Abstract
Aichi viruses (AiVs) have been proposed as a causative agent of human gastroenteritis potentially transmitted by fecal-oral routes through contaminated food or water. In the present study, we developed a TaqMan minor groove binder (MGB)-based reverse transcription-quantitative PCR (RT-qPCR) system that is able to quantify AiVs and differentiate between genotypes A and B. This system consists of two assays, an AiV universal assay utilizing a universal primer pair and a universal probe and a duplex genotype-specific assay utilizing the same primer pair and two genotype-specific probes. The primers and probes were designed based on multiple alignments of the 21 available AiV genome sequences containing the capsid gene. Using a 10-fold dilution of plasmid DNA containing the target sequences, it was confirmed that both assays allow detection and quantification of AiVs with a quantitative range of 1.0 × 101 to 1.0 × 107 copies/reaction, and the genotype-specific assay reacts specifically to each genotype. To validate the newly developed assays, 30 clinical stool specimens were subsequently examined with the assays, and the AiV RNA loads were determined to be 1.4 × 104 to 6.6 × 109 copies/g stool. We also examined 12 influent and 12 effluent wastewater samples collected monthly for a 1-year period to validate the applicability of the assays for detection of AiVs in environmental samples. The AiV RNA concentrations in influent and effluent wastewater were determined to be up to 2.2 × 107 and 1.8 × 104 copies/liter, respectively. Our RT-qPCR system is useful for routine diagnosis of AiVs in clinical stool specimens and environmental samples.
INTRODUCTION
Aichi viruses (AiVs), together with bovine and porcine kobuviruses, are members of the genus Kobuvirus in the family Picornaviridae (1–3). The prototype AiV strain A846/88 was first isolated in 1989 in Japan from a fecal sample from a patient with oyster-associated acute gastroenteritis (4). AiVs were subsequently detected from fecal samples of gastroenteritis patients in other countries in Asia (5–9), Europe (10–16), South America (14), and Africa (17, 18), suggesting the worldwide distribution of AiVs.
Several AiV seroprevalence studies performed in Japan (3), Germany (14), France (19), Spain (20), and Tunisia (21) demonstrated a high prevalence of AiV antibodies in adults (80 to 99%), indicating widespread exposure to AiVs. Our recent findings showing the high occurrence of AiVs in wastewater and river water also suggest that they are highly prevalent among humans (22). On the other hand, a number of clinical investigations demonstrated a low incidence of AiV infection in patients with either sporadic or epidemic gastroenteritis (5, 7, 8, 10, 12–18). These findings suggest that AiVs might be circulating without causing any symptoms or that AiVs could be responsible for a portion of subclinical gastroenteritis infections.
The AiV genome is a single-stranded positive-sense RNA of approximately 8.3 kb (23). AiVs have been divided into two genetically distinct genotypes (A and B) on the basis of nucleotide sequences of the 3C-3D (3CD) junction region (24). Recently, an AiV strain representing a novel genotype was identified and proposed to represent genotype C (10). The reverse transcription (RT)-PCR assay is a widely used method for AiV identification because of its high sensitivity and applicability for further genetic analysis to determine genotypes (24). In both clinical and environmental studies, the genotypes of AiVs have been determined based on nucleotide sequence analysis of the PCR products of the 3CD junction or the capsid region (5, 6, 8–12, 14, 16, 18, 22, 25); especially AiV detection in environmental samples requires cloning of the PCR products since usually multiple AiV strains exist in an environmental sample (22). However, these procedures are labor-intensive and time-consuming and require expensive reagents and equipment.
The RT-quantitative PCR (RT-qPCR) assay, which allows rapid, sensitive, and specific detection and can be used for quantitative analysis and for differentiation of genotypes using a multiplex format, has been widely used for detection of viruses and would be an attractive alternative for AiV detection and genotyping. In the present study, we aimed to establish a TaqMan minor groove binder (MGB)-based RT-qPCR system that would allow quantitative detection and genotyping of AiVs in clinical and environmental samples.
MATERIALS AND METHODS
Nucleotide sequence alignment of Aichi viruses.
Nucleotide sequences of 21 AiV genomes, of which 15 are genotype A and 6 are genotype B, were aligned using the program Bio Edit (Ibis Therapeutics, Carlsbad, CA). The accession numbers of the sequences used in this study were as follows: AB040749, NC_001918, AY747174, EU143286, EU143271, EU143272, EU143273, EU143278, EU143281, EU143282, EU143287, EU143288, EU143279, EU143277, EU143285, DQ028632, EU143274, EU143275, EU143276, EU143290, and AB712380.
Construction of the standard plasmid.
To construct standard plasmids of each AiV genotype, approximately 490 nucleotides (nt) of the partial viral protein (VP) 0 region of A846/88 (the prototype AiV strain) and N1277/91 strains belonging to genotypes A and B, respectively, were amplified by PCR with KOD-plus DNA polymerase (Toyobo, Osaka, Japan). Forward primer AiVp-AB-1702F (5′-TACCCCACCGCCACCC-3′, corresponding to nt 1702 to 1717 in strain A846/88 [accession no. AB040749]), and reverse primer AiVp-A-2188R (5′-GGACCTCTATGGTAGCGGTGTT-3′, corresponding to nt 2167 to 2188 in strain A846/88) were used for the A846/88 strain to construct the genotype A plasmid standard, and the forward primer AiVp-AB-1702F and reverse primer AiVp-B-2153 (5′-GGACCTCTATGGTGGCAGTGTT-3′, corresponding to nt 2153 to 2174 in the Goiania/GO/03/01/Brazil strain) were used for the N1277/91 strain to construct the genotype B plasmid standard. The PCR products were purified with the High Pure product purification kit (Roche Applied Science, Mannheim, Germany) and cloned into a pCR-Blunt II-TOPO vector (Invitrogen, Carlsbad, CA). The plasmid was purified with a QIAprep spin miniprep kit (Qiagen, Hilden, Germany), and the concentration of the purified plasmid was determined by measuring the optical density at 260 nm. The DNA sequence was confirmed by direct sequencing using a GenomeLab DTCS quick start kit and Beckman CEQ 8000 DNA sequencer system (Beckman Coulter, Fullerton, CA).
