Abstract
This review highlights the role of atrial monophasic action potential duration (APD) in understanding atrial electrical properties in paroxysmal, persistent, and permanent atrial fibrillation (AF) states. Alternans of APD and rate maladaptation in a spatially divergent way appear mechanistically involved in AF initiation, development, and persistence. The underlying pathophysiology warrants further investigation.
Keywords: Atrial fibrillation, Electrical remodelling, Monophasic action potential (MAP), Action potential duration (APD), Electrical restitution curve (ERC), Rate maladaptation, Action potential duration (APD) alternans, Alternans, Electrical heterogeneity
Introduction
Atrial fibrillation (AF) currently affects 1–2% of the population with an expectation to increase over the next several decades.1 Atrial fibrillation serves as an adverse prognostic marker and is associated with, among other things, increased rates of death, stroke, and heart failure events.1 Both the prevalence of AF and the number of strokes attributable to AF increases in each decade of life.2,3 It is predicted that the prevalence of AF-related hospitalizations in the USA will exceed 3.8 and 5.6 million by 2025 and 2050, respectively.4
Several mechanisms of AF have been described. The prevailing concept has focused on interplay between triggers that induce and substrate that maintain arrhythmia.5 Pulmonary vein triggers are currently thought to be integral in the development of AF. Studies suggest that AF can be initiated by pulmonary vein focal impulses that propagate to atrial tissue, and that radiofrequency ablation of these foci can terminate AF.6 This review focuses on the role of action potential duration (APD) alternans as a ubiquitous preamble to AF initiation.
The role of monophasic action potential recording in studying the substrate of atrial fibrillation
Electrical remodelling of the atria and other structural changes are recognized as substrates essential for persistence of AF. Recording of monophasic action potentials (MAPs) by the ‘Franz’ contact electrode catheter have facilitated the investigation of both normal properties and pathological states of the atria, including AF. Recently, investigation of atrial MAP recordings in human AF resulted in a theory positing APD alternans as a dynamic substrate for AF.7 Moreover, APD alternans was noted to precede all AF episodes and was absent when AF did not initiate. To appreciate the role of the human atrial action potential in the initiation of AF, it is appropriate to review the MAP and how its relationship with AF continues to evolve.
Monophasic action potential recording
Localized myocardial depolarization and repolarization in the human heart in situ can only be investigated by recording MAPs.8,9 Under optimal conditions, MAPs record the repolarization time course of transmembrane action potentials (TAPs) with high fidelity.10 Monophasic action potentials can be recorded from contact with cardiac surface in contrast to TAPs, which require tedious in vitro preparations. As the MAP electrode diameter is prohibitively large from entering a singe cardiac cell, the probable origination of the MAP is from the electrical gradient between the myocardium subjacent and adjacent to the catheter tip.10,11
Action potential duration
Precise measurement of total MAP duration is difficult because of the asymptotic end of repolarization. As a result, the MAP duration is usually determined at a repolarization level of 90% (or another fraction) with respect to the MAP amplitude. The MAP amplitude is defined as the distance from the baseline to the crest of the MAP plateau voltage, not its upstroke peak.10,12 The intersection between the diastolic baseline and a tangent placed on phase 3 of the action potential may also determine MAP duration, but typically yields more arbitrary results.
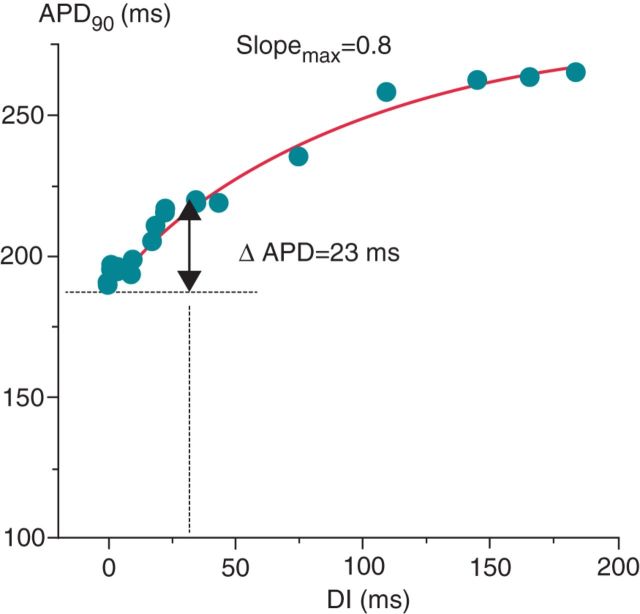
In normal states, APD is dependently related to changes in heart rate, for which physiological advantages exist. A longer cycle length (CL) causes a requisite APD lengthening, providing a longer period of excitation–contraction coupling. Conversely, a shorter CL causes APD shortening and maintenance of a sufficient diastolic interval (DI), preserving coronary flow and ventricular filling.13 This APD–CL relationship can be expressed by the electrical restitution curve (ERC), which describes APD recovery time course as a function of the DI or CL from the most premature beat to long intervals following a steady-state APD.14–17 An ERC is obtained by pacing at a constant S1–S1 interval, followed by an S2, a single premature stimulus. S1–S2 coupling interval is progressively shortened until block of the premature pulse, or the effective refractory period. Figure 1 exhibits an example of an ERC, a graphical relationship between APD 90% (or another fraction) to its preceding DI or CL.17–19 A modified technique for describing the APD–CL relationship, referred to as the ‘dynamic’ restitution curve, exhibits the relationship between APD and different steady CLs determined during the early phases of rate adaptation.15,17
Figure 1.
