Abstract
A prodrug is a compound that has negligible, or lower, activity against a specified pharmacological target than one of its major metabolites. Prodrugs can be used to improve drug delivery or pharmacokinetics, to decrease toxicity, or to target the drug to specific cells or tissues. Ester and phosphate hydrolysis are widely used in prodrug design because of their simplicity, but such approaches are relatively ineffective for targeting drugs to specific sites. The activation of prodrugs by the cytochrome P450 system provides a highly versatile approach to prodrug design that is particularly adaptable for targeting drug activation to the liver, to tumors or to hypoxic tissues.
Prodrugs
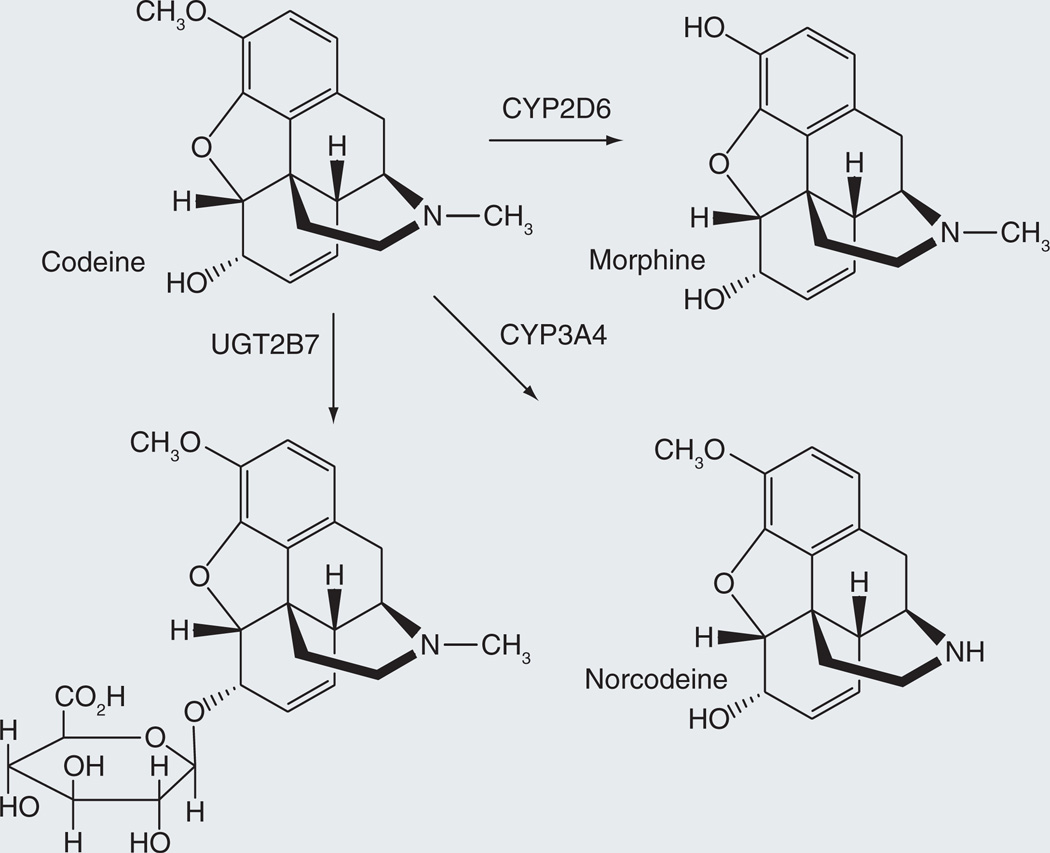
An ideal prodrug is a chemical entity that has no pharmacological activity against a designated physiological target, but is metabolically transformed into a compound with the desired activity. Prodrugs need not meet these ideal conditions, however. A compound with the desired biological activity, but at a lower level than one of its principal metabolites, is also a prodrug if the metabolite is a major contributor to the intended pharmacological response. An informative example is provided by codeine, which is a morphine prodrug to the extent that it can be oxidatively demethylated by cytochrome P450 CYP2D6 to give morphine (Figure 1) [1]. Detailed analysis has shown that codeine is largely (~80%) converted to the 6-glucuronide [2], a metabolite that is pharmacologically active and, on a dose basis, elicits approximately 60% of the analgesic effect of codeine itself [3]. Furthermore, <5% of the dose is normally converted to morphine. The remaining approximately 15% is demethylated by CYP3A4 to norcodeine, which is less potent than morphine. From these results, it has been proposed that codeine-6-glucuronide is the agent responsible for most of the analgesic action of codeine, in which case codeine is a prodrug of the glucuronide metabolite rather than of morphine [4,5]. This therapeutic scenario is complicated by the fact that O-demethylation to morphine plays a greater role in individuals with the extensive metabolizer phenotype associated with abnormally high CYP2D6 activity. Thus, enhanced morphine production was found to cause the death of a baby breast-feeding with a mother who was an extensive metabolizer and was taking codeine [6]. It has also been found to have lethal effects in CYP2D6 hypermetabolizer children treated with codeine [7]. Although the question of which compound is the principal analgesic agent of codeine may vary, the fact that codeine can act directly, or as a prodrug be converted to either the 6-glucuronide or morphine, illustrates the ambiguities that can arise when classifying a compound as a prodrug.
Figure 1.
Conversion of codeine to its glucuronide and morphine metabolites with higher biological activity.
Prodrugs can be utilized for a variety of purposes, including improvement of the bioavailability or pharmacokinetics of a drug, decreasing drug toxicity, facilitating administration of the drug, or delivering the drug to specific cells or tissues [8]. Some drugs, such as the antituberculosis agents isoniazid and ethionamide [9], have incidentally been found to be prodrugs. The same is true of some P450-activated prodrugs, which were not specifically designed as such, but were found to be prodrugs when they were characterized. Codeine is a case in point. However, the design of prodrugs is now a deliberate and useful approach in the pharmaceutical chemist’s repertoire [8]. The most commonly employed method of preparing prodrugs, in part because of its experimental simplicity, is to esterify a hydroxyl (or amine) group of the drug. Physiological esterases, amidases or phosphatases then release the drug from its prodrug form. Although less common, cytochrome P450 enzymes are also often targeted for the activation of prodrugs. This review focuses specifically on the activation of prodrugs by the cytochrome P450 system.
Cytochrome P450 system
The human genome encodes 57 cytochrome P450 enzymes, roughly a third of which are involved in the biosynthesis of essential sterols, signaling molecules or regulatory factors, a third of which are largely devoted to the metabolism of xenobiotics, and a third with functions that remain unclear [10]. The P450 enzymes primarily involved in the metabolism of xenobiotics are CYP1A2, −2B6, −2C8, −2C9, 2C19, −2D6,-2E1, −3A4 and −3A5. In general, the enzymes that oxidize drugs and xenobiotics are of primary interest for the activation of prodrugs. Of these, CYP3A4 is present in the highest concentration in the liver and is responsible for the oxidation of approximately two-thirds of all known drugs [11]. However, the other P450 enzymes listed are also important players in drug metabolism and prodrug activation.
The sites of expression and local concentrations differ for the various P450 enzymes. These are two of the reasons why cytochrome P450 enzymes may be exploited to selectively target drug activation to specific tissues. Notable examples are CYP1B1, CYP2S1 and CYP2W1, three enzymes that are preferentially expressed in tumors and/or hypoxic tissues [12–14]. Two factors must be kept in mind, however, with regard to the distribution and activities of drug metabolizing P450 enzymes. First, polymorphisms in the P450 genes can either decrease or elevate the activities of the individual enzymes, with higher levels of expression giving rise to higher metabolic capacity in individuals that are consequently described as ‘extensive’ or ‘hyper’ metabolizers [15,16]. Second, most of the drug-metabolizing enzymes are subject to induction by xenobiotics or environmental factors [17,18]. A high potential therefore exists for variability of prodrug activation in patients due to differences in the expression and activity of individual P450 enzymes.
Cytochrome P450 enzymes can:
-
▪
Insert an oxygen into a C-H or N-H bond to give the corresponding hydroxyl derivative;
-
▪
Catalyze the epoxidation of olefinic or aromatic π-bonds;
-
▪
Add an oxygen atom to the electron pair of a nitrogen or sulfur atom, resulting in the formation of an N-oxide or S-oxide [19].
Chemically, the most impressive of the P450 reactions is oxidation of an unactivated C-H bond to an alcohol. If the oxidation occurs within a hydrocarbon chain, the alcohol is the terminal product, but if the hydroxylation occurs at a C-H bond adjacent to a heteroatom, the product is usually chemically unstable. The resulting intermediate undergoes an internal elimination reaction that produces two fragments, one with a carbonyl group and the other with a hydroxyl, amine or thiol function, depending on the nature of the relevant heteroatom in the parent drug. This type of fragmentation reaction, or a variation of it, is the most common basis for the P450-catalyzed release of a drug from its prodrug form. The conversion of codeine into morphine and norcodeine, respectively, are examples of O-dealkylation and N-dealkylation (Figure 1). Nevertheless, other P450 reactions can also be employed in prodrug design. For example, as discussed later for a duocarmycin prodrug, the addition of a hydroxyl group to an aromatic ring via an epoxidation-rearrangement mechanism is a viable avenue for prodrug activation.
The catalytic turnover of cytochrome P450 enzymes requires two electrons per substrate oxidation event. For all the mammalian drug-metabolizing cytochrome P450 enzymes, these electrons are provided by NADPH cytochrome P450 reductase [20], although the second electron can sometimes be provided by cytochrome b5 or cytochrome b5 reductase. Cytochrome P450 reductase is a protein with two flavin prosthetic groups that uncouples the two electrons provided by NADPH and delivers them individually to the P450 heme group. Under anaerobic or hypoxic conditions, cytochrome P450 enzymes can accept the electrons from P450 reductase and use them to catalyze reductive reactions. This feature of P450 catalysis suggests that reductive cytochrome P450 reactions can be exploited for the reductive activation of prodrugs in hypoxic cells. Furthermore, cytochrome P450 reductase itself, as a one-electron donor, can directly promote the reduction of certain functionalities, such as nitro groups.
Anticancer prodrug activation
Prodrugs intended for the treatment of human tumors can be activated by cytochrome P450 through oxidative or reductive mechanisms. If the activation is through an oxidative mechanism, the prodrug will not be preferentially targeted to tumor tissues unless the activation is mediated by a cytochrome P450 enzyme that is selectively overexpressed in cancer cells. Although not yet exploited, the targeting of prodrugs for aerobic activation in cancer cells is now feasible due to the discovery that a number of P450 enzymes are selectively expressed in tumors. As already noted, reductive activation by the P450 system can also be used to target prodrugs to hypoxic cells such as those found in the poorly vascularized regions of solid tumors.
Oxidative activation
Cyclophosphamide and ifosfamide, two anticancer drugs that are extensively used in the clinic, derive from formulation of the prodrug concept by Druckrey in 1952 [21,22]. In addition to these two mainstream anticancer drugs, other agents have found clinical acceptance in cancer chemotherapy.
Cyclophosphamide/ifosfamide
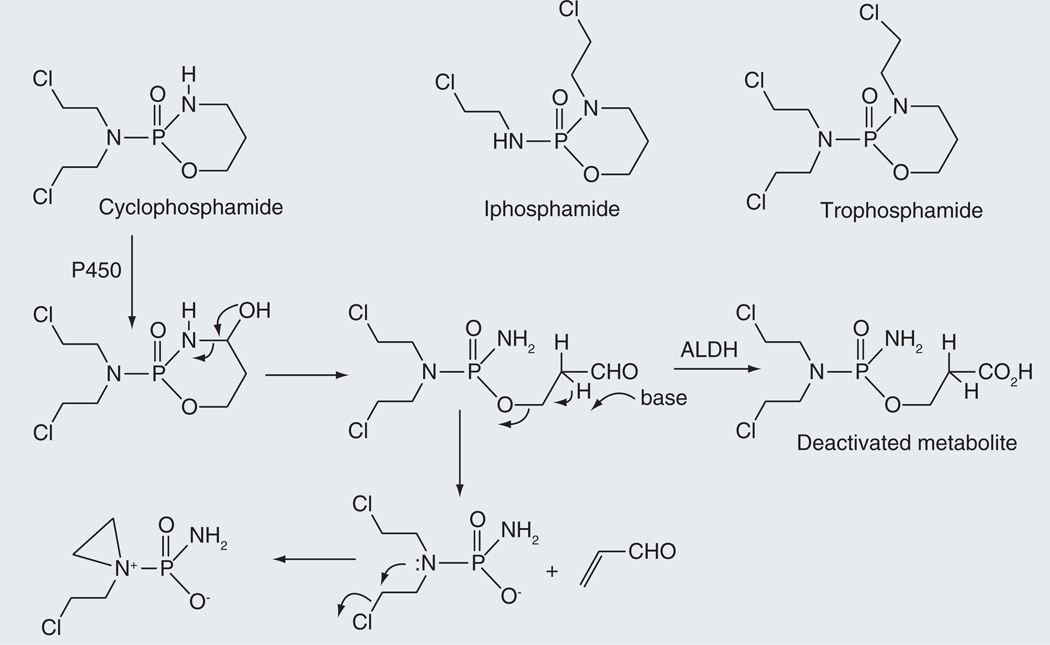
Cyclophosphamide is an alkylating prodrug with a relatively high therapeutic index that is effective against a range of human tumors [23]. It also has immunosuppressive activity and is employed clinically to treat auto immune diseases and in bone marrow and renal transplantation [23]. In vivo, cyclophosphamide is converted to a phosphoramide mustard that spontaneously cyclizes to an aziridinium DNA crosslinking agent (Figure 2) [24,25]. The release of the alkylating mustard involves cytochrome P450 hydroxylation adjacent to the ring nitrogen atom, reversible opening of the ring to give an aldophosphamide, and spontaneous decomposition of this intermediate to yield the phosphoramide mustard and acrolein. A competing reaction is the oxidation of the aldophosphamide aldehyde group to a carboxylic acid by an aldehyde dehydrogenase, a metabolite that is no longer able to release the active alkylating agent [26]. The drug is thus most effective in tissues with low aldehyde dehydrogenase activity, while its genotoxicity is attenuated in cells that have high titers of this enzyme. The acrolein side product is responsible for the kidney toxicity of cyclophosphamide, but the incidence of hemorrhagic cystitis can be attenuated by co-administration of sodium 2-sulfanylethanesulfonate (mesna: HSCH2CH2SO3−Na+), a hydrophilic, cell-impermeable thiol agent that adds to acrolein in the circulation and inactivates it [27].
