Abstract
Background
Agriculture is the single largest geo-engineering initiative that humans have initiated on planet Earth, largely through the introduction of unprecedented amounts of reactive nitrogen (N) into ecosystems. A major portion of this reactive N applied as fertilizer leaks into the environment in massive amounts, with cascading negative effects on ecosystem health and function. Natural ecosystems utilize many of the multiple pathways in the N cycle to regulate N flow. In contrast, the massive amounts of N currently applied to agricultural systems cycle primarily through the nitrification pathway, a single inefficient route that channels much of this reactive N into the environment. This is largely due to the rapid nitrifying soil environment of present-day agricultural systems.
Scope
In this Viewpoint paper, the importance of regulating nitrification as a strategy to minimize N leakage and to improve N-use efficiency (NUE) in agricultural systems is highlighted. The ability to suppress soil nitrification by the release of nitrification inhibitors from plant roots is termed ‘biological nitrification inhibition’ (BNI), an active plant-mediated natural function that can limit the amount of N cycling via the nitrification pathway. The development of a bioassay using luminescent Nitrosomonas to quantify nitrification inhibitory activity from roots has facilitated the characterization of BNI function. Release of BNIs from roots is a tightly regulated physiological process, with extensive genetic variability found in selected crops and pasture grasses. Here, the current status of understanding of the BNI function is reviewed using Brachiaria forage grasses, wheat and sorghum to illustrate how BNI function can be utilized for achieving low-nitrifying agricultural systems. A fundamental shift towards ammonium (NH4+)-dominated agricultural systems could be achieved by using crops and pastures with high BNI capacities. When viewed from an agricultural and environmental perspective, the BNI function in plants could potentially have a large influence on biogeochemical cycling and closure of the N loop in crop–livestock systems.
Keywords: AMO, ammonia mono-oxygenase, biological nitrification inhibition, BNI, BNI capacity, brachialactone, fatty acids, HAO, hydroxylamine oxidoreductase, high-nitrifying production systems, low-nitrifying production systems, nitrification, Nitrosomonas, nitrate leaching, synthetic nitrification inhibitors, nitrous oxide emissions, sustainability
INTRODUCTION
The biological oxidation of ammonium (NH4+) to nitrate (NO3−), a critical aerobic process, termed ‘nitrification’, evolved about 2·5 billion years ago (Berner, 2006). It is carried out by two groups of chemo-lithotrophic bacteria – ammonia-oxidizing bacteria (AOB; mainly Nitrosomonas spp. and Nitrobacter spp.) and ammonia-oxidizing archaea (AOA), that are ubiquitous components of the soil microbial population (Leninger et al., 2006; Taylor et al., 2010). In most agricultural soils, AOB and AOA are largely responsible for nitrification. Other soil bacterial spp. such as Nitrosocystus and Nitrosospira, and some heterotrophic fungi such as Aspergillus flavus, can play a significant role in the nitrification of selected forest ecosystems (Sommer et al., 1976). Inorganic N forms, i.e. NH4+ or NO3−, are predominantly the major source of nitrogen (N) uptake in agricultural systems. In some N-limited natural ecosystems of the Arctic, however, organic N forms, particularly free amino acids, can be absorbed directly by plant roots (Kielland, 2001). Nitrification and subsequent denitrification that reduce nitrate are critical parts of the processes used for removing excess N from organic wastes and aquatic ecosystems. Conversely, in agricultural systems, rapid and unregulated nitrification results in inefficient N use by crops, leading to increased N leakage and environmental pollution (Clark, 1962; Likens et al., 1969; Schlesinger, 2009). Most plants have the ability to use either NH4+ or NO3− as their N source, and thus are not dependent solely on NO3− (Haynes and Goh, 1978; Salsac et al., 1987; Boudsocq et al., 2012). Consequently, reducing nitrification rates in agricultural systems does not alter the intrinsic ability of plants to absorb N, but increases N retention time in the root zone as NH4+ is much less mobile than NO3−, providing additional time for the plants to absorb N. This in turn reduces the amount of N lost through leaching and denitrification (Hodge et al., 2000; Subbarao et al., 2012a).
Nitrogen cycle in intensified agricultural systems
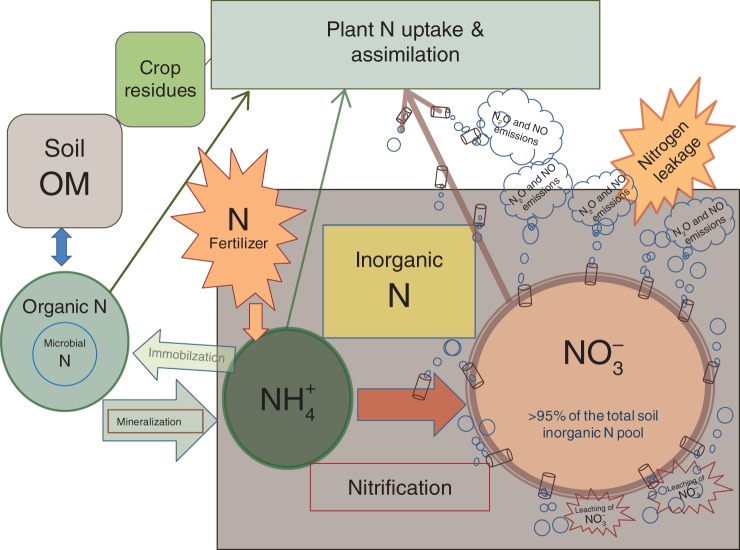
Nitrogen fixation, organic matter (OM) mineralization, ammonification, nitrification and denitrification are all important components of the soil N cycle in terrestrial ecosystems; however, their relative importance may vary. To limit N leakage, natural ecosystems exploit these multiple pathways to regulate N flows through the suppression of nitrification and by utilizing various N forms (both organic and inorganic forms) as N sources, restricting N flow through the nitrification path (Hari and Kulmala, 2008; Smolander et al., 2012). In contrast, nitrification is the process that dominates the N cycle in typical agricultural systems (i.e. neutral upland aerobic soils; Fig. 1), and NO3− accounts for >95 % of the total N uptake by crop plants, making N cycling in these systems inefficient and extremely leaky to the environment (Fig. 1; Sahrawat, 1982; Galloway et al., 2008; Schlesinger, 2009).
Fig. 1.
The nitrogen cycle in a typical agricultural system (i.e. upland aerobic soil with neutral reaction) dominated by the nitrification pathway in which >95 % of the N flows through, and NO3− remains the major inorganic N form for absorption and assimilation by plants (adapted from Subbarao et al., 2012a).
