Abstract
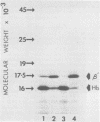
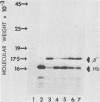
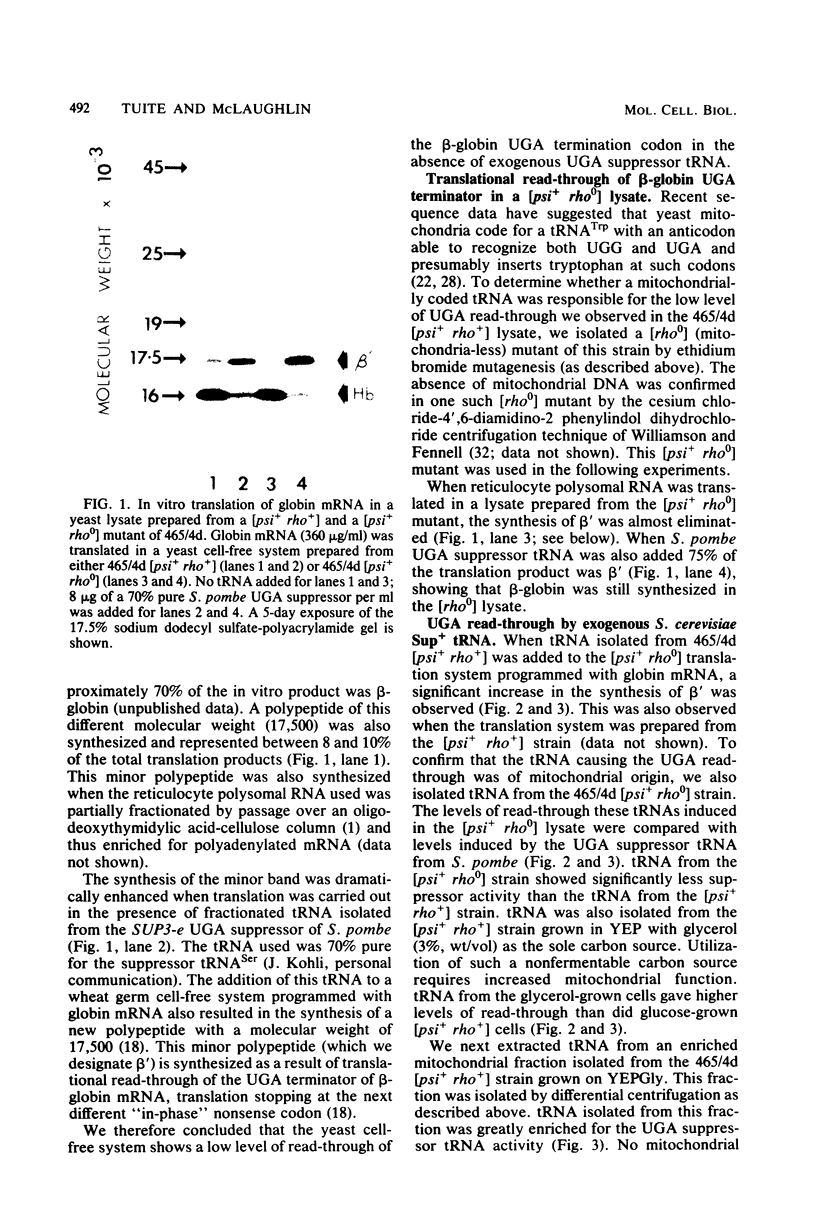
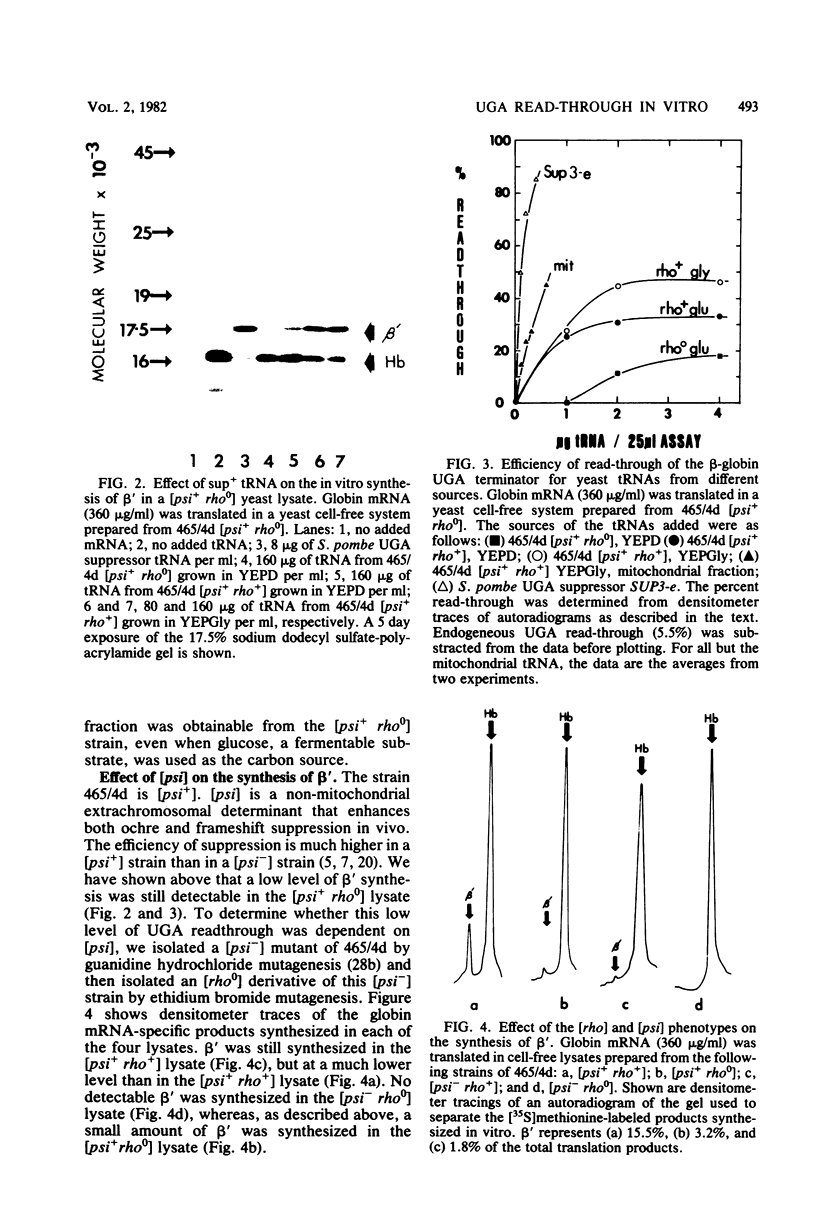
Globin mRNA, translated in a Saccharomyces cerevisiae cell-free protein synthesizing system prepared from a [psi+ rho+] strain, primarily directed the synthesis of alpha- and beta-globin. A third globin mRNA-specific polypeptide was also synthesized, representing approximately 10% of the total translation products. This polypeptide (beta') was synthesized by translational read-through of the beta- globin mRNA UGA terminator and was mediated primarily by an endogenous tRNA coded for by the mitochondria. This mitochondrial tRNA, when charged, could be preferentially bound, in high salt, to benzoylated DEAE-cellulose, a characteristic of a tRNATrp. The synthesis of beta- mediated by this mitochondrial tRNATrp was significantly reduced when the translation system was prepared from an isogenic [psi-] strain. Evidence for a nuclear-coded tRNA, also able to suppress the beta-globin mRNA UGA terminator in [psi+] but not [psi-] lysates, was also obtained. The presence of these endogenous UGA suppressor activities in the yeast cell-free system should allow successful in vitro translation of mitochondrial mRNAs.