Figure 2.
Cytochrome P450-catalyzed activation of cyclophosphamide to its aziridinium DNA alkylating metabolite.
Analysis of the human cytochrome P450 enzymes responsible for the activation of cyclophosphamide shows that CYP2A6, −2B6, −2C9, −2C18, −2C19, −3A4 and 3A5 have significant cyclophosphamide 4-hydroxylase activity, but CYP2B6 is the dominant player in human liver [28]. The Km and Vmax values of human CYP2B6 for cyclophosphamide are 4.9 µM and 62.5 mol/min/mol P450 [29]. CYP2B6 mutants with higher cyclophosphamide 4-hydroxylation activities have been prepared as potential catalysts to be transfected into human tumor cells in gene-directed enzyme prodrug therapy [7,30].
Ifosphamide and trophosphamide are anticancer drugs closely related to cyclophosphamide, although of these two only ifosphamide is used extensively (Figure 2). The activation of these drugs is similar to that of cyclophosphamide and is triggered by 4-hydroxylation of the drug, ring opening to give an aldehyde intermediate, elimination of acrolein to release the phosphamide and spontaneous formation of an aziridinium product that crosslinks DNA [24,25]. Studies of the cytochrome P450 specificity of ifosphamide 4-hydroxylation indicate that the most active enzyme in this case is CYP3A4 [28].
Dacarbazine/procarbazine
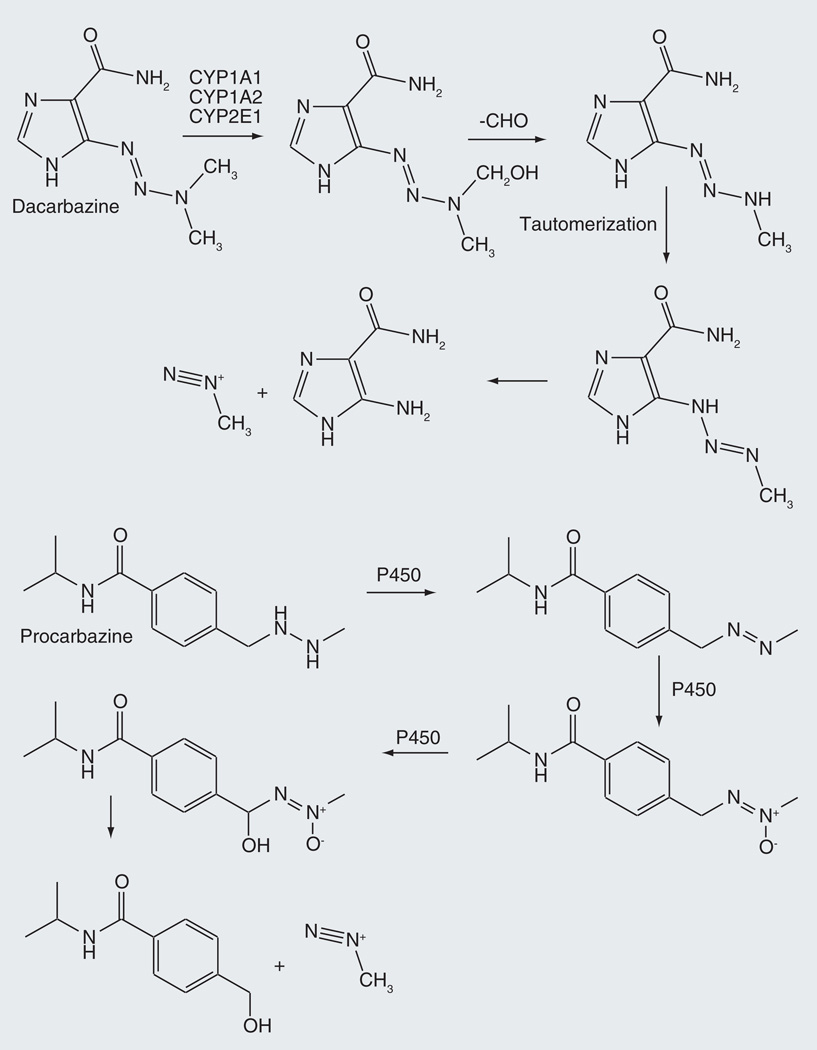
Dacarbazine is an anticancer alkylating prodrug employed in the treatment of Hodgkin’s lymphoma, metastatic melanoma, soft tissue sarcoma and non-Hodgkin’s lymphoma [31,32]. Cytochrome P450-catalyzed N-demethylation of dacarbazine initiates the release of methyl diazonium, which in turn methylates the O6 of guanine in DNA (Figure 3) [33,34]. The prodrug formulation makes possible the cellular delivery of a highly reactive chemical agent. This oxidative reaction can be catalyzed by CYP1A1, CYP1A2 or CYP2E1 [35]. In the context of possible gene-directed enzyme prodrug therapy, mutants of CYP1A1 have been developed that more efficiently oxidize dacarbazine to its active metabolite [36]. The requirement for activation by a cytochrome P450 enzyme has more recently been eliminated in the triazene prodrug temozolomide, which spontaneously decomposes to the same alkylating agent [37].
Figure 3.
Cytochrome P450-catalyzed oxidations of dacarbazine and procarbazine that produce the DNA alkylating agent methyl diazonium.
An N-dealkylation is also involved in activation of procarbazine, a related triazene prodrug (Figure 3). In this case, activation involves cytochrome P450 oxidation of the hydrazine to an azo linkage, further oxidation of the azo nitrogen to the azoxy state, and finally hydroxylation at the benzylic carbon [38–40]. This final metabolite spontaneously decomposes to produce methyl diazonium. Procarbazine is a substrate for CYP1A1 and −2B6 [41].
Tegafur (Ftorafur®)
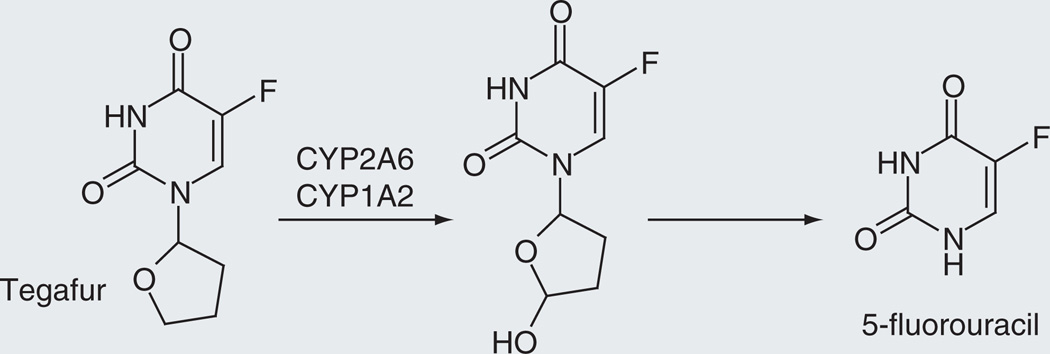
The anticancer prodrug tegafur (Figure 4) is converted to 5-fluorouracil, an agent that acts by inhibiting thymidylate synthase and through its incorporation into RNA [42,43]. The prodrug form of the drug circumvents the rapid degradation of 5-fluorouracil that occurs in the intestinal system and is also less toxic. The conversion of tegafur to 5-fluorouracil is mediated by both cytosolic thymidine phosphorylase and cytochrome P450, but a comparison of the two activation pathways indicates that the activation by cytochrome P450 predominates [44]. Incubations with 14 recombinantly expressed human P450 enzymes showed that CYP1A2, CYP2A6, CYP2E1 and CYP3A5 have high activities for the conversion of tegafur to 5-fluorouracil, but further studies with competitive substrates and antibodies suggest that the primary enzymes involved are CYP1A2, CYP2A6 and possibly CYP2C8 [45,46].
Figure 4.
Cytochrome P450-catalyzed release of 5-fluorouracil from tegafur.
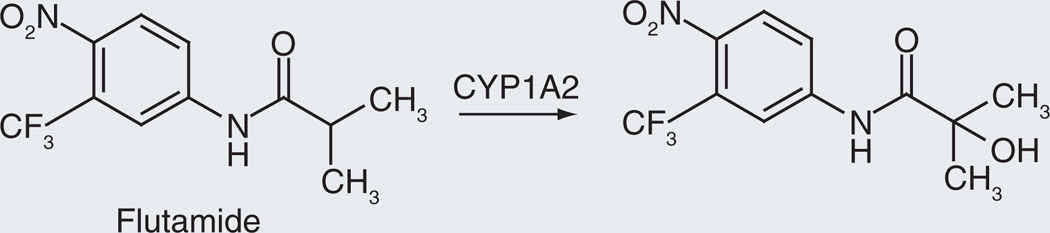
Flutamide
Flutamide is a nonsteroidal antiandrogen that is used in the treatment of prostate cancer [47]. It is a prodrug that is hydroxylated by cytochrome P450 to the 2-hydroxy derivative (Figure 5), the primary active form in vivo [48,49]. The hydroxylation can be catalyzed by CYP1A1 (Km = 103 µM), CYP1A2 (Km = 18.0 µM), and CYP1B1 (Km = 19 µM), with relative Vmax values of 15:20:1, respectively [50]. These values indicate that CYP1A2 is the primary enzyme involved in the 2-hydroxylation, although the tumor-specific enzyme CYP1B1 may contribute to catalysis of this reaction in tumors. The role of the 2-hydroxy metabolite as the pharmacologically important form of the drug is supported by the fact that: the parent compound is rapidly converted to the 2-hydroxylated metabolite; the 2-hydroxylated structure is the major metabolite; it has equal or higher activity as an antiandrogen [51].
Figure 5.
Oxidation of flutamide to it biologically active form.
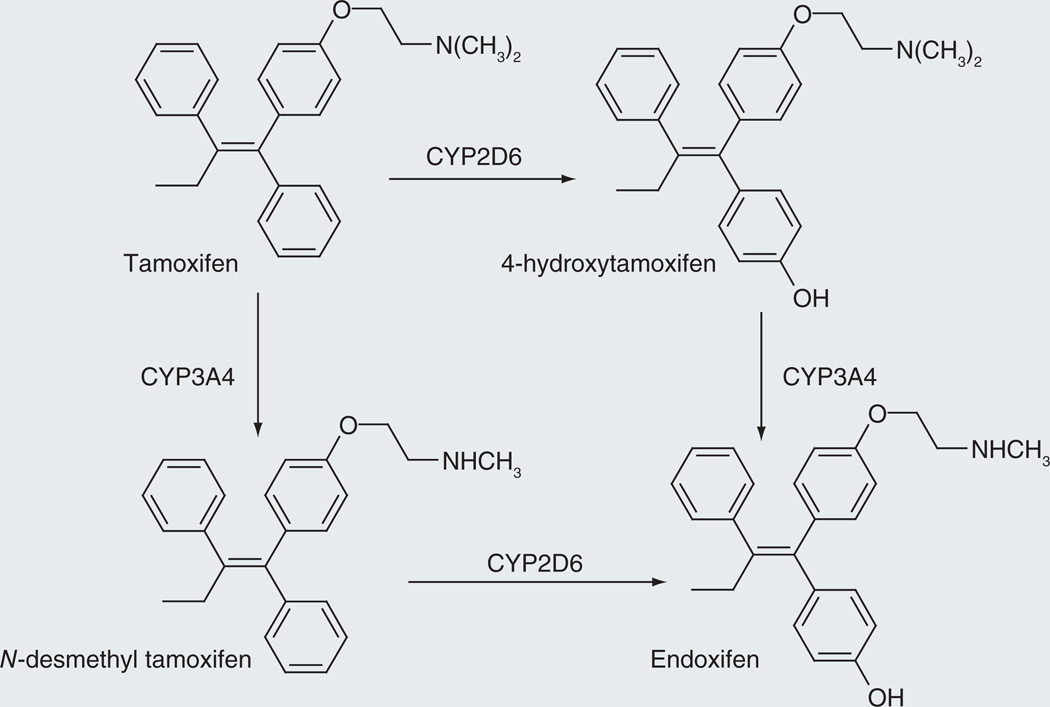
Tamoxifen
Tamoxifen (Figure 6) is employed in the treatment of breast cancer. It has intrinsic anticancer activity, but it is metabolized by phenyl ring hydroxylation and N-deamethylation to endoxifen, which is a more potent anticancer agent. The phenyl ring hydroxylation is mediated largely by CYP2D6, although other P450 enzymes can contribute to the reaction, and the N-dealkylation by CYP3A4 and CYP3A5 [52]. Individuals who are genetically deficient in CYP2D6 activity do not convert tamoxifen to endoxifen as efficiently and have poorer response to the drug than individuals with high CYP2D6 activity [53].
