Abstract
The lung is most common site for metastatic disease via hematogenous route. Tumor emboli of the vessels of the lung induces fibrocellular and fibromuscular intimal proliferation. These histopathological changes may cause pulmonary tumor trombotic microangiopaty. Few cases are diagnosed antemortem. We report a 60 year old woman with by metastatic epithelioid angiosarcoma involving the lung. Tumor cells were positive for VEGF and topoisomerase II. VEGF may be involved in the pathogenesis pulmonary tumor trombotic microangioapy and topoisomerase II positivity showed sensitivity against catalytic topoisomerase II inhibitors.
KEY WORDS : Pulmonary tumor thrombotic microangiopathy, Angiosarcoma, Vascular endothelial growth factor, Topoisomerase II
Introduction
The lungs are frequently the site of metastatic tumors because of vast capillary network and position in the circulation. Secondary tumors of the lung may present form of discrete nodules, diffuse lymphatic permeation, diffuse consolidation, endobronchial growth or massive tumor embolism (1). Pulmonary tumor embolism (PTE) is not an uncommon cause of respiratory failure in patients with cancer. It is observed in up to 26% all cancer autopsies (2). Tumor cell emboli may not only occulude the vessels but also activate both local and systemic coagulation system, leading to thrombosis, fibrocellular and fibromuscular intimal proliferation, luminal stenosis and finally occlusion. These caused pulmonary tumor thrombotic microangiopathy (PTTM) with malign tumors patients (3). Few cases are diagnosed antemortem. PTTM and PTE may cause dyspne, pulmonary hypertension, and right-side heart failure. The most common neoplasm associated with PTTM is adenocarcinoma and the most frequent primary site is the stomach (4).We described the case of a patient with PTE and PTTM due to metastatic epithelioid angiosarcoma, not previously reported with this association. The pathogenesis of this rare metastatic pattern was discussed.
Case report
A 60 year old woman was admitted with two month history dyspne, haemoptysis, weight lost, nausea and night sweats. A chest X-ray demonstrated bilateral nodular shadows (Figure 1). A computed tomographic scan showed a solid mass, 6.5 cm in diameter in right lower lobe and ill defined parenchymal nodules in both lungs (Figure 2). PET scan from vertex to knee was performed 1.5 hours after intravenous injection of F-18 labelled flurodeoxyglucose (FDG). PET scan showed hypermetabolic lesion in the bilaterally hiler lymphadenopaty (SUVmax: 8.0) and in the liver (SUVmax: 3.6). Multifocal abnormal FDG uptake were observed in bilaterally lung nodules and 6 cm diameter mass in the right lower lobe (SUVmax: 4.6) (Figure 3). In right ramus mandibula, left frontal bone, head of left humerus, C2T8 corpus vertabrae, T9 cervical transvers proces, T10 right transvers and spinous proces and corpus vertebrae, T10-12, l1-S vertebra, sacrum, bilaterally ischium, symphisis were observed hypermetabolic (SUVmax: 14.5) with PET scan (Figure4).
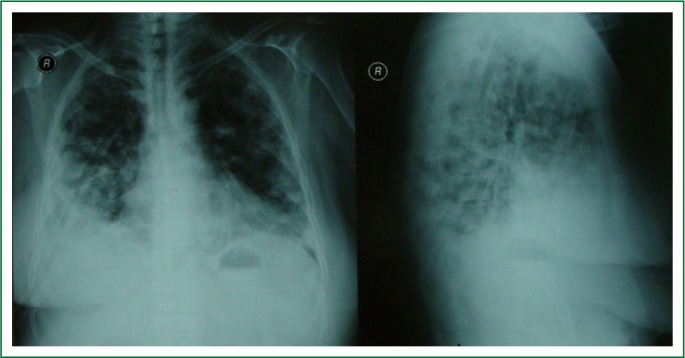
Figure 1.
A chest X-ray revealed bilateral nodules.
Figure 2.
