Abstract
Tumor metastasis is driven not only by the accumulation of intrinsic alterations in malignant cells, but also by the interactions of cancer cells with various stromal cell components of the tumor microenvironment. In particular, inflammation and infiltration of the tumor tissue by host immune cells, such as tumor-associated macrophages, myeloid-derived suppressor cells, and regulatory T cells have been shown to support tumor growth in addition to invasion and metastasis. Each step of tumor development, from initiation through metastatic spread, is promoted by communication between tumor and immune cells via the secretion of cytokines, growth factors and proteases that remodel the tumor microenvironment. Invasion and metastasis requires neovascularization, breakdown of the basement membrane, and remodeling of the extracellular matrix for tumor cell invasion and extravasation into the blood and lymphatic vessels. The subsequent dissemination of tumor cells to distant organ sites necessitates a treacherous journey through the vasculature, which is fostered by close association with platelets and macrophages. Additionally, the establishment of the pre-metastatic niche and specific metastasis organ tropism is fostered by neutrophils and bone marrow-derived hematopoietic immune progenitor cells and other inflammatory cytokines derived from tumor and immune cells, which alter the local environment of the tissue to promote adhesion of circulating tumor cells. This review focuses on the interactions between tumor cells and immune cells recruited to the tumor microenvironment, and examines the factors allowing these cells to promote each stage of metastasis.
Keywords: Tumor microenvironment, immune cell, metastasis, inflammation, stroma
Introduction
For decades it was believed that the primary, if not the sole function of the immune system during malignant growth, was to inhibit tumor development through immune surveillance. While certain immune cells such as cytotoxic T cells and natural killer (NK) cells are indeed important mediators of tumor clearance, it is now well accepted that the interactions between tumor and immune cells are more complex and dynamic than previously thought, as many subtypes of immune cells trafficking to the tumor microenvironment also possess potent tumor-promoting activity [1].
Once established, tumors are quite adept at preventing anti-tumor immune responses, and several defense mechanisms to circumvent immune detection have been described including antigen loss, down-regulation of major histocompatibility molecules (MHC), deregulation or loss of components of the endogenous antigen presentation pathway, and tumor-induced immune suppression mediated through cytokine secretion or direct interactions between tumor ligands and immune cell receptors [2]. These mechanisms contribute to the process of immunoediting in which tumor cell subpopulations susceptible to immune recognition are lysed and eliminated, while resistant tumor cells proliferate and increase their frequency in the developing neoplasia [3]. However, tumors not only effectively escape immune recognition, they also actively subvert the normal anti-tumor activity of immune cells to promote further tumor growth and metastasis.
During early stages of cancer development, infiltrating immune cell populations are primarily tumor suppressive, but depending on the presence of accessory stromal cells, the local cytokine milieu, and tumor-specific interactions, these immune cells can undergo phenotypic changes to enhance tumor cell dissemination and metastasis. For instance, CD4+ T cells, macrophages, and neutrophils have all been shown to possess opposing properties depending on the inflammatory state of the tumor environment, the tissue context, and other cellular stimuli intrinsic to the altered tumor cells [4, 5]. These features are dependent upon the inherent plasticity of immune cells in response to stimulatory or suppressive cytokines [6]. Notably, the switch from a Th1 tumor-suppressive phenotype such as CD4+ “helper” T cells, which aid cytotoxic CD8+ T cells in tumor rejection, to a Th2 tumor-promoting “regulatory” phenotype, which blocks CD8+ T-cell activity, is a characteristic outcome in the inflammatory, immune-suppressive tumor microenvironment [5, 7]. Likewise, M1 macrophages and N1 neutrophils are known to have pronounced anti-tumor activity; however, these immune cells are often subverted to a tumor-promoting M2 and N2 phenotype, respectively, in response to immune-suppressive cytokines secreted by tumor tissue [8].
Tumor metastasis is responsible for greater than 90% of cancer mortality yet remains the least understood stage of tumor development. Years of research have led to the development of the understanding that successful metastatic spread requires several steps including epithelial-to-mesenchymal transition (EMT) and local invasion, intravasation into the vasculature, transit through the circulatory system, extravasation and seeding at the pre-metastatic niche, and finally survival and growth at the metastatic site [9, 10]. Each of these stages of metastasis has been shown to require the close association and collaboration between cancer cells, immune cells, and other stromal and inflammatory components of the tumor microenvironment such as cancer-associated fibroblasts (CAFs) and bone marrow-derived cells (BMDCs) [9]. Here we review our current understanding of the complex interactions between tumor cells and infiltrating host immune cells, and how these associations drive tumor progression and metastasis. In particular, we will discuss tumor inflammation and the roles of myeloid-derived suppressor cells (MDSCs), macrophages, neutrophils, regulatory T lymphocytes (Treg cells), and suppressive dendritic cells (DCs) in metastasis. Involvement of immune cells such as macrophages, neutrophils, and platelets in the promotion of circulating tumor cell (CTC) dissemination and colonization will also be discussed as well as the role of the adaptive immune system in establishing the pre-metastatic niche and organotropism.
Inflammation and immune suppression during tumor progression
Inflammation induced by oncogenic stress in the tumor microenvironment, or from tumor extrinsic sources such as recurrent tissue damage or persistent infections, can contribute to the initiation, promotion, and progression of malignant disease. Inflammation can be described as acute or chronic; each of which can have vastly different effects on wound healing and tissue health. Acute inflammation is typically considered to be an innate, first-line defense against tissue damage and infection characterized by an initial influx of plasma and cytokine-secreting immune cells that are recruited to the site of injury to heal the damaged tissue [11]. Subsequently, this pro-inflammatory program must be terminated after healing to avoid negative effects of prolonged exposure to pro-inflammatory cytokines and factors which can further damage the tissue through aberrant cell proliferation and tissue remodeling [12]. Pathologic inflammation, such as chronic inflammation, can perpetuate a cycle of damage and healing that has been linked to many forms of cancer. This dynamic interplay between tumor cells and infiltrating immune cells has positive and negative affects on tumor growth and results in continuous proliferation and cell renewal that has been used to describe tumors as “wounds that do not heal [13].” It has been well established that patients suffering from prolonged inflammatory conditions such as pancreatitis, inflammatory bowel disease (IBD), prostatitis, and chronic obstructive pulmonary disease (COPD) will have greatly increased risk for developing cancer later on in these affected tissues [14, 15]. Not surprisingly, environmental triggers linked to chronic inflammation such as tobacco smoke, asbestos, and obesity have also been shown to contribute to cancer development and progression [16]. Additionally, many pathogens including bacteria and viruses such as Heliobactor pylori, hepatitis B (HBV) or C (HCV) viruses, and species of Schistosoma and Bacteroides can initiate an inflammatory response, which eventually progresses to chronic inflammation linked to gastric cancer, hepatocellular carcinoma, and bladder and colon cancer, respectively [17, 18].
The association between inflammation and cancer was first made in the nineteenth century by Rudolf Virchow when he observed the presence of leukocytes within tumor tissue and concluded that inflammation may be a driving force in promoting neoplastic growth [19]. Since then our understanding of the immune system and its critical role in contributing to the initiation, promotion, and progression of cancer has unveiled numerous immune pathways and molecules that could be utilized for therapeutic intervention [20]. While genetic damage, mutations, and deregulated signaling pathways arise during tumor cell development and significantly contribute to the commitment of progeny cells to a malignant course, many studies over the past decade have shed light on how the program of chronic inflammation specifically contributes to tumorigenesis [17].
Perhaps in most cases of solid malignancy, chronic inflammation does not initiate tumor growth, but rather fosters tumor progression and metastasis by providing a nurturing environment for invasion [14]. Cells with oncogenic stress can trigger inflammation by activating certain transcriptional programs that lead to remodeling of the tumor microenvironment. For instance, Ras and Myc family members have been shown to directly contribute to inflammation by Myc-mediated recruitment of mast cells to the tumor microenvironment, which promotes angiogenesis and tumor cell dissemination, while Ras-induced expression of interleukin-8 (IL-8) has been shown to promote neovascularization through CXCL-8/IL-8 signaling [21, 22]. Release of pro-inflammatory molecules such as IL-1 and HMGB1 by the primary tumor as it undergoes necrosis due to hypoxia has also been shown to promote angiogenesis with the release of growth factors [23]. Coinciding with the increase in tumor vasculature, immune cells with both anti- and pro-tumor activity have a new pathway to access the tumor microenvironment.
During the course of malignancy, the inflammatory microenvironment hijacks the innate and adaptive immune responses to promote tumor growth, by preventing the recruitment, survival, and function of anti-tumor immune effector cells [24]. Some of the key factors facilitating this immune suppression are an abundance of inflammatory chemokines and cytokines including GM-CSF, CCL2, CCL20, CXCL5, CXCL12, TNF-α, TGF-β, IL-1β, IL-6, IL-8, IL-10, and IL-23, which are secreted by tumor tissue as well as other immune and stromal cells to promote recruitment and suppression of many immune cell types [14]. For instance, IL-10 has been shown to inhibit the differentiation and activation of DCs, which are key activators of anti-tumor effector cells of the adaptive immune system, such as cytotoxic CD8+ T cells. Tumors also actively recruit Tregs, which are known to suppress both adaptive and innate immune responses. MDSCs and macrophages recruited to the tumor microenvironment from the bone marrow by tumor cells and Tregs are also potent suppressors of anti-tumor immunity when they are converted to an M2 suppressive phenotype by cytokines such as IL-10 and TGF-β. These recruited myeloid cells further suppress the anti-tumor immune response by releasing IL-10 to promote Treg function, and inhibit effector T and NK cell responses by mechanisms involving the release of arginase and reactive oxygen and nitrogen species (ROS and NOS) [25, 26]. Activation of NF-κB and STAT3 transcription factor signaling in tumor cells has also been shown to be a pivotal mechanism by which tumors evade immune system destruction by inhibiting DC maturation, and suppressing the anti-tumor immune response [27–29].
Similar to Tregs, regulatory B cells (Bregs) have also been shown to play a role in tumor progression to metastasis. Olkhanud, et al, demonstrated that a subset of B cells, or “tumorevoked Bregs,” induce the TGF-β-dependent conversion of resting CD4+ T cells to immune-suppressive Foxp3+ Tregs [30]. Likewise, in other studies, tumor promoting B cells have been shown to facilitate the conversion of M1 macrophages to a pro-tumoral M2 phenotype through IL-10 secretion [31]. Additionally, B cells are known to promote lymphangiogenesis and may indeed actively promote metastasis as has been shown for B-cell-mediated lymphangiogenic metastasis in lymphoma and melanoma [32, 33]. While B cells and DCs do play active roles in anti-tumor immunity, these and other studies have demonstrated the capacity of the tumor microenvironment to alter immune function to promote tumor progression. Interestingly, NK cells do not appear to play an active role in tumor promotion, however, induced natural killer T (iNKT) cells may be involved in tumor promotion through immune suppression in certain circumstances [34].
Overall, chronic inflammation modifies the local environment in the affected tissue by providing an abundant source of growth factors, cytokines, chemokines, prostaglandins, and reactive oxygen species, to promote angiogenesis and metastasis (Figure 1) [19, 35]. As a result of these mechanisms of inflammation the tumor microenvironment frequently contains an abundance of immune cells including innate immune cells, such as mast cells, tumor-associated macrophages (TAMs), and MDSCs, as well as adaptive immune cells including T and B lymphocytes and DCs that can have tumor-promoting properties [36]. Each of these cell types can affect immunosuppression and will be discussed in detail below as they relate to tumor progression during each stage of metastatic progression (Table I) [4].