Clinical stool specimens.
A total of 30 clinical stool specimens obtained from six nonbacterial acute gastroenteritis outbreaks (25 specimens) and sporadic cases (5 specimens) in Aichi Prefecture, Japan, were used to validate the qPCR. These specimens had previously been examined for AiVs using RT-PCR, and 20 of them were positive for AiVs; genotypes were determined by direct sequencing of the PCR products for 17 out of the 20 AiV-positive specimens (24, 25). The stool samples were prepared as a 10% homogenate in veal infusion broth with 0.5% bovine serum albumin (fraction V; Sigma Chemical, St. Louis, MO) and centrifuged at 10,000 × g for 20 min, and the supernatants were stored at −30°C until further analysis.
Wastewater samples.
Influent and effluent water samples were collected monthly, from March 2005 to February 2006, from a wastewater treatment plant (WWTP) utilizing a conventional activated sludge process followed by chlorination, located in an urbanized area in Japan. All samples were collected on sunny or cloudy days in plastic bottles, stored on ice, and transported to the laboratory. The wastewater samples were concentrated by the adsorption-elution method, using an electronegative membrane (catalog no. HAWP-090-00; Millipore, Tokyo, Japan) with an acid rinse procedure (26). Briefly, 2.5 M MgCl2 was added to the samples to obtain a final concentration of 25 mM. The samples (100 ml influent and 1,000 ml effluent) were then passed through the electronegative filter (Millipore) attached to a glass filter holder (Advantec, Tokyo, Japan). Magnesium ions were removed by passing 200 ml of 0.5 mM H2SO4 (pH 3.0) through the filter, and the viruses were eluted with 10 ml of 1.0 mM NaOH (pH 10.8). The eluate was recovered in a tube containing 50 μl of 100 mM H2SO4 (pH 1.0) and 100 μl of 100× Tris-EDTA buffer (pH 8.0) for neutralization, followed by further centrifugal concentration using a Centriprep YM-50 (Millipore) to obtain a final volume of 700 μl. The concentrates were stored at −80°C until further analysis.
These wastewater samples had previously been examined for AiVs by RT-nested PCR, and 12 influent (100%) and 11 effluent (92%) wastewater samples were positive for AiVs; their genotypes were determined by cloning and sequencing of the PCR product (22).
RNA extraction and reverse transcription.
For stool samples, viral RNA was extracted from 200 μl of the supernatant using the High Pure viral RNA kit (Roche Applied Science) to obtain a final volume of 50 μl. For wastewater samples, viral RNA was extracted from the 140 μl of the concentrated wastewater samples using a QIAamp viral RNA minikit (Qiagen). The RT reaction was performed using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Briefly, RNA (15 μl) was added to 15 μl of RT mixture containing RT buffer, and 75 U of MultiScribe reverse transcriptase (Applied Biosystems). The RT reaction mixture was incubated at 25°C for 10 min, then at 37°C for 120 min, and then at 85°C for 5 min to inactivate the enzyme.
qPCR. (i) AiV universal assay.
A qPCR that reacts with both genotypes A and B (the AiV universal assay) was performed in a 25-μl reaction volume containing 2.5 μl of template (plasmid DNA or cDNA), 12.5 μl of QuantiTect probe PCR master mix (a mixture of HotStarTaq DNA polymerase, QuantiTect probe PCR buffer, deoxynucleoside triphosphate [dNTP] mix, and ROX dye) (Qiagen), 400 nM each AiV universal primer (forward, AiV-AB-F; reverse, AiV-AB-R), and 300 nM AiV universal TaqMan MGB probe (AiV-AB-TP) (Table 1). PCR amplification was performed with an ABI Prism 7500 sequence detection system (Applied Biosystems) under the following conditions: initial denaturation at 95°C for 15 min to activate DNA polymerase, followed by 50 cycles of amplification with denaturation at 95°C for 15 s and annealing and extension at 60°C for 1 min. Amplification data were collected and analyzed with Sequence Detector software version 1.3 (Applied Biosystems). The threshold value (ΔRn) was adjusted to be 0.01 according to the instructions from manufacturer of the PCR master mix (Qiagen). A 10-fold serial dilution of standard plasmid DNA (101 to 107 copies) of either genotype A or B suspended in molecular-grade water was used for the quantification of viral copy numbers in the PCR tubes. The slope (S) of the linear regression curve correlates with efficiency (E) of the PCR according to the formula E = 10−1/S − 1.
Table 1.