Figure 6.
Two reaction sequences that convert tamoxifen to the more biologically active endoxifen.
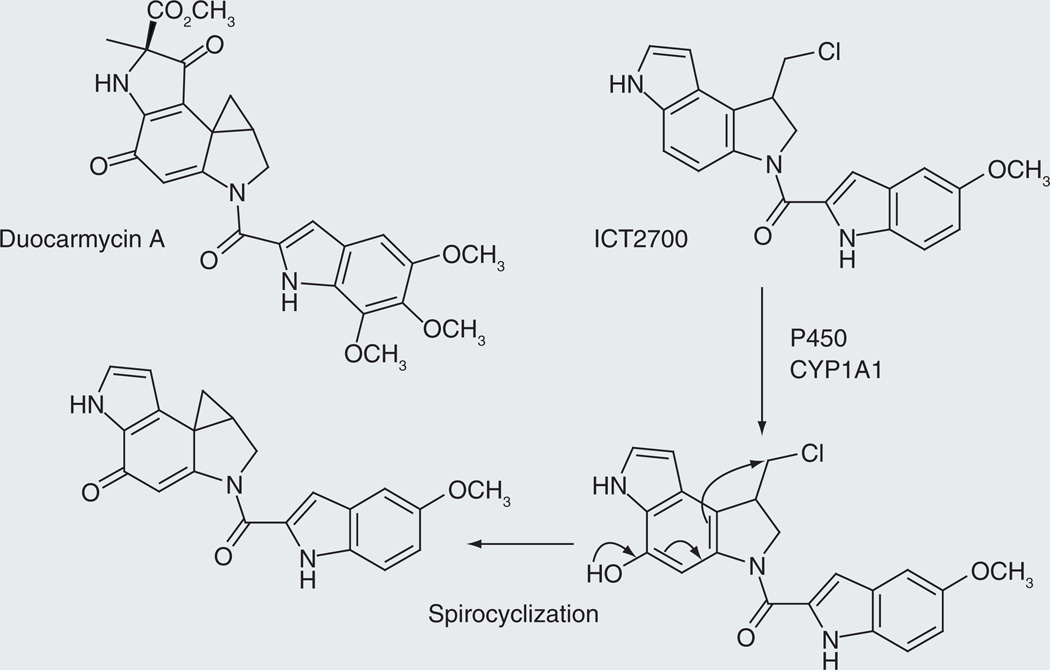
Duocarmycin/ICT2700
The duocarmycins (Figure 7) are exceptionally potent cytotoxins in which the cyclopropane ring of the drug adds to the N3 of adenine in AT-rich sites in the minor groove of DNA [54]. The cyclopropane ring is generated by intramolecular reaction of a phenol with a halogen-substituted side chain. Cytochrome P450 is not involved in the activation of these natural products. However, highly potent anticancer agents are often too toxic for clinical use. Increasing the specificity of the agent by constructing a prodrug that is released by cytochrome P450 enzymes overexpressed in tumors provides a potential mechanism for delivering the active form of the drug to the tumor while sparing nontumor tissues.
Figure 7.
Aromatic ring hydroxylation as a novel route for the conversion of a prodrug to its active form.
The synthesis and evaluation of ICT2700, a prodrug for a duocarmycin-like molecule, has recently been reported [55]. This molecule lacks the phenolic oxygen that is critical for intramolecular formation of the cyclopropane ring that alkylates DNA (Figure 7). However, CYP1A1 is able to efficiently introduce the required phenolic hydroxyl group, thus converting the prodrug ICT2700 to a molecule that forms the cyclopropane ring and alkylates DNA. In principle, ICT2700 would be activated to its cytotoxic form to a higher extent in tumor tissues if CYP1A1 is overexpressed, or induced to a greater extent, in cancer cells, conferring a therapeutic advantage on the molecule. However, although CYP1B1, CYP2J2, CYP2S1, CYP2W1 and CYP4Z1 are overexpressed in human tumors and thus may be suitable for the localized activation of prodrugs, CYP1A1 is not specifically associated with tumor cells [56–60].
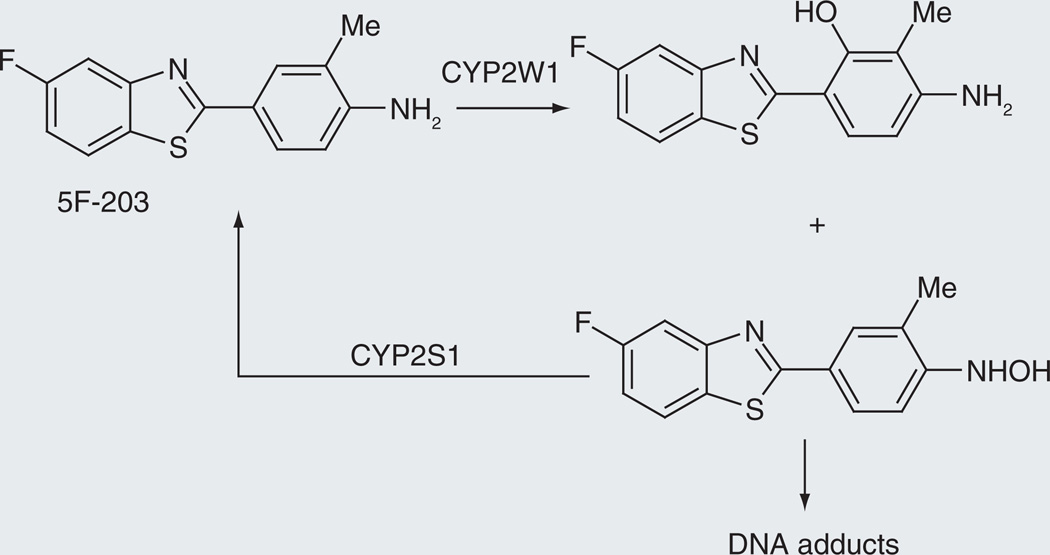
2-aryl-benzothiazoles
The agents 2-(4-amino-3-methylphenyl)-5-fluorobenzothiazole (5F 203) and 5-fluoro-2-(3,4-dimethoxyphenyl)benzothiazole (GW 610) are experimental cancer prodrugs that require cytochrome P450-catalyzed activation to form the DNA adducts responsible for their antitumor activity [61–64]. Analysis of the antitumor activity of GW-610 in breast cancer cell lines selectively depleted of CYP1A1, CYP2W1 or CYP2S1 indicated that CYP1A1 and CYP2W1 are involved in activation of the prodrug, whereas CYP2S1 mediates its inactivation [63]. A more recent detailed analysis of the metabolites formed from these drugs suggests that the activation–deactivation of SF 203 involves the formation of a hydroxylamine metabolite that can either be reduced back to the parent drug by CYP2S1 or progress to the formation of DNA adducts, as shown in Figure 8 [64].
Figure 8.
Activation of 5F-203 by CYP2W1 and deactivation by CYP2S1, two enzymes that are selectively expressed in tumor tissues.
Reductive
The quest for an anticancer targeted prodrug that is selectively activated in hypoxic tumor cells has not yet produced an approved drug. A number of efforts, including tyrapazamine and AQ4N, have fallen short during clinical trials. However, two new agents, TH-302 and PR-104, are currently in clinical trials. A number of prodrugs also exist that are reductively activated by electron transfer from reductase enzymes, including cytochrome P450 reductase. Due to space limitations, these are not covered here, but have been reviewed [65].
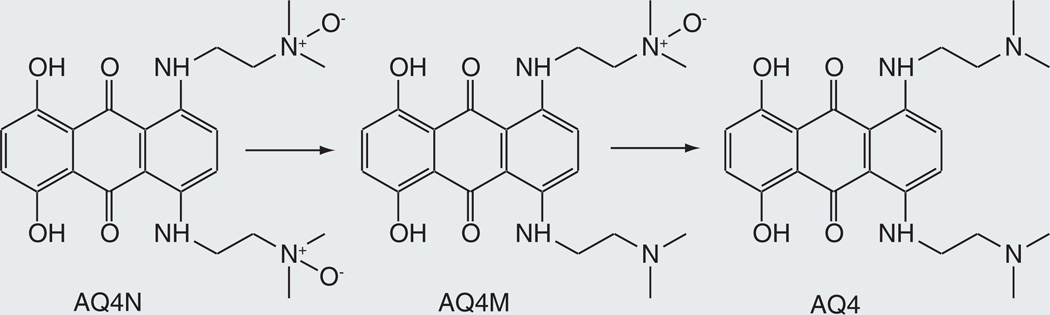
AQ4N
AQ4N is a di-N-oxide prodrug of AQ4, a potent topoisomerase II inhibitor that is too toxic to normal cells to be employed as an antitumor agent [66,67]. The di-N-oxide prodrug does not have topoisomerase inhibitory activity, but in a hypoxic environment such as that of solid tumors can be reduced to AQ4 by cytochrome P450 enzymes (Figure 9). The deoxygenation of N-oxides, based on earlier work [68,69], involves transfer of the oxygen to the reduced iron atom. The active drug can thus be targeted to hypoxic tissues while sparing normoxic cells. Initial screens showed that several P450 enzymes catalyze the anaerobic reduction of AQ4N to AQ4, but identified CYP3A4 as the most effective catalyst [70,71]. More recent work has demonstrated that CYP2S1 and CYP2W1, two P450 enzymes that are selectively expressed in tumors and/or hypoxic cells [13–15], are even better catalysts [72].
Figure 9.
Cytochrome P450-catalyzed reductive activation of AQ4N to AQ4 under hypoxic conditions.
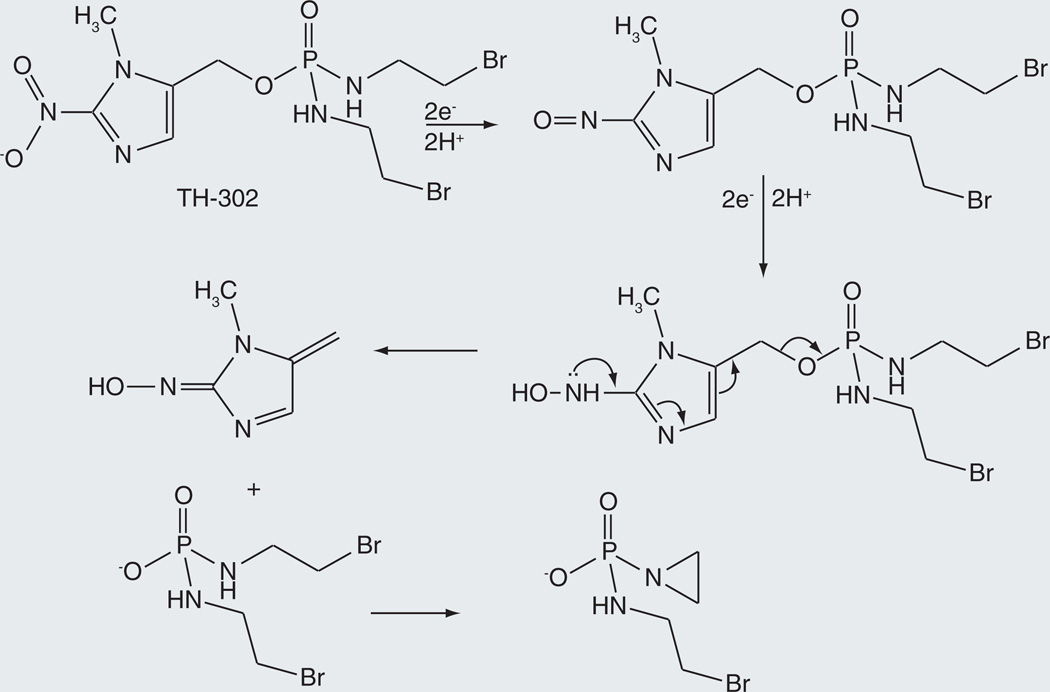
TH-302
A limitation of agents such as cyclophosphamide and ifosfamide (Figure 1) is that the activation reaction largely occurs in the liver rather than in the tumor, exposing all cells to the alkylating agent. To circumvent this shortcoming, TH-302 has been designed as an alkylating prodrug whose activation is largely restricted to hypoxic cells (Figure 10) [73]. The activation reaction does not actually require a cytochrome P450 enzyme, but does require NADPH cytochrome P450 reductase, the electron-donor partner of most mammalian P450 enzymes, or an alternative electron donor of appropriate redox potential. The activation reaction thus involves hypoxic sequential single-electron transfers to the nitro group to reduce it, first to the nitroso state and subsequently to the hydroxylamine (Figure 10). The nitrogen of the hydroxylamine can then extrude the alkylating agent by an intramolecular elimination reaction. In aerobic environments, the nitro radical anion produced by the first one-electron transfer donates the electron to molecular oxygen, producing superoxide and regenerating the original nitro group. The details of nitro activation in TH-302 have not been extensively studied, but a large amount of literature exists on the reduction of nitroaromatic compounds [74,75].