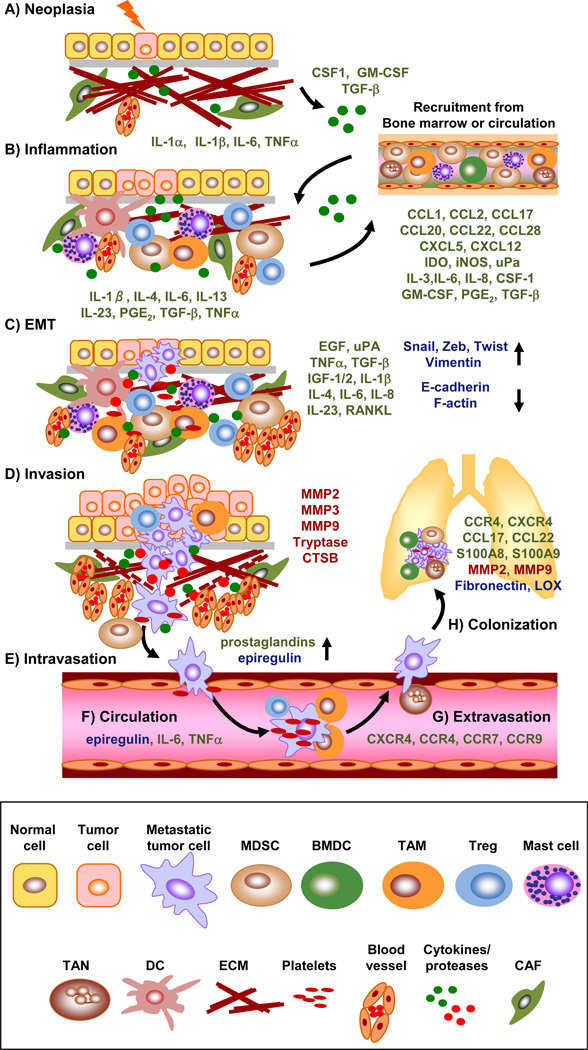
Figure 1. Recruitment of tumor-promoting immune cells contributes to each stage of tumor progression and metastasis.
A) Neoplasia: Genetic mutations arising from radiation, carcinogen exposure, or chronic infection initiates neoplastic growth and the initial inflammatory immune state. B) Inflammation: A recurring cycle of chronic inflammation, immune cell recruitment, and secretion of tumor promoting cytokines (green) promotes immunosuppression and tumor proliferation. Chemokines secreted from tumor cells and immune cells in the inflammatory tumor microenvironment that recruit tumor-promoting immune cell populations are listed below the depicted bone marrow compartment. C) EMT: Cytokines and factors produced by immune cells contribute to the initiation of an epithelial conversion to a mesenchymal state with concurrent induction of mesenchymal and reduction in epithelial proteins, respectively (blue). D) Invasion: Immune cells recruited to the tumor tissue secrete proteases (Red) and upregulate tumor-intrinsic factors that promote invasion through the basement membrane and surrounding ECM into the vasculature. E) Intravasation: Immune cells mediate prostaglandin and epiregulin expression to facilitate vascular permeability and migration into the circulation. F) Circulation: Platelets, neutrophils, lymphocytes, and inflammatory chemokines direct CTC travel and protect tumor cells from shear stress and immune-mediated recognition and lysis. G) Extravasation: cytokines secreted from immune cells and the inflammatory tumor microenvironment and direct contact with neutrophils increase expression of adhesion proteins such as ANGPTL4, ICAM-1, VCAM-1, and fibronectin on endothelial cells, which facilitates CTC vascular arrest and extravasation at the metastatic site. H) Colonization: BMDCs and myeloid cells help to establish the pre-metastatic niche by upregulating adhesion molecules, while platelets and neutrophils can also mediate CTC arrest and adhesion to tissue-specific endothelial cells. Green = cytokines/chemokines; Red = proteases; Blue = other metastasis related proteins (e.g. transcription factors and adhesion molecules).
Table I.
Metastasis-associated immune cells: their recruitment, activation, and contributions to tumor progression and metastasis
| Immune cell | Stage of metastasis involvement |
Recruiting tumor-derived cytokines |
Factors inducing pro- tumor activity |
Immune-secreted EMT/invasion factors |
Ref |
|---|---|---|---|---|---|
| Tumor- associated macrophage (TAM) |
Inflammation EMT Invasion Circulation Immunosuppression |
CSF-1 GM-CSF IL-3 CCL2 TGF-β1 |
IL-4, IL-10, IL-13 Arginase-1 CCL2, CCL22 TGF-β1 ROS |
TGF-β1 EGF IGF-1, IGF-2 MMP2, MMP9 uPA IL-6, IL-8 TNF-α |
[44, 66, 67, 104– 106, 111, 116, 212] |
| Myeloid- derived suppressor cell (MDSC) |
Inflammation EMT Invasion Immunosuppression |
IL-6 uPA/uPAR IPGE2 iNOS CXCL5 GM-CSF |
IL-6, IL-10 COX-2 PGE2 iNOS TGF-β1 |
TGF-β1 EGF MMP9 |
[67, 213– 217] |
| Suppressive and regulatory T cells (e.g. Treg) |
EMT Invasion Circulation Immunosuppression |
IDO CCL1 CCL17 CCL22 CCL28 |
IL-4, IL-6, IL-13, IL-23 TGF-β1 |
TGF-β1 MMP3 IL-6 IL-23 RANKL |
[44, 54, 67, 112, 116, 119, 218–220] |
| Dendritic cell (DC) |
EMT Invasion Immunosuppression |
N/A1 | CCL2 IL-10 PGE2 TGF-β1 TSP1 |
TGF-β1 MMP2 MMP3 MMP9 |
[56, 57, 67] |
| Tumor- associated neutrophil (TAN) |
Inflammation EMT Invasion Circulation Extravasation Immunosuppression |
IL-8 CXCL1 CXCL2 CXCL6 GM-CSF TGF-β1 |
TGF-β1 | MMP9 | [128, 129, 131, 221–223] |
| Platelets | EMT Circulation Extravasation |
ADP Thrombin |
ADP Thrombin |
TGF-β1 | [68, 119, 122, 224] |
| Mast cells | Invasion | VEGF-A | Tryptase | [103, 225] |
Suppressive dendritic cells do not appear to be actively recruited but are rather converted to an immunosuppressive state by local factors in the tumor microenvironment.
Inflammatory immune cells and cytokines contribute to each stage of metastasis
Epithelial-to-mesenchymal transition (EMT)
In the last decade there has been increasing evidence that malignant cells on the tumor boarder can develop invasive, mesenchymal characteristics that facilitate tumor detachment and acquisition of a migratory phenotype [37–39]. These EMT cells are characterized by a loss of epithelial features such as cell-to-cell adhesions, apical-basal polarity, and reorganization of cytoskeletal filaments with a concomitant induction of mesenchymal characteristics including increased migratory capacity and plasticity [39]. These phenotypic changes are driven by alterations in signal transduction pathways that activate EMT-associated transcription factors such as Snail, Zeb, and Twist family members, which directly alter epithelial gene expression programs, particularly suppression of Cdh1, which encodes the epithelial cell adhesion protein E-Cadherin [10, 40]. Reduction of E-cadherin results in loss of epithelial-to-epithelial cell connections at adherins junctions and allows for enhanced cell motility. Interestingly, malignant EMT has been associated with chronic inflammation, and likely represents a key convergence point between immune-mediated inflammation and cancer progression [41].
A wide range of tumor-infiltrating immune cells have been shown to secrete cytokines and chemokines that promote EMT including macrophages, MDSCs, granulocytes, and lymphocytes (Figure 1) [38, 42–44]. Tumor-infiltrating stromal cells can initiate EMT by activating several different signaling pathways in tumor cells including transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α), NF-κB, Notch, Wnt, and Hedgehog signaling pathways [45–48]. Leukocytes, macrophages, and CAFs in particular are some of the key components of the stroma that regulate the EMT phenotype in the chronically inflamed tumor environment [12]. For instance, TNF-α-stimulated MSCs have been shown to secrete CCL2, which was found to recruit CD11b(+)Ly6C(+) monocytes, F4/80(+) macrophages, and CD11b(+)Ly6G(+) neutrophils to the tumor [49]. This recruitment was shown to promote malignancy, largely through macrophage-dependent mechanisms, as macrophage depletion abrogated tumor growth. TNF-α secreted by TAMs has been shown to activate NF-κB-mediated transcription of Snail1 and Zeb, which leads to diminished E-cadherin expression on tumor cells [44, 50, 51]. Snail is a highly unstable protein, being rapidly targeted for degradation by GSK-3β phosphorylation and SCFβ-Trcp ubiquitylation; however, TNF-α induces Snail1 stabilization by activating COP9 signalosome 2 (CNS2), which inhibits Snail1 binding to GSK-3β and SCFβ-Trcp [50, 52]. Furthermore, Pikarsky et al., demonstrated that TNF-α blockade results in apoptosis and diminished hepatocellular carcinoma progression by suppressing NF-κB activation [27]. Notably, TNF-α signaling was found to be dispensable during early stages of tumor development, but was required for malignant conversion and progression in later stages of disease, indicating that NF-κB is essential for promoting inflammation-associated EMT.
Leukocyte-secreted cytokines such as IL-6 and IL-23 have also been shown to initiate EMT via the activation of STAT3 signaling resulting in Twist expression [53–55]. STAT protein signaling in the inflammatory environment is known to dictate whether the immune response generated is tumor-inhibitory or tumor-promoting. In this case, STAT3 signaling in response to inflammatory cytokines leads directly to inhibition of E-cadherin expression and loss of cell-cell adhesion. Furthermore, Snail1 itself has been shown to upregulate expression of certain pro-inflammatory cytokines such as IL-1, IL-6, and IL-8, which may establish a feedback loop to promote additional EMT events through enhanced inflammatory signals [41]. In melanoma, Snail overexpression leading to EMT has been shown to induce immunosuppression through CCL2 and TSP1 production [56, 57]. These tumor-derived cytokines were found to shift anti-tumor immune cell populations to regulatory lymphocytes and immune-suppressive DCs.
Tumor hypoxia can also promote EMT through the activation of inflammatory signals that trigger motility programs. For instance, HIF1-α and TGF-β expression during hypoxia has been shown to initiate Twist and SIP1 expression [41], while in other studies, hypoxia-induced inflammation signals triggered expression of NF-κB, which was directly shown to activate EMT by repressing E-cadherin expression through transcription of Snail and Zeb [58–61]. In fact, NF-κB is one of the most potent inducers of EMT that is also directly stimulated by inflammatory signals.
Specifically, NF-κB has been shown to initiate EMT by activating Akt [61], which regulates Snail expression, and Snail can further directly inhibit expression of certain tumor suppressor genes including the Raf kinase inhibitor protein (RKIP) in melanoma and PTEN in prostate cancer [62, 63]. Furthermore, NF-κB can also affect EMT programs by directly binding to the promoter of miR-488, which establishes a positive EMT feedback loop as miR-488 targets special AT-rich sequence-binding protein-1 (SATB1) mRNA for degradation, which in turn promotes EMT by increasing expression of Twist and NF-κB activation [64]. Once NF-κB is activated, other feedback loops can be established that maintain inflammatory signals in the tumor environment since NF-κB controls expression of several genes encoding pro-inflammatory cytokines (e.g. IL-1, IL-2, ILp6, TNFα), chemokines (e.g. IL-8, MIP-1 α, MCP-1, RANTES, eotaxin), adhesion molecules (e.g. ICAM, VCAM, E-selectin), and growth factors and enzymes such as COX-2 and iNOS [65]. Indeed, NF-κB signaling has been recognized as the most likely bridge between tumor-associated inflammation and cancer metastasis [41].
TGF-β1 is another critical inflammatory cytokine known to be a potent inducer of EMT, invasion, and metastatic spread [66]. The source of TGF-β in the tumor microenvironment is varied as this cytokine can be produced directly by tumor cells or by CAFs, however, infiltrating immune cells such as macrophages, MDSCs, and Tregs can also produce large quantities of TGF-β1 and are important inducers of EMT [67]. In addition, studies have shown that platelet-derived TGF-β1 can initiate EMT through TGF-β1/Smad and NF-κB signaling pathways in cancer cells [68]. In this study the authors demonstrated that blocking NF-κB signaling in cancer cells or ablating TGF-β1 selectively in platelets was sufficient to protect against lung metastasis in an in vivo model. Furthermore, recent studies have shown that STAT3 signaling in tumor cells stimulates TGF-β and IL-10 production, which inhibits the function of effector cytotoxic T cells and promotes Treg development, contributing to immunosuppression in the tumor environment [69]. Other inflammatory cytokines including TNF-α have also been shown to upregulate TGF-β expression by tumor stromal cells, such as through AP-1 signaling in lung fibroblasts [70]. While TGF-β1 is initially secreted to control cell proliferation and inflammation, tumor cells eventually lose the ability to respond to its pro-apoptotic and anti-proliferative effects through mutation and immune editing, and instead, its pro-EMT inducing activities prevail. Indeed, the association of TGF-β with oncogenic Ras and p53 has recently been shown to form a mutant p53/Smad complex that nullifies TGF-β tumor-suppressing effects, instead diverting its function to promote metastasis [71]. In accordance with this finding, TGF-β has also been shown to associate with oncogenic Ras to initiate transcription of Snail during EMT induction [72].