Primer and probe oligonucleotides used for RT-qPCR
| Primer or probe | Target genotype(s) | Sequence (5′→3′)a | Polarityb | Location |
|---|---|---|---|---|
| Primers | ||||
| AiV-AB-F | A and B | GTCTCCACHGACACYAAYTGGAC | + | 1882–1904d |
| AiV-AB-R | A and B | GTTGTACATRGCAGCCCAGG | − | 1970–1989d |
| Probesc | ||||
| AiV-AB-TPc | A and B | FAM-TTYTCCTTYGTGCGTGC-MGB-NFQ | + | 1939–1955d |
| AiV-A-TPc | A | FAM-ACCTCCAGTCCTACYGCA-MGB-NFQ | + | 1909–1926d |
| AiV-B-TPc | B | VIC-TCYACAAAYTCTCCCACCGC-MGB-NFQ | + | 1892–1911e |
Mixed bases in degenerate primers and probes are as follows: H represents A, C, or T, R represents A or G, and Y represents C or T.
+, sense; −, antisense.
Probes are labeled with 6-carboxyfluorescein (FAM) or 6-carboxyrhodamine (VIC) reporter dye at the 5′ end and with minor groove binder (MGB) and nonfluorescent quencher (NFQ) at the 3′ end of the oligonucleotide.
Corresponding nucleotide position of the prototype Aichi virus A846/88 strain complete genome (accession no. AB040749).
Corresponding nucleotide position of Goiania/GO/03/01/Brazil, a genotype B strain (accession no. DQ028632).
(ii) Genotype-specific assay.
A duplex qPCR that specifically reacts with each genotype was performed in a 25-μl reaction volume containing 2.5 μl of template (plasmid DNA or cDNA), 12.5 μl of QuantiTect probe PCR master mix, 400 nM each AiV universal primer (forward, AiV-AB-F; reverse, AiV-AB-R), and 300 nM each genotype-specific TaqMan MGB probe (AiV-A-TP and AiV-B-TP) (Table 1). Duplex PCR was performed with an ABI Prism 7500 real-time PCR system (Applied Biosystems) under conditions identical to those for the AiV universal assay.
RESULTS
Development of RT-qPCR.
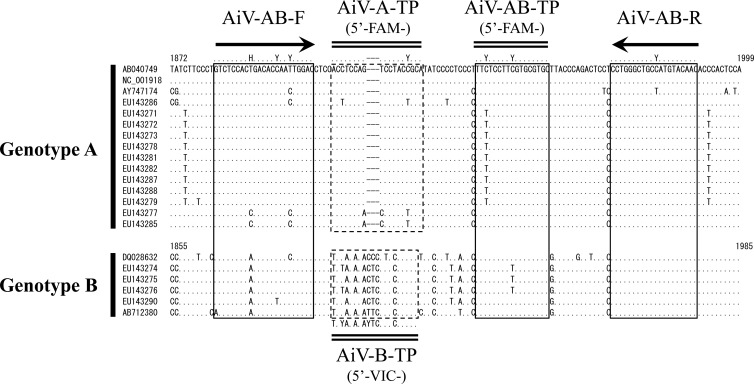
The nucleotide sequences of the 21 AiV strains were aligned, and a region (108 nt for genotype A and 111 nt for genotype B) located on the capsid region was selected as the target region of the RT-qPCR (Fig. 1). An AiV universal primer set (AiV-AB-F and AiV-AB-R) and an AiV universal TaqMan MGB probe (AiV-AB-TP) that reacts with both genotypes A and B were designed based on highly conserved sequences. The genotype-specific probes (AiV-A-TP and AiV-B-TP) were designed based on sequences that have significant nucleotide mismatches between the two genotypes, especially including a triple-nucleotide insertion (CCC, CTC, or TTC) located within the VP0 region of genotype B strains (corresponding to nt 1900 to 1902 in the Goiania/GO/03/01/Brazil strain) compared to those of genotype A strains. The locations of the primer and probe binding sites are illustrated in Fig. 1, and the nucleotide sequences are shown in Table 1.
Fig 1.
Alignment of 21 nucleotide sequences of the VP0 region of AiV. The nucleotide sequences corresponding to nt 1872 to 1999 of prototype AiV strain A846/88 (AB040749) are shown. The accession number is indicated on the left side of the sequence. Nucleotide sequences corresponding to the AiV universal primers (AiV-AB-F and AiV-AB-R) and AiV universal probe (AiV-AB-TP) are boxed in solid lines, and genotype-specific primers (AiV-A-TP and AiV-B-TP) are boxed in dashed lines. Arrows and double lines show the locations of the primers and probes, respectively.
A nucleotide BLAST search of each primer and probe showed no significant homology to non-AiV sequences, and we observed no cross-reactions with other viruses in the genus Kobuvirus (bovine and porcine kobuviruses), viruses in other genera in the family Picornaviridae (bovine enterovirus and poliovirus type 1), and other human gastroenteritis viruses (genogroup II [GII] norovirus, group A rotavirus, and adenovirus type 40) using the RT-qPCR (data not shown).
Dynamic range and standard curve.