Figure 10.
Release of a mustard alkylating agent from TH-302 as the result of electron transfer to the parent drug under hypoxic conditions.
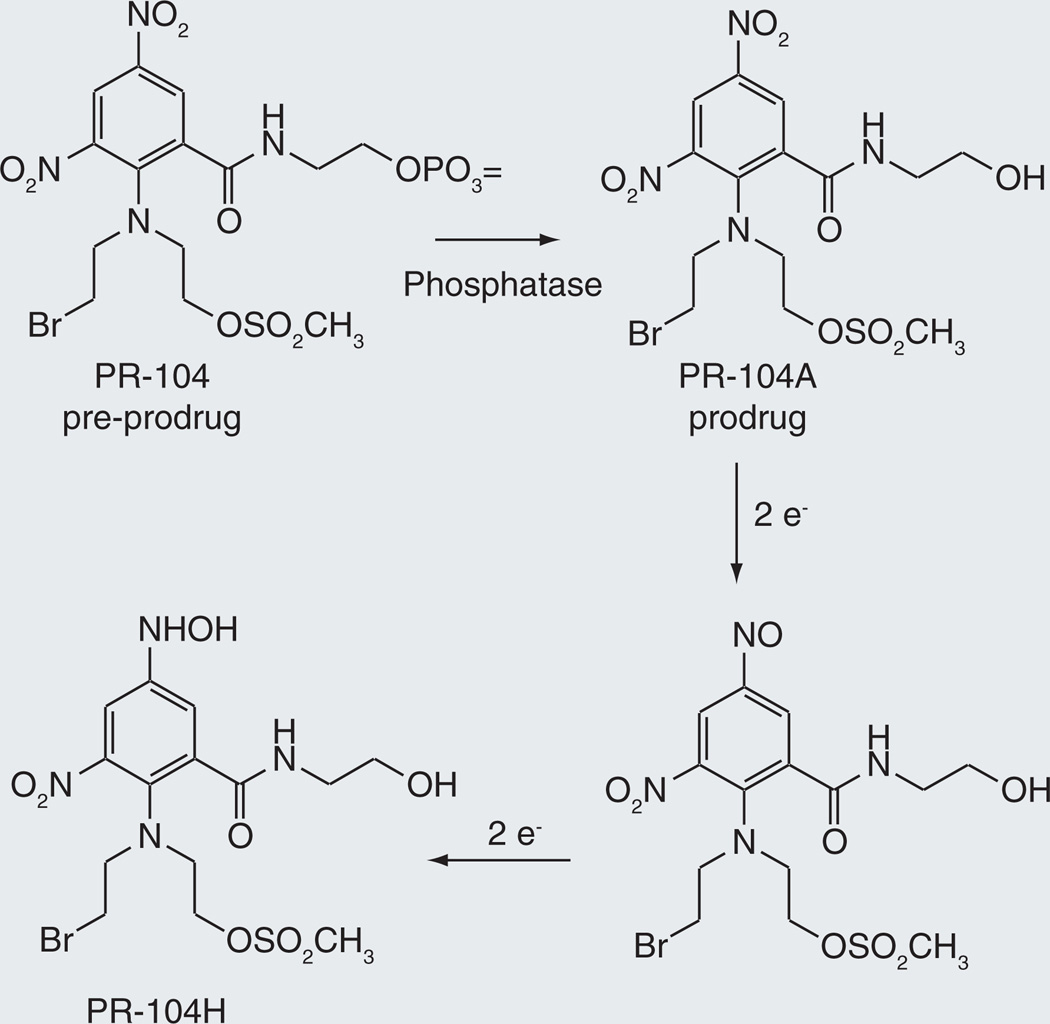
PR-104
Another reductive anticancer prodrug in clinical trials is PR-104, the active form of which is a DNA-crosslinking agent [76,77]. PR-104 is actually a pre-prodrug, in that a phosphate group introduced to enhance water solubility has to be removed by endogenous phosphatases before the reductive activation takes place (Figure 11). The resulting metabolite, termed PR-104A, undergoes reduction of a nitro group on the aromatic ring. Under hypoxic conditions, the sequential reduction steps are mediated by electron-donor proteins that may include cytochrome P450 reductase. In the presence of oxygen the reduction sequence is terminated after the first electron transfer, as the unpaired electron is donated to molecular oxygen, forming superoxide and regenerating the parent prodrug. However, in this instance, it has been found that the nitro compound can also be reduced aerobically through a two-electron transfer mechanism by aldo-keto reductase 1C3 [78]. This reduction is relatively specific, as ten other hypoxia-activated drugs, including TH-302 and AQ4N, were not subject to aerobic reduction by this enzyme. Aerobic reduction is likely to increase the toxicity of the drug to normal tissues, limiting the dose that can be utilized.
Figure 11.
Biological activation of PR-104 by phosphate hydrolysis followed by reduction of a nitro group under hypoxic conditions.
Prodrugs for other indications
P450-activated prodrugs have been designed for clinical targets other than cancer, including cardiovascular disorders, inflammation, parasitic infections, and as antihistamines for the treatment of allergies, hypertension and hepatitis.
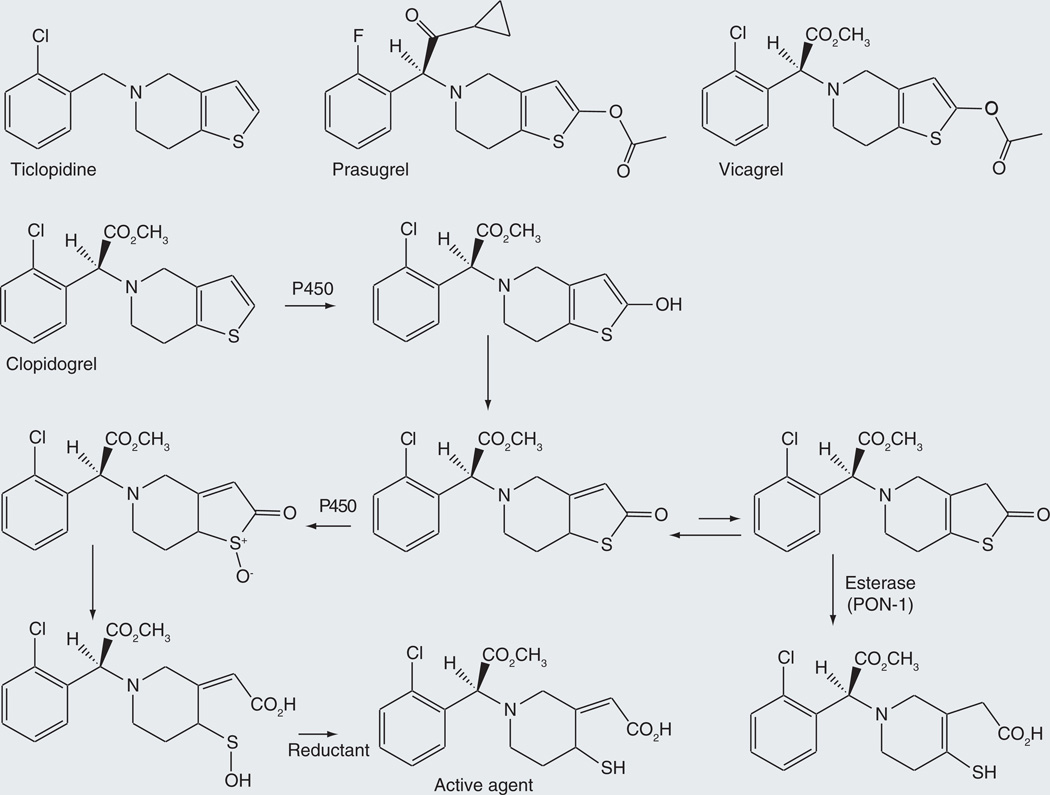
Ticlopidine/clopidogrel/prasugrel
Clopidogrel is a prodrug that releases an antagonist of the ADP P2Y12 receptor. It is employed clinically as an antiplatelet agent to prevent atherothrombotic episodes in patients with coronary artery disease that are being subjected to stent implantation. It was not designed as a prodrug, but its prodrug nature enables delivery of an unstable thiol agent to the site of therapeutic action. The generally agreed first step in the activation of clopidogrel is hydroxylation of the thiophene ring to give a 2-hydroxy and, after proton tautomerization, 2-oxo metabolite (Figure 12). However, two alternatives have been proposed for the second step of the activation process.
Figure 12.
Alternative pathways proposed for activation of the prodrug clopidogrel and of the closely related analogues ticlopidine, prasugrel and vicagrel.
In one activation mechanism, a second P450-catalyzed reaction oxidizes the sulfur atom to the sulfoxide, which then undergoes spontaneous hydrolysis to give a carboxy sulfenic acid (Figure 12). Reduction of the sulfenic acid by glutathione or another reducing agent generates the biologically active carboxy thiol metabolite [79]. Incubation of clopidogrel thiolactone with CYP1A2, −2C8, −2C9, −2C19, −2D6, −3A4 or −3A5, produced the sulfenic acid metabolite. Independent work led to the conclusion that CYP1A2, −2B6 and −2C19 contributed significantly to the first oxidative activation step, and CYP2B6, −2C9, −2C19 and −3A4 to the second one [80]. Taking into account the hepatic levels of the P450 enzymes, it was concluded that CYP2C19 and CYP3A4 were the most important catalysts of the first and second activation steps, respectively. These same studies confirmed the requirement for glutathione or a similar agent to complete the formation of the pharmacologically active thiol compound.
This second oxidative activation sequence has been questioned by work suggesting that the initial thiolactone metabolite is hydrolytically converted to the active agent by the thioesterase PON-1 rather than by a P450-catalyzed reaction [81]. Further investigation by Dansette et al. showed that the oxidative pathway produces one isomer of the pharmacologically active metabolite, whereas the PON-1 hydrolytic reaction produces a different isomer with the double bond shifted to another ring position (Figure 12) [82,83]. Their results suggest that the oxidative pathway is the dominant one. Nevertheless, based on a study of the drug in patients, the proponents of the PON-1 activation step have argued that it is, in fact, the critical one [84].
Prasugrel is a prodrug closely related in terms of structure and mechanism to the initial thiolactone metabolite of clopidogrel (Figure 12). In this molecule, the first activation step is hydrolysis of the exocyclic ester to produce a thiolactone analogous to that obtained oxidatively from clopidogrel. Conversion of this intermediate to the ring opened carboxy thiol product requires a P450 oxidation to cleave the ring and give a carboxy sulfenic acid product that is reductively converted to the pharmacologically active thiol drug [85]. The sulfenic acid can be chemically trapped [85–87]. As reported for clopidogrel, thioesterase opening of the thiolactone yields a thiol derivative with the double bond at a different ring position. Experiments carried out with 18O2 and H218O provide direct evidence that the thiolactone sulfoxide is opened by attack of the water molecule on the carbonyl group with sulfenic acid as the leaving group. As with clopidogrel, glutathione is required for formation of the final pharmacologically active agent.
Other members of this class of drugs are similarly activated. These include ticlopidine (Figure 12), the simplest of these antiplatelet drugs. It has a higher risk of thrombocytopenic purpuria and neutropenia than clopidogrel, which has largely replaced it in clinical practice. The same mechanism of activation proposed for clopidogrel and prasugrel applies to ticlopidine [79]. Vicagrel, a molecule with the same first activation step as prasugrel, but with the chloro and methyl ester substituents of clopidogrel, has recently been described. It, too, is assumed to undergo a second P450-catalyzed activation step [88].