The ability of TGF-β to activate EMT depends on both Smad-dependent and Smad-independent signaling pathways [73]. While the TGFβ/TGFβR/Smad2 signaling axis is required to maintain epigenetic silencing of EMT genes in breast cancer progression [74], TGF-β also signals through non-canonical Smad-independent pathways to regulate mammary epithelial cell (MEC) EMT programs including small GTP-binding proteins, MAPKs, PI3K, and NF-κB [75–81]. Investigation of TGF-β signaling during EMT is an active field of research and increasing evidence suggests that TGF-β can facilitate EMT induction through numerous targets. Studies in various cancer models have collectively shown that ZAG, Na, K-ATPase, SKIP, IGFBP3, Dab2, ROCK, TGF-β type I receptor (TβRI), LIMK, PIAS1, SNAIL, TWIST, Six1, and SIP1 can all be regulated by TGF-β during EMT [82–87]. Additionally, TGF-β signaling in tumor cells can be mediated by members of the miR-30 and miR-200 microRNA families, such as that observed in thyroid carcinoma [88]. Work conducted by our group and others has demonstrated that TGF-β can inhibit miR-200 expression in mammary epithelial cells, which results in the expression of ZEB1, ZEB2, and SIP1 leading to EMT through downregulation of E-cadherin [89–93]. Likewise, TGF-β inhibition of miR-21 and miR-155 has also been shown to activate an EMT phenotype in MCF-7 breast cancer cells and MEC cells, respectively [94, 95]. Overall, TGF-β secreted by tumor cells and infiltrating stromal cells can modulate both the genetic and epigenetic landscape of tumor cells to alter and maintain their differentiation into mesenchymal-like cells with invasive capabilities. While these findings are well accepted, some aspects of the linear progression model of metastasis are controversial and EMT-independent routes to invasion and metastasis should also be considered.
Invasion and intravasation
Migration of cells from the primary tumor requires the breakdown of the basement membrane and remodeling of the extracellular matrix (ECM). The proteolytic cleaving and degradation of ECM proteins during tumor invasion is coordinated by a number of enzymes including serine proteases, matrix metalloproteinases (MMPs), and cysteine cathepsins [9]. In fact, increased expression of these enzymes in human and murine tumor tissues is associated with lower survival rates and worse patient prognosis in a variety of tumor types including colorectal, ovarian, breast, and head and neck cancers [96, 97]. These proteolytic enzymes can be secreted by tumor cells, however, the majority are secreted by resident stromal cells such as fibroblasts and also infiltrating immune cells [98, 99]. Work by several groups has shown that mast cells, neutrophils, and macrophages can secrete proteases that remodel the extracellular matrix on the tumor boundary (Figure 1) [100–102]. Indeed, mast cells recruited to the inflammatory breast tumor environment have been shown to secrete tryptase, which results in enhanced rates of metastasis to the lymph nodes and higher-grade breast tumors [103]. Macrophages in particular have been shown to be key players in tumor cell migration, invasion, and metastasis [104]. In a polyoma middle T antigen (PyMT) model of breast cancer, Vasiljeva et al., demonstrated that macrophages exposed to IL-4 up-regulate expression of cysteine protease cathepsin B (CTSB) leading to increased lung metastases [105]. The secretion of proteases from macrophages in the tumor may in part be regulated by IL-6 production from neoplastic cells. Specifically, Mohamed et al., demonstrated that MDA-MB-231 breast tumor cells secrete IL-6, which in co-culture stimulated monocyte proliferation, expression, secretion, and activity of CTSB, MMP2, MMP9, IL-6, and insulin-like growth factor binding protein-1 (IGFBP-1) [106]. Likewise, in pancreatic islet cancers, cathepsin B expression by macrophages has been associated with a loss of E-cadherin on neighboring tumor cells [9]. TGF-β secretion by inflammatory cells has also been shown to promote neoplastic invasion by activating ILK, which leads to remodeling of renal epithelial cell attachment to the basement membrane through the activation of MMP2 and MMP9, which degrades collagen type IV in the basement membrane [107, 108]. Likewise, in a model of colorectal cancer, immature CCR1+ myeloid cells recruited from the bone marrow and expressing MMP2, MMP9, and CCR1 migrate toward the CCR1 ligand CCL9 expressed in the tumor epithelium and also promote tumor invasion [109].
In addition to myeloid cells, leukocytes are also conspicuous on the tumor periphery and may foster tumor invasion and metastasis [9]. IL-4 produced by T cells has been shown to stimulate cathepsin expression and activity by TAMs in multiple types of cancer [110]. These macrophages further promote tumor cell invasion by secreting factors to stimulate tumor cell migration such as migration-stimulating factor (MSF), which can be induced in TAMs by several cytokines found in tumor-conditioned media including macrophage-colony stimulating factor (CSF), IL-4, and TGF-β [111]. Lymphocytes on the tumor periphery may also promote invasion through expression of lymphotoxin and RANKL, both of which mediate IKKα activation in progressing breast and prostate cancer lesions [112]. IKKα accumulation in the nuclei of these cells subsequently results in a reduction of maspin, which has been shown to suppress metastasis.
While many of the proteolytic enzymes secreted by tumor-associated immune cells promote tumor cell motility through EMT and intravasation into the vasculature, some of these proteases can also enhance the presence and/or activity of anti-tumor leukocytes, B cells, and mast cells in the primary tumor, which can exhibit both tumor promoting and tumor suppressive activity [113]. This dual role of the immune system should be noted, as some proteases derived from lymphocytes, macrophages, and dendritic cells such as cathepsin E, can directly mediate apoptosis of cancer cells by cleaving tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) from the cell surface resulting in increased tumor apoptosis and diminished metastasis [114]. However, immune cells secrete other cytokines that promote invasion such as IL-1, TNF-α, and IL-6 that stimulate MMP expression in tumor cells through NF-κB and STAT3 signaling [69]. Furthermore Th17 and Th2 lymphocytes can program TAMs to foster a metastatic environment via secretion of several cytokines including IL-6 and IL-17 [115, 116]. Additionally, immunosuppressive CD4+ T cells recruited to the tumor environment can secrete IL-4 and IL-13 to convert tumor-suppressive M1 macrophages to a tumor-promoting M2 phenotype, which have been shown to support breast cancer metastasis to the lungs [116]. The tumor-promoting activity of TAMs is further strengthened by depletion studies, which demonstrate that loss of TAMs, or the associated suppressive CD4+ T cells, significantly reduces the frequency of lung metastases [9, 116]. Direct contact between carcinoma cells and TAMs can also mediate tumor metastasis, wherein pre-metastatic tumor cells direct TAM recruitment through the secretion of numerous chemokines or cytokines. In particular, breast cancer cells can recruit TAMs through the production of CSF1 and CXCL12 [9]. These recruited EGF+ myeloid cells were shown to induce breast cancer metastasis by binding the EGF receptor (EGFR)-expressing breast tumor cells. Similarly, Kim et al., found that Lewis lung carcinoma (LLC) cells were the most potent macrophage activators via TNF-α and IL-6 secretion, which signaled through toll-like receptor 2 (TLR2) and TLR6 to induce invasion and metastasis by versican production [117].
Intravasation of invasive cells into the blood or lymph vessels requires that tumor cells take on the amoeboid characteristics exemplified by immune cells such as lymphocytes, which are some of the only cells capable of intravasation and extravsation from tissue to vasculature in order to control inflammation and infection. Likely, the EMT phenotype that confers mesenchymal traits is prerequisite for tumor cells to efficiently intravasate into circulation. Investigation into tumor cell dissemination has provided abundant evidence that intravasation is regulated by prostaglandins (produced by COX2 signaling), cytokines (e.g. epigregulin, which increases cancer cell survival) and proteases such as MMP that clear ECM proteins blocking access to the endothelium [118]. Inflammation may also promote intravasation through the production of factors that increase vascular permeability.
Circulation and extravasation
It has been estimated that only about 0.01% of disseminating tumor cells that intravasate into the circulation will survive and establish micrometastases at distant sites [9]. Such low rates of survival for circulating CTCs is predominantly attributed to mechanical shear stresses, detachment-induced cell death or “anoikis,” and cell-mediated cytotoxicity occurring in microcirculation [119]. Factors that increase the probability of survival of CTCs include endogenous factors in the blood and inflammatory mediators released by cancer cells or cancer-associated stimuli [117, 120]. Many of these tumor-associated factors and cytokines released into the blood depend on activation of NF-κB and STAT3 activation in inflammatory immune cells or cancer cells [118]. For instance, a variety of cytokines secreted in the tumor microenvironment including TNF-α, epiregulin, and IL-6 have been shown to promote the survival of cancer cells in circulation [118]. Once CTCs intravasate into the circulation and leave the immunosuppressive tumor microenvironment with its milieu of cytokines and growth factors the tumor cells are no longer protected from immune-mediated recognition and lysis by host immune cells present in the circulation. However, some cytokines produced in the tumor microenvironment can directly link CTCs to macrophages, which can protect them during transit (Figure 1) [104]. It has even been proposed that CTCs might fuse with macrophages or other BMDCs as has been reported in some animal models, which may provide metastatic cells with myeloid traits that would be beneficial for their survival in circulation [121]. Also, direct association with lymphocytes and aggregation of platelets around CTCs has also been shown to protect circulating metastatic cells from NK cell-mediated lysis [119, 122]. Protection of CTCs from NK cell recognition was found by Palumbo et al., to rely on tumor cell-associated tissue factor (TF) expression (the receptor for coagulation factors VIIa and X on platelets [123]), which was dependent on prothrombin expression, platelet function, and fibrinogen expression in a transgenic mouse model lacking TF [124]. These CTC/platelet/fibrin clots may also reduce susceptibility to shear forces that could otherwise destroy the circulating cancer cells and may slow their arrest facilitating adhesion to endothelial cells at distant organ sites [9]. A high platelet count has been shown to be a associated with worse patient prognosis and decreased survival in many types of cancer including breast, colorectal, and lung cancer patients [125], and it has been conclusively demonstrated that treatment with anti-coagulants can decrease incidence of metastasis in rodent models and human patients [126].
The migration of metastatic cells in circulation is often dependent upon chemokine gradients, including CXCR4, CCR4, CCR7, and CCR9 that direct tumor cells through the vasculature [127]. The CTC’s journey through the vascular comes to an end upon adhesion to integrins expressed by endothelial cells. It has been shown that this integrin-dependent adhesion is mediated by molecules such as ANGPTL4, which can be upregulated by many cytokines, including TGF-β, that are present in the inflammatory environment, [118]. Furthermore, McDonald et al., found in a mouse model of liver metastasis that a systemic inflammatory state enhances CTC attachment in liver tissue through neutrophil-dependent upregulation of adhesion molecules on hepatic sinusoids [128]. Indeed, it has been found that several inflammatory cytokines can act in long distance manner to increase adhesion molecules on endothelial cells in target organs, and these cytokines are prevalent in the circulation of cancer patients [14]. This inflammation-induced expression of adhesion molecules on endothelial cells greatly increases metastatic cell attachment at distant organ sites.