High-nitrifying agricultural systems impact the global environment
The Green Revolution, largely based on the use of massive amounts of industrially fixed N for semi-dwarf rice and wheat cultivars, quadrupled global food grain production during 1960–2009, but at a large environmental cost (Broadbent and Rauschkolb, 1977; Matson et al., 1999; Tilman et al., 2001, 2002; Hungate et al., 2003; Sutton et al., 2011). Worldwide, chemical fertilizer consumption has increased 4-fold during the last 50 years (FAO, 2011). The high-nitrifying nature of these intensive production systems results in loss of nearly 70 % of the overall N-fertilizer inputs (Peterjohn and Schlesinger, 1990; Raun and Johnson, 1999; Vitousek and Howarath, 1991). With the worldwide N-fertilizer applications reaching 150 Tg year−1 (Galloway et al., 2008) and the cost of urea N ranging recently from US$0·80 to 0·54 kg−1 N, the direct annual economic loss is estimated at nearly US$81 billion (Subbarao et al., 2012a). Fertilizer N use is expected to double by 2050 to reach close to 300 Tg year−1 (Galloway et al., 2008; Schlesinger, 2009). This will further increase N leakage from agricultural systems, placing an even greater pollution load on the environment (Ju et al., 2009; Schlesinger, 2009; Tilman et al., 2001). The loss of NO3− from the root zone and NO3− contamination of ground and surface waters are major environmental concerns associated with nitrification (Tilman et al., 2001; Galloway et al., 2008; Schlesinger, 2009). Current estimates indicate that N lost by NO3− leaching from agricultural systems could reach 61·5 Tg N year−1 by 2050 (Schlesinger, 2009). Globally, agricultural systems contribute almost 30 % of nitric oxide (NO) and 70 % of N2O atmospheric emissions (Bremner and Blackmer, 1978; Smith et al., 1997, 2007; Hofstra and Bouwman, 2005). N2O is a powerful greenhouse gas having a GWP (global warming potential) 300 times greater than that of CO2 (Kroeze, 1994; IPCC, 2007), while the Earth's protective ozone layer is damaged by NOs that reach the stratosphere (Crutzen and Ehhalt, 1977). Current estimates indicate that nearly 17 Tg N year−1 is emitted into the atmosphere as N2O (Galloway et al., 2008; Schlesinger, 2009). By 2100, the global N2O emissions are projected to be four times greater than the current estimations, due largely to an increase in the use of N-fertilizers (Hofstra and Bouwman, 2005; Galloway et al., 2008; Burney et al., 2010; Kahrl et al., 2010).
Greenhouse gas (GHG) emissions (CO2, N2O and CH4) associated with N-fertilizer production
Nitrogen-fertilizers are largely responsible for N2O emissions in agricultural systems when they undergo nitrification/denitrification, as described earlier. In addition, substantial amounts of GHGs (eg. CO2, N2O and CH4) are emitted during the production of N-fertilizers, which can be expressed as CO2 equivalents per unit mass of fertilizer (g CO2-e kg−1 N-fertilizer) based on their GWP (IPCC, 1996). Synthesis of ammonia is a very energy-intensive process, and requires about 25–35 GJ t−1 of ammonia (Patyk, 1996; Kongshaug, 1998; Davis and Haglund, 1999). In addition, the production of nitric acid from ammonia, the feedstock for synthesis of complex N-fertilizers (such as NPK formulations), results in large-scale N2O emissions (IPCC, 2012). This emission factor is about 4 kg CO2-e kg−1 urea N and about 10 kg CO2-e kg−1 N in complex fertilizers (i.e. NPK; Kuesters and Jenssen, 1998; Davis and Haglund, 1999; Kramer et al., 1999). With the current levels of annual N-fertilizer application in agriculture (i.e. 150 Tg-N), this amounts to GHG emissions of 600–1500 Tg CO2-e associated with global N-fertilizer production (excluding GHG emissions during transportation of N-fertilizers from factory to farm). These GHG emissions are similar in magnitude to annual CO2-e emissions from motor vehicles, which are at 900 Tg CO2-e (Schafer and Victor, 1999; DeCicco and Fung, 2006). By the year 2050, GHG emissions from global N-fertilizer production will reach 1200–3000 Tg CO2-e (based on current estimates that N-fertilizer usage will reach 300 Tg by 2050; Galloway et al., 2008; Schlesinger, 2009). Currently, global CO2 emissions are at 34 000 Tg CO2-e (IPCC, 2012) and GHG emissions from N-fertilizer production currently accounts for about 2–4 % of global CO2 emissions.
Switching to low-nitrifying agricultural systems?
Nitrification plays a relatively minor role in many natural climax plant communities where only a small portion of the total N goes through the nitrification pathway (Rice and Pancholy, 1972, 1973, 1974; White, 1991; Nasholm et al., 1998; Paavolainen et al., 1998). In contrast, >90 % of the total N flow is through the nitrification pathway in most agricultural systems (Fig. 1; Sahrawat, 1982; Vitousek et al., 1997; Smolander et al., 1998; Subbarao et al., 2006a). Most modern agricultural systems have become high-nitrifying environments where nitrification is so rapid that most of the NH4+ that enters either from OM mineralization or from external N inputs (i.e. chemical N-fertilizer such as urea) is nitrified within a few weeks (often <2 weeks), making it vulnerable to loss through either leaching or denitrification (Sahrawat, 2008; Subbarao et al., 2012a).
Several changes in agricultural management practices during the 20th century have led to high-nitrifying agronomic environments. Current production systems depend heavily on industrially fixed N (i.e. N-fertilizer), and they have replaced the earlier production systems that relied primarily on N2-fixing legumes and/or animal wastes as their N sources (Dinnes et al., 2002; Foley et al., 2005). The separation of crop production from animal production has led to an even greater dependence on mineral N-fertilizers, bypassing classical agricultural systems for OM recycling. This has also resulted in the reduction of soil OM (SOM) levels in agricultural systems worldwide (Elliott, 1986; Ross, 1993; Tiessen et al., 1994; Celik, 2005; Foley et al., 2005; van Wesemael et al., 2010). The heavy dependence of modern agriculture on mineral N-fertilizers has contributed to the stimulation of nitrifier activity and the development of high-nitrifying soil environments (Poudel et al., 2002; Bellamy et al., 2005). In addition, installation of sub-surface drainage systems has further accelerated N losses from NO3− leaching and denitrification, leading to further declines in N-use efficiency (NUE; Clark, 1962; Pratt and Adriano, 1973; Dinnes et al., 2002).
As a cation, NH4+ is held electrostatically by negatively charged clay surfaces and functional groups of the SOM (Sahrawat, 1989). This bonding is sufficiently strong to reduce the leaching loss of NH4+ N. In contrast, NO3−, with its negative charge, does not readily bond to the soil, and is much more liable to be leached out of the root zone. Several heterotrophic soil bacteria denitrify NO3− under anaerobic or partially anaerobic conditions. This often coincides with temporary water logging of a soil after a heavy rainfall or irrigation in fields that have improper drainage (Bremner and Blackmer, 1978; Mosier et al., 1996). The N loss during and following nitrification reduces the effectiveness of N fertilization and at the same time causes serious N pollution (Clark, 1962; Jarvis, 1996).
Rapid conversion of NH4+ to NO3− in the soil results in inefficient use of both soil N and applied N, and, as soil organic N is also subject to nitrification, this makes it liable to N loss by the same pathways as fertilizer N (Clark, 1962; Barker and Mills, 1980; Dinnes et al., 2002). In addition, the assimilation of NH4+ is energetically more efficient than that of NO3− (20 mol of ATP per mol of NO3− vs. 5 mol of ATP per mol of NH4+; Salsac et al., 1987). Moreover, assimilation of NO3−, but not of NH4+, results in the direct emission of N2O from crop canopies, further reducing NUE (Smart and Bloom, 2001). Maintaining soil N in the NH4+ form thus is advantageous even after taking into consideration the potential negative effects of rhizosphere acidification from its uptake and assimilation (caused by H+ excretion; Britto et al., 2001). Also, by slowing the soil nitrification rates, NH4+ can move into the microbial pool where it becomes a slow-release N source (Vitousek and Matson, 1984; Hodge et al., 2000). Better utilization of NH4+ also depends on the N preference of plant species or cultivars. Many of these advantages associated with inhibiting nitrification in improving crop yield, grain quality and environmental quality have been demonstrated using chemical inhibitors (Huber et al., 1977; Slangen and Kerkhoff, 1984; Sahrawat, 1989; Subbarao et al., 2012a).