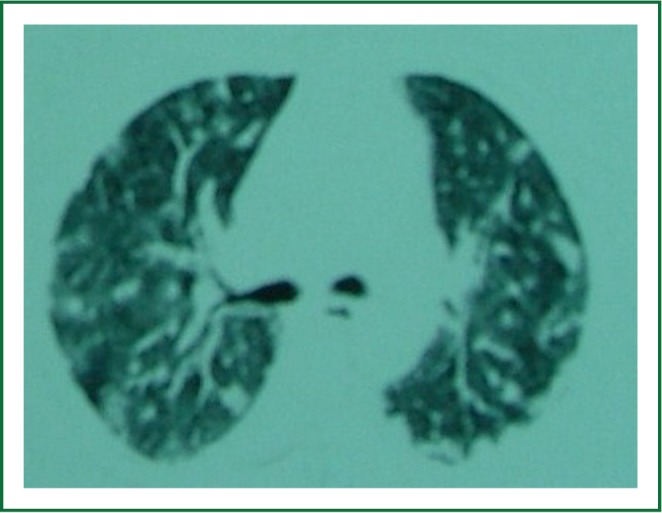
Chest computed tomography showed tickened interlobular septa and vascular structure.
Figure 3.
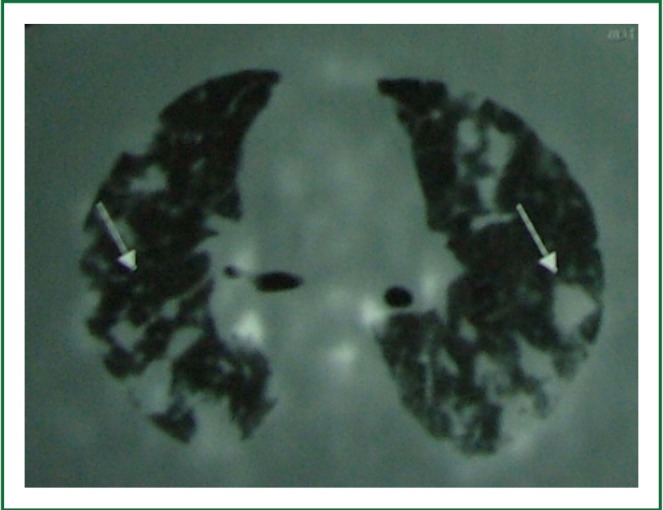
Fluorine-18 fluordeoxyglucose positron emission tomography showed increased glucose metabolism of multiple lung nodules and pathologic mediastinal lymph nodes.
Figure 4.
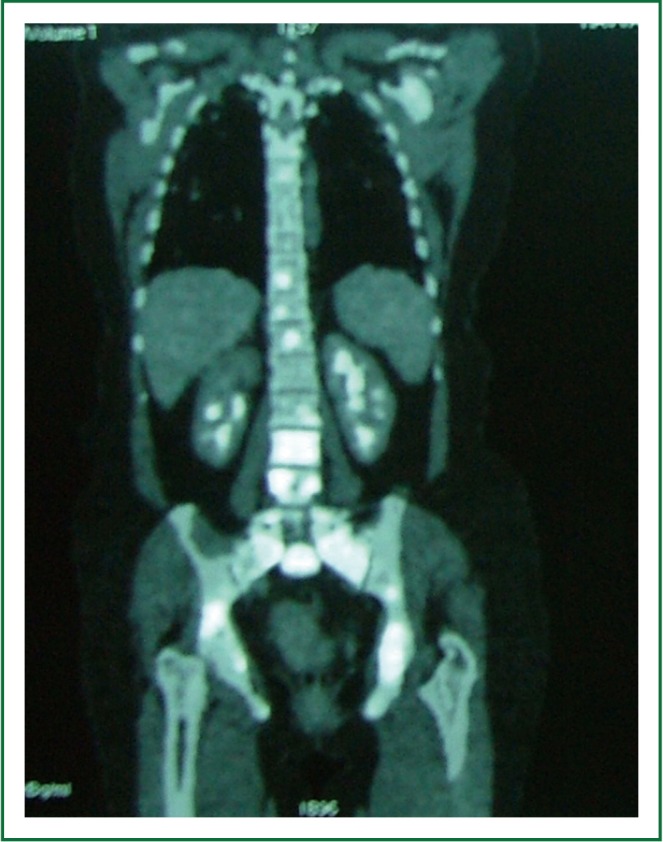
Fluorine-18 fluordeoxyglucose positron emission tomography showing increased glucose metabolism of the spine.
Broncoscopy was performed. Endobronchial lesion was not obseved. Patient was not accepted transthoracic byopsi. Hypermetabolic lesions in PET suggested metastatic malignancy. Cranium BT and abdominal USG, bilateral breast and thyroid USG were normal. Meedle lobe wedge resection and 5. rib exicion were performed with right thoracotomy.
