | Not determined |
| Ks for nitrite (μM) | 0.45 | 86 ± 4 | <5 | 0.2–3 | Not determined |
| Activation energy (kJ mol−1) | 81.4 ± 3 | 56 ± 3 | 70 | Not determined | Not determined |
| Protein content of biomass (g protein [g VSS]−1) | 0.64 | 0.61 | 0.6 | Not determined | Not determined |
| μmax (h−1) | 0.0020 | 0.0041 | 0.0027 | 0.0026–0.0035 | Not determined |
| Tolerance | |||||
| Nitrite (mM) | 7.5 | <16 | 7 | 13, 25 | Not determined |
| Ammonium (mM) | >16 | Not determined | Not determined | Not determined | Not determined |
Fig 3.
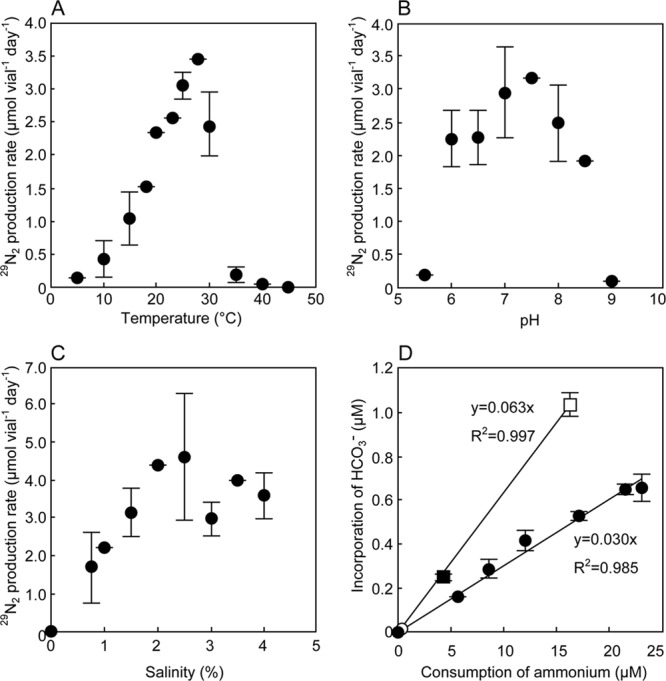
The results of batch experiments. (A to C) Influence of temperature, pH, and salinity on anammox activity determined by the 29N2 gas production rate. (D) Incorporation of [14C]bicarbonate and ammonium consumption by anammox bacteria. Incorporation of [14C]bicarbonate (vertical axis) increased with increases in the ammonium consumption rate (horizontal axis). Filled circles represent the value of “Candidatus Scalindua sp.” at 28°C, whereas an open circle represents the value at 37°C. Filled and open squares represent the values of “Candidatus Brocadia sinica” at 28 and 37°C, respectively. Biomass yields, 0.030 and 0.063 mol C (mol NH4+)−1, were obtained by dividing the amount of incorporated [14C]bicarbonate by the amounts of consumed ammonium obtained under different temperature conditions. All values are means ± standard deviations of the results of independent triplicate experiments.
The Ks values for nitrite and ammonium of “Candidatus Scalindua sp.” (Table 1; see also Fig. S3 in the supplemental material) were lower than those of other anammox bacteria, suggesting that the high affinity for nitrite is necessary for the bacteria survive in marine environments with extremely low levels of nitrite concentrations. Indeed, the ammonium, nitrite, and nitrate concentrations at the sediment sampling point (the sediment was used as the inoculum in this study) were 17.8, 2.1, and 5.7 μM, respectively (15), which suggests no occurrence of the substrate limitation for the growth of “Candidatus Scalindua sp.” in such marine environments.
Interestingly, the biomass yield of “Candidatus Scalindua sp.” was half the reported value (19, 23). The biomass yield of “Candidatus Scalindua sp.” was determined to be 0.030 mol C (mol NH4+)−1 at 28°C and 37°C (Fig. 3D). The biomass yield of “Candidatus Brocadia sinica” was also not dependent on temperature (0.063 mol C [mol NH4+]−1 at 28 and 37°C) (Fig. 3D). The reason for the low biomass yield is not clear at present. The maximum volumetric ammonium oxidation rate (qmax; 4.02 g N liter−1 day−1) was obtained in an up-flow column reactor after 50 days of operation (see Fig. S4 in the supplemental material). The biomass concentration at that point was 4.8 g volatile suspended solids (VSS) liter−1 (corresponding to 3.07 g protein liter−1). Based on the biomass yield, qmax, and biomass concentration, the μmax was calculated to be 0.0020 h−1 (doubling time = 14.4 days). The μmax of “Candidatus Scalindua sp.” (0.0020 h−1) was significantly lower than those of other anammox bacteria (0.0027 to 0.0041 h−1) (18, 22, 23). The low μmax of “Candidatus Scalindua sp.” was derived from the low biomass yield, as the qmax of “Candidatus Scalindua sp.” (65 μmol NH4+ [g protein]−1 min−1) was comparable to those of other anammox bacteria (3, 23, 26).