Options for nitrification control
Various N management strategies have been developed both to synchronize fertilizer N application with crop requirements to facilitate rapid uptake and to reduce N residence time in soil, thereby limiting losses associated with leaching and denitrification of NO3− (Newbould, 1989; Dinnes et al., 2002). There are a number of agronomic strategies involving rates and/or timing of fertilizer application (such as autumn vs. spring, basal vs. broadcast, deep vs. surface applications, point injection placement of solutions, foliar applications of urea) that have been used to minimize N losses from agricultural systems, with varying degrees of success in production agriculture. Often these agronomic strategies have intrinsic limitations such as additional labour requirements/costs that make them less desirable to adopt (Dinnes et al., 2002). In addition, specialty fertilizers with controlled N release in the soil such as polythene-coated urea (PCU) have been demonstrated to reduce nitrification (Shaviv and Mikkelsen, 1993; Zvomuya et al., 2003). Such specialty fertilizers can be used more effectively, and the rate of N application can be reduced up to 40 % without yield loss (Shoji and Kanno, 1994); however, these fertilizers are nearly 8-fold more expensive than normal urea, and thus have not been widely adopted in production agriculture.
Use of synthetic chemical inhibitors
Nitrification inhibitors (NIs) are compounds that delay the bacterial oxidation of NH4+ by suppressing nitrifying soil bacteria, which should improve NUE (Hendrickson et al., 1978; Bremner et al., 1981; Rodgers, 1986). Reducing nitrification rates during the initial crop establishment phase and increasing it during the rapid growing phase will reduce the risk of NO3− leaching from the rooting zone (Dinnes et al., 2002; Liao et al., 2004). Though several NIs have been proposed for use in production agriculture, only a few compounds, including nitrapyrin, DCD (dicyandiamide) and DMPP (3, 4-dimethyl pyrazole phosphate), have reached the agronomic evaluation stage (Guthrie and Bomke, 1980; Weiske et al., 2001; Zerulla et al., 2001; Subbarao et al., 2006a, 2012a). Nitrapyrin has been adopted for certain niche production systems such as winter wheat in North America. However, NIs are not widely used in production agriculture due to their limited biological stability, the non-availability of technology to deliver them to the sites of nitrification (due to differences in mobility of NIs and fertilizer N) and, equally important, their lack of cost-effectiveness (Sahrawat and Keeney, 1985; Subbarao et al., 2012a).
In the field, the effects of NIs are most likely to be greater on soils which are N rich and where the N losses due to leaching and denitrification are large. However, the expression of these effects through plant growth will depend on the soil N status, as limiting N losses on N-rich soils may have little effect on plant production. To determine the effectiveness of NIs, it is therefore important also to take into account other soil (texture, temperature, moisture content, OM content and pH) and climatic (temperature, rainfall intensity and frequency) parameters impacting the size of N losses. In particular, OM also releases NH4+ through mineralization, which goes through nitrification in the same way as does fertilizer N; targeting such OM-derived NH4+ for NIs could be even more challenging as this OM-derived NH4+ could be evenly distributed in the soil profile unlike fertilizer N (which is normally banded close to plant roots).
Lessons from natural systems for managing nitrification in agricultural systems
Unlike most agricultural systems, the climax ecosystems retain large amounts of N through its incorporation into SOM (immobilization), but the underlying mechanisms remain poorly understood (Jordan et al., 1979; Magill et al., 2000). Natural ecosystems have evolved a range of mechanisms allowing multiple pathways for N uptake and conservation (by closing the cycle; Vitousek and Matson, 1984; Hari and Kulmala, 2008; Smolander et al., 2012). They include direct uptake of organic N by plants (essentially short-circuiting mineralization) and suppressing nitrification to facilitate NH4+ uptake (Cooper, 1986; White, 1991; Steltzer and Bowman, 1998; Kielland, 2001; Weigelt et al., 2005; Barot et al., 2007; Harrison et al., 2007; Houlton et al., 2007; Aanderud and Bledsoe, 2009; Hewins and Hyatt, 2009; Ashton et al., 2010; Smolander et al., 2012). For example, in certain pine forest systems, polyphenols released from leaf litter form complexes with dissolved organic N (DON; Baldwin et al., 1983), and DON–polyphenol complexes resist mineralization, but can be taken up by certain ecto-mycorrhizae (that colonize pine root systems), where it is mineralized and supplied to the pine host, thereby tightly regulating the N flow within these ecosystems without much N loss (Northup et al., 1995; Smolander et al., 2012).
Researchers have observed substantial differences in soil nitrification potential among several ecosystems that are not associated with soil physical and chemical characteristics (Clark et al., 1960; Robertson et al., 1982a, b; Montagnini et al., 1989; Steltzer and Bowman, 1998; Lata et al., 2004). Often the levels of NH4+ exceed NO3− levels by a factor of ten, indicating that the availability of NH4+ is not the limiting factor for nitrification (Schimel and Bennett, 2004). The influence of vegetation in inhibiting nitrification has long been suspected, but not directly proven (Basaraba, 1964; Bate, 1981; Donaldson and Henderson, 1990a, b; Erickson et al., 2000; Smits et al., 2010a, b; Smolander et al., 2012). Certain forest trees, such as Arbutus unedo, have been reported to suppress soil nitrification and N2O emissions by releasing gallocatechin and catechin during the decomposition of leaf litter (Castaldi et al., 2009).
Since NH4+ assimilation in plants requires a quarter as much metabolic energy as that of NO3−, it is hypothesized that inhibition of nitrification could be an ecological driving force for the development of low-nitrifying climax ecosystems (Rice and Pancholy, 1972; Salsac et al., 1987; Lata et al., 2004). Slow rates of nitrification have been observed in several tropical grassland and forest ecosystems, and are often considered to be an indicator of ecosystem maturity (Vitousek and Matson, 1984; Cooper, 1986; Sylvester-Bradley et al., 1988; Lata et al., 1999; Ishikawa et al., 2003; Castaldi et al., 2009; Smits et al., 2010a, b; Smolander et al., 2012). This led to the hypothesis that plants may influence nitrification by releasing certain phytochemicals that interfere with the activity of soil nitrifiers (Subbarao et al., 2006a, 2012a; Fillery, 2007).
The hypothesis that plants can suppress or stimulate nitrification has been debated since the 1960s, and experimental evidence has proved elusive due to the lack of a suitable methodology to detect and assess any inhibitory activity of roots unequivocally (Munro, 1966a, b; Meiklejohn, 1968; Moore and Waid, 1971; Purchase, 1974; Rice and Pancholy, 1974; Lodhi, 1979, 1982; Sylvester-Bradley et al., 1988; Stienstra et al., 1994; Lata et al., 1999, 2004; Fillery, 2007; Smits et al., 2010a, b). By controlling nitrification, plants could increase N availability for their own survival in N-limiting environments (Hodge et al., 2000; Weigelt et al., 2005; Hewins and Hyatt, 2009). From an evolutionary viewpoint, the question remains of whether such nitrification inhibition ability [i.e. biological nitrification inhibition (BNI)] would provide a sufficient competitive advantage to outcompete other plants (Mouquet et al., 2002; Lata et al., 2004; Hawkes et al., 2005; Hewins and Hyatt, 2009; Rossiter-Rachor et al., 2009). Modelling studies on BNI function and in situ observations provide additional support to the hypothesis that BNI capacity may offer a sufficient competitive advantage to the observed invading/introduced tropical grasses from Africa into South America and Australia (Lata et al., 2004; Hawkes et al., 2005; Barot et al., 2007; Boudsocq et al., 2009, 2011, 2012; Hewins and Hyatt, 2009; Maire et al., 2009; Rossiter-Rachor et al., 2009).