On the macroscopic examination diffuse haemorrage and ill defined two nodules were observed. Nodules were constituted from ischemic necrosis. Around necrosis showed extensive haemorrage and malignant neoplasm. Exhibiting distinctive patterns of neoplasm were intraarterial (embolic), lymphatic, interstitial spread, focal neoplastic arteriopathy and tumoral pneumonia (Figures 5,6,7). Tumor cells were mildly pleomorphic, medium to large with pale eosinophilic and large vesicular nuclei with distinct nucleoli. They arranged loosely solid, scattered mitosis. Tumoral pneumonia composed of intraalveolar accumulation of the tumor cells admixed with fibrin and focal organisation of the fibrinous neoplastic exudate.
Figure 5.
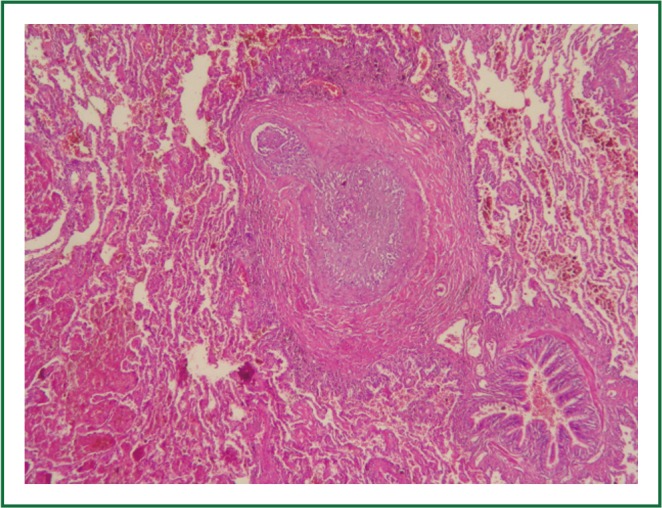
Tumor emboli showing admixed fibrin thrombi and tumor cells (HE ×400).
Figure 6.
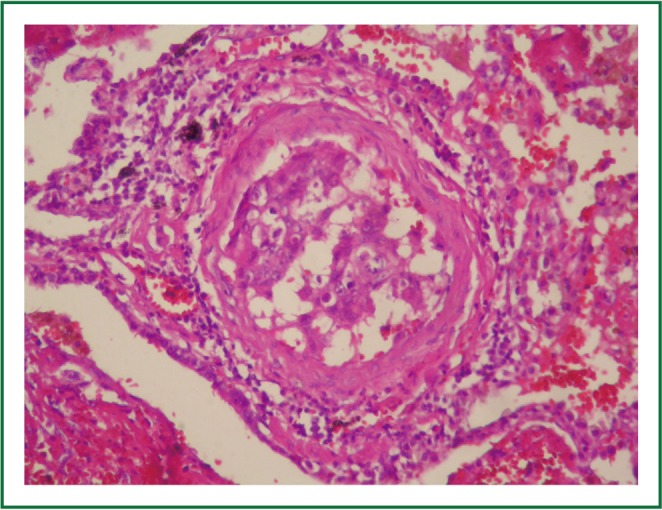
Intimal fibrocellular proliferation with tumor cells, causing marked luminal stenosis (HEX100).
Figure 7.
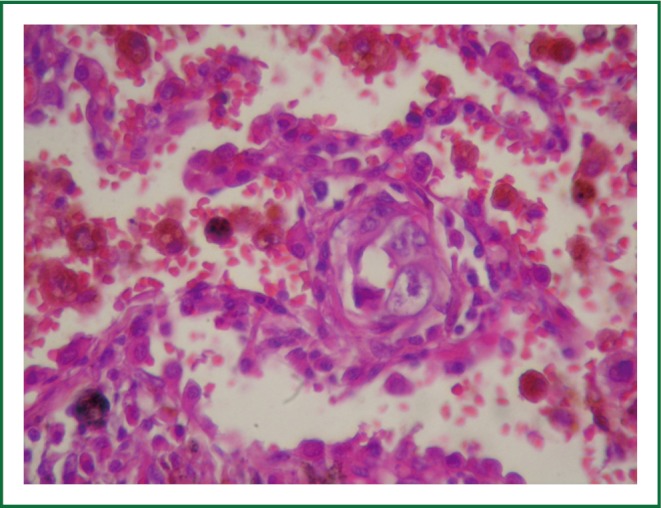
Interstitial infiltration (HE HE ×400).
