Abstract
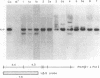
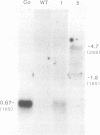
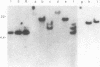
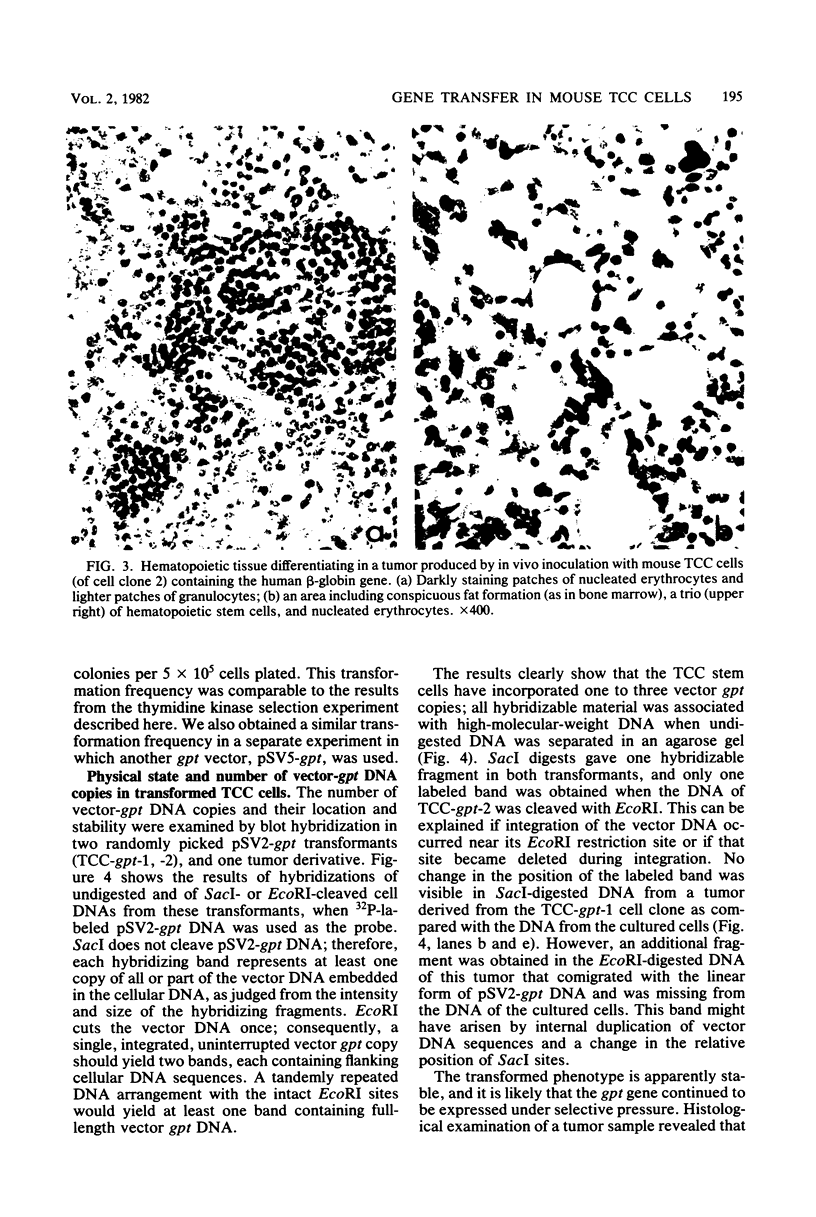
Teratocarcinoma (TCC) stem cells can function as vehicles for the introduction of specific recombinant genes into mice. Because most genes do not code for a selectable marker, we investigated the transformation efficiency of vectors with a linked selectable gene. In one series, TCC cells first selected for thymidine kinase deficiency were treated with DNA from the plasmid vector PtkH beta 1 containing the human genomic beta-globin gene and the thymidine kinase gene of herpes simplex virus. A high transformation frequency was obtained after selection in hypoxanthine-aminopterin-thymidine medium. Hybridization tests revealed that the majority of transformants had intact copies of the human gene among three to six total copies per cell. These were associated with cellular DNA sequences as judged from the presence of additional new restriction fragments and from stability of the sequences in tumors produced by injecting the cells subcutaneously. Total polyadenylate-containing RNA from cell cultures of two out of four transformants examined showed hybridization to the human gene probe: one RNA species resembled mature human beta-globin mRNA transcripts; the others were of larger size. In differentiating tumors, various tissues, including hematopoietic cells of TCC provenance could be found. In a second model set of experiments, wild-type TCC cells were used to test a dominant-selection scheme with pSV-gpt vectors. Numerous transformants were isolated, and their transfected DNA was apparently stably integrated. Thus, any gene of choice can be transferred into TCC stem cells even without mutagenesis of the cells, and selected cell clones can be characterized. Cells of interest may then be introduced into early embryos to produce new mouse strains with predetermined genetic changes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F., Killos L., Sanders-Haigh L., Kretschmer P. J., Diacumakos E. G. Replication and expression of thymidine kinase and human globin genes microinjected into mouse fibroblasts. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5399–5403. doi: 10.1073/pnas.77.9.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Feigelson P., Schutz G. Analysis of the complexity and diversity of mRNA from chicken liver and oviduct. Cell. 1976 Feb;7(2):247–254. doi: 10.1016/0092-8674(76)90024-6. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Colbere-Garapin F., Chousterman S., Horodniceanu F., Kourilsky P., Garapin A. C. Cloning of the active thymidine kinase gene of herpes simplex virus type 1 in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3755–3759. doi: 10.1073/pnas.76.8.3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronmiller C., Mintz B. Karyotypic normalcy and quasi-normalcy of developmentally totipotent mouse teratocarcinoma cells. Dev Biol. 1978 Dec;67(2):465–477. doi: 10.1016/0012-1606(78)90212-9. [DOI] [PubMed] [Google Scholar]
- Cudennec C. A., Johnson G. R. Presence of multipotential hemopoietic cells in teratocarcinoma cultures. J Embryol Exp Morphol. 1981 Feb;61:51–59. [PubMed] [Google Scholar]
- Cudennec C., Nicolas J. F. Blood formation in a clonal cell line of mouse teratocarcinoma. J Embryol Exp Morphol. 1977 Apr;38:203–210. [PubMed] [Google Scholar]
- Dewey M. J., Martin D. W., Jr, Martin G. R., Mintz B. Mosaic mice with teratocarcinoma-derived mutant cells deficient in hypoxanthine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5564–5568. doi: 10.1073/pnas.74.12.5564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Molecular cloning and characterization of the human beta-like globin gene cluster. Cell. 1980 Apr;19(4):959–972. doi: 10.1016/0092-8674(80)90087-2. [DOI] [PubMed] [Google Scholar]