BIOLOGICAL NITRIFICATION INHIBITION
The concept
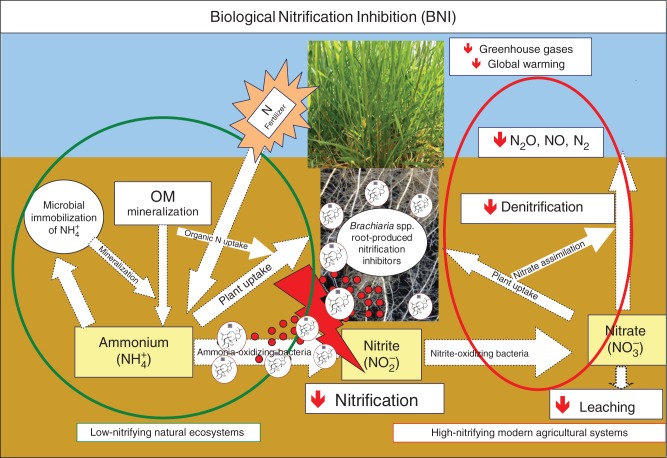
The ability to suppress soil nitrification through the release of nitrification inhibitors from plant roots is termed ‘biological nitrification inhibition’ (Subbarao et al., 2006a, b, 2009a, b, 2012a; Fig. 2). Nitrification is one of the most important processes determining N cycling efficiency (i.e. the proportion of N that stays in the ecosystem during a complete recycling loop); controlling nitrification thus will help in minimizing N leakage and facilitating N flow through NH4+ assimilation pathways.
Fig. 2.
Schematic representation of the biological nitrification inhibition (BNI) interfaces with the N cycle. The BNI exuded by the root inhibits nitrification that converts NH4+ to NO2−. In ecosystems with large amounts of BNI (e.g. brachialactone) such as Brachiaria grasses, the flow of nitrogen from NH4+ to NO3− is restricted, and it is NH4+ and microbial N rather than NO3− that accumulates in the soil and root systems. In systems with little or no BNI, such as modern agricultural systems, nitrification occurs at a rapid rate, converting NH4+ to NO3−, which is highly susceptible to loss by denitrification and leaching from the system (adapted from Subbarao et al., 2012a).
Nitrogen-use efficiency (NUEagronomic = grain yield per unit of applied N) is a function of both intrinsic NUE (NUEintrinsic = dry matter produced per unit N absorbed), harvest index (HI) and N uptake. The NUEintrinsic of a plant is a physiologically conserved function (Glass, 2003), and thus would not necessarily be easy to manipulate genetically. Improvements in NUEagronomic can therefore only come from improvements in crop N uptake (Finzi et al., 2007), which is largely related to recovery of applied N-fertilizer. Consequently, BNI function can positively influence NUEagronomic by improving N recovery by reducing N losses associated with nitrification and denitrification (Subbarao et al., 2012a). Recent modelling studies support this hypothesis, and are linked to previous in situ measurements in savanna systems indicating that grasses that inhibit nitrification exhibit a 2-fold greater productivity in above-ground biomass than those that lack such ability (Lata, 1999; Boudsocq et al., 2009, 2012).
Methodology to detect and determine nitrification inhibitory activity (i.e. BNI activity) in plant–soil systems
Lack of a suitable methodology to detect and quantify BNI activity in plant–soil systems has been a major hurdle for characterizing BNI function in plants (Subbarao et al., 2006a). With the development of a bioluminescence assay using a recombinant strain of Nitrosomonas europaea, it is now possible to detect and quantify BNI activity released from roots, a plant function termed ‘BNI capacity’ (Iizumi et al., 1998; Subbarao et al., 2006b). The recombinant strain of N. europaea carries an expression vector for the Vibrio harveyi lux AB genes (Fig. 3), and produces a distinct two-peak luminescence pattern during a 30 s analysis period (Subbarao et al., 2006b). The functional relationship between bioluminescence emission and nitrite production in the assay has been shown to be linear using a synthetic nitrification inhibitor, allylthiourea (AT; Subbarao et al., 2006b). The inhibition caused by 0·22 µm AT in the assay (about 80 % inhibition in bioluminescence and NO2− production) is defined as one allylthiourea unit (ATU; Subbarao et al., 2006b). Using the response to a concentration gradient of AT (i.e. a dose–response standard curve), the inhibitory effects of test samples (e.g. root exudates or plant or soil extracts) can be expressed and compared in ATUs. These recently developed research tools facilitated the characterization of BNI function in plants (Subbarao et al., 2006b). Soil-based assays to determine the changes in nitrification potential of rhizosphere soil complement this characterization of BNI capacity (based on BNI activity release from roots). The changes in potential soil nitrification (due to BNI function) can be determined by monitoring ammonia-oxidizing activity (Hart et al., 1994), and this methodology has been successfully deployed to assess the BNI capacity of Brachiaria grasses and in matgrass swards in the field (Subbarao et al., 2009a; Smits et al., 2010a). In addition, analysis of nitrifier populations [(AOB) and AOA)] in rhizosphere soil would provide further evidence for the changes in potential soil nitrification (Subbarao et al., 2009a).
Fig. 3.
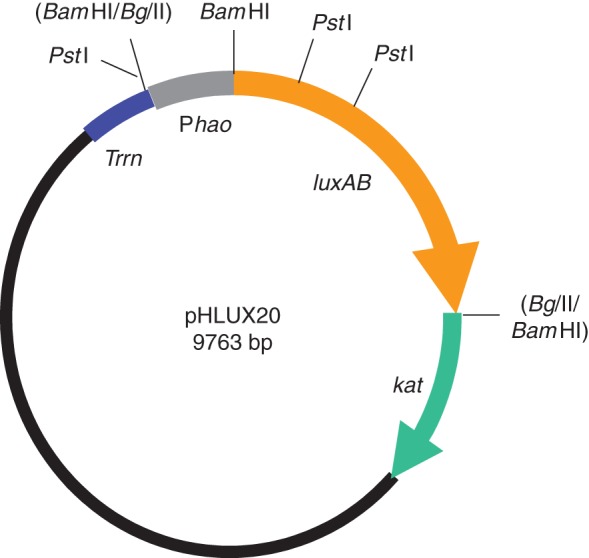
Map of recombinant luminous Nitrosomonas europaea (pHLUX20) developed to detect and quantify nitrification inhibitors in the plant–soil system (redrawn from Iizumi et al., 1998).
Evidence of BNI function in selected field crops and pasture grasses
Evaluation of selected tropical forage grasses, cereals and legume crops indicated a wide range in the BNI capacity of their root systems (Subbarao et al., 2007b). Forage grasses of Brachiaria humidicola and B. decumbens, which are highly adapted to the low-N production environments of South American savannas (Miles et al., 2004), showed the greatest BNI capacity among the tropical grasses evaluated (Subbarao et al., 2007b). In contrast, Panicum maximum, which is adapted to high-N availability environments, showed the least BNI capacity (Rao et al., 1996; Subbarao et al., 2007b). Among the cereal crops evaluated, only sorghum (Sorghum bicolor) showed significant BNI capacity (Subbarao et al., 2007b; 2012b). Other crops including rice (Oryza sativa), maize (Zea mays), wheat and barley (Hordeum vulgare) were found to lack BNI capacity in their root systems during initial screening studies (Subbarao et al., 2007b, 2012a; Zakir et al., 2008). Most legumes evaluated showed negative BNI activity in root exudates, indicating that they are likely to stimulate nitrification (Subbarao et al., 2007b). Inhibition of nitrification is likely to be part of an adaptation mechanism to conserve and use N efficiently in natural systems that are N limiting (Lata et al., 2004; Subbarao et al., 2007a). N-limiting environments thus could be one of the dominant forces driving the evolution of BNI function (Rice and Pancholy, 1972; Lata et al., 2004). It is not surprising then that legumes did not show much BNI capacity in their roots as it is likely that the BNI attribute may have no adaptive value due to their ability to fix N symbiotically; in addition, conserving N may not offer much of an advantage for legumes as it may attract non-legumes as competitors (Subbarao et al., 2009b, 2012a).