Electrical restitution curve by S1–S2. The decrease in APD90 with increasing prematurity resulted in an electrical restitution curve slope of 0.8. Adapted with permission from Kim et al.19
Substrate for arrhythmia
It is possible that many mechanisms combine to initiate arrhythmia.20 Cycle length-dependent changes in APD are suspected to participate in arrhythmogenesis and, in particular, fibrillation.15–17 As ERCs describe, progressive shortening of CL and DI accentuate changes in APD. Increasing APD ‘alternans’ leads to larger wavelength oscillations and points of wavebreak resulting in temporal heterogeneity and reentry. The mechanisms by which APD alternans may contribute to electrical instability have been described utilizing computer and numeric simulations.20–22 The demonstration of APD alternans at high stimulus rates provides mechanistic support for this concept.23–26 Action potential duration alternans has also been linked to ventricular fibrillation (VF) vulnerability.27–29 Notably, there is now compelling evidence that APD alternans may lead directly to AF and its transition from atrial flutter (Afl) in animal models,30,31 in numerical simulations32 and directly in humans.7,33–35
Pictorial assessment of APD alternans is typically performed spectrally. Utilizing validated software, APs of a contiguous selection are each denoted by beat number and time sample. A fast-Fourier transform is used to compute power spectra across beats for each time sample, and then summation of spectra across the AP is obtained.36 As shown in Figure 2, the magnitude of APD alternans is represented by the dimensionless k score utilizing the following equation: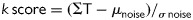 , where ΣT is the spectral magnitude at 0.5 cycles/beat, and μnoise and σnoise are the mean and standard deviation of noise. A k > 0 indicates that alternans exceeds noise.
, where ΣT is the spectral magnitude at 0.5 cycles/beat, and μnoise and σnoise are the mean and standard deviation of noise. A k > 0 indicates that alternans exceeds noise.
Figure 2.
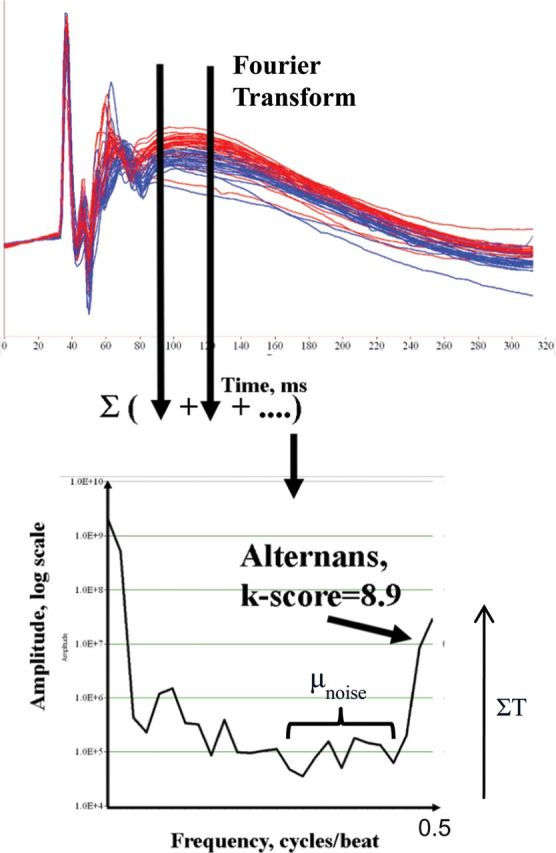
(Top) Action potential amplitude shows marked alternans, shown by the separation of even (blue line) and odd (red line) beats. (Bottom) Action potential amplitude alternans (k score 8.9, as peak magnitude at 0.5 cycles/beat.
It is postulated that the degree of APD alternans, which is related to the slope of the ERC, estimates wavebreak and the substrate for reentry. A steeper ERC slope represents a relatively small change in DI or CL yet renders large changes in APD and refractoriness. The ‘restitution hypothesis’ suggests that when the ERC slope > 1, augmented APD ‘alternans’ results in larger wavelength oscillations and points of wavebreak. Alternatively, when ERC slope < 1, oscillations progressively decrease and ultimately achieve a new steady state.16,27,35,37 In addition, studies have shown that drugs that flatten the restitution curve decrease the chance of VF induction and stabilize VF to ventricular tachycardia.38–40 These findings make this hypothesis quite an attractive explanation for the correlation between APD alternans and arrhythmogenesis, and suggest that a ‘flatter than normal’ ERC may be antiarrhythmic.
This raises the question, however, as to whether the steep early phase of the ERC is indeed hazardous and a predictor of VF (or AF). In normal myocardium, APD alternans in this early ERC phase dampens out over a few beats as a rapidly adapting change in APD follows a sudden change in rate.14 This physiological APD rate adaptation leads to shortening of the APD as it adapts to the new steady-state CL, which allows the DI to get longer and the APD to move quickly onto the flat portion of the ERC and away from the vulnerable window and threshold for greater alternans.16 The steep initial slope of the ERC may, in fact, be protective as it facilitates APD ‘movement’ to a flatter, safer portion of the ERC. Flattening of the ERC by medications will indeed flatten the slope and essentially extinguish APD alternans. This is, however, not physiological and may have other adverse effects on cardiac performance. Of note, myocardial ischaemia also flattens the ERC slope, which obviously is not an antiarrhythmic scenario.41
Atrial electrophysiological properties and atrial fibrillation
The normal atrial AP is triangular with a relatively short APD compared with the ventricular myocardial tissue.42,43 Depolarizing currents, both inactivating Na+ current (INa) and the L-type Ca2+ current (ICaL), are the same as in ventricular myocytes. Atrial myocytes, however, express certain ion channels and channel subunits nearly absent on ventricular myocytes, including ultrarapid delayed rectifier current (IKur), which initiates atrial repolarization faster than the ventricular myocyte predominant rapid delayed rectifier current (IKr).44 The transient outward K+ current (ITO), which greatly contributes to early rapid phase 1 repolarization, is more heavily distributed on normal atrial than ventricular myocytes and likely facilitates a shorter atrial APD.45 In addition, Ca2+-activated potassium channel (IKCa) was discovered to have greater effect and density in the atria compared with the ventricle.46 These and other atrial electrophysiological features afford complex conduction patterns at rapid atrial rates.9 Further, the atrial Ik-ATP channel is of great importance, but is beyond the scope of this review.