Migratory arrest depends both on the quality and quantity of adhesion molecules on endothelial cells, as well as the adhesion molecule repertoire on the CTCs themselves and the content of the underlying ECM in the local tissue environment. It has been demonstrated that the adhesion of melanoma cells to the blood vessel wall is mediated by platelets and neutrophils through the production of matrix attachment molecules including β2-integrin/intracellular adhesion molecule-1 (ICAM-1) as well as selectins [122, 128–130]. In another study Spicer et al., showed that neutrophils promote liver metastasis of LLC H-59 tumor cells by facilitating neutrophil Mac-1/ICAM-1-dependent adhesion to liver sinusoids [131]. Indeed, for multiple types of cancer, inflammation-inducible cell adhesion molecules (CAMs) are frequently associated with metastatic progression and adhesion to endothelial cell walls in distant organ sties [132]. In fact, recent studies demonstrate that VCAM-1 has multiple roles in promoting metastasis by facilitating immune evasion of metastatic cells, promoting recruitment of metastasis-promoting stromal cells, in addition to adhesion of metastatic cells [133]. VCAM-1 expressed on the surface of tumor cells was found to bind to its cognate ligand, α4β1, expressed by T cells, which decreased infiltration of lymphocytes into the tumor by mediating T-cell migration away from the source of VCAM-1 [134]. Furthermore, VCAM1 mediates the interaction between tumor cells and α4β1, positive macrophages and pre-osteoclasts to promotes both lung and bone metastasis [135, 136]
One final obstacle that must be surmounted by CTCs for efficient attachment to the endothelial wall is to inhibit or avoid cell death by anoikis [12]. Binding of CTC chemokine receptors CXCR4 and CCR7 to their respective ligands (CXCL12 and CCL21) on endothelial cell lumens not only helps to mediate tumor cell arrest, but also dampens the effects of anoikis by regulating pro- and anti-apoptotic Bmf and Bcl-xL proteins [137]. These interactions have been demonstrated to foster CTC cell attachment and block cell death.
Finally, it should be noted that the dissemination of circulating cancer cells from a primary tumor might not always follow a direct route to future metastatic sites in distant organs. It has long been a clinical criteria in disease staging and prognosis to evaluate the presence of lymph node metastases, and it remains controversial whether tumor cells metastasize to distant organs from the lymph nodes or whether the presence of tumor cells in the lymphatic system simply reflects the high invasive potential of these vessels and tissues [138]. Indeed, with no inter-endothelial tight junctions, periocytes, or basement membranes to traverse, metastatic seeding through lymphatics is much more passive than intravasation and extravasation within the blood vasculature [139]. The aggregation of metastatic cells in the lymph nodes or bone marrow may promote their survival and proliferation and provide sufficient time for these cells to adapt to the stresses encountered in the difficult journey to other organ sites. This may also provide additional time for the establishment of a “pre-metastatic niche” before the arrival of CTCs.
Pre-metastatic niche and organatropism
Several studies have shown that primary tumors can “prepare” the local environment of distant target organ sites by mobilizing BMDCs that prepare the tissue for colonization even before the arrival of disseminated tumor cells. [140]. Kaplan et al., demonstrated that vascular endothelial growth factor receptor (VEGFR) 1-expressing hematopoietic progenitor cells are primed by factors secreted from the primary tumor such as placental growth factor (PIGF) [141]. These progenitor cells subsequently trafficked to pre-metastatic sites and induced expression of fibronectin on resident fibroblasts by VLA4/fibronectin binding in the lungs of mice. The upregulation of fibronectin was ultimately found to increase metastatic seeding, presumably by enhancing adhesion of CTCs to tissue expressing these adhesion molecules in the lung. It is still unknown whether the resident fibroblasts that produce the fibronectin arise from local MSCs or if they originate from the bone marrow.
Additional studies have revealed that Gr1+CD11b+ myeloid cells localize to the lungs of tumor-bearing mice to mediate changes in the local tissue environment that promotes metastasis. For example, in a study modeling the metastatic spread of breast adenocarcinoma to the lungs, Yan et al., found that these immature myeloid cells suppress IFNγ production and increase inflammatory cytokines in the pre-metastatic niche, as well as induce expression of MMP9, which promoted vascular remodeling [142]. This study revealed that a progenitor immune cell population could not only remodel the pre-metastatic lung to enhance immune suppression, inflammation, and tumor proliferation, but could also secrete proteases to restructure the tumor vasculature. These changes create docking sites for circulating metastatic cells. In other studies, mammary tumor cells remotely activated expression of TARC/CCL17 and MDC/CCL22 in the lungs of mice injected with 4T1 breast tumor cells [143]. While the expression of these chemokines in lung tissue recruited both 4T1 breast tumor cells and other immune cells through CCR4-mediated chemotaxis, this remodeling of the pre-metastatic niche was insufficient to promote establishment of micrometastases as resident NK cells eliminated the migrating tumor cells. However, the authors also showed that adding CCR4+ Treg cells could promote the outgrowth of metastasis by killing the NK cells via β-galactoside-binding protein. In other model systems, researchers have shown that Mac1+ myeloid cells are recruited to pre-metastatic sites via TLR-4 by primary tumor induction of S100A8 and S100A9 secretion at secondary sites [144, 145]. These chemoattractants recruit Mac1+ myeloid cells to enhance the inflammatory state of the pre-metastatic niche, which promotes the migration of primary tumor cells to lung tissue.
Lysyl oxidase (LOX) is another tumor-derived factor secreted in response to hypoxia, which has been shown to foster the development of a pre-metastatic niche [146]. LOX accumulation in the lung has been found to act on ECM proteins by crosslinking collagen IV in epithelial basement membranes and by recruiting CD11b+ myeloid cells to the pre-metastatic tissue, which adhere to the crosslinked collagen IV [147]. These myloid cells in turn produce MMP2 and enhance the recruitment and invasive capability of BMDCs and CTCs.
While the concept of pre-metastatic niche is still evolving, an abundance of evidence does indicate that the establishment of an inflammatory microenvironment at distant organ sites, either prior to or at the time of metastatic tumor cell arrival, predisposes the development of metastasis through enhanced survival and proliferation of tumor cells. The establishment of micrometastases at distant organ sites is a highly regulated process involving numerous cell intrinsic and extrinsic factors that dictate organ-specific colonization. The barriers to metastatic organ colonization and the composition of the microenvironment are unique to each tissue [118]. Increasing evidence suggests that the outgrowth of initial metastatic seeds can exhibit a period of latency before detection of overt metastasis for some tumor types. The gap between CTC organ colonization and the detection of macrometastases can require decades as the tumor cells must circumvent immune detection and “educate” the local microenvironment to support their growth and survival. Certainly cell intrinsic factors and metastasis-progression genes dictate to a large degree how successful tumor cells will be in colonizing the parenchyma of a specific tissue. Genes that were selected for early on may confer a selective advantage for growth in one tissue type versus another. For instance, it has been shown that osteoclast-mobilizing factors, such as PTHRP and IL-11, provide a selective growth advantage to metastatic cells in the bone microenvironment, yet provide no growth advantage in the primary tumor [118]. Likewise, the CXCR4 signaling axis has been shown to be very important for breast cancer metastasis to specific tissues [148], while CX3CR1 expression by pancreatic tumor cells mediates tumor cell spread specifically to peripheral neurons expressing the ligand CX3CL1 [149].
Organotropism may be innately tied to the concept of the pre-metastatic niche as certain tumors prime specific organs to support metastatic growth by altering adhesion molecules and ECM components of endothelial cells and fibroblasts in secondary tumor sites through the secretion of inflammatory chemokines, cytokines, and proteases (Figure 1) [9, 150–152]. Platelets have also been shown to play a key role in this process as described above and recent evidence shows that platelet-secreted SDF1 may alter the migration of CXCR4-expressing tumor cells as well as BMDCs to specific sites of metastasis in distant organs [153]. The mechanisms contributing to these microenvironmental changes are poorly understood, however, growing evidence suggests a key role for immune pathways in this process [12]. For instance, during early liver metastasis by melanoma cells in a mouse model, Mendoza and colleagues demonstrated that vascular arrest of CTCs on liver sinusoids triggers a local inflammatory response, wherein tumor-secreted VEGF induces inflammatory cytokines and VCAM-1 expression by sinusoidal endothelial cells, which further promotes CTC arrest and adhesion of metastatic melanoma cells [154]. Likewise, tumor-activated inflammation, cytokine secretion, and release of factors supporting angiogenesis, proliferation, and invasion from liver stellate cells, hepatocytes, and myofibroblasts has been shown to support the survival of CTCs in the liver [155]. In melanoma, 69% of metastatic tumor cells disseminating to the liver have been shown to ectopically express the FcγRIIB inhibitory receptor for IgG, thus terminating humoral activation signals initiated by cross-linking the B cell receptor with its inhibitory ITIM motif [156]. By hijacking this immune pathway, melanoma cells were found to escape humoral immunity, and this mechanism was found to promote liver metastasis by providing a decoy for antibodydependent cellular cytotoxicity (ADCC) that is mediated by macrophages, neutrophils, and NK cells [157]. Thus, the liver-specific colonization of metastatic melanoma may be promoted by the acquisition of immune inhibitory molecules and traits that aid establishment of metastasis in a heavily immune-monitored tissue. Furthermore, our group has previously shown in xenograft models of breast cancer metastasis that CCL2 signaling can mediate the organ-specific metastasis of human MDA-MB-231 breast cancer cells to the lung and bone by promoting macrophage infiltration and osteoclast differentiation, respectively [158].
Immune cell-secreted cytokines such as IL-6, IL-11, and TNFα are often implicated with metastasis specifically to the bone and bone marrow [12]. For instance, breast cancer cells often secrete these immune cytokines in addition to parathyroid-hormone-related peptide (PTHRP) to activate osteoclasts through RANKL, which has been shown to be a key mechanism of breast cancer bone metastasis [159]. Similarly, IL-6 secretion from bone-marrow stromal cells has been shown to promote osteolysis through RANKL signaling in osteoblasts, which has been shown to promote metastasis in neuroblastoma [160], while NF-κB signaling in breast cancer cells has been shown to promote osteolysis and bone metastasis by signaling osteoclasts through GM-CSF [161]. Furthermore, tumor-recruited immune glial cells have also been shown to promote tissue-specific breast cancer metastasis selectively in the brain in both mice and humans through tumor-induced immune inflammatory programs [162]. Moreover, TGF-β secretion in the inflammatory tumor microenvironment has been shown to upregulate ANGPTL4 expression on breast cancer cells, thereby facilitating their adhesion and arrest on the endothelium of blood vessels specifically in the lung, promoting both extravasation and metastatic growth [163].
It should be noted that organ tropism during metastasis may not be entirely dependent upon active homing of tumor cells to specific tissues, but rather, this process could simply reflect the proximity of the primary tumor to the secondary organ site as disseminating tumor cells become entrapped in local capillary beds. For instance breast tumor cells that have detached from a primary tumor spread predominantly to the local lymph nodes, lung, and bone, while colon cancer usually metastasizes to the liver by transit through the hepatic-portal blood vessels [9]. Further research is necessary to delineate the specific contributions of each of these mechanisms to organ-specific tropism during metastasis, however, it is highly likely that a combination of many intrinsic and extrinsic factors contribute to this process and that these factors are likely to be varied depending on the tumor type and secondary organ site. The linear tumor progression model that has been highlighted in the metastatic cascade described above is but one possibility for metastatic spread. Another, but not mutually exclusive model for metastatic progression, is the parallel progression model of metastasis, wherein tumor cells disseminate to secondary sites early on during malignant progression. Evidence for this model is eloquently discussed in other comprehensive reviews [164, 165].
Other stromal components contributing to metastasis through inflammation and immunosuppression
Cancer associated fibroblasts and immune suppression
While CAFs are not part of the immune stroma, they are some of the most important tumor-infiltrating stromal cells contributing to EMT and cancer progression via their secretion of pro-inflammatory cytokines [166, 167]. Normal mesenchymal stem cells (MSCs) are immune stimulatory, however, MSCs recruited to tumor tissues typically become immune-suppressive, inhibiting tumor infiltration of anti-tumor effector immune cells and reinforcing the immune-suppressive program in the tumor microenvironment. In other cases, resident MSCs or fibroblasts have been shown to acquire a tumor-promoting phenotype through synergistic associations with malignant or pre-malignant epithelial cells that become stressed due to DNA damage or telomere malfunction [168]. Factors, such as cytokines and growth factors, secreted from these stressed epithelial cells have been shown to “activate” resident fibroblasts, which promote epithelial cell motility [168]. Immune suppressive MSCs found in tumor tissue have been shown to secrete TGF-β and HGF, which suppresses T-cell proliferation, indoleamine 2,3-dioxygenase, which mediates apoptosis of activated T cells, HLA-G5 found to promote the expansion of Tregs, and inhibit DC differentiation through the loss of TNF-α, IFNγ, and IL-12 [166]. Overall, CAFs clearly polarize the tumor microenvironment to an immunosuppressive Th2 phenotypic profile. Indeed, the elimination of CAFs in a 4T1 model of breast cancer metastasis has been shown to promote anti-tumor activity, shift the immune phenotype from Th2 to Th1, reduce macrophage, MDSC, and Treg recruitment, and inhibit angiogenesis and lymphangiogenesis [169].