Field validation of BNI function in suppressing soil nitrification and N2O emissions
Based on conservative estimates that the live root biomass from a long-term grass pasture is 1·5 Mg ha−1 (Fisher et al., 1994) with a BNI capacity of 17–70 ATU g−1 root d. wt d−1 (Subbarao et al., 2007a), it was estimated that BNI activity of 2·6 × 106–7·5 × 106 ATU ha−1 d−1 can potentially be released from B. humidicola roots (Subbarao et al., 2007a, 2009a). This estimate amounts to nitrification inhibitory potential equivalent to the application of about 6·2–18 kg of nitrapyrin ha−1 year−1 (based on 1 ATU being equal to 0·6 µg of nitrapyrin), which is large enough to have a significant influence on the function of soil nitrifier populations and nitrification rates (Subbarao et al., 2009a). Subsequently, field studies (Mollisol) at the International Center for Tropical Agriculture (CIAT) indicated a 90 % decline in soil ammonium oxidation rates (Fig. 4A) due to extremely small populations of nitrifiers [(AOB and AOA); determined as amoA genes] in B. humidicola plots within 3 years of establishment. Nitrous oxide emissions were also suppressed by >90 % in field plots of B. humidicola (CIAT 16888) compared with plots of soybean, which lacks BNI capacity in its roots, or control plots (plant-free field plots; Fig. 4B). Two other pasture grasses, P. maximum and Brachiaria spp. hybrid ‘Mulato’ that have a low to moderate level of BNI capacity (3–10 ATU g−1 root d. wt d−1), showed only an intermediate level of inhibitory effect on soil ammonium oxidation rates (Fig. 4A). A negative relationship was observed between the BNI capacity of roots of a species and N2O emissions, based on field monitoring of N2O emissions over a 3-year period in tropical pasture grasses having a wide range of BNI capacity in roots (Fig. 5).
Fig. 4.

(A) Soil ammonium oxidation rates (mg of NO2− N kg−1 soil d−1) in field plots planted to tropical pasture grasses (differing in BNI capacity) and soybean (lacking BNI capacity in roots) [covering 3 years from establishment of pastures (September 2004–November 2007); for soybean, two planting seasons every year and after six seasons of cultivation]. CON, control (plant-free) plots; SOY, soybean; PM, P. maximum; BHM, Brachiaria hybrid ‘Mulato’; BH-679, B. humidicola CIAT 679 (standard cultivar); BH-16888, B. humidicola accession CIAT 16888 (a germplasm accession). Values are means ± s.e. of three replications (adapted from Subbarao et al., 2009a). (B) Cumulative N2O emissions (mg of N2O N m−2 year−1) from field plots of tropical pasture grasses (monitored monthly over a 3-year period, from September 2004 to November 2007). Values are means ± s.e. of three replications (adapted from Subbarao et al., 2009a).
Fig. 5.
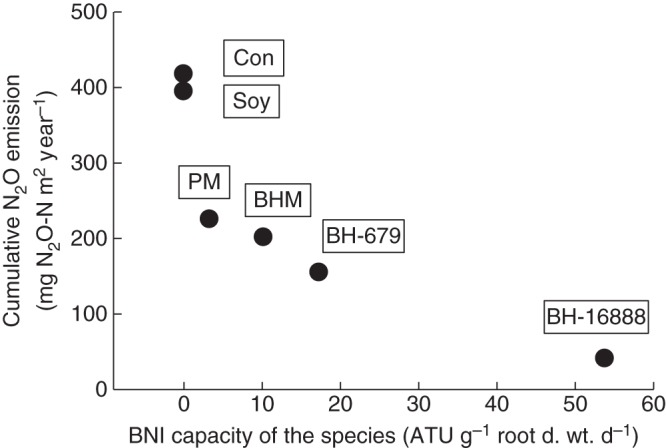
Relationships of the BNI capacity of plant species to N2O emissions from field plots (based on data from Fig. 4B). The N2O emissions were monitored over a period of 3 years (adapted from Subbarao et al., 2012a; see Fig. 4 for abbreviations and treatment details).
Regulation of BNI function
Synthesis and release of BNIs is a highly regulated attribute (Subbarao et al., 2007a; Zhu et al., 2012). The form of N applied (i.e. NH4+ or NO3−) has a major influence on the synthesis and release of BNIs in B. humidicola, sorghum and Leymus racemosus, wild wheat (Subbarao et al., 2007a, c, 2009c, 2012a, b; Zakir et al., 2008). Plants grown with NO3− as N source did not release BNIs from roots, whereas BNIs were released from plants grown with NH4+ as their N source (Subbarao et al., 2007a, 2009a; Zakir et al., 2008; Zhu et al., 2012). Despite high levels of BNIs detected in the root tissues of NH4+-grown plants, the release of BNIs was observed only when plant roots were directly exposed to NH4+ (Subbarao et al., 2007a, c, 2009a, b; Fig. 6A, B). In addition to the presence of NH4+ in the root zone, the rhizosphere pH may also influence the release of BNIs from roots. Recent results suggest that sorghum plants do not release BNIs from their roots in the presence of NH4+ when the rhizosphere pH is 7 or higher; maximum release of BNI activity was observed only at a rhizosphere pH ranging from 5·0 to 6·0 (Subbarao et al., 2012b). Such a tight control of rhizosphere pH on BNI release has implications regarding in which soils the BNI function is likely to be effective (Subbarao et al., 2012b). For example, the heavy black soils (Vertisols), which generally exhibit a soil pH of >7·0, have a large buffering capacity and thus resist changes in rhizosphere pH (Burford and Sahrawat, 1989). Sorghum grown on soils with pH in the alkaline range might thus not release BNIs, and hence such soil types might not be suitable for the expression of BNI function in sorghum. Perhaps light-textured soils with low buffering capacity and with moderate acidity (pH <6·0) might be better suited for the expression and exploitation of BNI function in sorghum (Subbarao et al., 2012a, b).
Fig. 6.
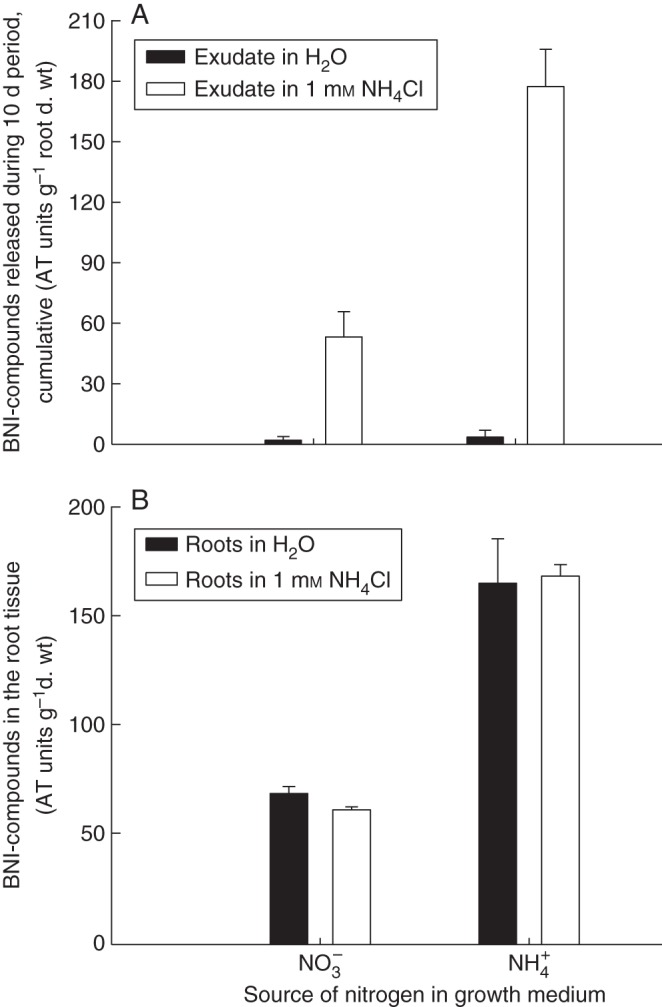
(A) BNI activity released from B. humidicola roots during a 10 d period (adapted from Subbarao et al., 2007a). (B) The BNI activity in the root tissue (ATU g−1 root d. wt) at the end of the 10 d root exudate collection (redrawn from Subbarao et al., 2007a).