Atrial electrophysiological remodelling as a consequence of AF was significantly advanced by work conducted in animal models.47,48 Further studies also suggested that AF induced atrial APD shortening and action potential plateau phase depression in animals49,50 as well as humans.51–53 Alterations in ion channels have been proposed to contribute to these electrophysiological observations.54 AF induces abnormal cellular calcium handling that may promote compensatory downregulation of inward L-type Ca2+ current (ICaL). 53,55,56 It is theorized that this reduction in ICaL activity mitigates the plateau phase and largely explains the accentuated shortening of atrial APD.45,57 Transient outward K+ current (ITO) is also reduced in chronic AF, 43,45,58,59 which seems puzzling but may help explain additional effects of AF on APD. Chronic AF also exhibits an increase in inward rectifying K+ current IK1,59,60 which primarily controls the resting potential of the atrial myocyte.61 In sum, a complex interplay of currents in atrial AF myocytes renders a shorter APD and mitigation of the plateau phase.
While the time course of the changes in atrial refractoriness may well be explained, at least in large part, by ionic changes, it typically occurs before the development of persistent AF.47,49 This suggests that factors other than atrial electrophysiological changes contribute to the development of AF.5,62 This ‘second factor’ hypothesis includes several potential contributors, including atrial dilation, stretch, and connexin and gap junction remodelling.5,63–65
An interesting phenomenon, first noted in goats by Wijffels et al.,47 was an alteration to physiological rate adaptation. As expected, normal goats in sinus rhythm exhibited atrial APD shortening at shorter pacing intervals. Goats artificially maintained in AF over 24–48 h, however, lost physiological adaptation and exhibited either constant or shorter APD at slower heart rates. Further, normal heart rate adaptation was restored within a few days after cardioversion to sinus rhythm. A later study by VanDerVelden et al.66 added that increasing pacing rates rendered longer APD in a post-cardioversion chronic AF goat model. This constitutes an AF-induced, paradoxical rate adaptation of the atrial APD to changes in heart rate. This maladaptation of the atrial APD had also been observed in prior human investigation,67,68 as well as animal49,69–71 models. More, recently, the studies by Narayan et al.7,34 also found this to be present in human atria.
What is the mechanism?
Ionic mechanisms for this rate-maladaptive response have been proposed. As noted earlier, ITO is heavily distributed in normal atrial myocytes but is significantly reduced in atrial myocytes affected by longer periods of AF. This decrease in ITO lengthens early rapid repolarization and may influence the rate maladaptive response.54 Downregulation of the αlc subunit of the L-type Ca2+ channel66 and general cytosolic Ca2+ overload72 may contribute while ryanodine may be protective,50 suggesting that abnormal intracellular Ca2+ handling and Ca2+ overload may be complicit in the maladaptive rate response.
Narayan et al.34 documented atrial APD alternans in humans during progressive disorganized alteration of Afl to AF, and noted that the presence of rate maladaptation leads to APD alternans preceding all such transitions. Conversely, much less APD alternans was seen in patients in whom AF did not develop. A critical observation, suggested in other investigations, is of spatial heterogeneity within the atria,73,74 and electrical heterogeneity of rate maladaptation in Afl and AF contributing to AF development and stability.50,69,75 Compared with other atrial sites, the isthmus represented an area in which faster atrial rates resulted in less robust APD shortening, a hallmark of maladaptation. In these maladaptive areas, pacing stimuli approached a partially or fully refractory myocardium more often than in ‘normal’ myocardium. The immediately following APD and DI then underwent compulsory lengthening, facilitating APD alternans in the isthmus. Similar observations subsequently were made in the left human atrium, near the pulmonary veins.35
It was also shown that continued incremental pacing enhanced APD alternans until beat-to-beat conduction was unable to be maintained physiologically, leading to 2 : 1 conduction block, which was also noted in prior investigation.19 Narayan et al. posited that, as a result of a maladaptive APD rate response, spatially heterogeneous APD alternans at increasing heart rates leads to wavefront fractionation and wavebreak, conduction block, and the eventual transition to AF.34
Narayan et al.7 further evaluated the concept of heterogeneity of APD maladaptation and alternans during initiation of AF in the electrophysiology laboratory in patients with and without clinical AF. As noted above, APD alternans is largely reported at fast heart rates and may conceptually be explained by restitution. His group hypothesized that AF-induced atrial remodelling may cause APD alternans at slower heart rates. Using an incremental pacing technique, the group studied left and right atrial APD90, APD restitution curve, APD alternans, and non-alternating complex APD oscillations on transitions to AF in 12 patients with persistent AF, 13 patients with paroxysmal AF, and 8 control patients without clinical AF.
The development of APD alternans was elicited at near-resting rates in patients with persistent AF, at intermediate rates in those with paroxysmal AF, and only at very rapid rates just prior to AF in controls. Moreover, the amplitude of the APD alternans also varied by group, and was greatest in those with permanent AF, intermediate in those with paroxysmal AF, and smallest just before AF induction in controls. A representative patient with persistent AF is shown in Figure 3, in whom marked left atrial APD alternans was seen at CL 600 ms (100 b.p.m.) and CL 500 ms (120 b.p.m., illustrated). At faster rates (shorter CLs), APD alternans disorganized to complex APD oscillation immediately preceding AF transition. In stark contrast, Figure 4 illustrates that APD alternans in control subjects did not arise until very fast rates (CL 200–250 ms), just prior to AF onset. No control subject had APD alternans at CL ≥250 ms and, when exhibited, alternans had very small magnitude. Moreover, the APD rate–response relationship and APD alternans directly indicated AF ‘substrates’, presumably caused by electrical remodelling. The CL onset of APD alternans and CL range varied among the three groups. As delineated in Figure 5, persistent and paroxysmal AF subjects exhibited APD alternans at all rates while controls only exhibited APD alternans around CL 250 ms.