Cytoplasmic features and vasotropic pattern was considered poorly differentiated carcinoma and melanoma. Intravascular B cell lymphoma and sarcoma with epithelioid features (rabdomyosarcoma, angiosarcoma and synovial sarcoma) were on our list of possible primary malignancies. A panel of immunohistochemical stains were performed, including CD45, CD31, CD34, TTF-1, CEA, pancytokeratin, Keratin20, bcl-2, actin, s-100, VEGF, Ki67 and topoisomeraseIIα (Figure 8). CD31 and VEGF were positive. Ki67 prolifaration index is high (>%90) . CD45, CD34, TTF-1, CEA, pancytokeratin, Keratin20, bcl-2, actin, s100 were negative. Topoisomerase II alpha was intranuclear positive. 5.rib contained microscopic tumor focus. We reported with these finding as metastatic epithelioid angiosarcoma. Patient was not accepted therapy. She died later 5 week.
Figure 8.
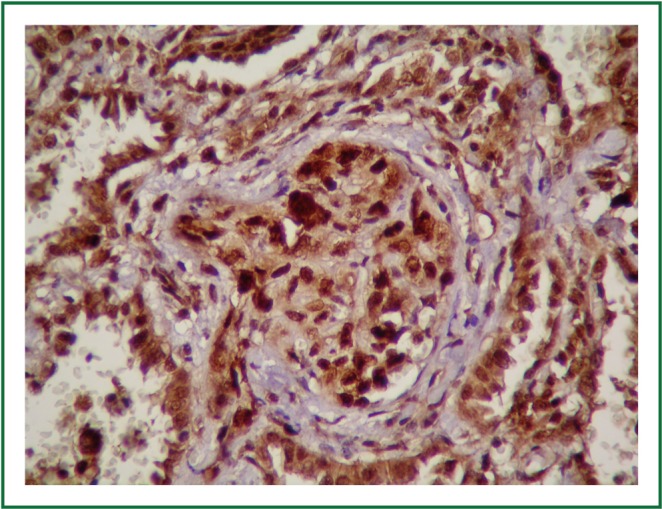
Angiosarcoma cells showed positive reactivity for topoisomerase IIα (topoisomeraseIIα ×400).
Discussion
The epithelioid form of angiosarcoma has been fully characterized only in the past decade. The tumor cells are typically large, amphophilic and have a large vesicular nucleus with a prominent nucleolus. It may contain intracytoplasmic lumen and erythrocytes. Mitosis, necrosis and hemorrhage are frequent. These features mimic malignant melanoma and carcinoma (5).
CD31 is most sensitive marker for angiosarcoma. Epithelioid angiosarcoma may be positive cytokeratin (6). But cytokeratin was negative in our case. In view of histolopathologic appearance, immunohistochemical phenotype, the metastatic tumor in our case is consistent with epithelioid angiosarcoma.
Pulmonary angiosarcomas are usually metastatic malignancies. There are only 20 primary pulmonary angiosarcomas in English literature (7). Heart is one of the most common primary sites for lung metastasis in noncutaneous angiosarcomas (6). There was no evidence of primary cardiac tumour as cardiomegaly, tachycardia, low blood pressure, reduced intensity of the heart tones in our case. Clinical features of our case was most consistent with metastatic disease. Hypermetabolic areas in bone appeared to be most possible source of metastatic disease. In literature one case with epithelioid angiosarcoma involving the lungs, soft tissue masses in the leg is possible primary foccus (8).
PTTM is a rare complication due to tumor dissemination. The tumor thrombi within small arteries and arterioles and associated fibrocellular and fibromuscular intimal proliferation are histological features of PTTM. The most common tumor associated with PTTM is gastric adenocarcinomas. Nongastric tumors are very rare. Esophageal squamous cell carcinoma, overian tumors, desmoplastic small cell tumor, salivary duct carcinoma ex pleomorphic adenoma and pulmonary adenocarcinoma with PTTM were reported in literature (9-15). We have summarized their features nongastric tumors with PTTM in Table 1.