In conclusion, the fundamental physiological characteristics of “Candidatus Scalindua sp.” enriched from the sediment of Hiroshima Bay, Japan, were investigated. This microorganism is a halophilic bacterium and exhibits higher affinity for nitrite and a lower growth rate than the known anammox species. “Candidatus Scalindua sp.” could maintain its anammox activity under low-temperature conditions. These physiological characteristics support the idea of the predominance of “Candidatus Scalindua sp.” in marine sediments. The findings contribute to our understanding of the niche adaptation of “Candidatus Scalindua sp.”
Nucleotide sequence accession numbers.
The 16S-23S rRNA gene sequence data were deposited in the GenBank/EMBL/DDBJ databases under accession numbers AB811945 to AB811948.
Supplementary Material
ACKNOWLEDGMENTS
This research was partially supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology, by JSPS Fellows (T.A.) from Japan Society for the Promotion of Science (JSPS), and by Core Research of Evolutional Science and Technology (CREST).
This study was partially conducted at the Analysis Center of Life Science, Hiroshima University.
Footnotes
Published ahead of print 12 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00056-13.
REFERENCES
- 1. Mulder A, van de Graaf AA, Robertson LA, Kuenen JG. 1995. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor. FEMS Microbiol. Ecol. 16:177–183 [Google Scholar]
- 2. van de Graaf AA, de Bruijn P, Robertson LA, Jetten MSM, Kuenen JG. 1996. Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142:2187–2196 [Google Scholar]
- 3. Strous M, Fuerst JA, Kramer EH, Logemann S, Muyzer G, van de Pas-Schoonen KT, Webb R, Kuenen JG, Jetten MSM. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446–449 [DOI] [PubMed] [Google Scholar]
- 4. Jetten MSM, Op den Camp HJM, Kuenen JG, Strous M. 2009. Family I. “Candidatus Brocadiaceae” fam. nov., p 918–925 In Krieg NR, Staley JT, Brown DR, Hedlund BP, Paster BJ, Ward NL, Ludwig W, Whitman WB. (ed), Bergey's manual of systematic bacteriology: the Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes, 2nd ed, vol 4 Springer-Verlag, New York, NY [Google Scholar]
- 5. Kuypers MMM, Sliekers AO, Lavik G, Schmid M, Jørgensen BB, Kuenen JG, Sinninghe Damsté JS, Strous M, Jetten MS. 2003. Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422:608–611 [DOI] [PubMed] [Google Scholar]
- 6. Amano T, Yoshinaga I, Okada K, Yamagishi T, Ueda S, Obuchi A, Sako Y, Suwa Y. 2007. Detection of anammox activity and diversity of anammox bacteria-related 16S rRNA genes in coastal marine sediment in Japan. Microbes Environ. 22:232–242 [Google Scholar]
- 7. Woebken D, Lam P, Kuypers MMM, Naqvi SWA, Kartal B, Strous M, Jetten MSM, Fuchs BM, Amann R. 2008. A microdiversity study of anammox bacteria reveals a novel Candidatus Scalindua phylotype in marine oxygen minimum zones. Environ. Microbiol. 10:3106–3119 [DOI] [PubMed] [Google Scholar]
- 8. Galán A, Molina V, Thamdrup B, Woebken D, Lavik G, Kuypers MMM, Ulloa O. 2009. Anammox bacteria and the anaerobic oxidation of ammonium in the oxygen minimum zone off northern Chile. Deep Sea Res. II 56:1021–1031 [Google Scholar]
- 9. Brandsma J, van de Vossenberg J, Risgaard-Petersen N, Schmid MC, Engström P, Eurenius K, Hulth S, Jaeschke A, Abbas B, Hopmans EC, Strous M, Schouten S, Jetten MSM, Damsté JSS. 2011. A multi-proxy study of anaerobic ammonium oxidation in marine sediments of the Gullmar Fjord, Sweden. Environ. Microbiol. Rep. 3:360–366 [DOI] [PubMed] [Google Scholar]
- 10. van de Vossenberg J, Woebken D, Maalcke WJ, Wessels HJCT, Dutilh BE, Kartal B, Janssen-Megens EM, Roeselers G, Yan J, Speth D, Gloerich J, Geerts W, van der Biezen E, Pluk W, Francoijs KJ, Russ L, Lam P, Malfatti SA, Tringe SG, Haaijer SCM, Op den Camp HJM, Stunnenberg HG, Amann R, Kuypers MMM, Jetten MSM. 9 May 2012. The metagenome of the marine anammox bacterium ‘Candidatus Scalindua profunda’ illustrates the versatility of this globally important nitrogen cycle bacterium. Environ. Microbiol. [Epub ahead of print.] 10.1111/j.1462-2920.2012.02774.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakajima J, Sakka M, Kimura T, Furukawa K, Sakka K. 2008. Enrichment of anammox bacteria from marine environment for the construction of a bioremediation reactor. Appl. Microbiol. Biotechnol. 77:1159–1166 [DOI] [PubMed] [Google Scholar]
