Abstract
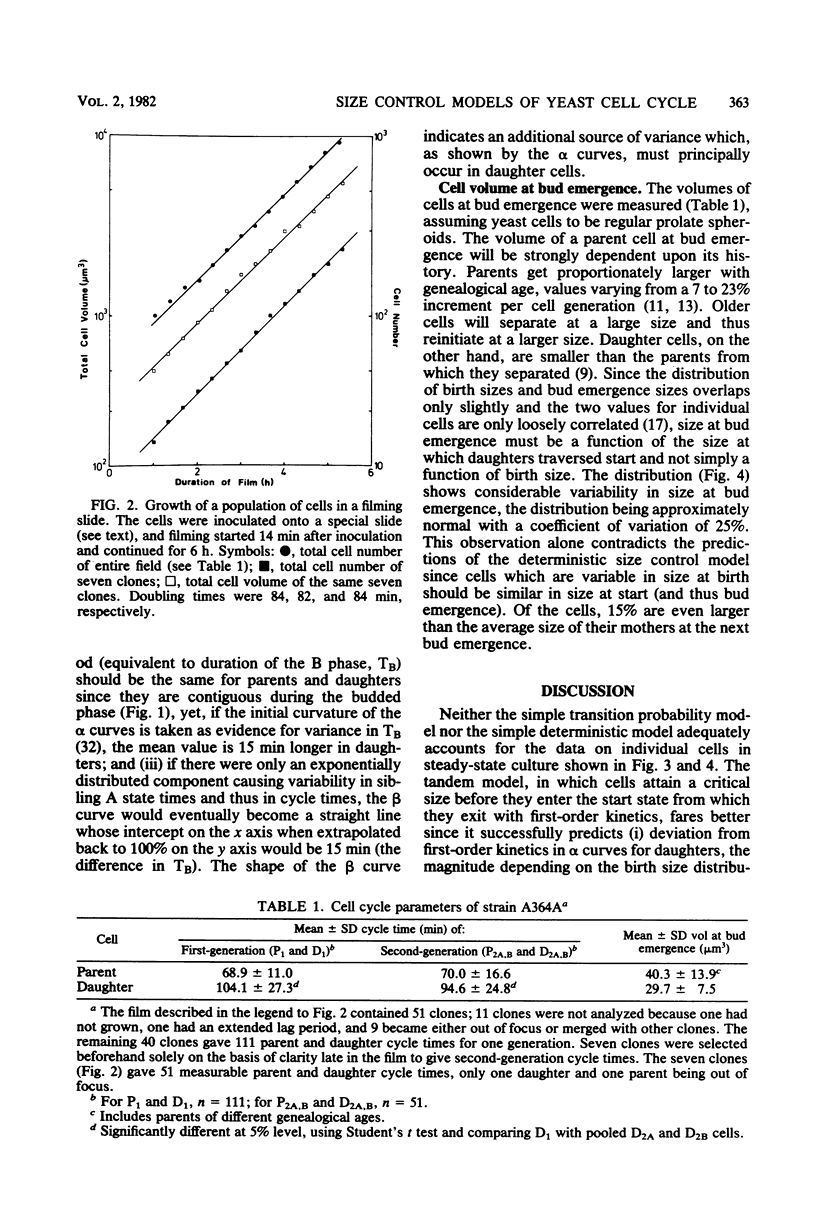
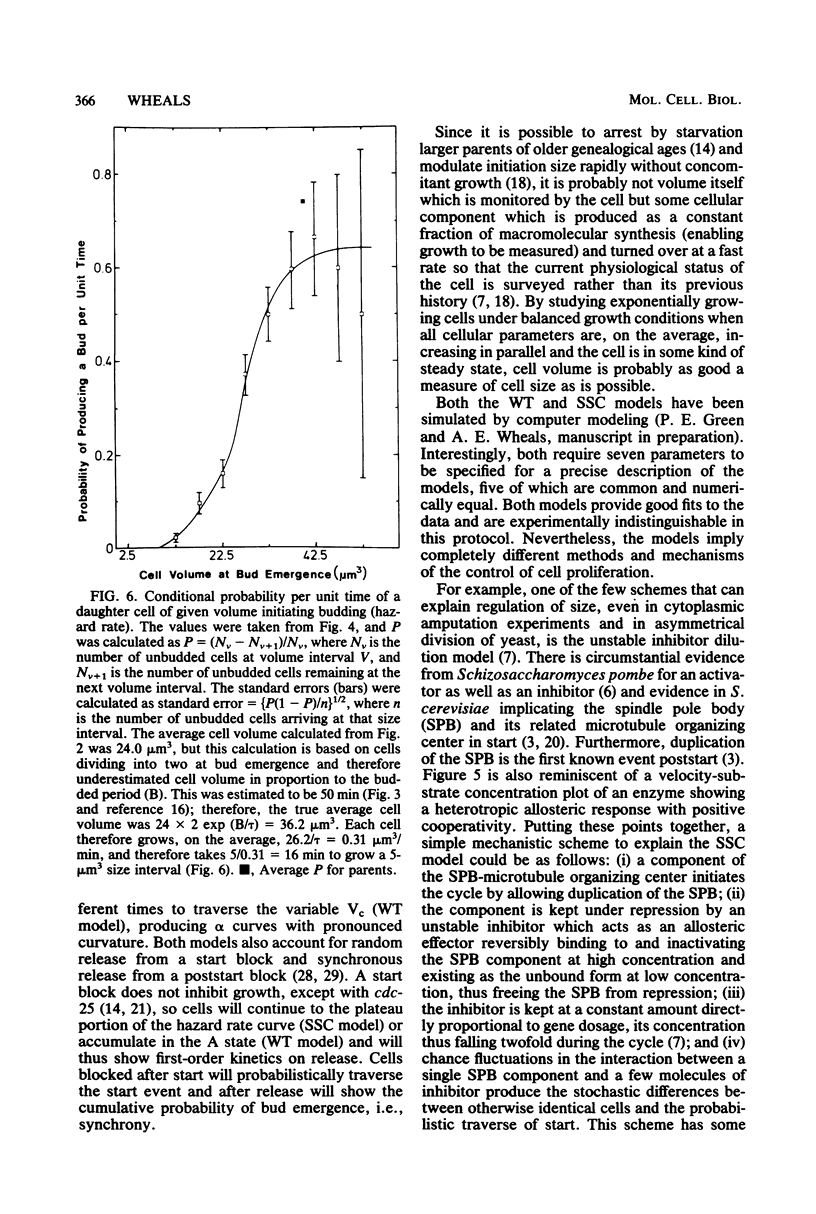
By using time-lapse photomicroscopy, the individual cycle times and sizes at bud emergence were measured for a population of saccharomyces cerevisiae cells growing exponentially under balanced growth conditions in a specially constructed filming slide. There was extensive variability in both parameters for daughter and parent cells. The data on 162 pairs of siblings were analyzed for agreement with the predictions of the transition probability hypothesis and the critical-size hypothesis of yeast cell proliferation and also with a model incorporating both of these hypotheses in tandem. None of the models accounted for all of the experimental data, but two models did give good agreement to all of the data. The wobbly tandem model proposes that cells need to attain a critical size, which is very variable, enabling them to enter a start state from which they exit with first order kinetics. The sloppy size control model suggests that cells have an increasing probability per unit time of traversing start as they increase in size, reaching a high plateau value which is less than one. Both models predict that the kinetics of entry into the cell division sequence will strongly depend on variability in birth size and thus will be quite different for daughters and parents of the asymmetrically dividing yeast cells. Mechanisms underlying these models are discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooks R. F., Bennett D. C., Smith J. A. Mammalian cell cycles need two random transitions. Cell. 1980 Feb;19(2):493–504. doi: 10.1016/0092-8674(80)90524-3. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol. 1974;38:123–131. doi: 10.1101/sqb.1974.038.01.016. [DOI] [PubMed] [Google Scholar]
- Cooper S. A unifying model for the G1 period in prokaryotes and eukaryotes. Nature. 1979 Jul 5;280(5717):17–19. doi: 10.1038/280017a0. [DOI] [PubMed] [Google Scholar]
- Elliott S. G., McLaughlin C. S. Rate of macromolecular synthesis through the cell cycle of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4384–4388. doi: 10.1073/pnas.75.9.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantes P. A. Control of cell size and cycle time in Schizosaccharomyces pombe. J Cell Sci. 1977 Apr;24:51–67. doi: 10.1242/jcs.24.1.51. [DOI] [PubMed] [Google Scholar]