Further, the release of BNIs from plant roots appears to be a highly regulated physiological function. The physiological consequences associated with the uptake of NH4+, such as activation of H+ pumps in the plasmalemma and acidification of the rhizosphere, facilitate BNI release from sorghum roots (Zhu et al., 2012). In addition, the release of BNIs from roots is a localized phenomenon (Subbarao et al., 2009a). The release of BNIs was confined to parts of the root system exposed to NH4+, and was not extended to the remaining parts of the root system. Such localized release of BNIs by roots ensures a relatively high concentration of BNIs in soil microsites where nitrifiers are active, which is often associated with the presence of NH4+ (Subbarao et al., 2009a). The availability of NH4+ in the soil either from soil organic N mineralization or through the application of N-fertilizers can thus enhance nitrifier activity (Robinson, 1963; Woldendorp and Laanbroek, 1989). The regulatory role of NH4+ in the synthesis and release of BNIs suggests a possible adaptive role in protecting NH4+ from nitrifiers, a key factor for the successful evolution of the BNI capacity as an adaptive trait (Subbarao et al., 2007a, 2009a, 2012a).
Stability of the BNIs in soil systems
The inhibitory activity of the BNIs was initially determined in an assay lasting only for a 30 min incubation exposure period to pure cultures of Nitrosomonas sp. (Subbarao et al., 2006b). For the BNIs to function effectively in the soil-based systems, the persistence of these compounds in the soil is a pre-requisite for the effectiveness of BNI function under field environments. This hypothesis was tested by adding extracted BNI activity (from root exudates of B. humidicola) to soil at different levels (0–20 ATU g−1 soil) along with an NH4+ source (200 mg N kg−1) and then incubating for 55 d at 20 °C. Results from these studies indicated that for the inhibitory activity to be effective, a threshold level of 5 ATU g−1 soil was needed; nearly 50 % inhibition was observed when the BNI activity level was 10 ATU g−1 soil and a nearly complete suppression of soil nitrification was achieved at 20 ATU g−1 soil (Subbarao et al., 2006a; Gopalakrishnan et al., 2009). Further, it was shown that certain BNIs [such as linoleic acid (LA) and α-linolenic acid (LN)] partially lost their effectiveness in the soil after 80 d, and their inhibitory effect was completely lost after 100 d (Subbarao et al., 2008). Preliminary measurements on mixed tropical savanna soils showed that the inhibitory effect in the soil can persist for a long period during natural air drying and storage of the soil in the dark (Lata, 1999). There is, however, still a paucity of information on the fate and efficacy of BNIs in the soil–plant systems. Thus, intensification of research is justified to generate information on emerging BNIs in different soil types under varying agro-climatic conditions relative to their persistence and effectiveness in soil–plant systems. Such knowledge will be helpful in targeting the use of BNIs to the most appropriate agro-ecosystems (Sahrawat, 1996; Wolt, 2004; Subbarao et al., 2006a).
Biological nitrification inhibitors and their modes of action
Several BNIs that belong to different chemical groups have been successfully isolated and identified from plant tissues or root exudates using bioassay-guided purification approaches (Fig. 7; Bremner and McCarty, 1988; Subbarao et al., 2006b, 2008, 2009a; Gopalakrishnan et al., 2007; Zakir et al., 2008). The compounds with BNI activity in the aerial parts of B. humidicola are unsaturated free fatty acids, LA and LN (Fig. 8; Subbarao et al., 2008). They are relatively weak inhibitors of nitrification, with IC50 values of 3 × 10−5 m; while the IC50 value of the synthetic nitrification inhibitor AT is 1 × 10−7 m. However, other free fatty acids differing in chain lengths or numbers of double bonds, e.g. stearic, oleic, arachidonic and cis-vaccenic acids, did not show much inhibitory activity (Subbarao et al., 2008). The inhibitory effect of LA was increased by its conversion to its methyl ester (LA-ME), but the activity was lost when converted into its ethyl ester (LA-EE; Table 1). In contrast, the inhibitory effect of LN was lost when converted to the methyl ester (LN-ME), indicating that there may be a high degree of specificity in the chemical structure needed to inhibit Nitrosomonas function (Subbarao et al., 2008). Both LA and LN inhibit Nitrosomonas through blocking of both AMO (ammonia mono-oxygenase) and HAO (hydroxylamine oxidoreductase) enzymatic pathways, which catalyse the essential reactions of ammonia oxidation (Subbarao et al., 2008). In addition, BNIs could also disrupt the electron transfer pathway from HAO to ubiquinone and cytochrome (which needs to be maintained to generate reducing power, i.e. NADPH), which is crucial to the metabolic functions of Nitrosomonas (Subbarao et al., 2009b). Most synthetic nitrification inhibitors (e.g. nitrapyrin, dicyandiamide, and DMPP) suppress Nitrosomonas activity by suppressing the AMO enzymatic pathway (McCarty, 1999; Subbarao et al., 2006a; Table 1). Also, our knowledge of the mechanisms of inhibition by BNIs is entirely based on AOBs (i.e. Nitrosomonas sp.). It is increasingly evident that AOA play a significant role in nitrification of most ecosystems and a dominant role in nitrification of certain ecosystems (Leninger et al., 2006). The AMO enzymatic pathway operates in both AOB and AOA; it is assumed that most BNIs that suppress nitrifier activity by blocking the AMO pathway (as mentioned above) are likely to be effective in inhibiting AOB and AOA. However, the second enzymatic pathway in nitrification (i.e. the HAO pathway) is only established for AOB; the existence of this second enzymatic pathway remains to be proven and established for AOA.
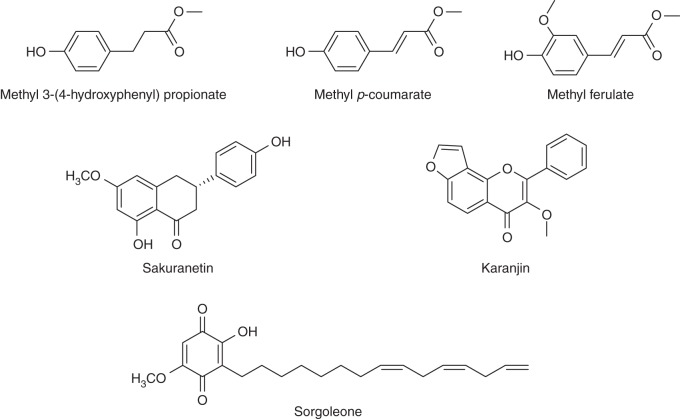
Fig. 7.
Chemical structure of BNIs belonging to different chemical functional groups isolated from plant tissues or root exudates.
Fig. 8.
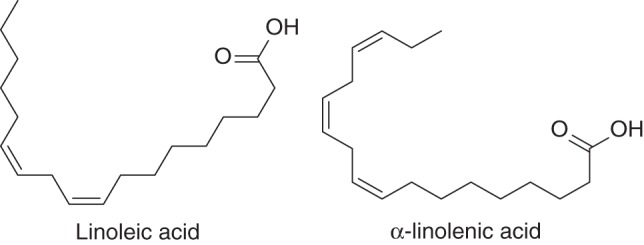
Chemical structure of linoleic acid and α-linolenic acid, the BNIs isolated from the aerial parts of B. humidicola.
Table 1.