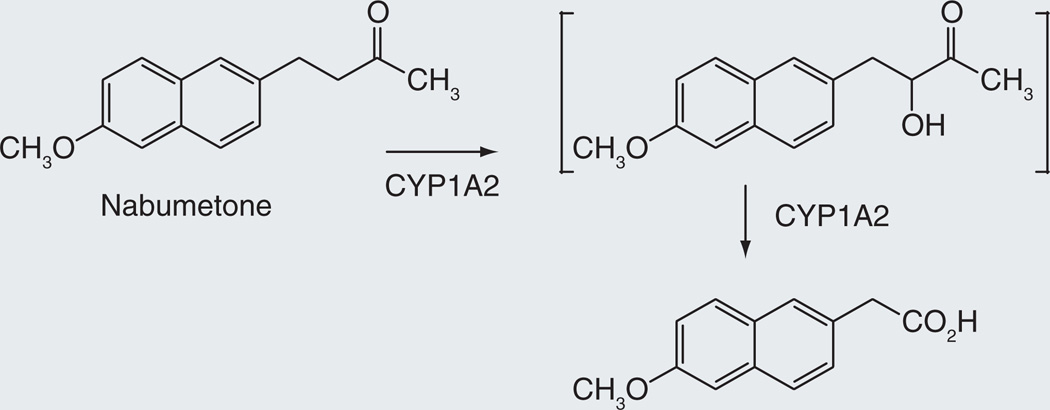
Nabumetone
Nabumetone, a popular NSAID prodrug, is used to relieve pain and swelling caused by osteoarthritis and rheumatoid arthritis [89]. Its primary advantages over NSAIDs like naproxen are its lower gastrointestinal irritation and its once-daily dosing. Approximately 35% of the prodrug is converted in the liver to 6-methoxy-2-naphthylacetic acid, the pharmacologically active agent that inhibits COX2 [90,91]. This transformation is catalyzed largely by CYP1A2. The compound was not designed as a P450-activated prodrug, but was subsequently found to be one. The mechanism of the activation reaction, which involves cleavage of a carbon–carbon bond, has not been elucidated. However, if it resembles that involved in the sterol C17–20 lyase reaction catalyzed by CYP17A1 [19], it may proceed via initial hydroxylation to give the α-hydroxyketone intermediate as shown in Figure 13.
Figure 13.
Hypothetical mechanism for the conversion of nabumetone to its biologically active carboxylic acid metabolite.
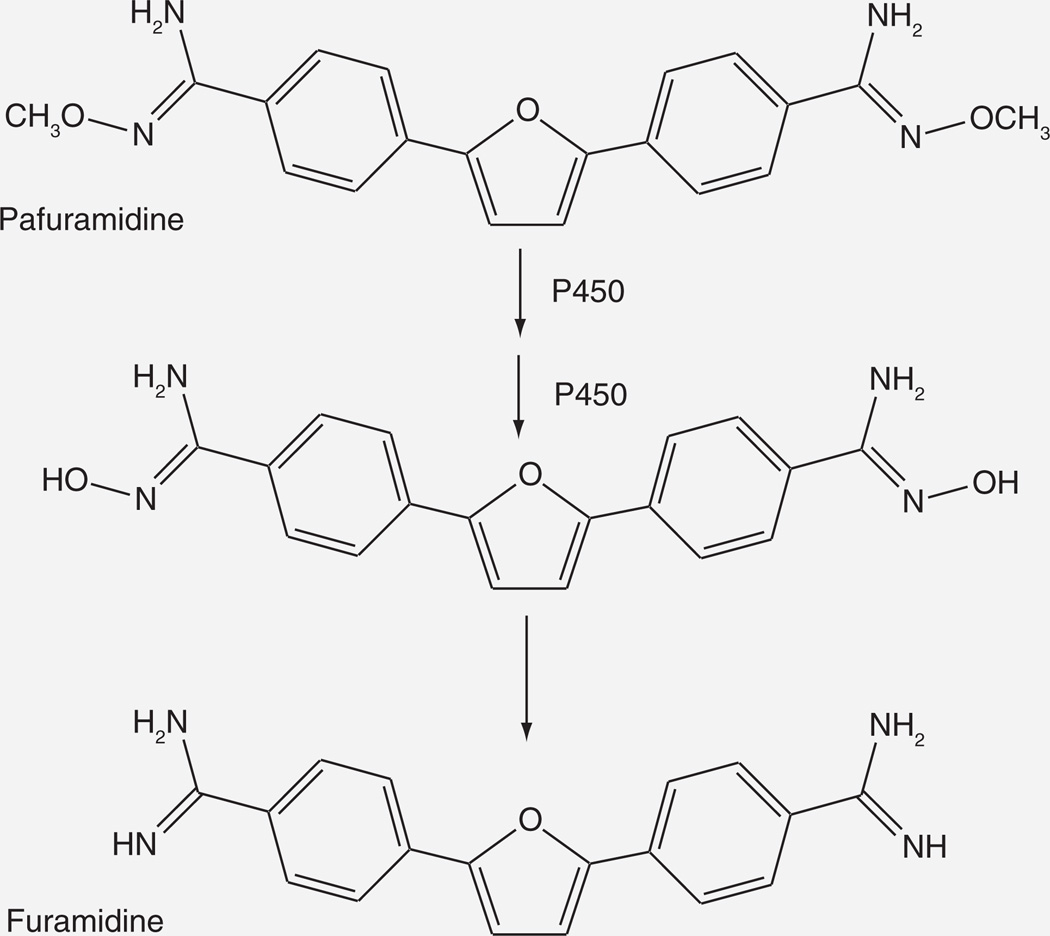
Pafuramidine/DB289
Pafuramidine (DB289) is a prodrug that is converted to the potent antiparasitic metabolite DB75 by two sequential O-demethylation reactions followed by an equal number of reductive dehydroxylation reactions Figure 14). The active agent DB75 has poor absorption due to its dicationic nature, whereas the prodrug DB289 has much better absorption properties. The prodrug is active against a variety of infections, including Pneumocystis pneumonia and trypanosomiasis.
Figure 14.
Cytochrome P450-catalyzed activation of pafuramidine to furamidine, the biologically active form.
The O-demethylations of DB289 are catalyzed by cytochrome P450 [92,93], whereas the dehydroxylation steps are mediated by cytochrome b5/NADH-cytochrome b5 reductase [94]. Screening with pure enzymes indicated that CY1A1, −1A2, −1B1, −2J2, −4F2 and −4F3B could catalyze the O-demethylation, but further work established that the primary catalysts in the enterohepatic system were CYP4F2 and CYP4F3B [92,93].
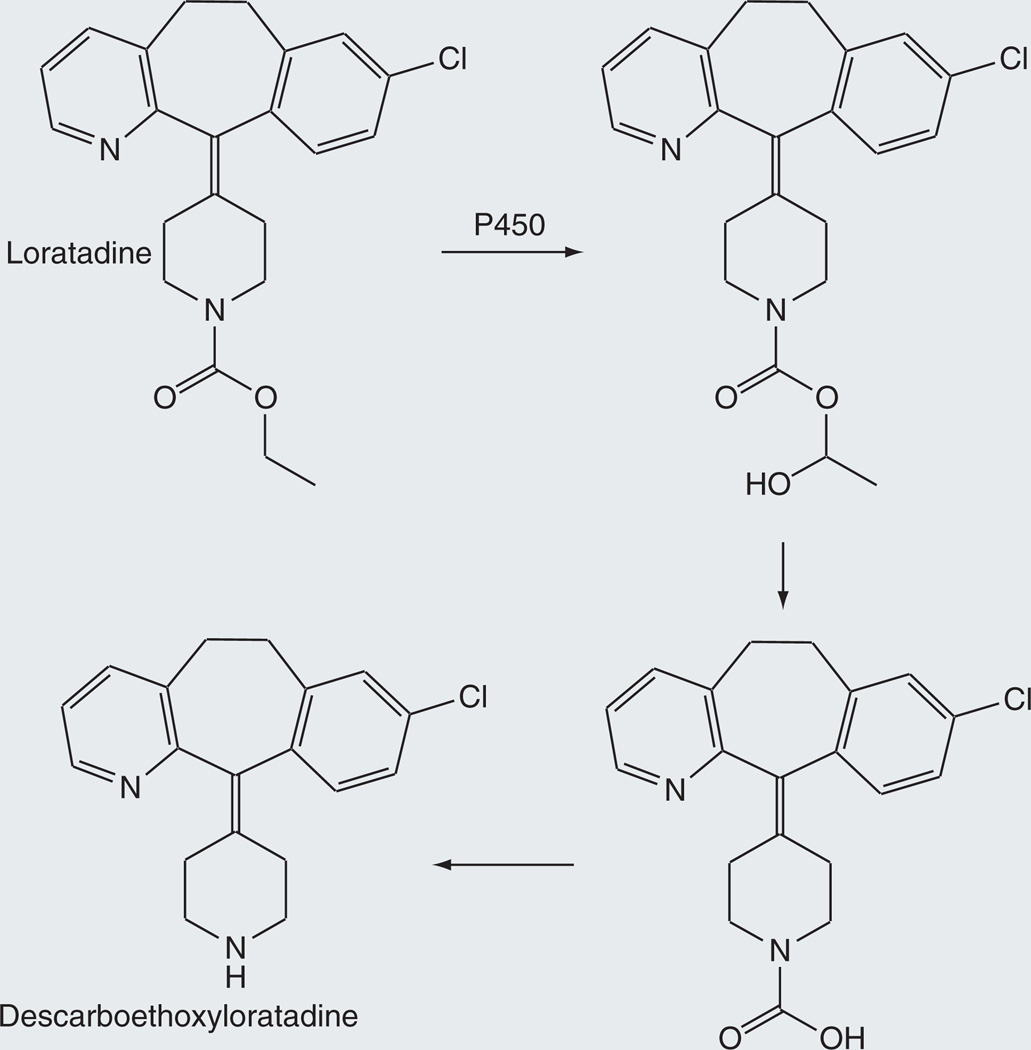
Loratadine
Loratadine, a nonsedating antihistamine employed for the relief of seasonal allergic symptoms, is marketed in the USA as Claritin® [95]. Loratadine is readily absorbed and undergoes extensive metabolism to give descarboethoxyloratadine, the principal pharmacologically active agent Figure 15 [96,97]. The cytochrome P450-catalyzed transformation of loratadine to descarboethoxyloratadine involves hydroxylation of the ethyl group adjacent to the oxygen atom, followed by sequential loss of ethanol and carbon dioxide. The role of cytochrome P450 rather than an esterase in this process is confirmed by the finding that in liver microsomes NADPH is required and esterase inhibitors do not interfere with the conversion [96]. Analysis of the transformation mediated by human liver microsomes indicates that CYP3A4 and CYP2D6 are the enzymes responsible for this reaction.
Figure 15.
Release of descarboethoxyloratadine from the prodrug loratadine by a hydroxylation-decarboxylation route.
Terfenadine
Terfenadine was marketed as an antihistamine until it was accidentally discovered to be a prodrug that was converted to the activated form by CPY3A4-catalyzed oxidation of one of the methyls of a tert-butyl group to a carboxylic acid. Unfortunately, terfenadine, the prodrug form, proved to cause a potentially fatal cardiac disorder known as torsades des pointes. It is normally converted rapidly in the liver to the acid derivative, now marketed as the drug fexofenadine, but in patients taking agents that inhibited CYP3A4 function this metabolism was impaired and resulted in patient deaths [98]. It is an example of what a prodrug should not be.
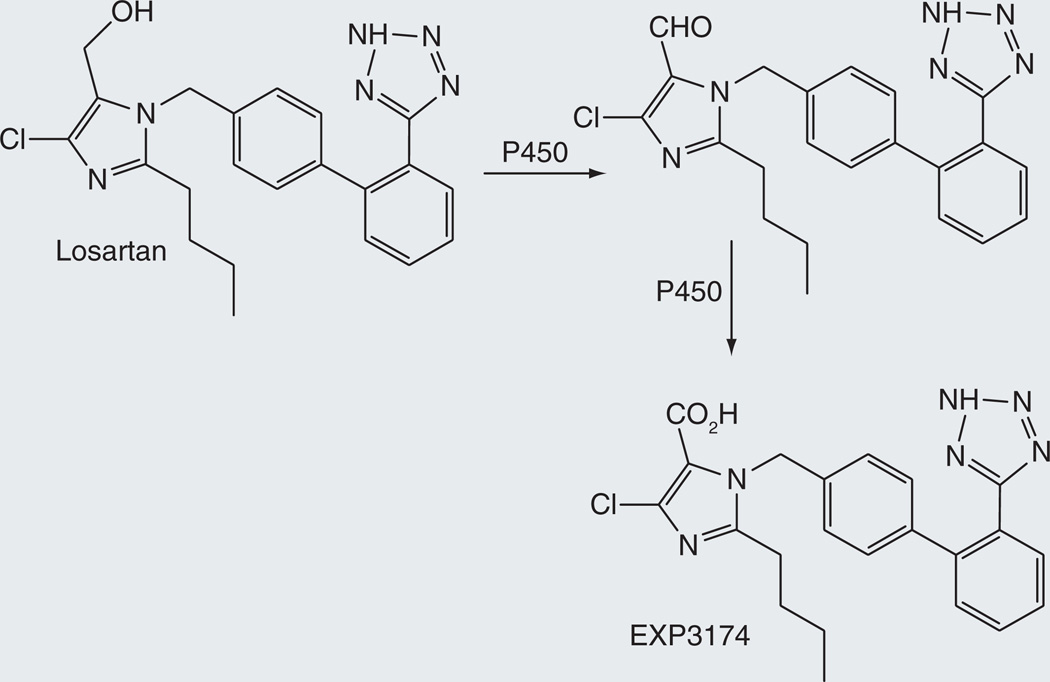
Losartan
Losartan is a highly selective, competitive angiotensin II receptor type 1 (AT1) antagonist that decreases total peripheral resistance and cardiac venous return [99]. Although losartan itself is pharmacologically active, it is oxidized by cytochrome P450 to its 5-carboxylic acid derivative (Figure 16). This metabolite, known as EXP3174, is 10–40 times more potent in blocking AT1 receptors and is longer acting, as shown by the T1/2 values of 1.5–2.5 and 3–9 h for elimination of losartan and E3174, respectively [100,101]. The oxidation of the losartan 5-methyl group to the aldehyde and subsequently to the carboxylic acid is mediated by CYP2C9 and CYP3A4 [102,103].
