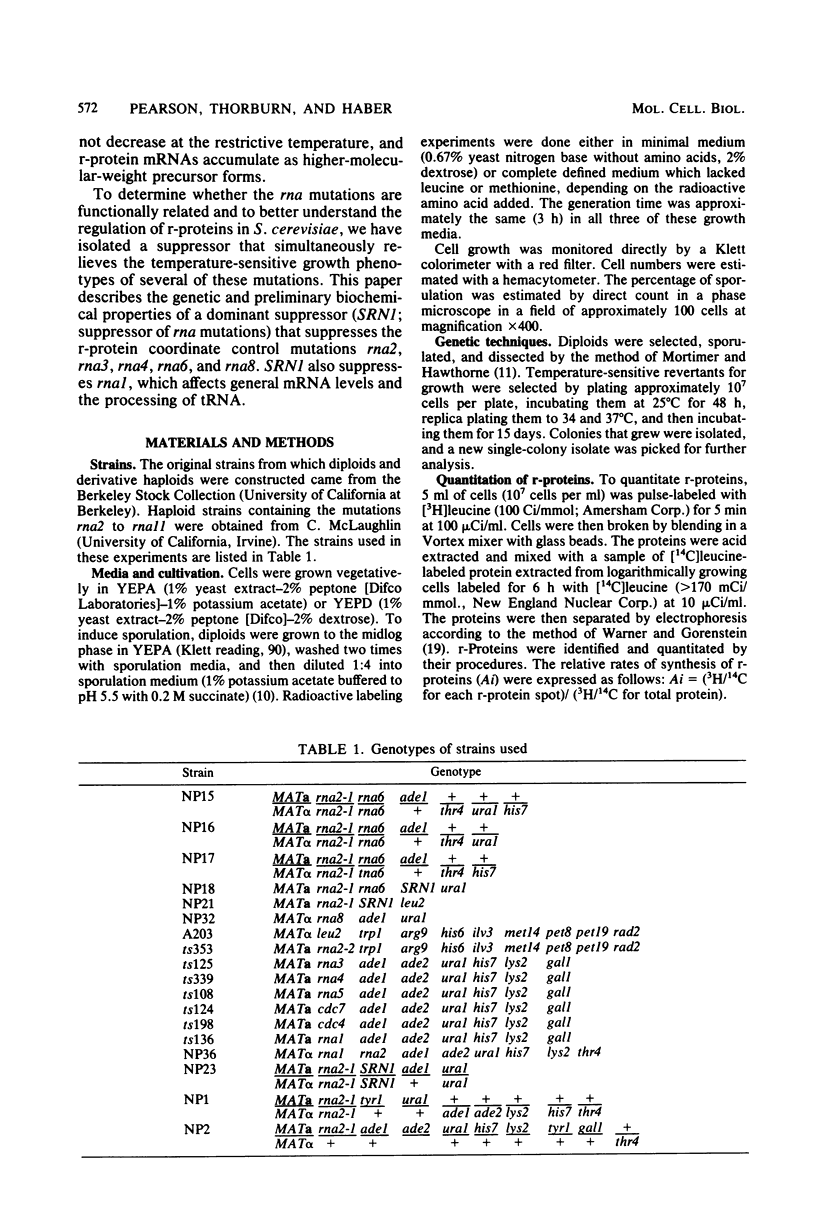
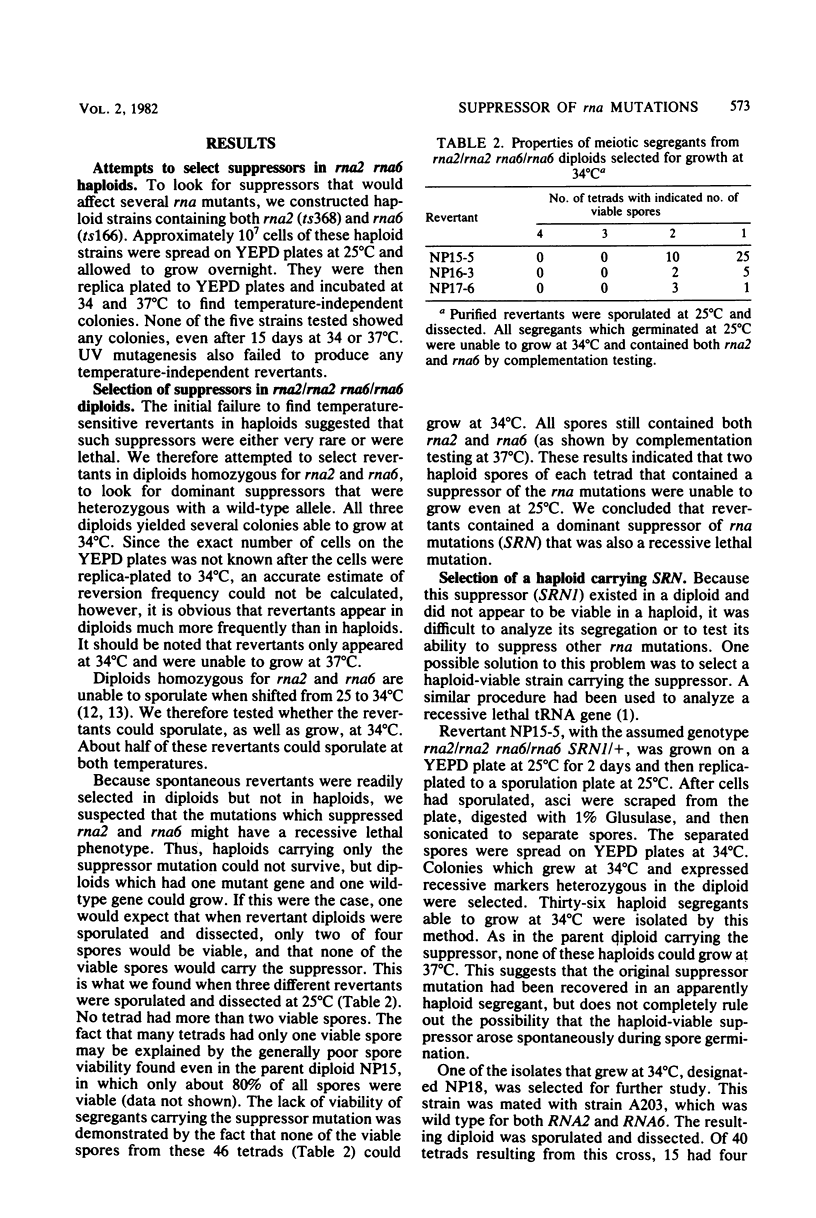
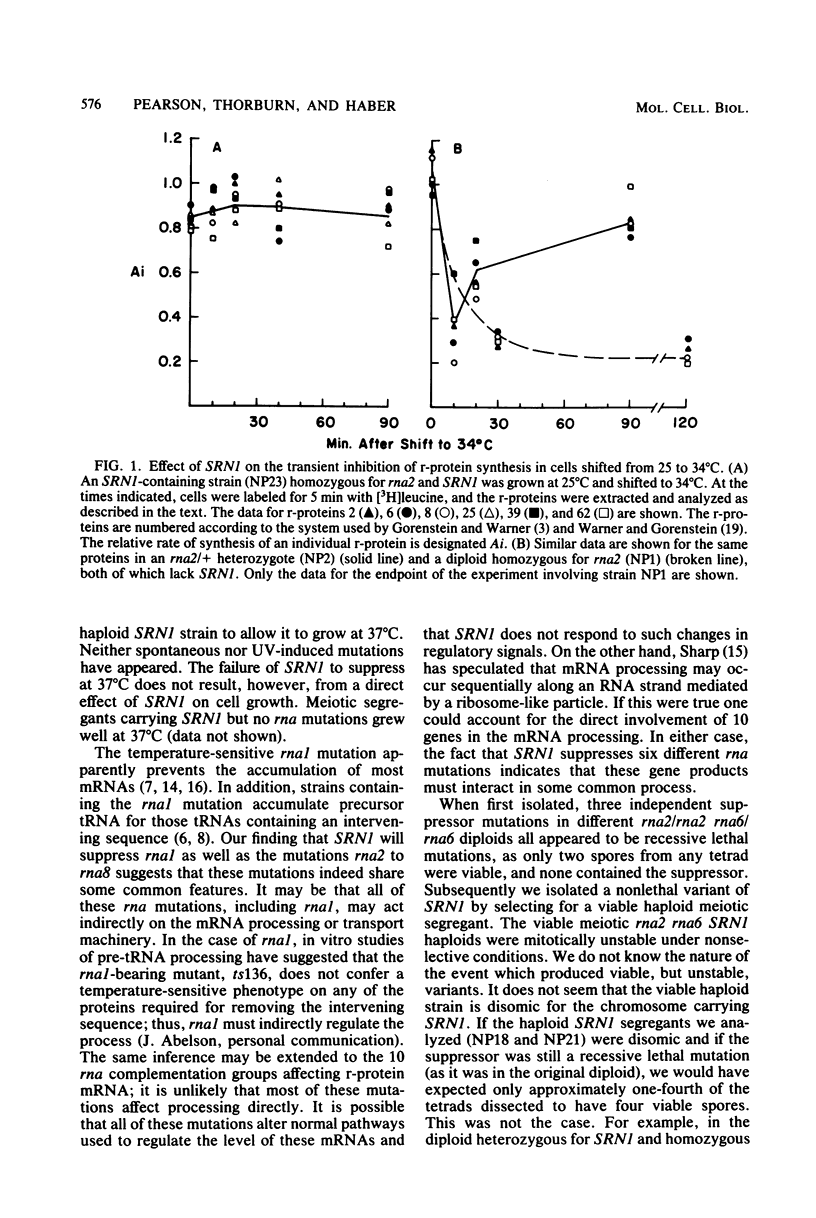
Abstract
We have isolated a dominant suppressor of rna mutation (SRN1) that relieves the temperature-sensitive inhibition of mRNA synthesis of ribosomal protein genes in the yeast Saccharomyces cerevisiae. The suppressor was selected for its ability to alleviate simultaneously the temperature-sensitive growth phenotypes of rna2 and rna6. Several independently isolated suppressors appeared to be recessive lethal mutations. One suppressor, SRN1, was recovered as viable in haploid strains. SRN1 can suppress rna2, rna3, rna4, rna5, rna6, and rna8 singly or in pairs, although some combinations of rna mutations are less well suppressed than others. The suppressor allows strains with rna mutations to grow at 34 degrees C but is unable to suppress at 37 degrees C; however, SRN1 does not, by itself, prevent growth at 37 degrees C. In addition, SRN1 suppresses the rna1 mutation which affects general mRNA levels and also leads to the accumulation of precursor tRNA for those tRNAs that have intervening sequences. SRN1 can suppress the rna1 mutation as well as the rna1 rna2 double mutation at 34 degrees C. The suppressor does not affect the temperature-sensitive growth of two unrelated temperature-sensitive mutations, cdc4 and cdc7.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandriss M. C., Soll L., Botstein D. Recessive lethal amber suppressors in yeast. Genetics. 1975 Apr;79(4):551–560. doi: 10.1093/genetics/79.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried H. M., Pearson N. J., Kim C. H., Warner J. R. The genes for fifteen ribosomal proteins of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10176–10183. [PubMed] [Google Scholar]