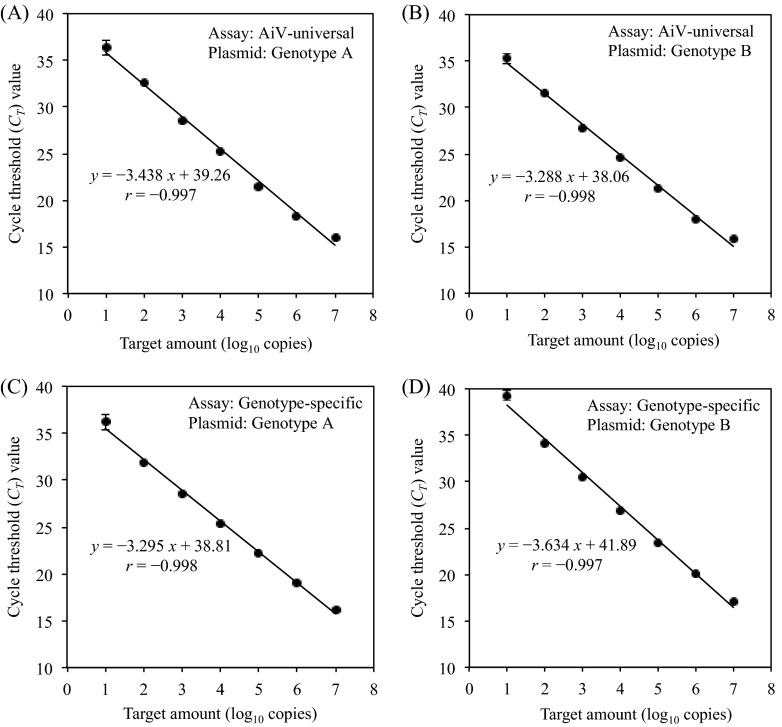
A 10-fold serial dilution of a standard plasmid DNA of either genotype A or B was tested with the AiV universal assay using the probe AiV-AB-TP (6-carboxyfluorescein [FAM] labeled), and individual standard curves for genotypes A and B were constructed based on the average cycle threshold (CT) values of the three reactions against the amount of the plasmid/reaction mixture. The AiV universal assay was able to amplify each plasmid standard between 1.0 × 101 and 1.0 × 107 copies/reaction (see Fig. S1A and B in the supplemental material), and the CT values were directly proportional to the log10 of the genome copies/reaction with correlation coefficients (r) of −0.997 and −0.998 for genotypes A and B, respectively (Fig. 2A and B). The slopes of the standard curves for genotypes A and B were −3.438 (E = 0.954) and −3.288 (E = 1.014), respectively (Fig. 2A and B). The lower quantification limit was estimated as 1.0 × 101 copies/reaction.
Fig 2.
Relationship between input copy numbers of the standard plasmids and CT values obtained from the AiV universal qPCR assay (A and B) and genotype-specific duplex qPCR assay (C and D) with a serial 10-fold dilution (107 to 101 copies/reaction) of a standard plasmid of AiV genotype A (A and C) or genotype B (B and D). The average CT value of the three reactions is plotted, and error bars indicate standard deviations.
The genotype-specific duplex assay using the genotype A-specific probe AiV-A-TP (FAM labeled) and genotype B-specific probe AiV-B-TP (6-carboxyrhodamine [VIC] labeled) was able to determine each plasmid standard between 1.0 × 101 and 1.0 × 107 copies/reaction (see Fig. S1C and D in the supplemental material). The genotype-specific assays reacted specifically to each genotype but did not cross-react with the other genotype. Although the genotype B-specific probe produced weak fluorescent signals against genotype A plasmid standard DNA, the fluorescent intensity was below the threshold value (ΔRn < 0.01) (see Fig. S1D). The slopes of the standard curves for genotypes A and B were −3.295 (E = 1.011; r = −0.998) and −3.634 (E = 0.884; r = −0.997), respectively (Fig. 2C and D). The standard curves generated from the duplex assay were similar to those generated from the singleplex assays (data not shown).
In order to evaluate the suitability of our system for the one-step RT-qPCR format, a one-step AiV universal RT-qPCR assay was performed against extracted AiV RNA of genotypes A and B, using the QuantiTect probe RT-PCR kit (Qiagen). For both genotypes A and B, the CT values of the one-step assay for each dilution were comparable to those of the two-step assay that was originally developed and optimized in the present study (see Fig. S2 in the supplemental material), showing that our RT-qPCR system can be implemented in a one-step RT-qPCR as well.
Detection of AiV RNA in stool specimens.
In order to validate the applicability of the newly developed RT-qPCR system for detection and genotyping of AiVs in clinical stool specimens, we examined a total of 30 specimens, among which 20 had previously been identified to be positive for AiVs by RT-PCR (24, 25). The AiV universal assay showed positive signals for a total of 20 specimens that included 19 out of 20 RT-PCR-positive specimens and 1 RT-PCR-negative specimen (stool code 1013/89), for which the AiV RNA load was relatively low, 4.5 × 101 copies/reaction (Table 2). The AiV RNA load determined by the AiV universal assay ranged from 1.6 × 101 to 7.3 × 106 copies/reaction (n = 20), corresponding to 1.4 × 104 to 6.6 × 109 copies/g stool (Table 2). In the genotype-specific duplex assay, the genotype A-specific probe produced positive signals for 16 specimens, including one specimen (stool code 942/89) that was negative by the AiV universal assay but positive for genotype B as well, while the genotype B-specific probe produced positive signals for 6 specimens that were positive for genotype B with RT-PCR followed by direct sequencing (Table 2). No fluorescent signal of either the AiV universal assay or genotype-specific assay was observed for the rest of the specimens (n = 9) that were negative by RT-PCR (24).
Table 2.