Full text
PDF

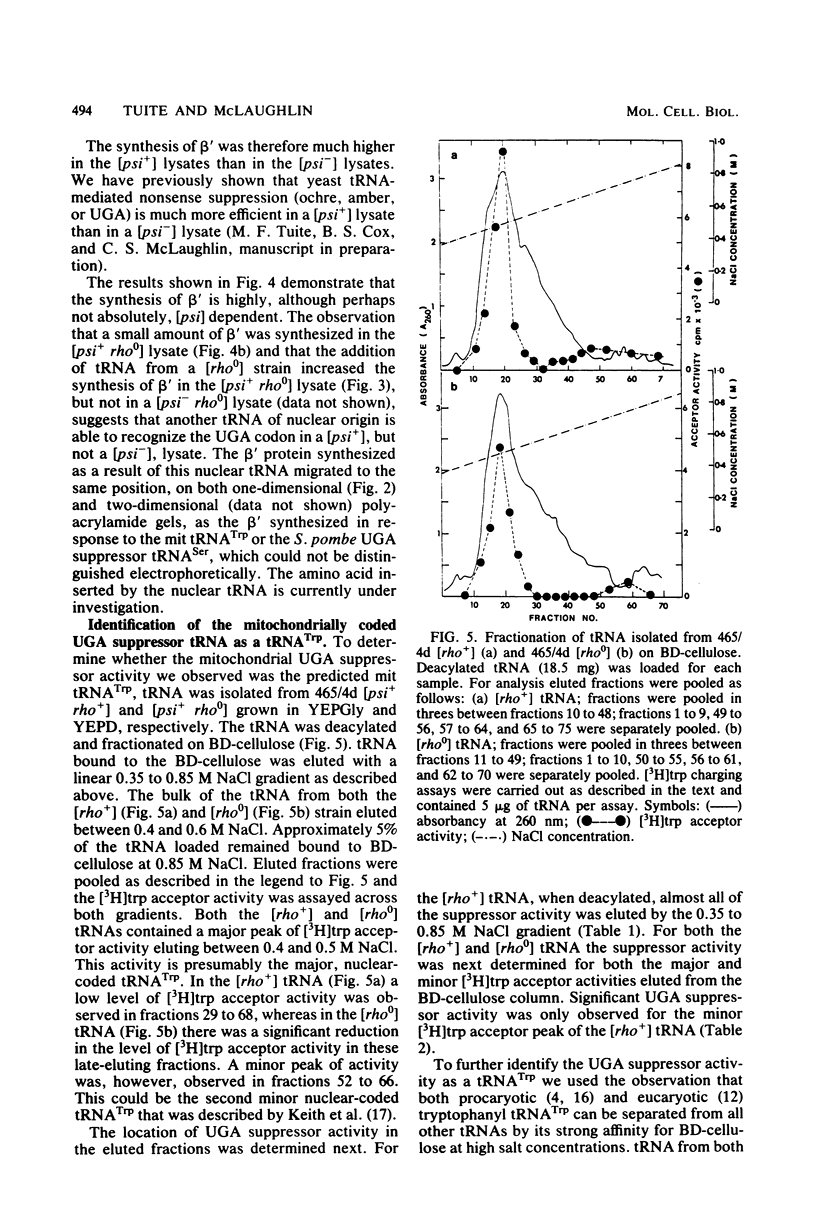
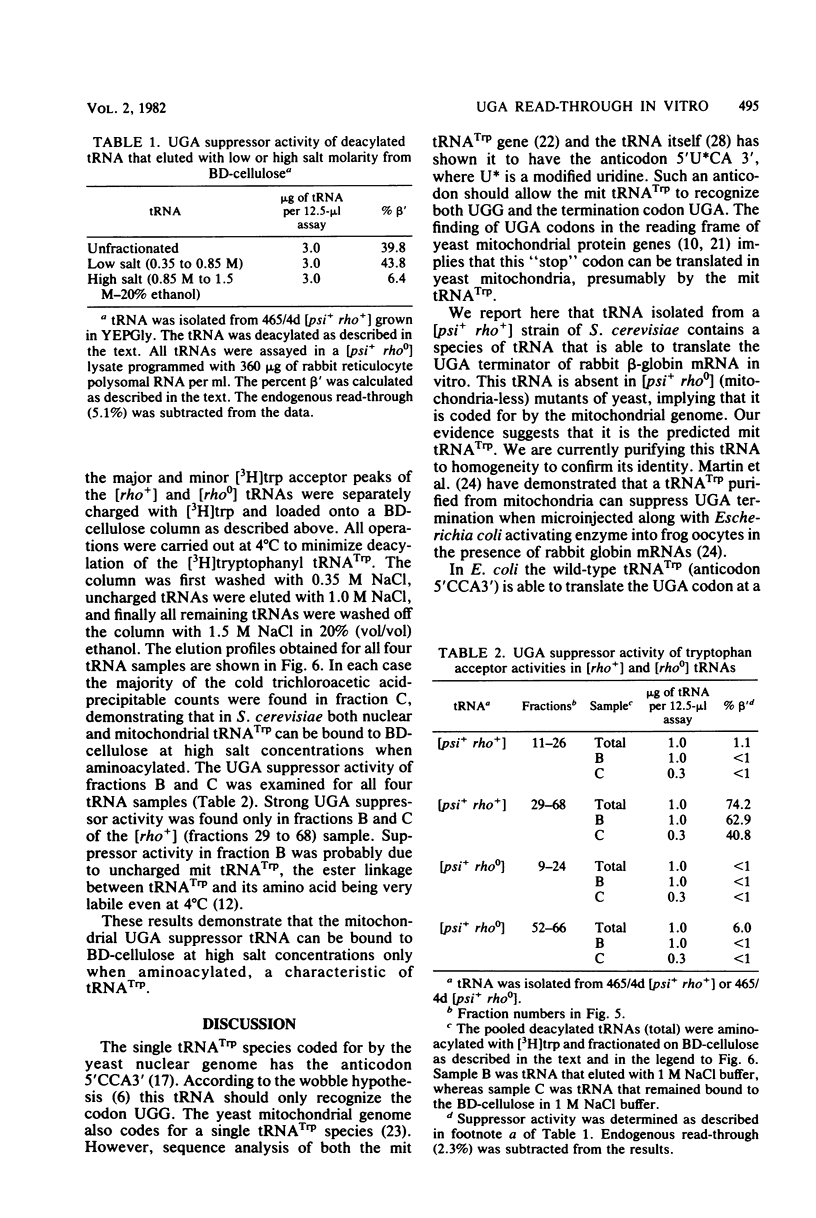
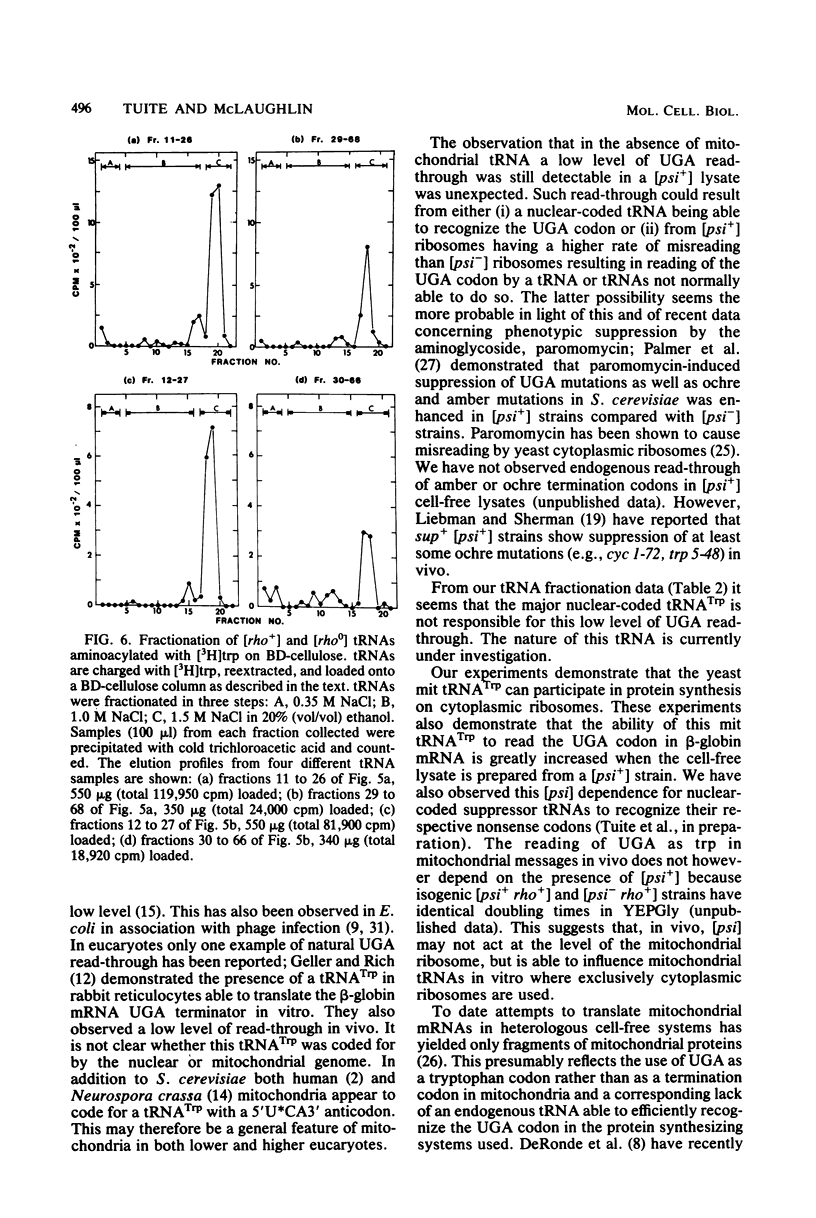

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Anderson S., Bankier A. T., de Bruijn M. H., Chen E., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A. Different pattern of codon recognition by mammalian mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3164–3166. doi: 10.1073/pnas.77.6.3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell B. G., Bankier A. T., Drouin J. A different genetic code in human mitochondria. Nature. 1979 Nov 8;282(5735):189–194. doi: 10.1038/282189a0. [DOI] [PubMed] [Google Scholar]
- Buckingham R. H. Anticodon conformation and accessibility in wild-type and suppressor tryptophan tRNA from E. coli. Nucleic Acids Res. 1976 Apr;3(4):965–975. doi: 10.1093/nar/3.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Culbertson M. R., Charnas L., Johnson M. T., Fink G. R. Frameshifts and frameshift suppressors in Saccharomyces cerevisiae. Genetics. 1977 Aug;86(4):745–764. doi: 10.1093/genetics/86.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ronde A., Van Loon A. P., Grivell L. A., Kohli J. In vitro suppression of UGA codons in a mitochondrial mRNA. Nature. 1980 Sep 25;287(5780):361–363. doi: 10.1038/287361a0. [DOI] [PubMed] [Google Scholar]
- Engelberg-Kulka H., Dekel L., Israeli-Reches M., Belfort M. The requirement of nonsense suppression for the development of several phages. Mol Gen Genet. 1979 Feb 26;170(2):155–159. doi: 10.1007/BF00337791. [DOI] [PubMed] [Google Scholar]
- Fox T. D. Five TGA "stop" codons occur within the translated sequence of the yeast mitochondrial gene for cytochrome c oxidase subunit II. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6534–6538. doi: 10.1073/pnas.76.12.6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior E., Herrera F., Sadnik I., McLaughlin C. S., Moldave K. The preparation and characterization of a cell-free system from Saccharomyces cerevisiae that translates natural messenger ribonucleic acid. J Biol Chem. 1979 May 25;254(10):3965–3969. [PubMed] [Google Scholar]