- Gorenstein C., Warner J. R. Coordinate regulation of the synthesis of eukaryotic ribosomal proteins. Proc Natl Acad Sci U S A. 1976 May;73(5):1547–1551. doi: 10.1073/pnas.73.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., McLaughlin C. S., Warner J. R. Identification of ten genes that control ribosome formation in yeast. Mol Gen Genet. 1970;109(1):42–56. doi: 10.1007/BF00334045. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Banks F. A yeast mutant which accumulates precursor tRNAs. Cell. 1978 Jun;14(2):211–219. doi: 10.1016/0092-8674(78)90108-3. [DOI] [PubMed] [Google Scholar]
- Hutchison H. T., Hartwell L. H., McLaughlin C. S. Temperature-sensitive yeast mutant defective in ribonucleic acid production. J Bacteriol. 1969 Sep;99(3):807–814. doi: 10.1128/jb.99.3.807-814.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp G., Beckmann J. S., Johnson P. F., Fuhrman S. A., Abelson J. Transcription and processing of intervening sequences in yeast tRNA genes. Cell. 1978 Jun;14(2):221–236. doi: 10.1016/0092-8674(78)90109-5. [DOI] [PubMed] [Google Scholar]
- McCusker J. H., Haber J. E. Efficient sporulation of yeast in media buffered near pH6. J Bacteriol. 1977 Oct;132(1):180–185. doi: 10.1128/jb.132.1.180-185.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Haber J. E. Changes in regulation of ribosomal protein synthesis during vegetative growth and sporulation of Saccharomyces cerevisiae. J Bacteriol. 1980 Sep;143(3):1411–1419. doi: 10.1128/jb.143.3.1411-1419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson N. J., Haber J. E. Changes in regulation of ribosome synthesis during different stages of the life cycle of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Dec 14;158(1):81–91. doi: 10.1007/BF00455122. [DOI] [PubMed] [Google Scholar]