Detection of AiVs in stool specimens by RT-qPCRa
| Stool codeb | Previous result byc: |
Result by RT-qPCR |
||||
|---|---|---|---|---|---|---|
| RT-PCR | Genotype | AiV universald |
Genotype-specific |
|||
| No. of copies/reaction | No. of copies/g stool | A | B | |||
| 1279/86 | + | A | 1.2 × 105 | 1.1 × 108 | + | − |
| 1280/86 | + | A | 4.9 × 105 | 4.4 × 108 | + | − |
| 1282/86 | + | A | 9.3 × 104 | 8.4 × 107 | + | − |
| 1284/86 | + | A | 1.8 × 104 | 1.6 × 107 | + | − |
| 1285/86 | + | A | 1.6 × 105 | 1.4 × 108 | + | − |
| 844/88 | + | ND | 2.9 × 106 | 2.6 × 109 | + | − |
| 846/88 | + | A | 7.3 × 106 | 6.6 × 109 | + | − |
| 847/88 | + | ND | 4.5 × 105 | 4.1 × 108 | + | − |
| 848/88 | + | A | 9.2 × 105 | 8.2 × 108 | + | − |
| 1013/89 | − | − | 4.5 × 101 | 4.0 × 104 | + | − |
| 1015/89 | + | A | 8.2 × 105 | 7.4 × 108 | + | − |
| 1468/96 | + | A | 5.6 × 106 | 5.0 × 109 | + | − |
| 1469/96 | + | A | 2.3 × 105 | 2.1 × 108 | + | − |
| 1471/96 | + | A | 2.9 × 106 | 2.6 × 109 | + | − |
| 1472/96 | + | ND | 2.0 × 103 | 1.8 × 106 | + | − |
| 942/89 | + | B | − | NA | + | + |
| 1277/91 | + | B | 1.2 × 106 | 1.1 × 106 | − | + |
| 166/92 | + | B | 2.2 × 104 | 2.0 × 107 | − | + |
| 628/92 | + | B | 2.5 × 105 | 2.3 × 108 | − | + |
| 139/98 | + | B | 2.2 × 101 | 2.0 × 104 | − | + |
| 414/98 | + | B | 1.6 × 101 | 1.4 × 104 | − | + |
Detection of AiV RNA in wastewater samples.
To further validate the applicability of the RT-qPCR system for detection and genotyping of AiVs in environmental samples, we also examined a total of 24 (12 influent and 12 effluent) wastewater concentrates, of which 23 samples were positive for AiVs by a RT-nested PCR (22). The AiV universal assay showed positive signals for all of the influent samples (n = 12) and 11 effluent samples (Table 3). The AiV RNA concentrations in influent and effluent wastewater samples were determined to be up to 9.5 × 103 and 7.4 × 101 copies/reaction, corresponding to 2.2 × 107 and 1.8 × 104 copies/liter, respectively; the highest AiV RNA concentrations for both influent and effluent wastewater were obtained from the samples in February 2006 (Table 3). The concentrations of AiV RNA in influent samples were significantly higher in winter and spring (March and April 2005 and from November 2005 to February 2006) than in summer and fall (from May to September 2005) (t test for log10 concentration, P < 0.01).
Table 3.
Detection of AiVs in wastewater samples by RT-nested PCR and RT-qPCR assays
| Yr and mo | Influent samples |
Effluent samples |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RT-nested PCR resulta | RT-qPCR |
RT-nested PCR result | RT-qPCR |
|||||||
| AiV universal |
Genotype specific |
AiV universalb |
Genotype specific |
|||||||
| No. of copies/reaction | No. of copies/liter | A | B | Copies/reaction | Copies/liter | A | B | |||
| 2005 | ||||||||||
| March | A | 1.1 × 103 | 2.7 × 106 | + | − | A | 5.4 × 101 | 1.3 × 104 | + | − |
| April | A | 2.8 × 102 | 6.7 × 105 | + | − | A | 2.6 × 101 | 4.9 × 103 | + | − |
| May | A | 2.2 × 102 | 5.2 × 105 | + | − | A | 2.0 × 101 | 4.9 × 103 | + | − |
| June | A | 5.6 × 101 | 1.4 × 105 | + | − | A | (9.5 × 100) | (2.3 × 103) | + | − |
| July | A | 1.4 × 102 | 3.3 × 105 | + | − | A | − | NA | − | − |
| August | A | 2.3 × 102 | 5.5 × 105 | + | − | A | (3.7 × 100) | (9.0 × 102) | + | − |
| September | A | 1.0 × 102 | 2.4 × 105 | + | − | A | (6.8 × 100) | (1.6 × 103) | + | − |
| October | A | 1.2 × 102 | 2.8 × 105 | + | − | − | (5.9 × 100) | (1.4 × 103) | + | − |
| November | A | 4.4 × 102 | 1.1 × 106 | + | − | A | (4.6 × 100) | (1.1 × 103) | + | − |
| December | A | 6.5 × 102 | 1.6 × 106 | + | − | A | 1.6 × 101 | 3.8 × 103 | + | − |
| 2006 | ||||||||||
| January | A | 1.0 × 103 | 2.4 × 106 | + | − | A | 2.1 × 101 | 4.9 × 103 | + | − |
| February | A + B | 9.5 × 103 | 2.2 × 107 | + | − | A | 7.4 × 101 | 1.8 × 104 | + | − |
Results for identification of AiV sequences using the RT-nested PCR assay followed by cloning and sequencing, which was described previously (22). A, genotype A; B, genotype B.
A number in parentheses indicates that the calculated copy number was below quantification limits (<1.0 × 101 copies/reaction), although fluorescent signals with CT values of no more than 40 were observed. NA, not available.
The wastewater samples were also examined with a genotype-specific duplex assay; samples that were positive by the AiV universal assay (n = 23) were determined to be positive for genotype A, and no positive signal of genotype B was obtained even from the influent sample from February 2006 that had previously been identified as being positive for both genotypes A and B, probably due to the relatively low abundance of genotype B in this sample (Table 3). One sample negative for the AiV universal assay (the effluent sample in July 2005) was also negative for both genotypes A and B (Table 3).