MSCs are recruited to malignant tissues by endocrine and paracrine signaling from tumor cells [170]. Although it is has been demonstrated that CXCR4, CXCR12, and CCL2 molecules play a major role in MSC tumor homing, the exact mechanism of tumor MSC recruitment is still not completely understood [171]. However, once MSCs are recruited to the tumor microenvironment, crosstalk between the tumor cells and stroma stimulates MSCs to differentiate into tumor-promoting CAFs. These stormal cells have been shown to secrete IL-6 [172, 173], angiopoietin 1 (Ang1) [174], and VEGF [175] that provide signals for growth, tissue regeneration, and angiogenesis, which promotes immune infiltration by providing new routes to the tumor environment. Simultaneously, CAFs also exhibit a strongly pro-inflammatory phenotype by actively secreting CCL5 (also called RANTES) and IL-17B[166]. Karnoub et al., demonstrated in a xenograft model of breast cancer that CCL-5 secreted from MSCs activates Akt signaling in tumor cells and induces their extravasation from the circulation allowing colonization at distal organ sites [176]. Likewise, TGF-β-induced IL-17B secretion by MSC-derived CAFs has been shown to also promote increased tumor cell migration, invasion and metastasis to the lungs and liver in xenograft mice inoculated with MDA-MB-231 and SUM315 breast cancer cells overexpressing ectopic IL-17B [177]. The function of IL-17B is largely unknown, however, high levels of IL-17B expression have been found in metastatic tissues and this expression is associated with worse patient prognosis[178]. There are five additional IL-17 family member proteins produced by various subsets of immune cells, which are structurally related and can possess both immune stimulatory and immune suppressive activity depending on the local cytokine milieu and the various immune cells on which they act [179, 180]. For instance, IL-17A+ CD4+ T cells recruited to the tumor microenvironment have been shown to promote angiogenesis and tumor growth, by increasing fibroblast expression of pro-angiogenic cytokines, stimulation of resident macrophages to produce pro-angiogenic cytokines in a TNFα-dependent manner, and IL-17A has been shown to independently increase the mitogenic activity of bEGF, HGF, and VEGF for vascular endothelial cells [181–183]. In contrast, IL-17B and C have been shown to induce neutrophil tumor infiltration by inducing TNF and IL-1β expression in monocytes [115, 184]. In addition to pro-angiogenic effects, IL-17A-secreting Th17 immune cells have been shown to recruit neutrophils to the tumor microvenvironment through IL-8 production, which is positively associated with tumor progression, lymph node invasion, and metastatic spread [185, 186]. The role of IL-17B is largely unknown, however, it does appear that MSC secretion of IL-17B could facilitate the metastatic process by promoting tumor angiogenesis and the recruitment of neutrophils to the metastatic site.
Immune-mediated angiogenesis
During progression to metastasis, tumor growth inevitably outpaces the available blood supply, thereby limiting access to oxygen and nutrients, which ultimately leads to tumor hypoxia. As the tumor becomes oxygen-starved, hypoxic signals are elicited to trigger neoangiogenesis. However, the developing tumor-associated blood vessels are poorly organized, lacking the classic hierarchical arteriole-to-capillary-to-venule formations, and are highly leaky, which results in an increase in hypoxia and interstitial fluid pressure and drop in pH [187–189]. The impact of this aberrant vasculature essentially inhibits the efficient extravasation of cytotoxic lymphocytes with effector functionality into the tumor microenvironment [188]. BMDCs such as macrophages, MDSCs, neutrophils, and mast cells have all been shown to contribute to angiogenesis through the production of cytokines, growth factors, and proteases, especially VEGF-A, PROK2, and MMPs [21, 100, 190–192].
Hypoxia also supports the development of angiogenesis by stimulating hypoxia-inducible factor 1 alpha (HIF1α) signaling in cancer cells to release vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF), which activate endothelial cells to initiate blood vessel growth in a chemokine-directed gradient [193]. Angiogenesis is also dependent upon recruitment of TAMs into cancer lesions, which sense hypoxic signals and produce chemokines and proangiogenic factors [147, 194]. Two key angiogenic mediatiors, angiopoetin 2 and VEGF, recruit both TAM progenitor cells and MDSCs, which regulate several important proangiogenesis genes including CXCL1, CXCL8, VEGF, IL-8, and HIF1α through NF-κB, STAT3, and AP-1 signaling [36, 194]. These findings are consistent with the clinical association found between high TAM infiltration and poor outcome in cancer patients [20].
Shojaei, et al, recently demonstrated in both murine models and human patients that the anti-angiogenic effect elicited by blocking VEGF-mediated angiogenesis was curtailed by infiltration of CD11b+Gr1+ myeloid cells into the tumor tissue [195]. These results suggested that refractory tumor growth in response to inhibition of angiogenesis is associated with tumor infiltration of TAM and MDSC myeloid-like cells or precursors. Indeed, a series of elegant studies by De Palma and Naldini have conclusively shown that Tie-2-expressing macrophages promote angiogenesis through the secretion of several pro-angiogenic enzymes including thymidine phosphorylase (TP) and cathepsin B (CTSB) [191, 196, 197]. Furthermore, the pro-angiogenic capacity of these myeloid cells was enhanced by exposure to tumor-derived angiopoietin-2 [196, 197]. Additional studies revealed that Tie-2-expressing monocytes/macrophages are also critical for maintaining the viability of newly-formed tumor blood vessels [198], and that this tumor-promoting activity could be blocked with IFN-α treatment [199]. Interestingly, studies by Lyden, et al, have demonstrated that endothelial and hematopoietic progenitor cells of myeloid origin contribute to the vasculature of growing tumors by direct incorporation into the growing blood vessels, promoting enhanced tumor growth and spread [200].
It is still not entirely clear whether the dominant driver of angiogenesis is hypoxic signals or factors secreted by infiltrating immune cells in response to inflammation-induced hypoxia. In either case, angiogenesis serves multiple purposes for the progression of tumor development as it not only supports primary tumor growth via increased availability to nutrients; it also allows greater access for tumor-promoting immune cell infiltration, and increases the probability of metastasis by providing new routes for intravasation into the circulation.
Potential for therapy and concluding remarks
The crosstalk that occurs between tumor and immune cells within the tumor microenvironment, the circulation, or at distant metastatic sites has been clearly shown to foster metastatic dissemination. Immune cells as well as the suppressive factors that they secrete represent potential targets for therapeutic intervention. Regardless of their source, cytokines, chemokines, proteases, and growth factors are some of the main factors contributing to immunosuppression and immune-mediated tumor progression. These molecules can be produced by immune, stromal, or malignant cells and can act in paracrine and autocrine fashion to promote each stage of tumor cell invasion and metastasis by enhancing inflammation, angiogenesis, tumor proliferation, and recruitment of additional immunosuppressive and tumor-promoting immune cells. These secreted factors provide the malignant cells with an abundant source of growth and survival signals that perpetuate a supportive microenvironment for tumor metastasis and represent some of the most attractive targets for directed anti-tumor therapy. Immune pathways provide numerous soluble targets for cancer treatment, and indeed, many drugs to target immune-suppressive molecules are moving forward in clinical trials. For instance, the anti-RANKL (Denosumab) antibody has been shown to effectively inhibit bone metastasis in prostate cancer patients [201], while a variety of neutralizing antibodies to IL-1β and IL-1 receptor have been shown to have efficacy in treating metastasis in pre-clinical animal models [202]. Several agents that target IL-1 or other immune-suppressive cytokines are already approved for the treatment of some inflammatory diseases and are prime candidates for human trails [202]. Additionally, other proteins involved in tumor progression that are induced directly or indirectly by immune cell populations, such as EMT-associated transcription factors, adhesion molecules, and tumor receptors and ligands which mediate immune suppression, could also be targeted with small molecules or blocking antibodies. Antibodies against two surface molecules expressed by suppressive lymphoid cells, anti-CTLA-4 (ipiliumimab) [203, 204] and anti-PD-1 have been recently gaining increasing support from clinical trials for their effective treatment for many forms of cancer including advanced melanoma and prostate cancer [205, 206]. Specifically, anti-CTLA-4 has been shown to be particularly efficacious in metastatic melanoma, while anti-PD-1 has only just begun a comprehensive evaluation in clinical trials [204, 207]. Likewise, non-steroidal anti-inflammatory drugs (NSAIDS) to prevent or treat chronic inflammation and lymphangiogenesis [208–210], and anti-coagulants to prevent platelet aggregation on circulating tumor cells [211] are just two examples of a multitude of therapeutic agents that could be utilized to prevent immune-mediated tumor progression at unique stages of metastasis. Of course, new methods or biomarkers for the detection of patients at risk of tumor progression or metastasis are also desperately needed to tailor personalized therapy for patients to obtain the best possible clinical outcome.
Thanks to diligent work by numerous researchers we now have a growing appreciation for the impact that the immune system can have on immunosuppression and tumor promotion during progression to metastasis. These cells and secreted factors may be ideal candidates for therapeutic targeting, however a difficulty arises in that these molecules can also be expressed by healthy cells and tissues and in some cases may help prevent other pathologic states such as autoimmune diseases. A better understanding of the recruitment and polarization of the immune stroma to a tumor-promoting and immune-suppressive phenotype will be critical in the treatment and prevention of metastatic progression. In particular, a greater understanding of the factors involved in the later stages of tumor progression, such as tumor cell circulation, extravasation, and colonization, as well as the establishment of the pre-metastatic niche, will be paramount in our ability to prevent the spread of malignant cells and improve the outcome for patients with this devastating stage of disease.
Footnotes
Disclosure
The authors declare that they have no conflict of interests.