- Rosbash M., Harris P. K., Woolford J. L., Jr, Teem J. L. The effect of temperature-sensitive RNA mutants on the transcription products from cloned ribosomal protein genes of yeast. Cell. 1981 Jun;24(3):679–686. doi: 10.1016/0092-8674(81)90094-5. [DOI] [PubMed] [Google Scholar]
- Sharp P. A. Speculations on RNA splicing. Cell. 1981 Mar;23(3):643–646. doi: 10.1016/0092-8674(81)90425-6. [DOI] [PubMed] [Google Scholar]
- Shiokawa K., Pogo A. O. The role of cytoplasmic membranes in controlling the transport of nuclear messenger RNA and initiation of protein synthesis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2658–2662. doi: 10.1073/pnas.71.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. The ribosomal proteins of Saccharomyces cerevisiae. Methods Cell Biol. 1978;20:45–60. doi: 10.1016/s0091-679x(08)62008-7. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. The synthesis of eucaryotic ribosomal proteins in vitro. Cell. 1977 May;11(1):201–212. doi: 10.1016/0092-8674(77)90331-2. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Gorenstein C. Yeast has a true stringent response. Nature. 1978 Sep 28;275(5678):338–339. doi: 10.1038/275338a0. [DOI] [PubMed] [Google Scholar]
- Warner J. R., Udem S. A. Temperature sensitive mutations affecting ribosome synthesis in Saccharomyces cerevisiae. J Mol Biol. 1972 Mar 28;65(2):243–257. doi: 10.1016/0022-2836(72)90280-x. [DOI] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Hereford L. M., Rosbash M. Isolation of cloned DNA sequences containing ribosomal protein genes from Saccharomyces cerevisiae. Cell. 1979 Dec;18(4):1247–1259. doi: 10.1016/0092-8674(79)90236-8. [DOI] [PubMed] [Google Scholar]