Figure 3.
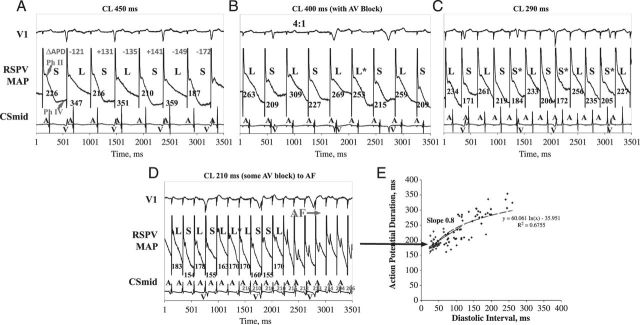
Action potential duration alternans at slow rates in persistent atrial fibrillation, disorganizing to complex oscillations just preceding atrial fibrillation. This patient showed substantial action potential duration alternans at 500 ms, (A) cycle length 450 ms (mean action potential duration 120 ms for 10 beats), (B) cycle length 400 ms during intermittent atrioventricular block, with a phase reversal (*), (C) Cycle length 290 ms with multiple phase reversals, and (D) cycle length 260 ms, with complex oscillations then atrial fibrillation. (E) Electrical restitution curve with maximum electrical restitution curve slope. Adapted with permission from Narayan et al.7
Figure 4.

Control subject with action potential duration alternans only at very fast rates preceding atrial fibrillation. This patient showed no action potential duration alternans at (A) cycle length 500 ms to (B) cycle length 240 ms, (C) action potential duration alternans appeared at cycle length 210 ms during 1 : 1 and 2 : 1 avtioventricular conduction, (D) at cycle length 180 ms, action potential duration alternans amplified to 6.5% of action potential duration immediately preceding AF initiation. Electrical restitution curve slope > 1 at the fast rates of action potential duration alternans onset. Adapted with permission from Narayan et al.7
Figure 5.
Action potential duration oscillation and cycle length. Atrial fibrillation patients show marked action potential duration oscillations (alternans at slow rates transitioning to complex oscillations at faster rate) whereas controls show action potential duration alternans only at very short cycle length. Adapted with permission from Narayan et al.7
The suggestive role of APD alternans and heart rate was also evaluated. The ‘restitution hypothesis’, described earlier, is the concept that an ERC slope > 1 indicates greater APD alternans at a faster rate and will facilitate wavebreak and arrhythmia development. This theory was supported by the initiation of APD alternans in some control subjects as well as a few paroxysmal AF subjects, in whom spontaneous premature atrial complexes triggered AF by increasing the ERC slope.
Groups with paroxysmal and permanent AF did not exhibit similar restitution kinetics. The ERC slope in all paroxysmal and persistent AF subjects was <1 at the onset of APD alternans, even if maximum slope was >1 at faster rates. The observation that persistent AF leads to APD alternans at slower heart rates with ERC slopes < 1 prompts inquiry into the mechanistic explanation of this phenomenon. As noted earlier, AF is thought to affect several aspects of atrial myocyte character and function, including abnormal intracellular Ca2+ handling, membrane ion current remodelling changes, and atrial conduction slowing. These and other mechanisms could potentially contribute to the observations of this study and require additional investigation.
Corroborating prior investigation,34 atrial APD alternans was noted to precede every AF transition, suggesting that APD alternans may be mechanistically complicit in transitions to AF. Accordingly, in addition to spontaneous premature atrial complexes, APD alternans amplification or complex oscillation preceding AF transition in fast rates may represent multiplying periods in non-linear systems, another suspected trigger for AF. Proposed mechanisms to elucidate this phenomenon are somewhat nebulous at this time and require further investigation.
Conclusion
Atrial fibrillation is common in our ageing population and is recognized as an adverse prognostic marker. The prevailing concept of AF mechanism focuses on interdependence of triggers and substrate, and MAP recordings illustrate differences between normal myocardial properties and AF substrate. While prior investigation suggests an arrhythmogenic nature of APD alternans in the ventricle, work highlighted in this review supports this concept in the atria and reports the role of APD alternans as a vital precursor to AF development. This idea, along with support from corroborating efforts, will likely alter our current notion of AF development, uncover new areas for exploration, and hopefully advance the formulation of therapeutic options.
Conflict of interest: S.M.N. reports being co-inventor on intellectual property owned by the University of California and licensed to Topera Medical, Inc. S.M.N. holds equity in Topera. Topera has not sponsored any research, including that presented here. S.M.N. also reports having received honoraria from Medtronic, St. Jude Medical and Biotronik Corporations and grant support from Biosense-Webster. All other authors have declared no conflict of interest.