DISCUSSION
AiV was first identified as a new cytopathic virus in BS-C-1 cells (4), and virus isolation using BS-C-1 or Vero cells (4, 7), the enzyme-linked immunosorbent assay (ELISA) (3), and conventional RT-PCR (6, 24, 27) have since been used to identify AiVs in clinical stool specimens. Isolation of AiVs by cell culture is time-consuming (about 4 to 6 weeks) (3), and both virus isolation and ELISA are less sensitive than conventional RT-PCR (24). Therefore, conventional RT-PCR has been commonly used as a method for AiV detection (6, 24, 27). qPCR methods have recently been introduced into clinical virology for the rapid and quantitative detection of viral genomes. qPCR even has many other advantages over conventional PCR, including lower risk of contamination, better specificity, and the capability of multiplex reaction using different reporter dyes.
At the time this study was conducted, only 5 AiV full-length sequences were available in GenBank, which made it difficult to design the qPCR assay. Since the AiV genotype has been determined based on sequence analysis of the 3CD junction region or the capsid gene, partial nucleotide sequences of these regions were also available for nucleotide sequence alignment. Based on the nucleotide sequence alignment of the full-length as well as partial genome of AiVs, we found that the VP0 region within the capsid gene has genotype-specific sequences, including a unique triple-nucleotide insertion of genotype B strains, sandwiched by highly conserved sequences (Fig. 1), as indicated by a previous study (6); Pham et al. (27) also designed a genotype B-specific seminested PCR primer, which anneals to this region, for differentiation of genotype B from genotype A. On the basis of this background, we chose this VP0 region as the target of the assay. From the highly conserved sequences, we designed a primer pair (AiV-AB-F and AiV-AB-R) and a TaqMan MGB probe (AiV-AB-TP) that react with a broad range of genetically diverse AiV strains. Genotype-specific probes (AiV-A-TP and AiV-B-TP) were designed to hybridize with nucleotides specific for each genotype. As a result, we successfully designed an RT-qPCR system that is specific for AiVs and is able to distinguish genotypes A and B with a genotype-specific duplex qPCR.
A recent study conducted in France found another AiV genotype (genotype C), which contains a sole strain, Rn48, using a conventional RT-PCR and nucleotide sequencing (10). However, since the VP0 region sequence of this strain is not available, we could neither design an assay targeting this novel genotype nor evaluate the reactivity of the newly developed RT-qPCR against it. In addition, at present genotype C may not be an essential target of our RT-qPCR system, because genotypes A and B are currently known as the predominant AiV genotypes (1, 24, 27), and genotype C, which has been identified in only one study (10), may be much less prevalent than the other genotypes.
For genotyping of AiVs, nucleotide sequence analysis of the 3CD junction region following RT-PCR amplification is the most commonly used method for both clinical and environmental samples (5, 8–10, 12, 14, 18, 22, 25). Previous studies demonstrated a high consistency between AiV genotypes determined based on the 3CD junction region and capsid gene (6, 8), which is supported by a fact reported by another previous study that no phylogenetic evidence of recombination was detected between AiV genotypes A and B in any part of the genome (28). These previous findings strongly suggest that AiV genotypes determined by our genotype-specific RT-qPCR assay based on the VP0 region are consistent with those determined by the conventional genotyping method based on the 3CD junction region. Although the nucleotide sequence analysis is still essential to explore the molecular origin of an outbreak or to determine the genetic heterogeneity or homogeneity of AiV strains in the environments, our genotype-specific qPCR assay can be a useful tool for rapid and high-throughput screening of AiV genotypes in both clinical and environmental samples.
The one-step RT-qPCR assay may be more beneficial than the two-step assay under certain circumstances, such as clinical surveillance, because the one-step assay can further reduce time, labor, and the risk of cross-contamination. Based on the comparison of the CT values of the two-step and one-step RT-qPCR assays against extracted AiV RNA (see Fig. S2 in the supplemental material), we demonstrated that our RT-qPCR system could be implemented not only in the two-step RT-qPCR format but also in the one-step format. The use of a one-step RT-qPCR assay will enable even-higher-throughput screening of AiV RNA in clinical specimens and potentially in environmental samples.
An RT-qPCR assay for AiVs targeting the highly conserved 5′-untranslated region, a common target region for broadly reactive assay for picornaviruses, has been reported recently (11). However, this assay does not distinguish the genotypes of AiVs. When we conducted a side-by-side comparison of CT values between our assay and the previously reported assay in detecting cDNA of genotype A and B strains, the CT values obtained from the two assays were comparable for both genotype A and B strains (see Table S1 in the supplemental material). The design of the newly developed assay based on the VP0 region has an advantage over the previously reported assay in that a single pair of primers is used not only to simplify the assay but also to enable simultaneous detection of two genotypes with a similar PCR efficiency.
To date, only one study has reported the AiV RNA load in clinical stool specimens (11). In the present study, 21 AiV-positive stool specimens were examined with the newly developed RT-qPCR assay to investigate the AiV RNA load. The AiV RNA load in the stool specimens determined in the present study using the AiV universal assay ranged from 1.4 × 104 to 6.6 × 109 copies/g stool, which falls within the range of those of the previous study determined by another RT-qPCR assay (1.1 × 102 to 1.3 × 1012 copies/g stool) (11), proving the validity of our study. For stool specimens, the results of our RT-qPCR assays were consistent with the previous genotyping results for all specimens except for one specimen (stool code 942/89) that was negative by the AiV universal assay but positive by the genotype-specific assay, probably due to one nucleotide mismatch to the AiV-AB-TP probe sequence that was found by direct sequencing of the RT-qPCR product (data not shown). The limitation of the primer or probe designation was due to the limited numbers of the VP0 region sequences available in the database. Although our RT-qPCR system has shown a reasonable capacity for quantifying and genotyping AiVs in clinical stool specimens, it should be further validated using large numbers of clinical AiV isolates with greater genetic variability.