- Fantes P. A., Grant W. D., Pritchard R. H., Sudbery P. E., Wheals A. E. The regulation of cell size and the control of mitosis. J Theor Biol. 1975 Mar;50(1):213–244. doi: 10.1016/0022-5193(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Culotti J., Pringle J. R., Reid B. J. Genetic control of the cell division cycle in yeast. Science. 1974 Jan 11;183(4120):46–51. doi: 10.1126/science.183.4120.46. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford L. M., Hartwell L. H. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J Mol Biol. 1974 Apr 15;84(3):445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Johnson B. F., Lu C. Morphometric analysis of yeast cells. IV. Increase of the cylindrical diameter of Schizosaccharomyces pombe during the cell cycle. Exp Cell Res. 1975 Oct 1;95(1):154–158. doi: 10.1016/0014-4827(75)90620-5. [DOI] [PubMed] [Google Scholar]
- Johnston G. C. Cell size and budding during starvation of the yeast Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):738–739. doi: 10.1128/jb.132.2.738-739.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Ehrhardt C. W., Lorincz A., Carter B. L. Regulation of cell size in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):1–5. doi: 10.1128/jb.137.1.1-5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Singer R. A., McFarlane S. Growth and cell division during nitrogen starvation of the yeast Saccharomyces cerevisiae. J Bacteriol. 1977 Nov;132(2):723–730. doi: 10.1128/jb.132.2.723-730.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord P. G., Wheals A. E. Asymmetrical division of Saccharomyces cerevisiae. J Bacteriol. 1980 Jun;142(3):808–818. doi: 10.1128/jb.142.3.808-818.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord P. G., Wheals A. E. Variability in individual cell cycles of Saccharomyces cerevisiae. J Cell Sci. 1981 Aug;50:361–376. doi: 10.1242/jcs.50.1.361. [DOI] [PubMed] [Google Scholar]
- Nurse P. Cell cycle control--both deterministic and probabilistic? Nature. 1980 Jul 3;286(5768):9–10. doi: 10.1038/286009a0. [DOI] [PubMed] [Google Scholar]