References
- 1.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12:1360–420. doi: 10.1093/europace/euq350. doi:10.1093/eurheartj/ehq278. [DOI] [PubMed] [Google Scholar]
- 2.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–8. doi: 10.1161/01.str.22.8.983. doi:10.1161/01.STR.22.8.983. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, et al. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the Anticoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. JAMA. 2001;285:2370–5. doi: 10.1001/jama.285.18.2370. doi:10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999: implications for primary prevention. Circulation. 2003;108:711–6. doi: 10.1161/01.CIR.0000083722.42033.0A. doi:10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 5.Allessie MA, Boyden PA, Camm AJ, Kleber AG, Lab MJ, Legato MJ, et al. Pathophysiology and prevention of atrial fibrillation. Circulation. 2001;103:769–77. doi: 10.1161/01.cir.103.5.769. doi:10.1161/01.CIR.103.5.769. [DOI] [PubMed] [Google Scholar]
- 6.Haissaguerre M, Jais P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. doi: 10.1056/NEJM199809033391003. doi:10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 7.Narayan SM, Franz MR, Clopton P, Pruvot EJ, Krummen DE. Repolarization alternans reveals vulnerability to human atrial fibrillation. Circulation. 2011;123:2922–30. doi: 10.1161/CIRCULATIONAHA.110.977827. doi:10.1161/CIRCULATIONAHA.110.977827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franz MR, Burkhoff D, Spurgeon H, et al. In vitro validation of a new cardiac catheter technique for recording monophasic action potentials. Eur Heart J. 1986;7:34–41. doi: 10.1093/oxfordjournals.eurheartj.a061954. [DOI] [PubMed] [Google Scholar]
- 9.Moore HJ, Franz MR. Monophasic action potential recordings in humans. J Cardiovasc Electrophysiol. 2007;18:787–90. doi: 10.1111/j.1540-8167.2006.00756.x. doi:10.1111/j.1540-8167.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 10.Franz MR. Current status of monophasic action potential recording: theories, measurements and interpretations. Cardiovasc Res. 1999;41:25–40. doi: 10.1016/s0008-6363(98)00268-5. doi:10.1016/S0008-6363(98)00268-5. [DOI] [PubMed] [Google Scholar]
- 11.Knollmann BC, Tranquillo J, Sirenko SG, Henriquez C, Franz MR. Microelectrode study of the genesis of the monophasic action potential by contact electrode technique. J Cardiovasc Electrophysiol. 2002;13:1246–52. doi: 10.1046/j.1540-8167.2002.01246.x. doi:10.1046/j.1540-8167.2002.01246.x. [DOI] [PubMed] [Google Scholar]
- 12.Franz MR. Long-term recording of monophasic action potentials from human endocardium. Am J Cardiol. 1983;51:1629–34. doi: 10.1016/0002-9149(83)90199-6. doi:10.1016/0002-9149(83)90199-6. [DOI] [PubMed] [Google Scholar]
- 13.Berger RD. Electrical restitution hysteresis: good memory or delayed response. Circ Res. 2004;94:567–9. doi: 10.1161/01.RES.0000124605.03595.E4. doi:10.1161/01.RES.0000124605.03595.E4. [DOI] [PubMed] [Google Scholar]
- 14.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988;82:972–9. doi: 10.1172/JCI113706. doi:10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knollmann BC, Schober T, Petersen AO, Sirenko SG, Franz MR. Action potential characterization in intact mouse heart: steady-state cycle length dependence and electrical restitution. Am J Physiol Heart Circ Physiol. 2007;292:H614–21. doi: 10.1152/ajpheart.01085.2005. doi:10.1152/ajpheart.01085.2005. [DOI] [PubMed] [Google Scholar]
- 16.Franz MR. The electrical restitution curve revisited: steep or flat slope—which is better? J Cardiovas Electrophysiol. 2003;14:S140–7. doi: 10.1046/j.1540.8167.90303.x. doi:10.1046/j.1540.8167.90303.x. [DOI] [PubMed] [Google Scholar]
- 17.Koller ML, Riccio ML, Gilmour RF., Jr Dynamic restitution of action potential duration during electrical alternans and ventricular fibrillation. Am J Physiol Heart Circ Physiol. 1998;54:840–50. doi: 10.1152/ajpheart.1998.275.5.H1635. [DOI] [PubMed] [Google Scholar]
- 18.Taggart P, Lab M. Cardiac mechano-electric feedback and electrical restitution in humans. Prog Biophys Mol Biol. 2008;97:452–60. doi: 10.1016/j.pbiomolbio.2008.02.021. doi:10.1016/j.pbiomolbio.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 19.Kim BS, Kim YH, Hwang GS, Pak HN, Lee SC, Shim WJ, et al. Action potential duration restitution kinetics in human atrial fibrillation. J Am Coll Cardiol. 2002;39:1329–36. doi: 10.1016/s0735-1097(02)01760-6. doi:10.1016/S0735-1097(02)01760-6. [DOI] [PubMed] [Google Scholar]
- 20.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. doi:10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 21.Qu Z, Garfinkel A, Chen PS, Weiss JN. Mechanisms of discordant alternans and induction of reentry in simulated cardiac tissue. Circulation. 2000;102:1664–70. doi: 10.1161/01.cir.102.14.1664. doi:10.1161/01.CIR.102.14.1664. [DOI] [PubMed] [Google Scholar]