References
- 1.Ruffell B, DeNardo DG, Affara NI, Coussens LM. Lymphocytes in cancer development: polarization towards pro-tumor immunity. Cytokine & growth factor reviews. 2010;21:3–10. doi: 10.1016/j.cytogfr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Lora A, Algarra I, Garrido F. MHC class I antigens, immune surveillance, and tumor immune escape. Journal of cellular physiology. 2003;195:346–355. doi: 10.1002/jcp.10290. [DOI] [PubMed] [Google Scholar]
- 3.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes? Current opinion in immunology. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nature reviews Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 5.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Current opinion in genetics & development. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galli SJ, Borregaard N, Wynn TA. Phenotypic and functional plasticity of cells of innate immunity: macrophages, mast cells and neutrophils. Nature immunology. 2011;12:1035–1044. doi: 10.1038/ni.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakayamada S, Takahashi H, Kanno Y, O'Shea JJ. Helper T cell diversity and plasticity. Current opinion in immunology. 2012;24:297–302. doi: 10.1016/j.coi.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanca T, Silva-Santos B. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. 2012;1:717–725. doi: 10.4161/onci.20068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Lowe DB, Storkus WJ. Chronic inflammation and immunologic-based constraints in malignant disease. Immunotherapy. 2011;3:1265–1274. doi: 10.2217/imt.11.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erez N, Coussens LM. Leukocytes as paracrine regulators of metastasis and determinants of organ-specific colonization. International journal of cancer Journal international du cancer. 2011;128:2536–2544. doi: 10.1002/ijc.26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England journal of medicine. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 14.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 15.Waldner MJ, Neurath MF. Colitis-associated cancer: the role of T cells in tumor development. Seminars in immunopathology. 2009;31:249–256. doi: 10.1007/s00281-009-0161-8. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 17.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 18.Wu S, Rhee KJ, Albesiano E, Rabizadeh S, Wu X, Yen HR, Huso DL, Brancati FL, Wick E, McAllister F, et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature medicine. 2009;15:1016–1022. doi: 10.1038/nm.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 20.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soucek L, Lawlor ER, Soto D, Shchors K, Swigart LB, Evan GI. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nature medicine. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 22.Sparmann A, Bar-Sagi D. Ras-induced interleukin-8 expression plays a critical role in tumor growth and angiogenesis. Cancer cell. 2004;6:447–458. doi: 10.1016/j.ccr.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 23.Vakkila J, Lotze MT. Inflammation and necrosis promote tumour growth. Nature reviews Immunology. 2004;4:641–648. doi: 10.1038/nri1415. [DOI] [PubMed] [Google Scholar]
- 24.Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annual review of immunology. 2007;25:267–296. doi: 10.1146/annurev.immunol.25.022106.141609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nature reviews Immunology. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostrand-Rosenberg S. Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer immunology, immunotherapy : CII. 2010;59:1593–1600. doi: 10.1007/s00262-010-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 28.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, et al. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nature medicine. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 29.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature medicine. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 30.Olkhanud PB, Damdinsuren B, Bodogai M, Gress RE, Sen R, Wejksza K, Malchinkhuu E, Wersto RP, Biragyn A. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4(+) T cells to T-regulatory cells. Cancer research. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sica A, Porta C, Riboldi E, Locati M. Convergent pathways of macrophage polarization: The role of B cells. European journal of immunology. 2010;40:2131–2133. doi: 10.1002/eji.201040736. [DOI] [PubMed] [Google Scholar]
- 32.Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. The American journal of pathology. 2007;170:774–786. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruddell A, Harrell MI, Furuya M, Kirschbaum SB, Iritani BM. B lymphocytes promote lymphogenous metastasis of lymphoma and melanoma. Neoplasia. 2011;13:748–757. doi: 10.1593/neo.11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjordahl RL, Gapin L, Marrack P, Refaeli Y. iNKT cells suppress the CD8+ T cell response to a murine Burkitt's-like B cell lymphoma. PloS one. 2012;7:e42635. doi: 10.1371/journal.pone.0042635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nature reviews Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 38.Kalluri R. EMT: when epithelial cells decide to become mesenchymal-like cells. The Journal of clinical investigation. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stockinger A, Eger A, Wolf J, Beug H, Foisner R. E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. The Journal of cell biology. 2001;154:1185–1196. doi: 10.1083/jcb.200104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang Y, Massague J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–279. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 41.Zhou C, Liu J, Tang Y, Liang X. Inflammation linking EMT and cancer stem cells. Oral oncology. 2012;48:1068–1075. doi: 10.1016/j.oraloncology.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 43.Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741–4751. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lopez-Novoa JM, Nieto MA. Inflammation and EMT: an alliance towards organ fibrosis and cancer progression. EMBO molecular medicine. 2009;1:303–314. doi: 10.1002/emmm.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell. 2011;145:926–940. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes & development. 1996;10:2462–2477. doi: 10.1101/gad.10.19.2462. [DOI] [PubMed] [Google Scholar]
- 47.Bates RC, Mercurio AM. Tumor necrosis factor-alpha stimulates the epithelial-to-mesenchymal transition of human colonic organoids. Molecular biology of the cell. 2003;14:1790–1800. doi: 10.1091/mbc.E02-09-0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, Isaacs JT, Berman DM, Beachy PA. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 49.Ren G, Zhao X, Wang Y, Zhang X, Chen X, Xu C, Yuan ZR, Roberts AI, Zhang L, Zheng B, et al. CCR2-Dependent Recruitment of Macrophages by Tumor-Educated Mesenchymal Stromal Cells Promotes Tumor Development and Is Mimicked by TNFalpha. Cell stem cell. 2012 doi: 10.1016/j.stem.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu Y, Deng J, Rychahou PG, Qiu S, Evers BM, Zhou BP. Stabilization of snail by NF-kappaB is required for inflammation-induced cell migration and invasion. Cancer cell. 2009;15:416–428. doi: 10.1016/j.ccr.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maier HJ, Schmidt-Strassburger U, Huber MA, Wiedemann EM, Beug H, Wirth T. NF-kappaB promotes epithelial-mesenchymal transition, migration and invasion of pancreatic carcinoma cells. Cancer letters. 2010;295:214–228. doi: 10.1016/j.canlet.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC. Dual regulation of Snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nature cell biology. 2004;6:931–940. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 53.Cheng GZ, Zhang WZ, Sun M, Wang Q, Coppola D, Mansour M, Xu LM, Costanzo C, Cheng JQ, Wang LH. Twist is transcriptionally induced by activation of STAT3 and mediates STAT3 oncogenic function. The Journal of biological chemistry. 2008;283:14665–14673. doi: 10.1074/jbc.M707429200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature reviews Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, Hall BM. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28:2940–2947. doi: 10.1038/onc.2009.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y. Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer cell. 2009;15:195–206. doi: 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 57.Kudo-Saito C, Shirako H, Ohike M, Tsukamoto N, Kawakami Y. CCL2 is critical for immunosuppression to promote cancer metastasis. Clinical & experimental metastasis. 2012 doi: 10.1007/s10585-012-9545-6. [DOI] [PubMed] [Google Scholar]
- 58.Bachelder RE, Yoon SO, Franci C, de Herreros AG, Mercurio AM. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. The Journal of cell biology. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26:711–724. doi: 10.1038/sj.onc.1209808. [DOI] [PubMed] [Google Scholar]
- 60.Barbera MJ, Puig I, Dominguez D, Julien-Grille S, Guaita-Esteruelas S, Peiro S, Baulida J, Franci C, Dedhar S, Larue L, et al. Regulation of Snail transcription during epithelial to mesenchymal transition of tumor cells. Oncogene. 2004;23:7345–7354. doi: 10.1038/sj.onc.1207990. [DOI] [PubMed] [Google Scholar]
- 61.Julien S, Puig I, Caretti E, Bonaventure J, Nelles L, van Roy F, Dargemont C, de Herreros AG, Bellacosa A, Larue L. Activation of NF-kappaB by Akt upregulates Snail expression and induces epithelium mesenchyme transition. Oncogene. 2007;26:7445–7456. doi: 10.1038/sj.onc.1210546. [DOI] [PubMed] [Google Scholar]
- 62.Lin K, Baritaki S, Militello L, Malaponte G, Bevelacqua Y, Bonavida B. The Role of B-RAF Mutations in Melanoma and the Induction of EMT via Dysregulation of the NF-kappaB/Snail/RKIP/PTEN Circuit. Genes & cancer. 2010;1:409–420. doi: 10.1177/1947601910373795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baritaki S, Chapman A, Yeung K, Spandidos DA, Palladino M, Bonavida B. Inhibition of epithelial to mesenchymal transition in metastatic prostate cancer cells by the novel proteasome inhibitor, NPI-0052: pivotal roles of Snail repression and RKIP induction. Oncogene. 2009;28:3573–3585. doi: 10.1038/onc.2009.214. [DOI] [PubMed] [Google Scholar]
- 64.Li QQ, Chen ZQ, Cao XX, Xu JD, Xu JW, Chen YY, Wang WJ, Chen Q, Tang F, Liu XP, Xu ZD. Involvement of NF-kappaB/miR-448 regulatory feedback loop in chemotherapy-induced epithelial-mesenchymal transition of breast cancer cells. Cell death and differentiation. 2011;18:16–25. doi: 10.1038/cdd.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blackwell TS, Christman JW. The role of nuclear factor-kappa B in cytokine gene regulation. American journal of respiratory cell and molecular biology. 1997;17:3–9. doi: 10.1165/ajrcmb.17.1.f132. [DOI] [PubMed] [Google Scholar]
- 66.Massague J. TGFbeta in Cancer. Cell. 2008;134:215–230. doi: 10.1016/j.cell.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fuxe J, Karlsson MC. TGF-beta-induced epithelial-mesenchymal transition: a link between cancer and inflammation. Seminars in cancer biology. 2012;22:455–461. doi: 10.1016/j.semcancer.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 68.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature reviews Immunology. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 70.Sullivan DE, Ferris M, Nguyen H, Abboud E, Brody AR. TNF-alpha induces TGF-beta1 expression in lung fibroblasts at the transcriptional level via AP-1 activation. Journal of cellular and molecular medicine. 2009;13:1866–1876. doi: 10.1111/j.1582-4934.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 72.Peinado H, Quintanilla M, Cano A. Transforming growth factor beta-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. The Journal of biological chemistry. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 73.de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Devedec SE, Smit VT, van der Wal A, et al. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6340–6345. doi: 10.1073/pnas.0913360107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Papageorgis P, Lambert AW, Ozturk S, Gao F, Pan H, Manne U, Alekseyev YO, Thiagalingam A, Abdolmaleky HM, Lenburg M, Thiagalingam S. Smad signaling is required to maintain epigenetic silencing during breast cancer progression. Cancer research. 2010;70:968–978. doi: 10.1158/0008-5472.CAN-09-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bakin AV, Rinehart C, Tomlinson AK, Arteaga CL. p38 mitogen-activated protein kinase is required for TGFbeta-mediated fibroblastic transdifferentiation and cell migration. Journal of cell science. 2002;115:3193–3206. doi: 10.1242/jcs.115.15.3193. [DOI] [PubMed] [Google Scholar]
- 76.Perlman R, Schiemann WP, Brooks MW, Lodish HF, Weinberg RA. TGF-beta-induced apoptosis is mediated by the adapter protein Daxx that facilitates JNK activation. Nature cell biology. 2001;3:708–714. doi: 10.1038/35087019. [DOI] [PubMed] [Google Scholar]
- 77.Zavadil J, Bitzer M, Liang D, Yang YC, Massimi A, Kneitz S, Piek E, Bottinger EP. Genetic programs of epithelial cell plasticity directed by transforming growth factor-beta. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:6686–6691. doi: 10.1073/pnas.111614398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arsura M, Panta GR, Bilyeu JD, Cavin LG, Sovak MA, Oliver AA, Factor V, Heuchel R, Mercurio F, Thorgeirsson SS, Sonenshein GE. Transient activation of NF-kappaB through a TAK1/IKK kinase pathway by TGF-beta1 inhibits AP-1/SMAD signaling and apoptosis: implications in liver tumor formation. Oncogene. 2003;22:412–425. doi: 10.1038/sj.onc.1206132. [DOI] [PubMed] [Google Scholar]
- 79.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nature cell biology. 2010;12:286–293. doi: 10.1038/ncb2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neil JR, Schiemann WP. Altered TAB1:I kappaB kinase interaction promotes transforming growth factor beta-mediated nuclear factor-kappaB activation during breast cancer progression. Cancer research. 2008;68:1462–1470. doi: 10.1158/0008-5472.CAN-07-3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park JI, Lee MG, Cho K, Park BJ, Chae KS, Byun DS, Ryu BK, Park YK, Chi SG. Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene. 2003;22:4314–4332. doi: 10.1038/sj.onc.1206478. [DOI] [PubMed] [Google Scholar]