We also applied the newly developed RT-qPCR system for quantification and genotyping of AiVs in wastewater samples. These samples had been tested previously for the detection of noroviruses and sapoviruses, which are major causes of human gastroenteritis, by RT-qPCR (29, 30). Interestingly, the viral RNA concentration of AiVs in the influent wastewater samples (up to 2.2 × 107 copies/liter) was higher than that of noroviruses (up to 1.5 × 106 and 9.1 × 105 copies/liter for genogroup I [GI] and GII, respectively) (30) or sapoviruses (up to 9.3 × 104 copies/liter) (29). In addition, the concentration of AiV RNA in wastewater was significantly higher in winter and spring than in summer and fall (Table 3). These results suggest that AiVs are more prevalent among human populations than human caliciviruses with higher prevalence in winter and spring seasons in Japan. Since the seasonal pattern of AiV infection remains unknown due to limited clinical and epidemiological studies on the prevalence of AiV among humans (1), our findings on the seasonality of the AiV RNA concentration in wastewater provide important insights into their epidemiology. Actually, the presence of AiVs in wastewater more accurately reflects the actual prevalence of AiVs in the community rather than clinical reports, because wastewater contains viruses shed from all populations in a service area regardless of their health status.
The RT-qPCR system developed in the present study will be an efficient tool for routine diagnosis of AiVs in clinical stool specimens as well as in environmental samples, because of its ability for quantification and genotyping of AiVs. Further studies on AiVs using this system are recommended for a better understanding of their clinical significance, genotype distribution, and behavior in the environment.
Supplementary Material
ACKNOWLEDGMENT
This work was supported in part by Core Research for Evolutional Science and Technology (CREST) from the Japan Science and Technology Agency (JST).
Footnotes
Published ahead of print 19 April 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00820-13.
REFERENCES
- 1. Reuter G, Boros A, Pankovics P. 2011. Kobuviruses—a comprehensive review. Rev. Med. Virol. 21:32–41 [DOI] [PubMed] [Google Scholar]
- 2. Yamashita T, Ito M, Kabashima Y, Tsuzuki H, Fujiura A, Sakae K. 2003. Isolation and characterization of a new species of kobuvirus associated with cattle. J. Gen. Virol. 84:3069–3077 [DOI] [PubMed] [Google Scholar]
- 3. Yamashita T, Sakae K, Ishihara Y, Isomura S, Utagawa E. 1993. Prevalence of newly isolated, cytopathic small round virus (Aichi strain) in Japan. J. Clin. Microbiol. 31:2938–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamashita T, Kobayashi S, Sakae K, Nakata S, Chiba S, Ishihara Y, Isomura S. 1991. Isolation of cytopathic small round viruses with BS-C-1 cells from patients with gastroenteritis. J. Infect. Dis. 164:954–957 [DOI] [PubMed] [Google Scholar]
- 5. Pham NT, Khamrin P, Nguyen TA, Kanti DS, Phan TG, Okitsu S, Ushijima H. 2007. Isolation and molecular characterization of Aichi viruses from fecal specimens collected in Japan, Bangladesh, Thailand, and Vietnam. J. Clin. Microbiol. 45:2287–2288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pham NT, Trinh QD, Khamrin P, Nguyen TA, Dey SK, Phan TG, Hoang L, Maneekarn PN, Okitsu S, Mizuguchi M, Ushijima H. 2008. Sequence analysis of the capsid gene of Aichi viruses detected from Japan, Bangladesh, Thailand, and Vietnam. J. Med. Virol. 80:1222–1227 [DOI] [PubMed] [Google Scholar]
- 7. Yamashita T, Sakae K, Kobayashi S, Ishihara Y, Miyake T, Mubina A, Isomura S. 1995. Isolation of cytopathic small round virus (Aichi virus) from Pakistani children and Japanese travelers from Southeast Asia. Microbiol. Immunol. 39:433–435 [DOI] [PubMed] [Google Scholar]
- 8. Yang S, Zhang W, Shen Q, Yang Z, Zhu J, Cui L, Hua X. 2009. Aichi virus strains in children with gastroenteritis, China. Emerg. Infect. Dis. 15:1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verma H, Chitambar SD, Gopalkrishna V. 2011. Circulation of Aichi virus genotype B strains in children with acute gastroenteritis in India. Epidemiol. Infect. 139:1687–1691 [DOI] [PubMed] [Google Scholar]
- 10. Ambert-Balay K, Lorrot M, Bon F, Giraudon H, Kaplon J, Wolfer M, Lebon P, Gendrel D, Pothier P. 2008. Prevalence and genetic diversity of Aichi virus strains in stool samples from community and hospitalized patients. J. Clin. Microbiol. 46:1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Drexler JF, Baumgarte S, de Souza Luna LK, Eschbach-Bludau M, Lukashev A, Drosten C. 2011. Aichi virus shedding in high concentrations in patients with acute diarrhea. Emerg. Infect. Dis. 17:1544–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kaikkonen S, Räsänen S, Rämet M, Vesikari T. 2010. Aichi virus infection in children with acute gastroenteritis in Finland. Epidemiol. Infect. 138:1166–1171 [DOI] [PubMed] [Google Scholar]