- 22.Watanabe MA, Fenton FH, Evans SJ, Hastings HM, Karma A. Mechanisms for discordant alternans. J Cardiovasc Electrophysiol. 2001;12:196–206. doi: 10.1046/j.1540-8167.2001.00196.x. doi:10.1046/j.1540-8167.2001.00196.x. [DOI] [PubMed] [Google Scholar]
- 23.Pastore JM, Girouard SD, Laurita KR, Akar FG, Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–94. doi: 10.1161/01.cir.99.10.1385. doi:10.1161/01.CIR.99.10.1385. [DOI] [PubMed] [Google Scholar]
- 24.Cao JM, Qu Z, Kim YH, Wu TJ, Garfinkel A, Weiss JN, et al. Spatiotemporal heterogeneity in the induction of ventricular fibrillation by rapid pacing: importance of cardiac restitution properties. Circ Res. 1999;84:1318–31. doi: 10.1161/01.res.84.11.1318. doi:10.1161/01.RES.84.11.1318. [DOI] [PubMed] [Google Scholar]
- 25.Walker ML, Rosenbaum DS. Cellular alternans as mechanism of cardiac arrhythmogenesis. Heart Rhythm. 2005;2:1383–6. doi: 10.1016/j.hrthm.2005.09.009. doi:10.1016/j.hrthm.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Hayashi H, Shiferaw Y, Sato D, Nihei M, Lin SF, Chen PS, et al. Dynamic origin of spatially discordant alternans in cardiac tissue. Biophys J. 2007;92:448–60. doi: 10.1529/biophysj.106.091009. doi:10.1529/biophysj.106.091009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weiss JN, Qu Z, Chen PS, Lin SF, Karagueuzian HS, Hayashi H, et al. The dynamics of cardiac fibrillation. Circulation. 2005;112:1232–40. doi: 10.1161/CIRCULATIONAHA.104.529545. doi:10.1161/CIRCULATIONAHA.104.529545. [DOI] [PubMed] [Google Scholar]
- 28.Ten Tusscher KH, Panfilov AV. Alternans and spiral breakup in a human ventricular tissue model. Am J Physiol Heart Circ Physiol. 2006;291:H1088–100. doi: 10.1152/ajpheart.00109.2006. doi:10.1152/ajpheart.00109.2006. [DOI] [PubMed] [Google Scholar]
- 29.Keldermann RH, ten Tusscher KH, Nash MP, Hren R, Taggart P, Panfilov AV. Effect of heterogeneous APD restitution on VF organization in a model of the human ventricles. Am J Physiol Heart Circ Physiol. 2008;294:H764–74. doi: 10.1152/ajpheart.00906.2007. doi:10.1152/ajpheart.00906.2007. [DOI] [PubMed] [Google Scholar]
- 30.Pruvot E, Jousset F, Ruchat P, Vesin JM, Prudat Y, Zerm T, et al. Propagation velocity kinetics and repolarization alternans in a free-behaving sheep model of pacing-induced atrial fibrillation. Europace. 2007;9:83–8. doi: 10.1093/europace/eum211. doi:10.1093/europace/eul179. [DOI] [PubMed] [Google Scholar]
- 31.Lu Z, Cui B, He B, Hu X, Wu W, Wu L, et al. Distinct restitution properties in vagally mediated atrial fibrillation and six-hour rapid pacing-induced atrial fibrillation. Cardiovasc Res. 2011;89:834–42. doi: 10.1093/cvr/cvq334. doi:10.1093/cvr/cvq334. [DOI] [PubMed] [Google Scholar]
- 32.Gong Y, Xie F, Stein KM, Garfinkel A, Culianu CA, Lerman BB, et al. Mechanism underlying initiation of paroxysmal atrial flutter/atrial fibrillation by ectopic foci: a simulation study. Circulation. 2007;115:2094–102. doi: 10.1161/CIRCULATIONAHA.106.656504. doi:10.1161/CIRCULATIONAHA.106.656504. [DOI] [PubMed] [Google Scholar]
- 33.Hiromoto K, Shimizu H, Furukawa Y, Kanemori T, Mine T, Masuyama T, et al. Discordant repolarization alternans induced atrial fibrillation is suppressed by verapamil. Circ J. 2005;69:1368–73. doi: 10.1253/circj.69.1368. doi:10.1253/circj.69.1368. [DOI] [PubMed] [Google Scholar]
- 34.Narayan SM, Bode F, Karasik PL, Franz MR. Alternans of atrial action potentials during atrial flutter as a precursor to atrial fibrillation. Circulation. 2002;106:1968–73. doi: 10.1161/01.cir.0000037062.35762.b4. doi:10.1161/01.CIR.0000037062.35762.B4. [DOI] [PubMed] [Google Scholar]
- 35.Narayan SM, Kazi D, Krummen DE, Rappel WJ. Repolarization and activation restitution near human pulmonary veins and atrial fibrillation initiation: a mechanism for the initiation of atrial fibrillation by premature beats. J Am Coll Cardiol. 2008;52:1222–30. doi: 10.1016/j.jacc.2008.07.012. doi:10.1016/j.jacc.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan SM, Bayer JD, Lalani G, Trayanova NA. Action potential dynamics explain arrhythmic vulnerability in human heart failure a clinical and modeling study implicating abnormal calcium handling. J Am Coll Cardiol. 2008;52:1782–92. doi: 10.1016/j.jacc.2008.08.037. doi:10.1016/j.jacc.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gilmour RF, Jr, Chialvo DR. Electrical restitution, critical mass, and the riddle of fibrillation. J Cardiovasc Electrophysiol. 1999;10:1087–9. doi: 10.1111/j.1540-8167.1999.tb00281.x. doi:10.1111/j.1540-8167.1999.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 38.Riccio ML, Koller ML, Gilmour RF., Jr Electrical restitution and spatiotemporal organization during ventricular fibrillation. Circ Res. 1999;84:955–63. doi: 10.1161/01.res.84.8.955. doi:10.1161/01.RES.84.8.955. [DOI] [PubMed] [Google Scholar]
- 39.Omichi C, Zhou S, Lee MH, Naik A, Chang CM, Garfinkel A, et al. Effects of amiodarone on wave front dynamics during ventricular fibrillation in isolated swine right ventricle. Am J Physiol Heart Circ Physiol. 2002;282:1063–70. doi: 10.1152/ajpheart.00633.2001. [DOI] [PubMed] [Google Scholar]