- Geller A. I., Rich A. A UGA termination suppression tRNATrp active in rabbit reticulocytes. Nature. 1980 Jan 3;283(5742):41–46. doi: 10.1038/283041a0. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Wolfner M., Grisafi P., Fink G., Botstein D., Roth J. R. Yeast suppressors of UAA and UAG nonsense codons work efficiently in vitro via tRNA. Cell. 1976 Mar;7(3):381–390. doi: 10.1016/0092-8674(76)90167-7. [DOI] [PubMed] [Google Scholar]
- Heckman J. E., Sarnoff J., Alzner-DeWeerd B., Yin S., RajBhandary U. L. Novel features in the genetic code and codon reading patterns in Neurospora crassa mitochondria based on sequences of six mitochondrial tRNAs. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3159–3163. doi: 10.1073/pnas.77.6.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh D., Gold L. Translation of the UGA triplet in vitro by tryptophan transfer RNA's. J Mol Biol. 1971 Jun 14;58(2):459–468. doi: 10.1016/0022-2836(71)90363-9. [DOI] [PubMed] [Google Scholar]
- Joseph D. R., Muench K. H. Tryptophanyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification of the enzyme and of tryptrophan transfer ribonucleic acid. J Biol Chem. 1971 Dec 25;246(24):7602–7609. [PubMed] [Google Scholar]
- Keith G., Roy A., Ebel J. P., Dirheimer G. The nucleotide sequences of two tryptophane-tRNAs from brewer's yeast. FEBS Lett. 1971 Oct 1;17(2):306–308. doi: 10.1016/0014-5793(71)80171-0. [DOI] [PubMed] [Google Scholar]
- Kohli J., Kwong T., Altruda F., Söll D., Wahl G. Characterization of a UGA-suppressing serine tRNA from Schizosaccharomyces pombe with the help of a new in vitro assay system for eukaryotic suppressor tRNAs. J Biol Chem. 1979 Mar 10;254(5):1546–1551. [PubMed] [Google Scholar]