Relative effectiveness of free fatty acids, fatty acid esters and standard chemical nitrification inhibitors on Nitrosomonas in an in vitro bioassay (based on Subbarao et al., 2008)
| Compound | ED80* |
|---|---|
| Synthetic nitrification inhibitors | |
| Nitrapyrin© | 4·0 |
| Dicyandiamide© | 185·0 |
| Free fatty acids | |
| Linoleic acid (LA) | 16·0 |
| Linolenic acid (LN) | 16·0 |
| Fatty acid esters | |
| Methyl linoleate (LA-ME) | 8·0 |
| Ethyl linoleate (LA-EE) | 400·0 |
| Methyl linolenate (LN-ME) | >2000·0 |
*Effective dose (μg mL−1) for 80 % inhibition of Nitrosomonas function (i.e. bioluminescence) in an in vitro bioassay system.
A phenyl propanoid from root exudates of hydroponically grown sorghum, methyl 3-(4-hydroxyphenyl) propionate (MHPP), has been identified as the BNI component of the inhibitory activity released from roots (Fig. 7; Zakir et al., 2008). The IC50 value for MHPP is 9 × 10−6 m (Zakir et al., 2008). The mode of inhibitory action for MHPP is solely through the AMO enzymatic pathway, and it has no inhibitory effect on the HAO enzymatic pathway in Nitrosomonas (Zakir et al., 2008). It was discovered more recently that sorgoleone, a p-benzoquinone (Fig. 7) exuded from sorghum roots, has a strong inhibitory effect on Nitrosomonas sp. activity and that this compound contributes significantly to the BNI capacity in sorghum (Subbarao et al., 2009c). Two phenyl propanoids, methyl-p-coumarate and methyl ferulate, were identified as responsible for the BNI activity in root tissues of B. humidicola (Fig. 7; Gopalakrishnan et al., 2007). The IC50 values for methyl-p-coumarate and methyl ferulate are 2 × 10−5 and 4 × 10−6 m, respectively (Gopalakrishnan et al., 2007). The corresponding free phenolic acids, namely p-coumaric acid and ferulic acid, which are involved in lignin biosynthesis, showed no inhibitory activity at concentrations <1 × 10−2 m (Gopalakrishnan et al., 2007). It was suggested that nitrification inhibitors released through exudation and from the root tissues during decomposition/turnover can potentially play a major role in modifying the soil nitrification potential in B. humidicola pasture ecosystems (Subbarao et al., 2006b; Gopalakrishnan et al., 2007).
Karanjin (3-methoxy furano-2,3,7,8-flavone or 3-methoxy-2-phenyl furo-[2,3-h]chromen-4-one; Fig. 7), isolated from seeds of Pongamia glabra Vent., shows a strong inhibitory effect on soil nitrifier activity, and karanjin was found to be effective in suppressing soil nitrification (Sahrawat and Mukerjee, 1977; Sahrawat, 1981). The furan ring present in the molecule appears to be critical for the biological activity (i.e. nitrification inhibition; Sahrawat et al., 1977). Several isothiocyanates (2-propenyl-glucosinolate, methyl-isothiocyanate, 2-propenyl-isothiocyanate, butyl-isothiocyanate, phenyl-isothiocyanate, benzyl-isothiocyanate and phenethyl-isothiocyanate; Fig. 9) are formed during the degradation of cruciferous tissues reported to have inhibitory effects on soil nitrification (Fenwick et al., 1983; Bending and Lincoln, 2000). Preliminary evaluation of these compounds shows inhibitory activity in the bioassay (G. V. Subbarao, unpubl. res.), indicating the possibility of incorporating cruciferous crop residues as a means to control nitrification.
Fig. 9.
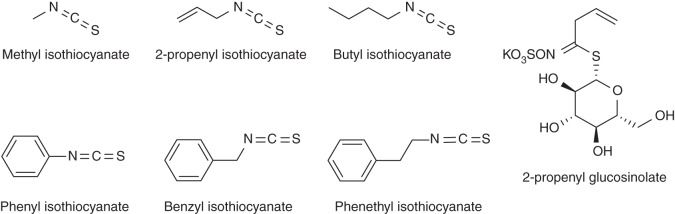
Chemical structure of isothiocyanates (mostly found in crucifer tissues) that show BNI function (based on Fenwick et al., 1983; Bending and Lincoln, 2000).
Discovery of brachialactone as a BNI compound
The major nitrification inhibitor released from roots of B. humidicola, a cyclic diterpene (Fig. 10), has been discovered and named ‘brachialactone’. This compound has a dicyclopenta[a,d]cyclooctane skeleton (5-8-5 ring system) with a γ-lactone ring bridging one of the five-membered rings and the eight-membered ring (Subbarao et al., 2009a). Similarly, 5-8-5 tricyclic terpenoids (ophiobolanes and fusicoccanes) are found in both fungi and plants (Muromtsev et al., 1994; Toyomasu et al., 2007). However, to the best of our knowledge, a compound or a derivative having a lactone ring appears to be a novel addition to the nitrification inhibitory groups. Fusicoccin-type cyclic diterpenes are biologically synthesized from geranylgeranyl diphosphate by a two-step cyclization catalysed by terpene cyclases (Toyomasu et al., 2007). The inhibition of nitrification in an in vitro assay with pure cultures of N. europaea was linearly related to the brachialactone concentrations in the range of 1·3–13·3 µm (Fig. 11A). Brachialactone with an ED80 (effective dose for 80 % inhibition) of 10·6 µm should be considered as one of the most potent nitrification inhibitors comparable with nitrapyrin or dicyandiamide, two of the synthetic nitrification inhibitors most commonly used in agriculture (ED80 of 5·8 µm for nitrapyrin and 2200 µm for dicyandiamide). Brachialactone inhibits Nitrosomonas sp. by blocking both AMO and HAO enzymatic functions, but appears to have a relatively stronger effect on the AMO than on the HAO pathway (Subbarao et al. 2009a). About 60–90 % of the inhibitory activity released from roots of B. humidicola is due to brachialactone (Fig. 11B); its release from roots is triggered by NH4+ in the rhizosphere. Also, brachialactone release is confined to root regions where NH4+ is present, and is mostly localized in nature (Subbarao et al., 2009a; Fig. 12A–C). Currently efforts are underway to understand the brachialactone biosynthetic pathway (K. Nakahara, JIRCAS, pers. comm.).
Fig. 10.

Chemical structure of brachialactone, the major nitrification inhibitor isolated from root exudates of B. humidicola (from Subbarao et al., 2009a).
Fig. 11.
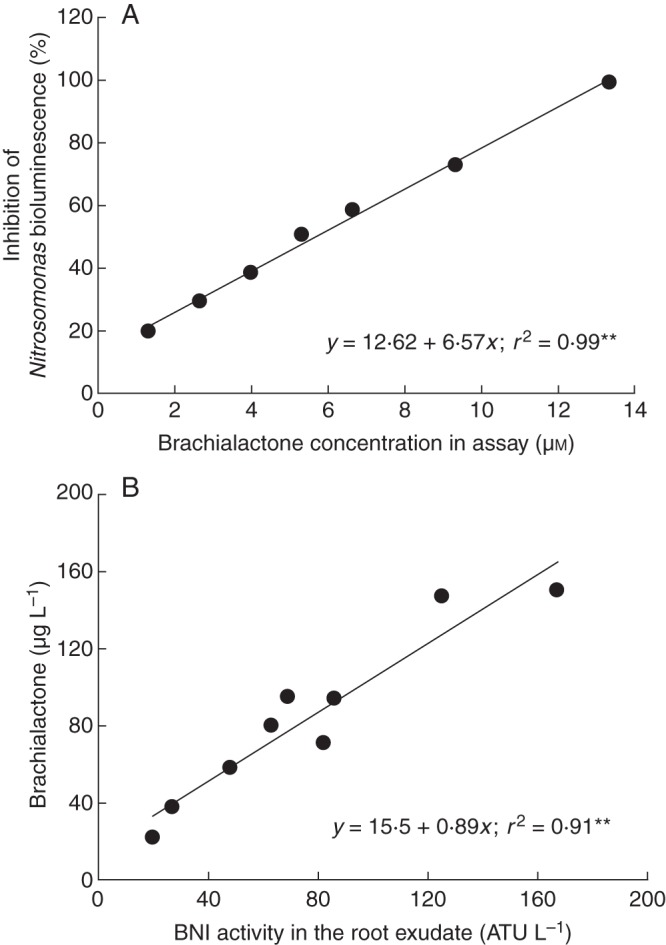
Inhibition of nitrification by brachialactone and the contribution of brachialactone to the BNI activity released from roots. (A) Inhibitory effect of brachialactone on N. europaea in an in vitro assay. (B) Contribution of brachialactone to the BNI activity released from roots (i.e. in root exudates) of B. humidicola. Root exudates were collected from intact plants using 1 L of aerated solution of 1 mm NH4Cl with 200 µm CaCl2 over 24 h. Each data point represents root exudates collected from hydroponically grown plants in a glasshouse during March–May of 2007 and 2008 (adapted from Subbarao et al., 2009a).