- 82.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 83.Rajasekaran SA, Huynh TP, Wolle DG, Espineda CE, Inge LJ, Skay A, Lassman C, Nicholas SB, Harper JF, Reeves AE, et al. Na,K-ATPase subunits as markers for epithelial-mesenchymal transition in cancer and fibrosis. Molecular cancer therapeutics. 2010;9:1515–1524. doi: 10.1158/1535-7163.MCT-09-0832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nature reviews Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 85.Natsuizaka M, Ohashi S, Wong GS, Ahmadi A, Kalman RA, Budo D, Klein-Szanto AJ, Herlyn M, Diehl JA, Nakagawa H. Insulin-like growth factor-binding protein-3 promotes transforming growth factor-{beta}1-mediated epithelial-to-mesenchymal transition and motility in transformed human esophageal cells. Carcinogenesis. 2010;31:1344–1353. doi: 10.1093/carcin/bgq108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kong B, Michalski CW, Hong X, Valkovskaya N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H, Kleeff J. AZGP1 is a tumor suppressor in pancreatic cancer inducing mesenchymal-to-epithelial transdifferentiation by inhibiting TGF-beta-mediated ERK signaling. Oncogene. 2010;29:5146–5158. doi: 10.1038/onc.2010.258. [DOI] [PubMed] [Google Scholar]
- 87.Micalizzi DS, Wang CA, Farabaugh SM, Schiemann WP, Ford HL. Homeoprotein Six1 increases TGF-beta type I receptor and converts TGF-beta signaling from suppressive to supportive for tumor growth. Cancer research. 2010;70:10371–10380. doi: 10.1158/0008-5472.CAN-10-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braun J, Hoang-Vu C, Dralle H, Huttelmaier S. Downregulation of microRNAs directs the EMT and invasive potential of anaplastic thyroid carcinomas. Oncogene. 2010;29:4237–4244. doi: 10.1038/onc.2010.169. [DOI] [PubMed] [Google Scholar]
- 89.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. The Journal of biological chemistry. 2008;283:14910–14914. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature cell biology. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 91.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, et al. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Molecular biology of the cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO reports. 2008;9:582–589. doi: 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & development. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu S, Si ML, Wu H, Mo YY. MicroRNA-21 targets the tumor suppressor gene tropomyosin 1 (TPM1) The Journal of biological chemistry. 2007;282:14328–14336. doi: 10.1074/jbc.M611393200. [DOI] [PubMed] [Google Scholar]
- 95.Kong W, Yang H, He L, Zhao JJ, Coppola D, Dalton WS, Cheng JQ. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Molecular and cellular biology. 2008;28:6773–6784. doi: 10.1128/MCB.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- 97.Berdowska I. Cysteine proteases as disease markers. Clinica chimica acta; international journal of clinical chemistry. 2004;342:41–69. doi: 10.1016/j.cccn.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 98.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 100.Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes & development. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xiang M, Gu Y, Zhao F, Lu H, Chen S, Yin L. Mast cell tryptase promotes breast cancer migration and invasion. Oncology reports. 2010;23:615–619. doi: 10.3892/or_00000676. [DOI] [PubMed] [Google Scholar]
- 104.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 105.Vasiljeva O, Papazoglou A, Kruger A, Brodoefel H, Korovin M, Deussing J, Augustin N, Nielsen BS, Almholt K, Bogyo M, et al. Tumor cell-derived and macrophage-derived cathepsin B promotes progression and lung metastasis of mammary cancer. Cancer research. 2006;66:5242–5250. doi: 10.1158/0008-5472.CAN-05-4463. [DOI] [PubMed] [Google Scholar]
- 106.Mohamed MM, Cavallo-Medved D, Rudy D, Anbalagan A, Moin K, Sloane BF. Interleukin-6 increases expression and secretion of cathepsin B by breast tumor-associated monocytes. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2010;25:315–324. doi: 10.1159/000276564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li Y, Yang J, Dai C, Wu C, Liu Y. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. The Journal of clinical investigation. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Role of basic fibroblast growth factor-2 in epithelial-mesenchymal transformation. Kidney international. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 109.Kitamura T, Kometani K, Hashida H, Matsunaga A, Miyoshi H, Hosogi H, Aoki M, Oshima M, Hattori M, Takabayashi A, et al. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nature genetics. 2007;39:467–475. doi: 10.1038/ng1997. [DOI] [PubMed] [Google Scholar]
- 110.Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, Berman T, Joyce JA. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes & development. 2010;24:241–255. doi: 10.1101/gad.1874010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 112.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 113.Sevenich L, Werner F, Gajda M, Schurigt U, Sieber C, Muller S, Follo M, Peters C, Reinheckel T. Transgenic expression of human cathepsin B promotes progression and metastasis of polyoma-middle-T-induced breast cancer in mice. Oncogene. 2011;30:54–64. doi: 10.1038/onc.2010.387. [DOI] [PubMed] [Google Scholar]
- 114.Kawakubo T, Okamoto K, Iwata J, Shin M, Okamoto Y, Yasukochi A, Nakayama KI, Kadowaki T, Tsukuba T, Yamamoto K. Cathepsin E prevents tumor growth and metastasis by catalyzing the proteolytic release of soluble TRAIL from tumor cell surface. Cancer research. 2007;67:10869–10878. doi: 10.1158/0008-5472.CAN-07-2048. [DOI] [PubMed] [Google Scholar]
- 115.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. The Journal of experimental medicine. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–106. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nature reviews Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 119.Gassmann P, Haier J. The tumor cell-host organ interface in the early onset of metastatic organ colonisation. Clinical & experimental metastasis. 2008;25:171–181. doi: 10.1007/s10585-007-9130-6. [DOI] [PubMed] [Google Scholar]
- 120.Luo JL, Maeda S, Hsu LC, Yagita H, Karin M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer cell. 2004;6:297–305. doi: 10.1016/j.ccr.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 121.Pawelek JM, Chakraborty AK. Fusion of tumour cells with bone marrow-derived cells: a unifying explanation for metastasis. Nature reviews Cancer. 2008;8:377–386. doi: 10.1038/nrc2371. [DOI] [PubMed] [Google Scholar]
- 122.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 123.Im JH, Fu W, Wang H, Bhatia SK, Hammer DA, Kowalska MA, Muschel RJ. Coagulation facilitates tumor cell spreading in the pulmonary vasculature during early metastatic colony formation. Cancer research. 2004;64:8613–8619. doi: 10.1158/0008-5472.CAN-04-2078. [DOI] [PubMed] [Google Scholar]
- 124.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Hu Z, Barney KA, Degen JL. Tumor cell-associated tissue factor and circulating hemostatic factors cooperate to increase metastatic potential through natural killer cell-dependent and-independent mechanisms. Blood. 2007;110:133–141. doi: 10.1182/blood-2007-01-065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jurasz P, Alonso-Escolano D, Radomski MW. Platelet--cancer interactions: mechanisms and pharmacology of tumour cell-induced platelet aggregation. British journal of pharmacology. 2004;143:819–826. doi: 10.1038/sj.bjp.0706013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nash GF, Turner LF, Scully MF, Kakkar AK. Platelets and cancer. The lancet oncology. 2002;3:425–430. doi: 10.1016/s1470-2045(02)00789-1. [DOI] [PubMed] [Google Scholar]
- 127.Bonecchi R, Galliera E, Borroni EM, Corsi MM, Locati M, Mantovani A. Chemokines and chemokine receptors: an overview. Frontiers in bioscience : a journal and virtual library. 2009;14:540–551. doi: 10.2741/3261. [DOI] [PubMed] [Google Scholar]
- 128.McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE. Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. International journal of cancer Journal international du cancer. 2009;125:1298–1305. doi: 10.1002/ijc.24409. [DOI] [PubMed] [Google Scholar]
- 129.Slattery MJ, Dong C. Neutrophils influence melanoma adhesion and migration under flow conditions. International journal of cancer Journal international du cancer. 2003;106:713–722. doi: 10.1002/ijc.11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Slattery MJ, Liang S, Dong C. Distinct role of hydrodynamic shear in leukocyte-facilitated tumor cell extravasation. American journal of physiology Cell physiology. 2005;288:C831–839. doi: 10.1152/ajpcell.00439.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P, Ferri LE. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer research. 2012;72:3919–3927. doi: 10.1158/0008-5472.CAN-11-2393. [DOI] [PubMed] [Google Scholar]
- 132.Oppenheimer SB. Cellular basis of cancer metastasis: A review of fundamentals and new advances. Acta histochemica. 2006;108:327–334. doi: 10.1016/j.acthis.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 133.Wu TC. The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer research. 2007;67:6003–6006. doi: 10.1158/0008-5472.CAN-07-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lin KY, Lu D, Hung CF, Peng S, Huang L, Jie C, Murillo F, Rowley J, Tsai YC, He L, Kim DJ, Jaffee E, Pardoll D, Wu TC. Ectopic expression of vascular cell adhesion molecule-1 as a new mechanism for tumor immune evasion. Cancer research. 2007;67:1832–1841. doi: 10.1158/0008-5472.CAN-06-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chen Q, Zhang XH, Massague J. Macrophage binding to receptor VCAM-1 transmits survival signals in breast cancer cells that invade the lungs. Cancer cell. 2011;20:538–549. doi: 10.1016/j.ccr.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M, Yan J, Hua Y, Tiede BJ, Haffty BG, et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging alpha4beta1-positive osteoclast progenitors. Cancer cell. 2011;20:701–714. doi: 10.1016/j.ccr.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kochetkova M, Kumar S, McColl SR. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell death and differentiation. 2009;16:664–673. doi: 10.1038/cdd.2008.190. [DOI] [PubMed] [Google Scholar]
- 138.Das S, Skobe M. Lymphatic vessel activation in cancer. Annals of the New York Academy of Sciences. 2008;1131:235–241. doi: 10.1196/annals.1413.021. [DOI] [PubMed] [Google Scholar]
- 139.Saharinen P, Tammela T, Karkkainen MJ, Alitalo K. Lymphatic vasculature: development, molecular regulation and role in tumor metastasis and inflammation. Trends in immunology. 2004;25:387–395. doi: 10.1016/j.it.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 140.Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the 'pre-metastatic niche': within bone and beyond. Cancer metastasis reviews. 2006;25:521–529. doi: 10.1007/s10555-006-9036-9. [DOI] [PubMed] [Google Scholar]
- 141.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yan HH, Pickup M, Pang Y, Gorska AE, Li Z, Chytil A, Geng Y, Gray JW, Moses HL, Yang L. Gr-1+CD11b+ myeloid cells tip the balance of immune protection to tumor promotion in the premetastatic lung. Cancer research. 2010;70:6139–6149. doi: 10.1158/0008-5472.CAN-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Olkhanud PB, Baatar D, Bodogai M, Hakim F, Gress R, Anderson RL, Deng J, Xu M, Briest S, Biragyn A. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer research. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature cell biology. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 145.Hiratsuka S, Watanabe A, Sakurai Y, Akashi-Takamura S, Ishibashi S, Miyake K, Shibuya M, Akira S, Aburatani H, Maru Y. The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a pre-metastatic phase. Nature cell biology. 2008;10:1349–1355. doi: 10.1038/ncb1794. [DOI] [PubMed] [Google Scholar]
- 146.Erler JT, Bennewith KL, Nicolau M, Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS, Giaccia AJ. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 147.Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer metastasis reviews. 2010;29:285–293. doi: 10.1007/s10555-010-9224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, Zhou X, Xia W, Hortobagyi GN, Yu D, Hung MC. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 149.Marchesi F, Piemonti L, Fedele G, Destro A, Roncalli M, Albarello L, Doglioni C, Anselmo A, Doni A, Bianchi P, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer research. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 150.Psaila B, Kaplan RN, Port ER, Lyden D. Priming the 'soil' for breast cancer metastasis: the pre-metastatic niche. Breast disease. 2006;26:65–74. doi: 10.3233/bd-2007-26106. [DOI] [PubMed] [Google Scholar]
- 151.Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Seminars in cancer biology. 2011;21:139–146. doi: 10.1016/j.semcancer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 152.Rucci N, Sanita P, Angelucci A. Roles of metalloproteases in metastatic niche. Current molecular medicine. 2011;11:609–622. doi: 10.2174/156652411797536705. [DOI] [PubMed] [Google Scholar]
- 153.Jin DK, Shido K, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nature medicine. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mendoza L, Valcarcel M, Carrascal T, Egilegor E, Salado C, Sim BK, Vidal-Vanaclocha F. Inhibition of cytokine-induced microvascular arrest of tumor cells by recombinant endostatin prevents experimental hepatic melanoma metastasis. Cancer research. 2004;64:304–310. doi: 10.1158/0008-5472.can-03-1829. [DOI] [PubMed] [Google Scholar]