Table 1. Rare causes of pulmonary thrombotic microangiopathy.
| Type of tumor | Number of cases | The time of diagnosis | References |
|---|---|---|---|
| Low grade ovarian serous carcinoma | 2 | postmortem | (10) |
| Ovarian clear cell carcinoma | 1 | postmortem | (4) |
| Desmoplastic small round cell tumor | 1 | postmortem | (11) |
| Esophageal squamous cell carcinoma | 1 | Antemortem thransbronchial lung biopsy | (9) |
| Cervix carcinoma | 1 | Antemortem thransbronchial lung biopsy | (12) |
| Salivary duct carcinoma ex pleomorphic adenoma | 1 | postmortem | (13) |
| Gallbladder carcinoma | 1 | postmortem | (14) |
| Lung adenocarcinoma | 5 | 2-antemortem | (15) |
| 3- postmortem | |||
| Epitheloid angiosarcoma | 1 | Antemortem | Current case |
| Wedge resection |
Very few cases in the literature have been diagnosed antemortem (9,12). The conventional radiologic findings are often minimal or nonspecific. Franquet et al. reported tree - in-bud pattern on high resolution CT of a patient with PTTM (16). But we didn’t detect tree-in-bud pattern in our case. PET/CT imaging with F-18 FDG helped for detection in metastatic angiosarcoma (17-19). SUV max were 4,6 and 8.0 in the bilaterally pulmonary and hiler lymphadenopaty, respectively in our case. Our findings supported that FDG-PET can help the diagnosis of PTTM as the intensity FDG accumulation. But in our case metastatic tumor in 5. rib was not hypermetabolic in PET/CT. Because the resolution of a PET/CT system is about 5-7 mm.
The nuclear enzyme DNA topoisomerase IIα is a major target for antineoplastic agents used in the treatment of breast, lung, and prostate cancer, sarcomas and hematological malignancies (10,20). Topoisomerase IIα positivity in our case showed sensitivity against catalytic topoisomerase IIα inhibitors.
Molecular mechanisms of PTTM is unclear, but some studies have suggested that tissue factor, osteopontin and vascular endothelial growth factor (VEGF) expressed by tumor cells (4,21). In addition to activated alveolar macrophages in the PTTM lesion has a critical role in te onset and progression of PTTM via expression of Platelet- Derived Growth Factor (22). Immunohistochemical expression of VEGF and their receptors in angiosarcomas were showed by Itakura et al. (23). We observed VEGF positivity in our case . This finding supported that VEGF may be involved in the pathogenesis of PTTM and new therapeutic approaches including anti-angiogenic agents.
In conclusion, epithelioid appearance sarcomas as epithelioid angiosarcoma may cause pulmonary tumor thrombotic microangiopathy. PET may not detect microscopic epithelioid angiosarcoma. Topoisomerase IIα and VEGF positivity showed sensitivity against catalytic topoisomerase IIα inhibitors and antiangiogenic agents.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Corrin B, Nicholson AG. Pathology of the Lungs, pp682, third ed, Elsevier, China, 2011. [Google Scholar]
- 2.Chatkin JM, Fritscher LG, Fiterman J, et al. Microscopic pulmonary neoplastic emboli: report of a case with respiratory failure but normal imaging. Primary Care Respiratory Journal 2007;16:115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao DX, Flieder DB, Hoda SA. Pulmonary tumor thrombotic microangiopathy. An often missed antemortem diagnosis. Arch Pathol Lab Med 2001;125:304-5 [DOI] [PubMed] [Google Scholar]
- 4.Chinen K, Fujino T, Horita A, et al. Pulmonary tumor thrombotic microangiopathy caused by an ovarian cancer expressing tissue factor and vascular endothelial growth factor. Pathol Res Pract 2009;205:63-8 [DOI] [PubMed] [Google Scholar]
- 5.Fletcher CD, Beham A, Bekir S, et al. Epithelioid angiosrcoma of deep soft tissue: a distinctive tumor readily mistaken for an epithelial neoplasm. Am J Surg Pathol 1991;15:915-24 [DOI] [PubMed] [Google Scholar]