Figure 16.
Two-step oxidation of losartan to the biologically active metabolite EXP3174.
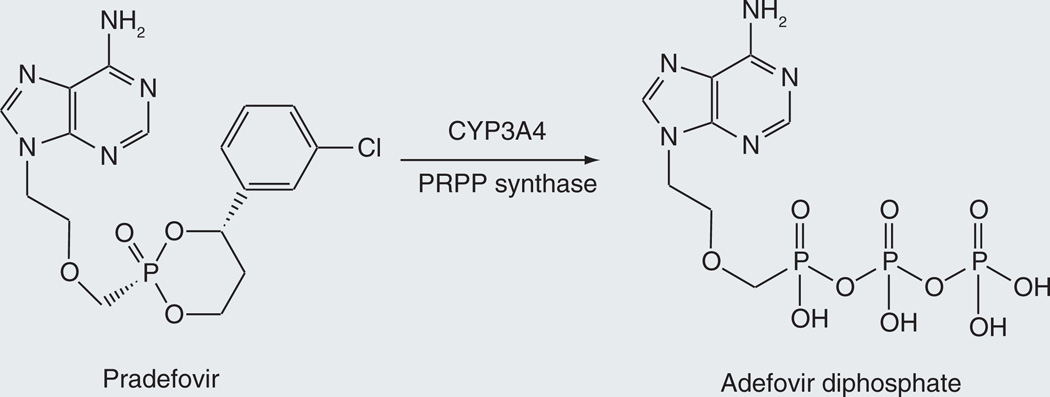
Pradefovir
Adefovir dipivoxyl, a drug that is clinically employed for the treatment of hepatitis B, is administered at a suboptimal dose due to renal toxicity of the drug. Efforts to circumvent this limitation led to the development of the prodrug pradefovir (Figure 17) [104]. The prodrug is activated by CYP3A4 hydroxylation of the ring in a manner similar to that for cyclophosphamide (Figure 3) [105]. The monophosphate thus released is phosphorylated in situ by PRPP synthase, producing adefovir. The prodrug preferentially delivers adefovir to the liver, as indicated by a 12-fold higher liver/kidney ratio of adefovir with the prodrug versus the parent agent [105].
Figure 17.
Conversion of pradefovir to adefovir diphosphate.
Future perspective
Prodrugs provide an avenue for the redesign of old drugs or the invention of new ones. A diversity of enzymatic processes can be exploited for the physiological activation of prodrugs and the selection of the activation mechanism depends on the structure of the drug and the purpose of the prodrug. The use of cytochrome P450 enzymes to activate prodrugs greatly expands the type of functionality that can be used in prodrug design due to the versatility of cytochrome P450 catalysis. Another advantage of cytochrome P450 catalysis is that it can, in principle, provide a mechanism for selective prodrug fragmentation within specific cells or tissues. At a basic level, this can occur in tissues with high P450 concentrations, such as the liver or lungs. At a more sophisticated level, prodrugs can theoretically be targeted to individual P450 enzymes whose cellular and organ distributions then determine the site(s) of prodrug activation. This cytochrome P450-specific targeting of prodrugs is in its infancy, but the targeting of prodrugs to P450 enzymes overexpressed in tumor tissues is a promising area for future work. The tumor-selective or -specific release of anticancer drugs can lower systemic toxicity and thus allow the use of higher doses, or of much more cytotoxic molecules, in cancer therapy. An alternative approach is to design prodrugs that are activated in hypoxic tissues, whether by a P450 enzyme or by its electron-donor partner. This approach, which has been under investigation for some time, is currently being tested by clinical trials with two drugs. Of course, one can envision a prodrug that is activated under hypoxic conditions by a tumor-specific P450 enzyme, increasing the constraints that limit its cytotoxic activity to the tumor tissue.
Three limitations, however, must be kept in mind with respect to the cytochrome P450-catalyzed activation of prodrugs. The first is that cytochrome P450 enzyme activities are subject to considerable variation due to isoform-specific polymorphisms, induction and other phenomena. This variation can result in different levels of prodrug activation in different individuals. The second is that cytochrome P450 enzymes are relatively slow, so that release of the drug from the prodrug usually take time.Finally, as drug-metabolizing forms of cytochrome P450 are involved in the metabolism of most drugs, a potential exists for drug–drug interactions at the level of prodrug release. These limitations are by no means prohibitive, but must be considered in the design and use of a P450-activated prodrug.
Executive summary.
Prodrugs
-
▪
A prodrug is a compound that has little or no activity on a desired pharmacological target, but is converted to an active, or more active, entity by an endogenous metabolic reaction.
-
▪
Prodrugs can fulfill many purposes, including improving pharmacokinetics, decreasing toxicity, or facilitating delivery of the drug to specific tissues or cells.
Cytochrome P450 system
-
▪
Cytochrome P450 enzymes are involved in the metabolism of most drugs and xenobiotics, and therefore, of most prodrugs.
-
▪
Cytochrome P450 enzymes are highly versatile oxidative catalysts for the activation of prodrugs.
-
▪
Cytochrome P450 enzymes can have distinct organ and cellular locations.
-
▪
The levels of cytochrome P450 activity can be altered in an enzyme-specific fashion by genetic and environmental factors.
-
▪
The selective location of several P450 enzymes in tumor or hypoxic tissues is of particular interest in the context of anticancer drug development.
-
▪
Under hypoxic conditions, reductive reactions can also be catalyzed by P450 enzymes or by cytochrome P450 reductase, their electron-donor partner.
Anticancer prodrug activation
-
▪
Anticancer agents are a particularly interesting area for prodrug development.
-
▪
The oxidative activation of prodrugs is embodied by the clinically used drugs cyclophosphamide, ifosfamide, dacarbazine, and procarbazine, all of which are alkylating agents.
-
▪
Oxidative activation is also involving in the activation of flutamide and the experimental agents ICT2700, a duocarmycin prodrug, and 5F 203.
-
▪
As shown by tamoxifen, polymorphisms in specific P450 enzymes can influence the activation of prodrugs.
-
▪
Targeting of solid tumors by alkylating prodrugs activated by the P450 system under hypoxic conditions is the basis for the experimental prodrugs AQ4N, TH-302 and PR-104.
Prodrugs for other indications
-
▪
P450-activated prodrugs exist for diseases other than cancer.
-
▪
P450 is involved in the activation of the prodrugs ticlopidine, clopidogrel and prasugrel involved in treatment of cardiovascular disorders.
-
▪
Nafumetone is a P450-activated nonsteroidal anti-inflammatory prodrug with lower gastric effects than other NSAID agents.
-
▪
The antiparasitic prodrug pafuramidine circumvents the poor absorption properties of the parent drug DB75.
-
▪
A nonsedating antihistamine, loratadine, is a P450-activated prodrug.
Acknowledgments
The research work of the author described in this review and preparation of the review were supported by NIH grant GM25515. The author is a co-founder of Cyterix Pharmaceuticals, a company with interests in the development of anticancer targeted prodrugs, and currently serves as a consultant to the company.
Key Term
- CYP
The prefix that denotes a cytochrome P450 enzyme. It is followed by a number for the family, a letter for the subfamily, and a number denoting the specific P450 enzyme in the subfamily. Enzymes with approximately 40% amino acid identity are classified in the same family and with approximately 55% identity in the same subfamily. Thus CYP1A2 denotes the second P450 enzyme of family 1, subfamily A.
- Gene-directed enzyme prodrug therapy
Experimental approach in which the concentration of a specific P450 enzyme that activates a prodrug is enhanced in a tumor by transfection before administration of the prodrug.
- Stent implantation
Insertion of a mesh tube into a constricted artery to widen it and improve blood flow.
Footnotes
Financial & competing interests disclosure
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Dayer P, Desmeules J, Leemann T, Striberni R. Bioactivation of the narcotic drug codeine in human liver is mediated by the polymorphic monooxygenase catalyzing debrisoquine 4-hydroxylation (cytochrome P-450 dbl/bufI) Biochem. Biophys. Res. Commun. 1988;152:411–416. doi: 10.1016/s0006-291x(88)80729-0. [DOI] [PubMed] [Google Scholar]
- 2.Vree TB, Verwey-van Wissen CP. Pharmacokinetics and metabolism of codeine in humans. Biopharm. Drug Dispos. 1992;13:445–460. doi: 10.1002/bdd.2510130607. [DOI] [PubMed] [Google Scholar]
- 3.Srinivasan V, Wielbo D, Tebbett IR. Analgesis effects of codeine-6-glucuronide after intravenous administration. Eur. J. Pain. 1997;1:185–190. doi: 10.1016/s1090-3801(97)90103-8. [DOI] [PubMed] [Google Scholar]
- 4.Vree TB, Van Dongren RT, Koopman-Kimenai PM. Codeine analgesia is due to codeine-6-glucuronide, not morphine. Int. J. Clin. Pract. 2000;54:395–398. [PubMed] [Google Scholar]
- 5.Armstrong SC, Cozza KL. Pharmacokinetic drug interactions of morphine, codeine, and their derivatives: theory and clinical reality, part II. Psychosomatics. 2003;44:515–520. doi: 10.1176/appi.psy.44.6.515. [DOI] [PubMed] [Google Scholar]
- 6.Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- 7.Kelly LE, Rieder M, van den Anker J, et al. More codeine fatalities after tonsillectomy in North American children. Pediatrics. 2012;129:e1343–e1347. doi: 10.1542/peds.2011-2538. [DOI] [PubMed] [Google Scholar]
- 8.Rautio J, Kumpulainen H, Heimbach T, et al. Prodrugs: design and clinical applications. Nat. Rev. Drug Discov. 2008;7:255–270. doi: 10.1038/nrd2468. [DOI] [PubMed] [Google Scholar]
- 9.Janin YL. Antituberculosis drugs: ten years of research. Bioorg. Med. Chem. 2007;15:2479–2513. doi: 10.1016/j.bmc.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Guengerich FP. Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. USA: Kluwer/Plenum/ Elsevier, NY; 2005. pp. 377–530. [Google Scholar]
- 11.Evans WE, Relling MV. Pharmacogenomics: Translating functional genomics into rational therapeutics. Science. 1999;286:487–491. doi: 10.1126/science.286.5439.487. [DOI] [PubMed] [Google Scholar]
- 12. Rivera SP, Wang F, Saarikoski ST, et al. A novel promoter element containing multiple overlapping xenobiotic and hypoxia response elements mediates induction of P4502S1 by both dioxin and hypoxia. J. Biol. Chem. 2007;282:10881–10893. doi: 10.1074/jbc.M609617200. ▪ Identification of regulatory elements in CYP2S1 that result in its overexpression in hypoxic tissues.
- 13.Downie D, McFadyen MCE, Rooney PH, et al. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clin. Cancer Res. 2005;11:7369–7375. doi: 10.1158/1078-0432.CCR-05-0466. [DOI] [PubMed] [Google Scholar]
- 14. Karlgren M, Gomez A, Stark K, et al. Tumor-specific expression of the novel cytochrome P450 enzyme, CYP2W1. Biochem. Biophys. Res. Commun. 2006;341:451–458. doi: 10.1016/j.bbrc.2005.12.200. ▪ Demonstration that CYP2W1 is a tumor-specific cytochrome P450 enzyme that may be exploited to target prodrugs to cancer tissues.