- 155.Vidal-Vanaclocha F. The prometastatic microenvironment of the liver. Cancer microenvironment : official journal of the International Cancer Microenvironment Society. 2008;1:113–129. doi: 10.1007/s12307-008-0011-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown KS, Blair D, Reid SD, Nicholson EK, Harnett MM. FcgammaRIIb-mediated negative regulation of BCR signalling is associated with the recruitment of the MAPkinasephosphatase, Pac-1, and the 3'-inositol phosphatase, PTEN. Cellular signalling. 2004;16:71–80. doi: 10.1016/s0898-6568(03)00113-x. [DOI] [PubMed] [Google Scholar]
- 157.Cohen-Solal JF, Cassard L, Fournier EM, Loncar SM, Fridman WH, Sautes-Fridman C. Metastatic melanomas express inhibitory low affinity fc gamma receptor and escape humoral immunity. Dermatology research and practice. 2010;2010:657406. doi: 10.1155/2010/657406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lu X, Kang Y. Chemokine (C-C motif) ligand 2 engages CCR2+ stromal cells of monocytic origin to promote breast cancer metastasis to lung and bone. The Journal of biological chemistry. 2009;284:29087–29096. doi: 10.1074/jbc.M109.035899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nature reviews Cancer. 2002;2:584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 160.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46:1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T, Gu K, Shah V, Pei L, Zarbo RJ, et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nature medicine. 2007;13:62–69. doi: 10.1038/nm1519. [DOI] [PubMed] [Google Scholar]
- 162.Fitzgerald DP, Palmieri D, Hua E, Hargrave E, Herring JM, Qian Y, Vega-Valle E, Weil RJ, Stark AM, Vortmeyer AO, Steeg PS. Reactive glia are recruited by highly proliferative brain metastases of breast cancer and promote tumor cell colonization. Clinical & experimental metastasis. 2008;25:799–810. doi: 10.1007/s10585-008-9193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Husemann Y, Geigl JB, Schubert F, Musiani P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmuller G, Klein CA. Systemic spread is an early step in breast cancer. Cancer cell. 2008;13:58–68. doi: 10.1016/j.ccr.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 164.Klein CA. Parallel progression of primary tumours and metastases. Nature reviews Cancer. 2009;9:302–312. doi: 10.1038/nrc2627. [DOI] [PubMed] [Google Scholar]
- 165.Sethi N, Kang Y. Unravelling the complexity of metastasis - molecular understanding and targeted therapies. Nature reviews Cancer. 2011;11:735–748. doi: 10.1038/nrc3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koh BI, Kang Y. The pro-metastatic role of bone marrow-derived cells: a focus on MSCs and regulatory T cells. EMBO reports. 2012;13:412–422. doi: 10.1038/embor.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tlsty TD, Coussens LM. Tumor stroma and regulation of cancer development. Annual review of pathology. 2006;1:119–150. doi: 10.1146/annurev.pathol.1.110304.100224. [DOI] [PubMed] [Google Scholar]
- 168.Fordyce CA, Patten KT, Fessenden TB, Defilippis R, Hwang ES, Zhao J, Tlsty TD. Cell-extrinsic consequences of epithelial stress: activation of protumorigenic tissue phenotypes. Breast cancer research : BCR. 2012;14:R155. doi: 10.1186/bcr3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PloS one. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Stagg J. Mesenchymal stem cells in cancer. Stem cell reviews. 2008;4:119–124. doi: 10.1007/s12015-008-9030-4. [DOI] [PubMed] [Google Scholar]
- 171.Dwyer RM, Potter-Beirne SM, Harrington KA, Lowery AJ, Hennessy E, Murphy JM, Barry FP, O'Brien T, Kerin MJ. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:5020–5027. doi: 10.1158/1078-0432.CCR-07-0731. [DOI] [PubMed] [Google Scholar]
- 172.Klopp AH, Gupta A, Spaeth E, Andreeff M, Marini F., 3rd Concise review: Dissecting a discrepancy in the literature: do mesenchymal stem cells support or suppress tumor growth? Stem Cells. 2011;29:11–19. doi: 10.1002/stem.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Oh JY, Kim MK, Shin MS, Lee HJ, Ko JH, Wee WR, Lee JH. The anti-inflammatory and anti-angiogenic role of mesenchymal stem cells in corneal wound healing following chemical injury. Stem Cells. 2008;26:1047–1055. doi: 10.1634/stemcells.2007-0737. [DOI] [PubMed] [Google Scholar]
- 174.Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M, Bianco P. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell. 2007;131:324–336. doi: 10.1016/j.cell.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 175.Beckermann BM, Kallifatidis G, Groth A, Frommhold D, Apel A, Mattern J, Salnikov AV, Moldenhauer G, Wagner W, Diehlmann A, et al. VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. British journal of cancer. 2008;99:622–631. doi: 10.1038/sj.bjc.6604508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 177.Goldstein RH, Reagan MR, Anderson K, Kaplan DL, Rosenblatt M. Human bone marrow-derived MSCs can home to orthotopic breast cancer tumors and promote bone metastasis. Cancer research. 2010;70:10044–10050. doi: 10.1158/0008-5472.CAN-10-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dunn L, Demichele A. Genomic predictors of outcome and treatment response in breast cancer. Molecular diagnosis & therapy. 2009;13:73–90. doi: 10.1007/BF03256317. [DOI] [PubMed] [Google Scholar]
- 179.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 180.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 181.Numasaki M, Fukushi J, Ono M, Narula SK, Zavodny PJ, Kudo T, Robbins PD, Tahara H, Lotze MT. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 2003;101:2620–2627. doi: 10.1182/blood-2002-05-1461. [DOI] [PubMed] [Google Scholar]
- 182.Numasaki M, Lotze MT, Sasaki H. Interleukin-17 augments tumor necrosis factoralpha-induced elaboration of proangiogenic factors from fibroblasts. Immunology letters. 2004;93:39–43. doi: 10.1016/j.imlet.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 183.Takahashi H, Numasaki M, Lotze MT, Sasaki H. Interleukin-17 enhances bFGF-, HGF- and VEGF-induced growth of vascular endothelial cells. Immunology letters. 2005;98:189–193. doi: 10.1016/j.imlet.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 184.Letuve S, Lajoie-Kadoch S, Audusseau S, Rothenberg ME, Fiset PO, Ludwig MS, Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. The Journal of allergy and clinical immunology. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 185.Iida T, Iwahashi M, Katsuda M, Ishida K, Nakamori M, Nakamura M, Naka T, Ojima T, Ueda K, Hayata K, et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncology reports. 2011;25:1271–1277. doi: 10.3892/or.2011.1201. [DOI] [PubMed] [Google Scholar]
- 186.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 187.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nature reviews Clinical oncology. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 188.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 189.Qian CN, Huang D, Wondergem B, Teh BT. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer. 2009;115:2282–2289. doi: 10.1002/cncr.24238. [DOI] [PubMed] [Google Scholar]
- 190.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA, Qian H, Xue XN, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer research. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 191.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 192.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Ivy SP, Wick JY, Kaufman BM. An overview of small-molecule inhibitors of VEGFR signaling. Nature reviews Clinical oncology. 2009;6:569–579. doi: 10.1038/nrclinonc.2009.130. [DOI] [PubMed] [Google Scholar]
- 194.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. The Journal of clinical investigation. 2008;118:3367–3377. doi: 10.1172/JCI35213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature biotechnology. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 196.Coffelt SB, Tal AO, Scholz A, De Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer research. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 197.Lewis CE, De Palma M, Naldini L. Tie2-expressing monocytes and tumor angiogenesis: regulation by hypoxia and angiopoietin-2. Cancer research. 2007;67:8429–8432. doi: 10.1158/0008-5472.CAN-07-1684. [DOI] [PubMed] [Google Scholar]
- 198.Capobianco A, Monno A, Cottone L, Venneri MA, Biziato D, Di Puppo F, Ferrari S, De Palma M, Manfredi AA, Rovere-Querini P. Proangiogenic Tie2(+) macrophages infiltrate human and murine endometriotic lesions and dictate their growth in a mouse model of the disease. The American journal of pathology. 2011;179:2651–2659. doi: 10.1016/j.ajpath.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 200.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, et al. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nature medicine. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 201.Vallet S, Smith MR, Raje N. Novel bone-targeted strategies in oncology. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:4084–4093. doi: 10.1158/1078-0432.CCR-10-0600. [DOI] [PubMed] [Google Scholar]
- 202.Dinarello C. Why not treat human cancer with interleukin-1 blockade? Cancer metastasis reviews. 2010;29:317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Grosso JF, Jure-Kunkel MN. CTLA-4 blockade in tumor models: an overview of preclinical and translational research. Cancer immunity. 2013;13:5. [PMC free article] [PubMed] [Google Scholar]
- 204.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Dulos J, Carven GJ, van Boxtel SJ, Evers S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P, Punt CJ, et al. PD-1 blockade augments Th1 and Th17 and suppresses Th2 responses in peripheral blood from patients with prostate and advanced melanoma cancer. J Immunother. 2012;35:169–178. doi: 10.1097/CJI.0b013e318247a4e7. [DOI] [PubMed] [Google Scholar]
- 206.Simeone E, Ascierto PA. Immunomodulating antibodies in the treatment of metastatic melanoma: the experience with anti-CTLA-4, anti-CD137, and anti-PD1. Journal of immunotoxicology. 2012;9:241–247. doi: 10.3109/1547691X.2012.678021. [DOI] [PubMed] [Google Scholar]
- 207.Lipson EJ, Drake CG. Ipilimumab: an anti-CTLA-4 antibody for metastatic melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6958–6962. doi: 10.1158/1078-0432.CCR-11-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Karnezis T, Shayan R, Fox S, Achen MG, Stacker SA. The connection between lymphangiogenic signalling and prostaglandin biology: a missing link in the metastatic pathway. Oncotarget. 2012;3:893–906. doi: 10.18632/oncotarget.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Sanz-Motilva V, Martorell-Calatayud A, Nagore E. Non-steroidal anti-inflammatory drugs and melanoma. Current pharmaceutical design. 2012;18:3966–3978. doi: 10.2174/138161212802083680. [DOI] [PubMed] [Google Scholar]
- 210.Clevers H. Colon cancer--understanding how NSAIDs work. The New England journal of medicine. 2006;354:761–763. doi: 10.1056/NEJMcibr055457. [DOI] [PubMed] [Google Scholar]
- 211.Lee DY, Park K, Kim SK, Park RW, Kwon IC, Kim SY, Byun Y. Antimetastatic effect of an orally active heparin derivative on experimentally induced metastasis. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:2841–2849. doi: 10.1158/1078-0432.CCR-07-0641. [DOI] [PubMed] [Google Scholar]
- 212.Schmid MC, Varner JA. Myeloid cells in tumor inflammation. Vascular cell. 2012;4:14. doi: 10.1186/2045-824X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Ilkovitch D, Carrio R, Lopez DM. uPA and uPA-Receptor Are Involved in Cancer-associated Myeloid-derived Suppressor Cell Accumulation. Anticancer research. 2012;32:4263–4270. [PubMed] [Google Scholar]
- 214.Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunological investigations. 2012;41:635–657. doi: 10.3109/08820139.2012.695417. [DOI] [PubMed] [Google Scholar]
- 215.Wu CT, Hsieh CC, Lin CC, Chen WC, Hong JH, Chen MF. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J Mol Med (Berl) 2012;90:1343–1355. doi: 10.1007/s00109-012-0916-x. [DOI] [PubMed] [Google Scholar]
- 216.Jayaraman P, Parikh F, Lopez-Rivera E, Hailemichael Y, Clark A, Ma G, Cannan D, Ramacher M, Kato M, Overwijk WW, et al. Tumor-expressed inducible nitric oxide synthase controls induction of functional myeloid-derived suppressor cells through modulation of vascular endothelial growth factor release. J Immunol. 2012;188:5365–5376. doi: 10.4049/jimmunol.1103553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, Prevost-Blondel A, Thiery JP, Abastado JP. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS biology. 2011;9:e1001162. doi: 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Facciabene A, Santoro S, Coukos G. Know thy enemy: Why are tumor-infiltrating regulatory T cells so deleterious? Oncoimmunology. 2012;1:575–577. doi: 10.4161/onci.19401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wainwright DA, Balyasnikova IV, Chang AL, Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y, Lesniak MS. IDO Expression in Brain Tumors Increases the Recruitment of Regulatory T Cells and Negatively Impacts Survival. Clinical cancer research : an official journal of the American Association for Cancer Research. 2012;18:6110–6121. doi: 10.1158/1078-0432.CCR-12-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, Biota C, Doffin AC, Durand I, Olive D, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer research. 2009;69:2000–2009. doi: 10.1158/0008-5472.CAN-08-2360. [DOI] [PubMed] [Google Scholar]
- 221.Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer cell. 2009;16:173–174. doi: 10.1016/j.ccr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 222.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. The American journal of pathology. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 223.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 224.Bambace NM, Holmes CE. The platelet contribution to cancer progression. Journal of thrombosis and haemostasis : JTH. 2011;9:237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 225.Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, Rossi FW, Basolo F, Ugolini C, de Paulis A, et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene. 2010;29:6203–6215. doi: 10.1038/onc.2010.348. [DOI] [PubMed] [Google Scholar]