- 40.Garfinkel A, Kim YH, Voroshilovsky O, Qu Z, Kil JR, Lee MH, et al. Preventing ventricular fibrillation by flattening cardiac restitution. Proc Natl Acad Sci USA. 2000;97:6061–6. doi: 10.1073/pnas.090492697. doi:10.1073/pnas.090492697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurz RW, Ren XL, Franz MR. Dispersion and delay of electrical restitution in the globally ischaemic heart. Eur Heart J. 1994;15:547–54. doi: 10.1093/oxfordjournals.eurheartj.a060541. [DOI] [PubMed] [Google Scholar]
- 42.Bode F, Kilborn M, Karasik P, Franz MR. The repolarization-excitability relationship in the human right atrium is unaffected by cycle length, recording site and prior arrhythmias. J Am Coll Cardiol. 2001;37:920–5. doi: 10.1016/s0735-1097(00)01189-x. doi:10.1016/S0735-1097(00)01189-X. [DOI] [PubMed] [Google Scholar]
- 43.Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011;91:265–325. doi: 10.1152/physrev.00031.2009. doi:10.1152/physrev.00031.2009. [DOI] [PubMed] [Google Scholar]
- 44.Mays DJ, Foose JM, Philipson LH, Tamkun MM. Localization of the Kv1.5K channel protein in explanted cardiac tissue. J Clin Invest. 1995;96:282–92. doi: 10.1172/JCI118032. doi:10.1172/JCI118032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Wagoner DR. Electrophysiological remodeling in human atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:1572–5. doi: 10.1046/j.1460-9592.2003.t01-1-00234.x. doi:10.1046/j.1460-9592.2003.t01-1-00234.x. [DOI] [PubMed] [Google Scholar]
- 46.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, et al. Molecular identification and functional roles of a Ca2+ activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–94. doi: 10.1074/jbc.M307508200. doi:10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 47.Wijffels MC, Kirchhof CJ, Dorland R, Allessie MA. Atrial fibrillation begets atrial fibrillation: a study in awake chronically instrumented goats. Circulation. 1995;92:1954–68. doi: 10.1161/01.cir.92.7.1954. doi:10.1161/01.CIR.92.7.1954. [DOI] [PubMed] [Google Scholar]
- 48.Morillo CA, Klein GJ, Jones DL, Guiraudon CM. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation. 1995;91:1588–95. doi: 10.1161/01.cir.91.5.1588. doi:10.1161/01.CIR.91.5.1588. [DOI] [PubMed] [Google Scholar]
- 49.Gaspo R, Bosch RF, Talajic M, Nattel S. Functional mechanisms underlying tachycardia-induced sustained atrial fibrillation in a chronic dog model. Circulation. 1997;96:4027–35. doi: 10.1161/01.cir.96.11.4027. doi:10.1161/01.CIR.96.11.4027. [DOI] [PubMed] [Google Scholar]
- 50.Hara M, Shvilkin A, Rosen MR, Danilo P, Jr, Boyden PA. Steady-state and non-steady-state action potentials in fibrillating canine atrium: abnormal rate adaptation and its possible mechanisms. Cardiovasc Res. 1999;42:455–69. doi: 10.1016/s0008-6363(99)00044-9. doi:10.1016/S0008-6363(99)00044-9. [DOI] [PubMed] [Google Scholar]
- 51.Daoud EG, Bogun F, Goyal R, Harvey M, Man KC, Strickberger SA, et al. Effect of atrial fibrillation on atrial refractoriness in humans. Circulation. 1996;94:1600–6. doi: 10.1161/01.cir.94.7.1600. doi:10.1161/01.CIR.94.7.1600. [DOI] [PubMed] [Google Scholar]
- 52.Franz MR, Karasik PL, Li C, Moubarak J, Chavez M. Electrical remodeling of the human atrium: similar effects in patients with chronic atrial fibrillation and atrial flutter. J Am Coll Cardiol. 1997;30:1785–92. doi: 10.1016/s0735-1097(97)00385-9. doi:10.1016/S0735-1097(97)00385-9. [DOI] [PubMed] [Google Scholar]
- 53.Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM. Atrial L-type Ca2+ currents and human atrial fibrillation. Circ Res. 1999;85:428–36. doi: 10.1161/01.res.85.5.428. doi:10.1161/01.RES.85.5.428. [DOI] [PubMed] [Google Scholar]
- 54.Schoonderwoerd BA, Van Gelder IC, Van Veldhuisen DJ, Van den Berg MP, Crijns HJ. Electrical and structural remodeling: role in the genesis and maintenance of atrial fibrillation. Prog Cardiovasc Dis. 2005;48:153–68. doi: 10.1016/j.pcad.2005.06.014. doi:10.1016/j.pcad.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 55.Yue L, Feng J, Gaspo R, Li GR, Wang Z, Nattel S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res. 1997;81:512–25. doi: 10.1161/01.res.81.4.512. doi:10.1161/01.RES.81.4.512. [DOI] [PubMed] [Google Scholar]
- 56.Hatem SN, Coulombe A, Balse E. Specificities of atrial electrophysiology: clues to a better understanding of cardiac function and the mechanisms of arrhythmias. J Mol Cell Cardiol. 2010;48:90–5. doi: 10.1016/j.yjmcc.2009.08.029. doi:10.1016/j.yjmcc.2009.08.029. [DOI] [PubMed] [Google Scholar]
- 57.Escande D, Coulombe A, Faivre JF, Coraboeuf E. Characteristics of the time- independent slow inward current in adult human atrial single myocytes. J Mol Cell Cardiol. 1986;18:547–51. doi: 10.1016/s0022-2828(86)80920-8. doi:10.1016/S0022-2828(86)80920-8. [DOI] [PubMed] [Google Scholar]
- 58.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ currents and Kv1.5K+ channels are reduced in chronic human atrial fibrillation. Circ Res. 1997;80:772–81. doi: 10.1161/01.res.80.6.772. doi:10.1161/01.RES.80.6.772. [DOI] [PubMed] [Google Scholar]