- 15.Wijnen PA, O den Buijsch RA, Drent M, et al. The prevalence and clinical relevance of cytochrome P450 polymorphisms. Aliment. Pharmacol. Ther. 2007;26(Suppl. 2):211–219. doi: 10.1111/j.1365-2036.2007.03490.x. [DOI] [PubMed] [Google Scholar]
- 16.Ingelman-Sundberg M, Sim SC, Gomez A, Rodriguez-Antona C. influence of cytochrome P450 polymorphisms on drug therapies: pharmacogenetic, pharmacoepigenetic and clinical aspects. Pharmacol. Ther. 2007;116:496–526. doi: 10.1016/j.pharmthera.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Pelkonen O, Maenpaa J, Taavitsainen P, Rautio A, Raunio H. Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica. 1998;28:1203–1253. doi: 10.1080/004982598238886. [DOI] [PubMed] [Google Scholar]
- 18.Tompkins LM, Wallace AD. Mechanisms of cytochrome P450 induction. J. Biochem. Mol. Toxicol. 2007;21:176–181. doi: 10.1002/jbt.20180. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz de Montellano PR, De Voss JJ. Substrate oxidation by cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry. Springer, NY, USA: 2005. pp. 183–245. [Google Scholar]
- 20.Iyanagi T, Xia C, Kim JP. NADPH-cytochrome P450 oxidoreductase: prototypic member of the diflavin reductase family. Arch. Biochem. Biophys. 2012;528:72–89. doi: 10.1016/j.abb.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Druckrey H. Experimentelle grundlagen der chemotherapie des krebses. Dtsch. Med. Wochenschr. 1952;77:1534–1537. doi: 10.1055/s-0028-1117290. [DOI] [PubMed] [Google Scholar]
- 22.Brock N. The history of the oxazaphorphorine cytostatics. Cancer. 1995;78:542–547. doi: 10.1002/(SICI)1097-0142(19960801)78:3<542::AID-CNCR23>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 23.Friedman OM, Myles A, Colvin M. Cyclophosphamide and phosphoramide mustards. Adv. Cancer Chemother. 1979;1:143–204. [Google Scholar]
- 24.Colvin M, Hilton J. Pharmacology of cyclophosphamide and metabolites. Cancer Treat. Rep. 1981;65(Suppl. 3):89–95. [PubMed] [Google Scholar]
- 25.Sladek NE. Metabolism of oxazaphosphorines. Pharmacol. Ther. 1988;37:301–355. doi: 10.1016/0163-7258(88)90004-6. [DOI] [PubMed] [Google Scholar]
- 26.Bunting KD, Townsend AJ. Protection by transfected rat or human class 3 aldehyde dehydrogenase against the cytotoxic effects of oxazaphosphorine alkylating agents in hamster V79 cell lines. Demonstration of aldophosphamide metabolism by the human cytosolic class 3 isozyme. J. Biol. Chem. 1996;271:11891–11896. doi: 10.1074/jbc.271.20.11891. [DOI] [PubMed] [Google Scholar]
- 27.Mace JR, Keohan ML, Bernardy H, et al. Crossover randomized comparison of intravenous versus intravenous/oral mesna in soft tissue sarcoma treated with high-dose ifosfamide. Clin. Cancer Res. 2003;9:5829–5834. [PubMed] [Google Scholar]
- 28.Roy P, Yu LJ, Crespi CL, Waxman DJ. Development of a substrate activity based approach to identify the major human liver P-450 catalysts of cyclophosphamide and ifosfamide activation based on cDNA-expressed activities and liver microsomal P-450 Profiles. Drug Metab. Dispos. 1999;27:655–666. [PubMed] [Google Scholar]
- 29.Nguyen T, Tychopoulos M, Bichat F, et al. Improvement of cyclophosphamide activation by CY2B6 mutants: from in silico to ex vivo . Mol. Pharmacol. 2008;73:1122–1133. doi: 10.1124/mol.107.042861. [DOI] [PubMed] [Google Scholar]
- 30.Waxman DJ, Chen L, Hecht JE, Jounaidi Y. Cytochrome P450-based cancer gene therapy: recent advances and future prospects. Drug Metab. Rev. 1999;31:503–522. doi: 10.1081/dmr-100101933. [DOI] [PubMed] [Google Scholar]
- 31.Legha SS. Current therapy for malignant melanoma. Semin. Oncol. 1989;16:34–44. [PubMed] [Google Scholar]
- 32.Marchesi F, Turriziani M, Tortorelli G, Avvisati G, Torino F, De Vecchis L. Triazene compounds: mechanism of action and related DNA repair systems. Pharmacol. Res. 2007;56:275–287. doi: 10.1016/j.phrs.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Kolar GF, Maurer M, Wildschütte M. 5-(3-Hydroxymethyl-3-methyl-1-triazeno imidazole-4-carboxamide is a metabolite of 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DIC, DTIC NSC-45388) Cancer Lett. 1980;10:235–241. doi: 10.1016/0304-3835(80)90076-2. [DOI] [PubMed] [Google Scholar]
- 34.Meer L, Janzer RC, Kleihues P, Kolar GF. In vivo metabolism and reaction with DNA of the cytostatic agent, 5-(3,3-dimethyl-1-triazeno)imidazole-4-carboxamide (DTIC) Biochem. Pharmacol. 1986;35:3243–3247. doi: 10.1016/0006-2952(86)90419-3. [DOI] [PubMed] [Google Scholar]
- 35.Reid JM, Kuffel MJ, Miller JK, Rios R, Ames MM. Metabolic activation of dacarbazine by human cytochromes P450: the role of CYP1A1, CYP1A2 and CYP2E1. Clin. Cancer Res. 1999;5:2192–2197. [PubMed] [Google Scholar]
- 36.Lewis BC, Mackenzie PI, Miners JO. Application of homology modeling to generate CYP1A1 mutants with enhanced activation of the cancer chemotherapeutic prodrug dacarbazine. Mol. Pharmacol. 2011;80:879–888. doi: 10.1124/mol.111.072124. [DOI] [PubMed] [Google Scholar]
- 37.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin. Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 38.Swaffar DS, Horstman MG, Jaw J, et al. Methylazoxyprocarbazine, the active metabolite responsible for the anticancer activity of procarbazine against L1210 leukemia. Cancer Res. 1989;49:2442–2447. [PubMed] [Google Scholar]
- 39.Cummings SW, Guengerich FP, Prough RA. The characterization of N-isopropyl-p-hydroxymethylbenzamide formed during the oxidative metabolism of azo-procarbazine. Drug Metab. Dispos. 1982;10:459–464. [PubMed] [Google Scholar]
- 40.Weinkam RJ, Shiba DA. Metabolic activation of procarbazine. Life Sci. 1978;22:937–946. doi: 10.1016/0024-3205(78)90358-2. [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez-Antona C, Ingelman-Sundberg M. Cytochrome P450 pharmacogenetics and cancer. Oncogene. 2006;25:1679–1691. doi: 10.1038/sj.onc.1209377. [DOI] [PubMed] [Google Scholar]
- 42.Au JL, Sadee W. The pharmacology of Ftorafur (R,S-1-(tetrahydro-2-furanyl)−5-fluorouracil) Recent Results Cancer Res. 1981;76:100–114. doi: 10.1007/978-3-642-81565-2_9. [DOI] [PubMed] [Google Scholar]
- 43.Rooseboom M, Commandeur JNM, Vermeulen NPE. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 44.Komatsu T, Yamazaki H, Shimada N, et al. Involvement of microsomal cytochrome P450 and cytosolic thymidine phosphorylase in 5-fluorouracil formation from tegafur in human liver. Clin. Cancer Res. 2001;7:675–681. [PubMed] [Google Scholar]
- 45.Komatsu T, Yamazaki H, Shimada N, Nakajima M, Yokoi T. Roles of cytochrome P450 1A2, 2A6, and 2C8 in 5-fluorouracil formation from tegafur, an anticancer prodrug, in human liver microsomes. Drug Metab. Dispos. 2000;28:1457–1463. [PubMed] [Google Scholar]
- 46.Ikeda K, Yoshisue K, Matsushima E, et al. Bioactivation of tegafur to 5-fluorouracil is catalyzed by cytochrome P-450 2A6 in human liver microsomes in vitro . Clin. Cancer Res. 2000;6:4409–4415. [PubMed] [Google Scholar]
- 47.Singh SM, Gauthier S, Labrie F. Androgen receptor antagonists (antiandrogens) structure–activity relationships. Curr. Med. Chem. 2000;7(2):211–247. doi: 10.2174/0929867003375371. [DOI] [PubMed] [Google Scholar]
- 48.Katchen B, Buxbaum S. Disposition of a new, nonsteroid, antiandrogen, α,α,α-trifluoro-2-methyl- 4´-nitro-m-propionotoluidide (flutamide), in men following a single oral 200 mg dose. J. Clin. Endocrinol. Metab. 1975;41:373–379. doi: 10.1210/jcem-41-2-373. [DOI] [PubMed] [Google Scholar]
- 49.Shet MS, McPhaul M, Fisher CW, Stallings NR, Estabrook RW. Metabolism of the antiandrogenic drug (flutamide) by human CYP1A2. Drug Metab. Dispos. 1997;25:1298–1303. [PubMed] [Google Scholar]
- 50.Rochat B, Morsman JM, Murray GI, Figg WD, McLeod HL. Human CYP1B1 and anticancer agent metabolism: mechanism for tumor-specific drug inactivation? J. Pharmacol. Exp. Therap. 2001;296:537–541. [PubMed] [Google Scholar]
- 51.Morris JJ, Hughes LR, Glen AT, Taylor PJ. Non-steroidal antiandrogens Design of novel compounds based on an infrared study of the dominant conformation and hydrogen-bonding properties of a series of anilide antiandrogens. J. Med. Chem. 1991;34:447–455. doi: 10.1021/jm00105a067. [DOI] [PubMed] [Google Scholar]
- 52.Desta Z, Ward BA, Soukhova NV, Flockhart DA. Comprehensive evaluation of tamoxifen sewuential biotransformation by the human cytochrome P450 system in vitro: prominent roles for CYP3A4 and CYP2D6. J. Pharmacol. Exp. Therap. 2004;310:1062–1075. doi: 10.1124/jpet.104.065607. [DOI] [PubMed] [Google Scholar]
- 53.Beverage JN, Sissung TM, Sion AM, Danesi R, Figg WD. CYP2D6 polymorphism and the impact on tamoxifen therapy. J. Pharm. Sci. 2007;96:2224–2231. doi: 10.1002/jps.20892. [DOI] [PubMed] [Google Scholar]
- 54.Boger DL, Johnson DS. CC-1065 and the duocarmycins: unraveling the keys to a new class of naturally derived DNA alkylating agents. Proc. Natl Acad. Sci. USA. 1995;92:3642–3649. doi: 10.1073/pnas.92.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pors K, Loadman PM, Shnyder SD, et al. Modification of the duocarmycin pharmacophore enables CYP1A1 targeting for biological activity. Chem. Commun. 2011;47:12062–12064. doi: 10.1039/c1cc15638a. ▪▪ Novel use of a cytochrome P450-catalyzed reaction to produce a highly toxic duocarmycin analogue in situ in an effort to avoid the general toxicity of duocarmycin itself.
- 56. Murray GI, Melvin WT, Greenlee WF, Burke MD. Regulation, function, and tissue-specific expression of cytochrome P450 CYP1B1. Annu. Rev. Pharmacol. 2001;41:297–316. doi: 10.1146/annurev.pharmtox.41.1.297. ▪▪ Review summarizing the properties of CYP1B1, which is selectively localized in tumor tissues and is suitable for targeted activation of prodrugs in tumors.
- 57.Jiang J, Chen C, Card JW, et al. Cytochrome P450 2J2 promotes the neoplastic phenotype of carcinoma cells and is up-regulated in human tumors. Cancer Res. 2005;65:4707–4715. doi: 10.1158/0008-5472.CAN-04-4173. [DOI] [PubMed] [Google Scholar]
- 58.Rieger MA, Ebner R, Bell DR, et al. Identification of a novel mammary-restricted cytochrome P450, CYP4Z1, with overexpression in breast carcinoma. Cancer Res. 2004;64:2357–2364. doi: 10.1158/0008-5472.can-03-0849. [DOI] [PubMed] [Google Scholar]
- 59.Downie D, McFadyen MCE, Rooney PH, et al. Profiling cytochrome P450 expression in ovarian cancer: identification of prognostic markers. Clin. Cancer Res. 2005;11:7369–7375. doi: 10.1158/1078-0432.CCR-05-0466. [DOI] [PubMed] [Google Scholar]
- 60.Saarikosi ST, Wikman HA, Smith G, Wolff CHJ, Husgafvel-Pursiainen K. Localization of cytochrome CYP2S1 expression in human tissues by in situ hybridization and immunohistochemistry. J. Histochem. Cytochem. 2005;53:549–556. doi: 10.1369/jhc.4C6576.2005. [DOI] [PubMed] [Google Scholar]
- 61.Mortimer CG, Wells G, Crochard J-P, et al. Antitumor benzothiazoles. 26. 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW 610, NSC 721648), a simple fluorinated 2-arylbenzo-thiazole, shows potent and selective inhibitory activity against lung, colon, and breast cancer cell lines. J. Med. Chem. 2006;49:179–185. doi: 10.1021/jm050942k. [DOI] [PubMed] [Google Scholar]
- 62.Brantley E, Trapani V, Alley MC, et al. Fluorinated 2-(4-amino-3-methylphenyl) benzothiazoles induce CYP1A1 expression, become metabolized, and bind to macromolecules in sensitive human cancer cell lines. Drug Metab. Dispos. 2004;32:1392–1401. doi: 10.1124/dmd.104.001057. [DOI] [PubMed] [Google Scholar]
- 63. Tan BS, Tiong KH, Muruhadas A, et al. CYP2S1 and CYP2W1 mediate 2-(3,4-dimethoxyphenyl)-5-fluorobenzothiazole (GW-610, NSC 721648) sensitivity in breast and colorectal cancer cells. Mol. Cancer Ther. 2011;10:1982–1992. doi: 10.1158/1535-7163.MCT-11-0391. ▪ Demonstration that two P450 enzymes selectively expressed in tumors are involved in the specific activation and deactivation of experimental anticancer prodrugs.