- Kantor J. A., Turner P. H., Nienhuis A. W. Beta Thalassemia: mutations which affect processing of the beta-Globin mRNA precursor. Cell. 1980 Aug;21(1):149–157. doi: 10.1016/0092-8674(80)90122-1. [DOI] [PubMed] [Google Scholar]
- Lewis W. H., Srinivasan P. R., Stokoe N., Siminovitch L. Parameters governing the transfer of the genes for thymidine kinase and dihydrofolate reductase into mouse cells using metaphase chromosomes or DNA. Somatic Cell Genet. 1980 May;6(3):333–347. doi: 10.1007/BF01542787. [DOI] [PubMed] [Google Scholar]
- Linnenbach A., Huebner K., Croce C. M. DNA-transformed murine teratocarcinoma cells: regulation of expression of simian virus 40 tumor antigen in stem versus differentiated cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4875–4879. doi: 10.1073/pnas.77.8.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantei N., Boll W., Weissmann C. Rabbit beta-globin mRNA production in mouse L cells transformed with cloned rabbit beta-globin chromosomal DNA. Nature. 1979 Sep 6;281(5726):40–46. doi: 10.1038/281040a0. [DOI] [PubMed] [Google Scholar]
- Mintz B., Cronmiller C. METT-1: a karyotypically normal in vitro line of developmentally totipotent mouse teratocarcinoma cells. Somatic Cell Genet. 1981 Jul;7(4):489–505. doi: 10.1007/BF01542992. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene expression in neoplasia and differentiation. Harvey Lect. 1978;71:193–246. [PubMed] [Google Scholar]
- Mintz B., Illmensee K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3585–3589. doi: 10.1073/pnas.72.9.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B. Teratocarcinoma cells as vehicles for introducing mutant genes into mice. Differentiation. 1979;13(1):25–27. doi: 10.1111/j.1432-0436.1979.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicer A., Wagner E. F., el-Kareh A., Dewey M. J., Reuser A. J., Silverstein S., Axel R., Mintz B. Introduction of a viral thymidine kinase gene and the human beta-globin gene into developmentally multipotential mouse teratocarcinoma cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2098–2102. doi: 10.1073/pnas.77.4.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perucho M., Hanahan D., Wigler M. Genetic and physical linkage of exogenous sequences in transformed cells. Cell. 1980 Nov;22(1 Pt 1):309–317. doi: 10.1016/0092-8674(80)90178-6. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robins D. M., Ripley S., Henderson A. S., Axel R. Transforming DNA integrates into the host chromosome. Cell. 1981 Jan;23(1):29–39. doi: 10.1016/0092-8674(81)90267-1. [DOI] [PubMed] [Google Scholar]
- Segal S., Khoury G. Differentiation as a requirement for simian virus 40 gene expression in F-9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5611–5615. doi: 10.1073/pnas.76.11.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stewart T. A., Mintz B. Successive generations of mice produced from an established culture line of euploid teratocarcinoma cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6314–6318. doi: 10.1073/pnas.78.10.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. F., Stewart T. A., Mintz B. The human beta-globin gene and a functional viral thymidine kinase gene in developing mice. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5016–5020. doi: 10.1073/pnas.78.8.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Dewey M. J., Mintz B. Teratocarcinoma cells as vehicles for introducing specific mutant mitochondrial genes into mice. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5113–5117. doi: 10.1073/pnas.75.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Wold B., Wigler M., Lacy E., Maniatis T., Silverstein S., Axel R. Introduction and expression of a rabbit beta-globin gene in mouse fibroblasts. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5684–5688. doi: 10.1073/pnas.76.11.5684. [DOI] [PMC free article] [PubMed] [Google Scholar]