- Liebman S. W., Sherman F. Extrachromosomal psi+ determinant suppresses nonsense mutations in yeast. J Bacteriol. 1979 Sep;139(3):1068–1071. doi: 10.1128/jb.139.3.1068-1071.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Stewart J. W., Sherman F. Serine substitutions caused by an ochre suppressor in yeast. J Mol Biol. 1975 Jun 5;94(4):595–610. doi: 10.1016/0022-2836(75)90324-1. [DOI] [PubMed] [Google Scholar]
- Macino G., Coruzzi G., Nobrega F. G., Li M., Tzagoloff A. Use of the UGA terminator as a tryptophan codon in yeast mitochondria. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3784–3785. doi: 10.1073/pnas.76.8.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N. C., Pham H. D., Underbrink-Lyon K., Miller D. l., Donelson J. E. Yeast mitochondrial tRNATrp can recognize the nonsense codon UGA. Nature. 1980 Jun 19;285(5766):579–581. doi: 10.1038/285579a0. [DOI] [PubMed] [Google Scholar]
- Martin N. C., rabinowitz M. Mitochondrial transfer RNAs in yeast: identification of isoaccepting transfer RNAs. Biochemistry. 1978 May 2;17(9):1628–1634. doi: 10.1021/bi00602a008. [DOI] [PubMed] [Google Scholar]
- Martin R. P., Sibler A. P., Dirheimer G., de Henau S., Grosjean H. Yeast mitochondrial tRNATrp injected with E. coli activating enzyme into Xenopus oocytes suppresses UGA termination. Nature. 1981 Sep 17;293(5829):235–237. doi: 10.1038/293235a0. [DOI] [PubMed] [Google Scholar]
- Masurekar M., Palmer E., Ono B. I., Wilhelm J. M., Sherman F. Misreading of the ribosomal suppressor SUP46 due to an altered 40 S subunit in yeast. J Mol Biol. 1981 Apr 15;147(3):381–390. doi: 10.1016/0022-2836(81)90490-3. [DOI] [PubMed] [Google Scholar]