- 64.Wang K, Guengerich FP. Bioactivation of fluorinated 2-aryl-benzothiazole antitumor molecules by hiuman cytochrome P450s 1A1 and 2W1 and deactivation by cytochrome P450 2S1. Chem. Res. Toxicol. 2012;25:1740–1751. doi: 10.1021/tx3001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rooseboom M, Commandeur JNM, Vermeulen NPE. Enzyme-catalyzed activation of anticancer prodrugs. Pharmacol. Rev. 2004;56:53–102. doi: 10.1124/pr.56.1.3. [DOI] [PubMed] [Google Scholar]
- 66.Patterson LH. Bioreductively activated antitumor N-oxides: the case of AQ4N, a unique approach to hypoxia-activated cancer chemotherapy. Drug Metab. Rev. 2002;34:581–592. doi: 10.1081/dmr-120005659. [DOI] [PubMed] [Google Scholar]
- 67.Lalani AS, Alters SE, Wong A, Albertella MR, Cleland JL, Henner WD. Selective tumor targeting by the hypoxia-activated prodrug AQ4N blocks tumor growth and metastasis in preclinical models of pancreatic cancer. Clin. Cancer. Res. 2007;13:2216–2225. doi: 10.1158/1078-0432.CCR-06-2427. [DOI] [PubMed] [Google Scholar]
- 68.Heimbrook DC, Murray RE, Egeberg KD, Sligar SG, Nee MW, Bruice TC. Demethylation of N,N-dimethylaniline and p-cyano-N,N-dimethylaniline and their N-oxides by cytochromes P450LM2 and P450cam . J. Am. Chem. Soc. 1984;106:1514–1515. [Google Scholar]
- 69.Seto Y, Guengerich FP. Partitioning between N-dealkylation and N-oxygenation in the oxidation of N,N-dialkylarylamines catalyzed by cytochrome P450 2B1. J. Biol. Chem. 1993;268:9986–9997. [PubMed] [Google Scholar]
- 70.Raleigh SM, Wanogho E, Burke MD, McKeown SR, Patterson LH. Involvement of human cytochromes P450 (CYP) in the reductive metabolism of AQ4N, a hypoxia activated anthraquinone di-N-oxide prodrug. Int. J. Radiation Oncol. Biol. Phys. 1998;42:763–767. doi: 10.1016/s0360-3016(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 71.Yakkundi A, McErlane V, Murray M, et al. Tumour selective drug activation: a GDEPT approach utilizing cytochrome P450 1A1 and AQ4N. Cancer Gene Therapy. 2006;13:598–605. doi: 10.1038/sj.cgt.7700933. [DOI] [PubMed] [Google Scholar]
- 72.Nishida CR, Lee M, Ortiz de Montellano PR. Efficient hypoxic activation of the anticancer agent AQ4N by CYP2S1 and CYP2W1. Mol. Pharmacol. 2010;78:497–502. doi: 10.1124/mol.110.065045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Duan J, Jiao H, Kaizerman J, et al. Potent and highly selective hypoxia-activated achiral phosphoroamidate mustards as anticancer drugs. J. Med. Chem. 2008;51:2412–2420. doi: 10.1021/jm701028q. [DOI] [PubMed] [Google Scholar]
- 74.Hadley WM. A Review of the Literature on Enzymatic Reduction of Nitrocompounds. Springfield, VA, USA: Inhalation Toxicology Research Institute, Lovelace Biomedical and Environmental Research Institute; 1983. pp. 1–20. [Google Scholar]
- 75.Spain JC. Biodegradation of nitroaromatic compounds. Annu. Rev. Microbiol. 1995;49:523–555. doi: 10.1146/annurev.mi.49.100195.002515. [DOI] [PubMed] [Google Scholar]
- 76.Patterson AV, Ferry DM, Edmunds SJ, et al. Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA cross-linking agent PR-104. Clin. Cancer Res. 2007;13:3922–3932. doi: 10.1158/1078-0432.CCR-07-0478. [DOI] [PubMed] [Google Scholar]
- 77.Singleton RS, Guise CP, Ferry DM, et al. DNA cross-links in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism, and cytotoxicity. Cancer Res. 2009;69:3884–3891. doi: 10.1158/0008-5472.CAN-08-4023. [DOI] [PubMed] [Google Scholar]
- 78.Guise CP, Abbattista MR, Singleton RS, et al. The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res. 2010;70:1573–1584. doi: 10.1158/0008-5472.CAN-09-3237. [DOI] [PubMed] [Google Scholar]
- 79.Dansette PM, Libraire J, Bertho G, Mansuy D. Metabolic oxidative cleavage of thioesters: evidence for the formation of sulfenic acid intermediates in the bioactivation of the antithrombotic prodrugs ticlopidine and clopidogrel. Chem. Res. Toxicol. 2009;22:369–373. doi: 10.1021/tx8004828. [DOI] [PubMed] [Google Scholar]
- 80.Kazui M, Nishiya Y, Ishizuka T, et al. Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab. Dispos. 2010;38:92–99. doi: 10.1124/dmd.109.029132. [DOI] [PubMed] [Google Scholar]
- 81.Bouman HJ, Schömig E, van Werkum JW, et al. Paraosonase-1 is a major determinant of clopidogrel efficacy. Nat. Med. 2011;17:110–116. doi: 10.1038/nm.2281. [DOI] [PubMed] [Google Scholar]
- 82.Dansette PM, Rosi J, Bertho G, Mansuy D. Paraoxonase-1 and clopidogrel efficacy. Nat. Med. 2011;17(9):1040–1041. doi: 10.1038/nm.2436. [DOI] [PubMed] [Google Scholar]
- 83. Dansette PM, Rosi J, Bertho G, Mansuy D. Cytochromes P450 catalyze both steps of the major pathway of clopidogrel bioactivation, whereas paraoxonase catalyzes the formation of a minor thiol metabolite isomer. Chem. Res. Tox. 2012;25:348–356. doi: 10.1021/tx2004085. ▪▪ Latest of a series of papers analyzing the mechanism of activation of clopidogrel in which a distinction is made between a P450-catalyzed or thioesterase-catalyzed second activation step.
- 84.Bouman HJ, Schömig E, van Werkum JW, et al. Reply to: ‘Paraoxonase-1 and clopidogrel efficacy’. Nat. Med. 2011;17(9):1042–1044. doi: 10.1038/nm.2367. [DOI] [PubMed] [Google Scholar]
- 85.Dansette PM, Rosi J, Devernardi J, Bertho G, Mansuy D. Metabolic activation of prasugrel: nature of the two competitive pathways resulting in the opening of its thiophene ring. Chem. Res. Toxicol. 2012;25:1058–1065. doi: 10.1021/tx3000279. [DOI] [PubMed] [Google Scholar]
- 86.Hagihara K, Kazui M, Kurihara A, et al. Biotransformation of prasugrel, a novel thienopyridine antiplatelet agent, to the pharmacologically active metabolite. Drug Metab. Dispos. 2010;38:898–904. doi: 10.1124/dmd.110.032086. [DOI] [PubMed] [Google Scholar]
- 87.Dansette PM, Thébault S, Bertho G, Mansuy D. Formation and fate of a sulfenic acid intermediate in metabolic activation of the antithrombotic prodrug prasugrel. Chem. Res. Toxicol. 2010;23:1268–1274. doi: 10.1021/tx1001332. [DOI] [PubMed] [Google Scholar]
- 88.Shan J, Zhang B, Zhu Y, et al. Overcoming clopidogrel resistance: discovery of vicagrel as a highly potent and orally bioavailable antiplatelet agent. J. Med. Chem. 2012;55:3342–3352. doi: 10.1021/jm300038c. [DOI] [PubMed] [Google Scholar]
- 89.Hedner T, Samulesson O, Währborg P, Wadenvik H, Ung KA, Ekbom A. Nabumetone: therapeutic use and safety profile in the management of osteoarthritis and rheumatoid arthritis. Drugs. 2004;64:2315–2343. doi: 10.2165/00003495-200464200-00004. [DOI] [PubMed] [Google Scholar]
- 90.Haddock RE, Jeffery DJ, Lloyd JA, Thawley AR. Metabolism of nabumetone (BRL 14777) by various species including man. Xenobiotica. 1984;14:327–337. doi: 10.3109/00498258409151419. [DOI] [PubMed] [Google Scholar]
- 91.Turpeinen M, Hofmann U, Klein K, Mürder T, Schwab M, Zanger UM. A predominate role of CYP1A2 for the metabolism of nabumetone to the active metabolite, 6-methoxy-2-naphthylacetic acid, in human liver microsomes. Drug Metab. Dispos. 2009;37:1017–1024. doi: 10.1124/dmd.108.025700. [DOI] [PubMed] [Google Scholar]
- 92.Wang MZ, Saulter JY, Usuki E, et al. CYP4F enzymes are the major enzymes in human liver microsomes that catalyze the O-demethylation of the antiparasitic prodrug DB289 [2,5-bis(4-amidinophenyl)furan-bis-O-methylamidoxime] Drug Metab. Dispos. 2006;34:1985–1994. doi: 10.1124/dmd.106.010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang MZ, Wu JQ, Bridges AS, et al. Human enteric microsomal CYP4F enzymes O-demethylate the antiparasitic prodrug pafuramidine. Drug Metab. Dispos. 2007;35:2067–2075. doi: 10.1124/dmd.107.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saulter JY, Kurian JR, Trepanier LA, et al. Unusual dehydroxylation of antimicrobial amidoxime prodrugs by cytochrome b5 and NADH cytochrome b5 reductase. Drug Metab. Dispos. 2005;33:1886–1893. doi: 10.1124/dmd.105.005017. [DOI] [PubMed] [Google Scholar]
- 95.Haria M, Fitton A, Peters DH. Loratadine. A reappraisal of its pharmacological properties and therapeutic use in allergic disorders. Drugs. 1994;48:617–637. doi: 10.2165/00003495-199448040-00009. [DOI] [PubMed] [Google Scholar]
- 96.Yumibe N, Huie K, Chen K, Snow M, Clement RP, Cayen MN. Identification of human liver cytochrome P450 enzymes that metabolize the nonsedating antihistamine loratadine. Formation of descarboethoxyloratadine by CYP3A4 and CYP2D6. Biochem. Pharmacol. 1996;51:165–172. doi: 10.1016/0006-2952(95)02169-8. [DOI] [PubMed] [Google Scholar]
- 97.Berthon B, Taudou G, Combettes L, et al. In vitro inhibition, by loratadine and descarboxyethoxyloratadine, of histamine release from human basophils, and of histamine release and intracellular calcium fluxes in rat basophilic leukemia cells (RBL-2H3) Biochem. Pharmacol. 1994;47:789–794. doi: 10.1016/0006-2952(94)90478-2. [DOI] [PubMed] [Google Scholar]
- 98.Renwick AG. The metabolism of antihistamines and drug interactions: the role of cytochrome P450 enzymes. Clin. Exper. Allergy. 1999;29(Suppl. 3):116–124. doi: 10.1046/j.1365-2222.1999.0290s3116.x. [DOI] [PubMed] [Google Scholar]
- 99.Timmermans PB, Carini JD, Chiu AT, et al. The discovery of a new class of highly specific nonpeptide angiotensin II receptor antagonists. Am. J. Hypertens. 1991;4:275S–281S. doi: 10.1093/ajh/4.4.275s. [DOI] [PubMed] [Google Scholar]
- 100.Wong PC, Price WA, Jr., Chiu AT, et al. Nonpeptide angiotensin II receptor antagonists. XI. Pharmacology of EXP3174: an active metabolite of DuP 753, an orally active antihypertensive agent. J. Pharmacol. Exp. Therap. 1990;255:211–217. [PubMed] [Google Scholar]
- 101.Stearns RA, Miller RR, Doss GA, et al. The metabolism of DuP 753, a nonpeptide angiotensin II receptor antagonist, by rat, monkey, and human liver slices. Drug Metab. Dispos. 1992;20:281–287. [PubMed] [Google Scholar]
- 102.Stearns RA, Chakravarty PK, Chen R, Chiu SH. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab. Dispos. 1995;23:207–215. [PubMed] [Google Scholar]
- 103.Iwamura A, Fukami T, Hosomi H, Nakaajima M, Yokoi T. CYP2C9-mediated metabolic activation of losartan detected by a highly sensitive cell-based screening assay. Drug. Metab. Dispos. 2011;39:838–846. doi: 10.1124/dmd.110.037259. [DOI] [PubMed] [Google Scholar]
- 104. Reddy KR, Matelich MC, Ugarkar BG, et al. Pradefovir: a prodrug that targets adefovir to the liver for the treatment of hepatitis B. J. Med. Chem. 2008;51:666–676. doi: 10.1021/jm7012216. ▪▪ Development of a P450-activated prodrug for a liver disease that is activated by P450 enzymes that are in highest concentration in the liver.
- 105.Lin C, Fang C, Benetton S, Xu G, Yeh L. Metabolic activation of pradefovir by CYP3A4 and its potential as an inhibitor or inducer. Antimicrob. Agents Chemother. 2006;50:2926–2931. doi: 10.1128/AAC.01566-05. [DOI] [PMC free article] [PubMed] [Google Scholar]