- 6.Bocklage T, Leslie K, Yousem S, et al. Extracutaneous angiosarcomas metastatic to the lungs: Clinical and pathologic features of twenty-one cases. Mod Pathol 2001;14:1216-25 [DOI] [PubMed] [Google Scholar]
- 7.Yang CF, Chen TW, Tseng GC, et al. Primary pulmonary epithelioid angiosarcoma presenting as a solitary pulmonary nodule on image. Pathology International 2012;62:424-8 [DOI] [PubMed] [Google Scholar]
- 8.Lund L, Amre R.Epithelioid angiosarcom involving the lungs. Arch Pathol Lab Med 2005;129:e7-10 [DOI] [PubMed] [Google Scholar]
- 9.Ueda A, Fuse N, Fujii S, et al. Pulmonary tumor thrombotic microangiopathy associated with esopahageal squamous cell carcinoma. Inter Med 2011;50:2807-10 [DOI] [PubMed] [Google Scholar]
- 10.Gru AA, Pai RK, Roma AA. Pulmonary tumor thrombotic microangiopathy in patients with low grade ovarian serous neoplasm: a clinicopathologic review of 2 cases a previously unknown assocition. Int J Gynecol Pathol 2012;31:438-42 [DOI] [PubMed] [Google Scholar]
- 11.Sadimin ET, Collier AG, Gaffney JW, et al. Pulmonary tumor thrombotic microangiopathy with cor pulmonale due to desmolastic small round cell tumor. J Pediatr 2012;160:697-9 [DOI] [PubMed] [Google Scholar]
- 12.Sekiyama T, Mizamura K, Kobayashi T, et al. A pulmonary tumor embolism which mimicked pulmonary tumor thrombotic microangiopathy caused by cervical cancer. Nihon Kokyuki Gakkai Zasshi 2010;48:595-9 [PubMed] [Google Scholar]
- 13.Uruga H, Fujii T, Kurosaki A, et al. A case of pulmonary tumor thrombotic caused by carcinoma (salivary duct carcinoma) ex pleomorphic adenoma. Nihon Kokyuki Gakkai Zasshi 2010;48:463-8 [PubMed] [Google Scholar]
- 14.Malani AK, Gupta C, Kutty AV, et al. Pulmonary tumor thrombotic microangiopathy from metastatic gallbladder carcinoma: an unusual cause of severe pulmonary hypertension. Dig Dis Sci 2007;52:555-7 [DOI] [PubMed] [Google Scholar]
- 15.Hotta M, Ishida M, Kojima F, et al. Pulmonary tumor thrombotic caused by lung adenocarcinoma: case report and with review of the literature. Oncol Lett 2011;2:435-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franquet T, Giménez A, Prats R, et al. Thrombotic microangiopathy of pulmonary tumors: A vascular cause of tree-in-bud pattern on CT. AJR Am J Roentgenol 2002;179:897-9 [DOI] [PubMed] [Google Scholar]
- 17.Vasanawala MS, Wang Y, Quon A, et al. F-18 fluorodeoxyglucose PET/CT as an imaging tool for staging and restaging cutaneous angiosarcoma of the scalp. Clin Nucl Med 2006;31:534-7 [DOI] [PubMed] [Google Scholar]
- 18.Cicone F, Del Forno M, Papa A, et al. Fatal pulmonary tumour thrombotic microangiopathy: Do typical FDG-PET findings exist? Nuklearmedizin 2012;51:N6-9 [PubMed] [Google Scholar]
- 19.Tashima Y, Abe K, Matsuo Y, et al. Pulmonary tumor thrombotic microangiopathy: PET/CT findings. Clin Nucl Med 2009;34:175-7 [DOI] [PubMed] [Google Scholar]
- 20.Larsen AK, Escargueil AE, Skladanowski A. Catalytic topoisomerase II inhibitors in cancer therapy. Pharmacology&Therapeutics 2003;99:167-81 [DOI] [PubMed] [Google Scholar]
- 21.Okubo Y, Nakayama M, Kitahara K, et al. Pulmonary tumor thrombotic microangiopathy induced by gastric carcinoma: Morphometric and immunohistochemical analysis of six autopsy cases. Diagnostic Pathology 2011;6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yokomine T, Hirakawa H, Ozawa E, et al. Pulmonary thrombotic microangiopathy caused by gastric carcinoma. J Clin Pathol 2010;63:367-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Itakura E, Yamamoto H, Oda Y, et al. Detection and characterization of Vascular Endothelial Growth Factors and their receptors in a series of angiosarcomas. J Surg Oncol 2008;97:74-81 [DOI] [PubMed] [Google Scholar]