- Samokhin G. P., Lizlova L. V., Bespalova J. D., Titov M. I., Smirnov V. N. The effect of alpha-factor on the rate of cell-cycle initiation in Saccharomyces cerevisiae: alpha-factor modulates transition probability in yeast. Exp Cell Res. 1981 Feb;131(2):267–275. doi: 10.1016/0014-4827(81)90231-7. [DOI] [PubMed] [Google Scholar]
- Shields R., Brooks R. F., Riddle P. N., Capellaro D. F., Delia D. Cell size, cell cycle and transition probability in mouse fibroblasts. Cell. 1978 Oct;15(2):469–474. doi: 10.1016/0092-8674(78)90016-8. [DOI] [PubMed] [Google Scholar]
- Shields R., Smith J. A. Cells regulate their proliferation through alterations in transition probability. J Cell Physiol. 1977 Jun;91(3):345–355. doi: 10.1002/jcp.1040910304. [DOI] [PubMed] [Google Scholar]
- Shields R. Transition probability and the origin of variation in the cell cycle. Nature. 1977 Jun 23;267(5613):704–707. doi: 10.1038/267704a0. [DOI] [PubMed] [Google Scholar]
- Shilo B., Riddle V. G., Pardee A. B. Protein turnover and cell-cycle initiation in yeast. Exp Cell Res. 1979 Oct 15;123(2):221–227. doi: 10.1016/0014-4827(79)90462-2. [DOI] [PubMed] [Google Scholar]
- Shilo B., Shilo V., Simchen G. Cell-cycle initiation in yeast follows first-order kinetics. Nature. 1976 Dec 23;264(5588):767–770. doi: 10.1038/264767a0. [DOI] [PubMed] [Google Scholar]
- Shilo B., Simchen G., Pardee A. B. Regulation of cell-cycle initiation in yeast by nutrients and protein synthesis. J Cell Physiol. 1978 Nov;97(2):177–187. doi: 10.1002/jcp.1040970207. [DOI] [PubMed] [Google Scholar]
- Singer R. A., Johnston G. C. Nature of the G1 phase of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1981 May;78(5):3030–3033. doi: 10.1073/pnas.78.5.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Martin L. Do cells cycle? Proc Natl Acad Sci U S A. 1973 Apr;70(4):1263–1267. doi: 10.1073/pnas.70.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompayrac L., Maaloe O. Autorepressor model for control of DNA replication. Nat New Biol. 1973 Jan 31;241(109):133–135. doi: 10.1038/newbio241133a0. [DOI] [PubMed] [Google Scholar]
- Transition probability and cell-cycle initiation in yeast. Nature. 1977 Jun 16;267(5612):647–649. doi: 10.1038/267647b0. [DOI] [PubMed] [Google Scholar]