- 13. Le Guyader FS, Le Saux JC, Ambert-Balay K, Krol J, Serais O, Parnaudeau S, Giraudon H, Delmas G, Pommepuy M, Pothier P, Atmar RI. 2008. Aichi virus, norovirus, astrovirus, enterovirus, and rotavirus involved in clinical cases from a French oyster-related gastroenteritis outbreak. J. Clin. Microbiol. 46:4011–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh DY, Silva PA, Hauroeder B, Diedrich S, Cardoso DD, Schreier E. 2006. Molecular characterization of the first Aichi viruses isolated in Europe and in South America. Arch. Virol. 151:1199–1206 [DOI] [PubMed] [Google Scholar]
- 15. Räsänen S, Lappalainen S, Kaikkonen S, Hämäläinen M, Salminen M, Vesikari T. 2010. Mixed viral infections causing acute gastroenteritis in children in a waterborne outbreak. Epidemiol. Infect. 138:1227–1234 [DOI] [PubMed] [Google Scholar]
- 16. Reuter G, Boldizsár A, Papp G, Pankovics P. 2009. Detection of Aichi virus shedding in a child with enteric and extraintestinal symptoms in Hungary. Arch. Virol. 154:1529–1532 [DOI] [PubMed] [Google Scholar]
- 17. Sdiri-Loulizi K, Gharbi-Khélifi H, de Rougemont A, Chouchane S, Sakly N, Ambert-Balay K, Hassine M, Guédiche MN, Aouni M, Pothier P. 2008. Acute infantile gastroenteritis associated with human enteric viruses in Tunisia. J. Clin. Microbiol. 46:1349–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sdiri-Loulizi K, Hassine M, Gharbi-Khelifi H, Sakly N, Chouchane S, Guediche MN, Pothier P, Aouni M, Ambert-Balay K. 2009. Detection and genomic characterization of Aichi viruses in stool samples from children in Monastir, Tunisia. J. Clin. Microbiol. 47:2275–2278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goyer M, Aho LS, Bour JB, Ambert-Balay K, Pothier P. 2008. Seroprevalence distribution of Aichi virus among a French population in 2006–2007. Arch. Virol. 153:1171–1174 [DOI] [PubMed] [Google Scholar]
- 20. Ribes JM, Montava R, Téllez-Castillo CJ, Fernández-Jiménez M, Buesa J. 2010. Seroprevalence of Aichi virus in a Spanish population from 2007 to 2008. Clin. Vaccine Immunol. 17:545–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sdiri-Loulizi K, Hassine M, Bour JB, Ambert-Balay K, Mastouri M, Aho LS, Gharbi-Khelifi H, Aouni Z, Sakly N, Chouchane S, Neji-Guédiche M, Pothier P, Aouni M. 2010. Aichi virus IgG seroprevalence in Tunisia parallels genomic detection and clinical presentation in children with gastroenteritis. Clin. Vaccine Immunol. 17:1111–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kitajima M, Haramoto E, Phanuwan C, Katayama H. 2011. Prevalence and genetic diversity of Aichi viruses in wastewater and river water in Japan. Appl. Environ. Microbiol. 77:2184–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamashita T, Sakae K, Tsuzuki H, Suzuki Y, Ishikawa Y, Takeda N, Miyamura T, Yamazaki S. 1998. Complete nucleotide sequence and genetic organization of Aichi virus, a distinct member of the Picornaviridae associated with acute gastroenteritis in humans. J. Virol. 72:8408–8412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yamashita T, Sugiyama M, Tsuzuki H, Sakae K, Suzuki Y, Miyazaki Y. 2000. Application of a reverse transcription-PCR for identification and differentiation of Aichi virus, a new member of the Picornavirus family associated with gastroenteritis in humans. J. Clin. Microbiol. 38:2955–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yamashita T, Sakae K. 2003. Molecular biology and epidemiology of Aichi virus and other diarrhoeogenic enteroviruses, p 645–657 In Desselberger U, Gray J. (ed), Perspectives in medical virology, vol 9 Viral gastroenteritis. Elsevier, Amsterdam, The Netherlands: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Katayama H, Shimasaki A, Ohgaki S. 2002. Development of a virus concentration method and its application to detection of enterovirus and Norwalk virus from coastal seawater. Appl. Environ. Microbiol. 68:1033–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pham NT, Trinh QD, Nguyen TA, Dey SK, Phan TG, Hoang L, Khamrin PP, Maneekarn N, Okitsu S, Mizuguchi M, Ushijima H. 2009. Development of genotype-specific primers for differentiation of genotypes A and B of Aichi viruses. J. Virol. Methods 156:107–110 [DOI] [PubMed] [Google Scholar]
- 28. Lukashev AN, Drexler JF, Belalov IS, Eschbach-Bludau M, Baumgarte S, Drosten C. 2012. Genetic variation and recombination in Aichi virus. J. Gen. Virol. 93:1226–1235 [DOI] [PubMed] [Google Scholar]
- 29. Kitajima M, Haramoto E, Phanuwan C, Katayama H. 2011. Genotype distribution of human sapoviruses in wastewater in Japan. Appl. Environ. Microbiol. 77:4226–4229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kitajima M, Haramoto E, Phanuwan C, Katayama H, Furumai H. 2012. Molecular detection and genotyping of human noroviruses in influent and effluent water at a wastewater treatment plant in Japan. J. Appl. Microbiol. 112:605–613 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.