- 12. van de Vossenberg J, Rattray JE, Geerts W, Kartal B, van Niftrik L, van Donselaar EG, Damsté JSS, Strous M, Jetten MSM. 2008. Enrichment and characterization of marine anammox bacteria associated with global nitrogen gas production. Environ. Microbiol. 10:3120–3129 [DOI] [PubMed] [Google Scholar]
- 13. Kawagoshi Y, Nakamura Y, Kawashima H, Fujisaki K, Furukawa K, Fujimoto A. 2010. Enrichment of marine anammox bacteria from seawater-related samples and bacterial community study. Water Sci. Technol. 61:119–126 [DOI] [PubMed] [Google Scholar]
- 14. Kindaichi T, Awata T, Suzuki Y, Tanabe K, Hatamoto M, Ozaki N, Ohashi A. 2011. Enrichment using an up-flow column reactor and community structure of marine anammox bacteria from coastal sediment. Microbes Environ. 26:67–73 [DOI] [PubMed] [Google Scholar]
- 15. Kindaichi T, Awata T, Tanabe K, Ozaki N, Ohashi A. 2011. Enrichment of marine anammox bacteria in Hiroshima Bay sediments. Water Sci. Technol. 63:964–969 [DOI] [PubMed] [Google Scholar]
- 16. Awata T, Tanabe K, Kindaichi T, Ozaki N, Ohashi A. 2012. Influence of temperature and salinity on microbial structure of marine anammox bacteria. Water Sci. Technol. 66:958–964 [DOI] [PubMed] [Google Scholar]
- 17. Kartal B, Koleva M, Arsov R, van der Star W, Jetten MSM, Strous M. 2006. Adaptation of a freshwater anammox population to high salinity wastewater. J. Biotechnol. 126:546–553 [DOI] [PubMed] [Google Scholar]
- 18. Strous M, Heijnen JJ, Kuenen JG, Jetten MSM. 1998. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 50:589–596 [Google Scholar]
- 19. Strous M, Kuenen JG, Jetten MSM. 1999. Key physiology of anaerobic ammonium oxidation. Appl. Environ. Microbiol. 65:3248–3250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dapena-Mora A, Fernandez I, Campos JL, Mosquera-Corral A, Mendez R, Jetten MSM. 2007. Evaluation of activity and inhibition effects on Anammox process by batch tests based on the nitrogen gas production. Enzyme Microb. Technol. 40:859–865 [Google Scholar]
- 21. van der Star WRL, van de Graaf MJ, Kartal B, Picioreanu C, Jetten MSM, van Loosdrecht MCM. 2008. Response of anaerobic ammonium-oxidizing bacteria to hydroxylamine. Appl. Environ. Microbiol. 74:4417–4426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Star WRL, Miclea AI, van Dongen UGJM, Muyzer G, Picioreanu C, van Loosdrecht MCM. 2008. The membrane bioreactor: a novel tool to grow anammox bacteria as free cells. Biotechnol. Bioeng. 101:286–294 [DOI] [PubMed] [Google Scholar]
- 23. Oshiki M, Shimokawa M, Fujii N, Satoh H, Okabe S. 2011. Physiological characteristics of the anaerobic ammonium-oxidizing bacterium ‘Candidatus Brocadia sinica.’ Microbiology 157:1706–1713 [DOI] [PubMed] [Google Scholar]
- 24. van Niftrik L, Geerts WJC, van Donselaar EG, Humbel BM, Yakushevska A, Verkleij AJ, Jetten MSM, Strous M. 2008. Combined structural and chemical analysis of the anammoxosome: a membrane-bounded intracytoplasmic compartment in anammox bacteria. J. Struct. Biol. 161:401–410 [DOI] [PubMed] [Google Scholar]
- 25. van Niftrik L, Geerts WJC, van Donselaar EG, Humbel BM, Webb RI, Fuerst JA, Verkleij AJ, Jetten MSM, Strous M. 2008. Linking ultrastructure and function in four genera of anaerobic ammonium-oxidizing bacteria: cell plan, glycogen storage, and localization of cytochrome c proteins. J. Bacteriol. 190:708–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Egli K, Fanger U, Alvarez PJJ, Siegrist H, van der Meer JR, Zehnder AJB. 2001. Enrichment and characterization of an anammox bacterium from a rotating biological contactor treating ammonium-rich leachate. Arch. Microbiol. 175:198–207 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.