- 59.Bosch RF, Zeng X, Grammer JB, Popovic K, Mewis C, Kühlkamp V. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. doi:10.1016/S0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 60.Bosch RF, Nattel S. Cellular electrophysiology of atrial fibrillation. Cardiovasc Res. 2002;54:259–69. doi: 10.1016/s0008-6363(01)00529-6. doi:10.1016/S0008-6363(01)00529-6. [DOI] [PubMed] [Google Scholar]
- 61.Koumi S, Backer CL, Arentzen CE. Characterization of inwardly rectifying K+ channel in human cardiac myocytes. Alterations in channel behavior in myocytes isolated from patients with idiopathic dilated cardiomyopathy. Circulation. 1995;92:164–74. doi: 10.1161/01.cir.92.2.164. doi:10.1161/01.CIR.92.2.164. [DOI] [PubMed] [Google Scholar]
- 62.Nattel S, Shiroshita-Takeshita A, Cardin S, Pelletier P. Mechanisms of atrial remodeling and clinical relevance. Curr Opin Cardiol. 2005;20:21–5. doi: [PubMed] [Google Scholar]
- 63.Schotten U, Neuberger H-R, Allessie MA. The role of atrial dilatation in the domestication of atrial fibrillation. Prog Biophys Mol Biol. 2003;82:151–62. doi: 10.1016/s0079-6107(03)00012-9. doi:10.1016/S0079-6107(03)00012-9. [DOI] [PubMed] [Google Scholar]
- 64.Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, et al. Reverse structural and gap-junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. 2003;107:2051–8. doi: 10.1161/01.CIR.0000062689.04037.3F. doi:10.1161/01.CIR.0000062689.04037.3F. [DOI] [PubMed] [Google Scholar]
- 65.Tsai C, Chiang F, Tseng MD, Tseng C, Yu CC, Wang YC, et al. Mechanical stretch of atrial myocyte monolayer decreases sarcoplasmic reticulum calcium adenosine triphosphatase expression and increases susceptibility to repolarization alternans. J Am Coll Cardiol. 2011;58:2106–15. doi: 10.1016/j.jacc.2011.07.039. doi:10.1016/j.jacc.2011.07.039. [DOI] [PubMed] [Google Scholar]
- 66.VanDerVelden HM, VanDerZee L, Wijffels MC, van Leuven C, Dorland R, Vos MA, et al. Atrial fibrillation in the goat induces changes in monophasic action potential and mRNA expression of ion channels involved in repolarization. J Cardiovasc Electrophysiol. 2000;11:1262–9. doi: 10.1046/j.1540-8167.2000.01262.x. doi:10.1046/j.1540-8167.2000.01262.x. [DOI] [PubMed] [Google Scholar]
- 67.Attuel P, Childers R, Cauchemez B, Poveda J, Mugica J, Coumel P. Failure in the rate adaptation of the atrial refractory period: its relationship to vulnerability. Int J Cardiol. 1982;2:179–97. doi: 10.1016/0167-5273(82)90032-8. doi:10.1016/0167-5273(82)90032-8. [DOI] [PubMed] [Google Scholar]
- 68.Boutjdir M, Le Heuzey J, Lavergne T, Chauvaud S, Guize L, Carpentier A, et al. Inhomogeneity of cellular refractoriness in human atrium: factor of arrhythmia? Pacing Clin Electrophysiol. 1986;9:1095–100. doi: 10.1111/j.1540-8159.1986.tb06676.x. doi:10.1111/j.1540-8159.1986.tb06676.x. [DOI] [PubMed] [Google Scholar]
- 69.Fareh S, Villemaire C, Nattel S. Importance of repolarization heterogeneity in the enhanced vulnerability to atrial fibrillation induction caused by tachycardia-induced atrial electrical remodeling. Circulation. 1998;98:2202–9. doi: 10.1161/01.cir.98.20.2202. doi:10.1161/01.CIR.98.20.2202. [DOI] [PubMed] [Google Scholar]
- 70.Lee SH, Lin FY, Yu WC, Cheng JJ, Kuan P, Hung CR, et al. Regional differences in the recovery course of tachycardia-induced changes of atrial electrophysiological properties. Circulation. 1999;99:1255–64. doi: 10.1161/01.cir.99.9.1255. doi:10.1161/01.CIR.99.9.1255. [DOI] [PubMed] [Google Scholar]
- 71.Shan Z, Yan J, Zhou J, Shi X, Guo J, Yuan H, et al. Role of progressive widening of the temporal excitable gap for perpetuation of atrial fibrillation in the goat. Circ J. 2010;74:655–63. doi: 10.1253/circj.cj-09-0596. doi:10.1253/circj.CJ-09-0596. [DOI] [PubMed] [Google Scholar]
- 72.Goette A, Honeycutt C, Langberg JJ. Electrical remodeling in atrial fibrillation: time course and mechanisms. Circulation. 1996;94:2968–74. doi: 10.1161/01.cir.94.11.2968. doi:10.1161/01.CIR.94.11.2968. [DOI] [PubMed] [Google Scholar]
- 73.Pan Q, He Y, Zhang Z, Liu Y, Zhou Q, Li J, et al. Spatial heterogeneity of muscarinic type 2 receptors in the atrium. Int J Cardiol. 2008;127:427–9. doi: 10.1016/j.ijcard.2007.04.190. doi:10.1016/j.ijcard.2007.04.190. [DOI] [PubMed] [Google Scholar]
- 74.Roberts-Thomson KC, Stevenson IH, Kistler PM, Haqqani HM, Goldblatt JC, Sanders P, et al. Anatomically determined functional conduction delay in the posterior left atrium: relationship to structural heart disease. J Am Coll Cardiol. 2008;51:856–62. doi: 10.1016/j.jacc.2007.11.037. doi:10.1016/j.jacc.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 75.Tse HF, Lau CP, Ayers GM. Heterogeneous changes in electrophysiologic properties in the paroxysmal and chronically fibrillating human atrium. J Cardiovasc Electrophysiol. 1999;10:125–35. doi: 10.1111/j.1540-8167.1999.tb00653.x. doi:10.1111/j.1540-8167.1999.tb00653.x. [DOI] [PubMed] [Google Scholar]