- Moorman A. F., Van Ommen G. J., Grivell L. A. Transcription in yeast mitochondria: isolation and physical mapping of messenger RNAs for subunits of cytochrome c oxidase and ATPase. Mol Gen Genet. 1978 Mar 20;160(1):13–24. doi: 10.1007/BF00275114. [DOI] [PubMed] [Google Scholar]
- Palmer E., Wilhelm J. M., Sherman F. Variation of phenotypic suppression due to the psi+ and psi- extrachromosomal determinants in yeast. J Mol Biol. 1979 Feb 15;128(1):107–110. doi: 10.1016/0022-2836(79)90311-5. [DOI] [PubMed] [Google Scholar]
- Sibler A. P., Bordonné R., Dirheimer G., Martin R. Structure primaire d'un tryptophane-tRNA de mitochondrie de levure capable de traduire le codon de terminaison U-G-A. C R Seances Acad Sci D. 1980 Mar 17;290(11):695–698. [PubMed] [Google Scholar]
- Tuite M. F., Cox B. S., McLaughlin C. S. An homologous in vitro assay for yeast nonsense suppressors. J Biol Chem. 1981 Jul 25;256(14):7298–7304. [PubMed] [Google Scholar]
- Tuite M. F., Mundy C. R., Cox B. S. Agents that cause a high frequency of genetic change from [psi+] to [psi-] in Saccharomyces cerevisiae. Genetics. 1981 Aug;98(4):691–711. doi: 10.1093/genetics/98.4.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuite M. F., Plesset J., Moldave K., McLaughlin C. S. Faithful and efficient translation of homologous and heterologous mRNAs in an mRNA-dependent cell-free system from Saccharomyces cerevisiae. J Biol Chem. 1980 Sep 25;255(18):8761–8766. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiner A. M., Weber K. Natural read-through at the UGA termination signal of Q-beta coat protein cistron. Nat New Biol. 1971 Sep 15;234(50):206–209. doi: 10.1038/newbio234206a0. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Fennell D. J. The use of fluorescent DNA-binding agent for detecting and separating yeast mitochondrial DNA. Methods Cell Biol. 1975;12:335–351. doi: 10.1016/s0091-679x(08)60963-2. [DOI] [PubMed] [Google Scholar]