Fig. 12.
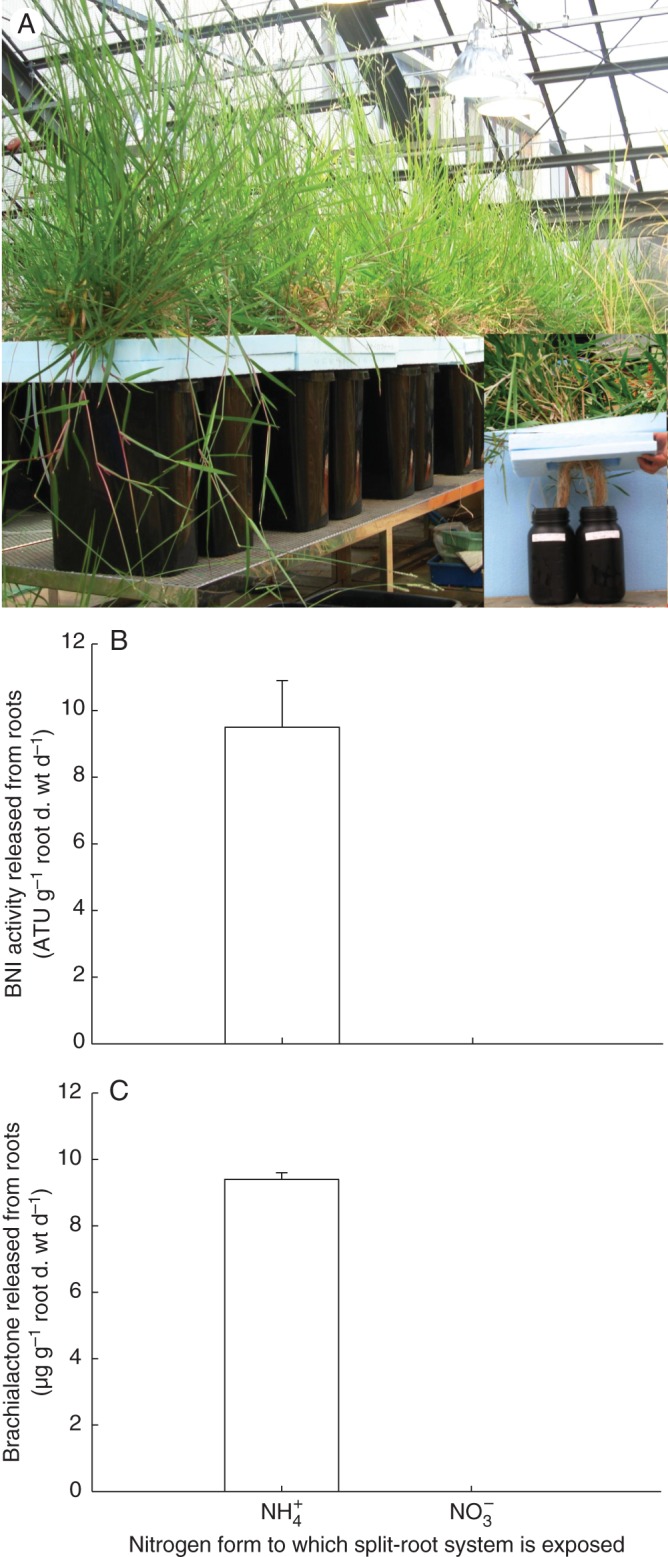
(A) Split-root system of B. humidicola. (B) Influence of nitrogen form (NH4+ vs. NO3−) in the exudate collection solutions on the release of BNI activity. (C) Release of brachialactone from the roots of B. humidicola in a split-root system (adapted from Subbarao et al., 2009a).
Potential for genetic manipulation of BNI capacity in cereals and pasture grasses
Several biologically active molecules with diverse chemical structures that belong to phenolic acids, hydroxamic acids, alkaloids, quinines, mono-terpenoids, di-terpenoids, fatty acids and isothiocyanates are released from root systems of cereal crops, crucifer members and tropical pasture grasses through exudation (Bertin et al., 2003; Frank and Groffman, 2009; Raaijmakers et al., 2009). These biologically active compounds exuded from roots have a wide range of functions that define the rhizosphere chemical and biological environment, and their biological roles range from nutrient acquisition, facilitating symbiotic associations with bacteria and fungi, to defending roots from pests and pathogens (Walker et al., 2003; Rengel and Marschner, 2005). Some of these compounds (such as the recently discovered di-terpenoid, brachialactone, released from Brachiaria roots, sorgoleone released from sorghum roots, and isothiocyanates released from degraded cruciferous tissues) have BNI function and thus could become important targets for genetic improvement through conventional and molecular breeding approaches. Such efforts in the future should become an important part of breeding programmes developing genetic and management strategies to exploit biologically active molecules with BNI function to regulate nitrification in production agriculture, where most of the fertilizer N is applied worldwide (Philippot and Hallin, 2011). Some examples of deployable genetic tools for improvement of the BNI capacity follow below
Tropical pasture grasses
Availability of genetic variability is a prerequisite for the genetic improvement of any plant trait using a conventional and/or molecular breeding programme. Significant genetic variability exists for the BNI capacity in B. humidicola (Subbarao et al., 2007b). Specific BNI activity (ATU g−1 root d. wt d−1) ranged from 7·1 to 46·3, indicating a significant potential for genetic improvement of BNI capacity by selection and recombination (Subbarao et al., 2007b). Using two ecotypes of a tropical grass Hyparrhenia diplandra (high- and low-nitrification ecotype), it was shown that nitrification can be stimulated or suppressed depending on the ecotype, suggesting that the suppression of soil nitrification in these grasses could be a genetic attribute (Lata et al., 2000, 2004). The ongoing Brachiaria breeding programme at CIAT in collaboration with JIRCAS plans to identify genetic regions associated with BNI function through quantitative trait locus (QTL) analysis, using a mapping population derived from crosses between apomictic and sexual germplasm accessions of B. humidicola, that have contrasting BNI capacity. Also, recent findings indicate substantial genetic variability for brachialactone release among germplasm accessions of B. humidicola, and several genetic stocks with contrasting ability (nearly 10-fold differences) for brachialactone release have been identified (G. V. Subbarao and K. Nakahara, JIRCAS, unpubl. res.), suggesting the possibility of breeding for high brachialactone release in B. humidicola (i.e. high BNI capacity).
Sorghum
Preliminary investigations indicate a substantial variability in the release of sorgoleone (a major component of hydrophobic root exudate determining the BNI capacity in sorghum) among sorghum genotypes (G. V. Subbarao and C. T. Hash, unpubl. res.). This is in agreement with earlier reports of nearly 30-fold variation in sorgoleone among 25 sorghum varieties (Nimbal et al., 1996). Sorgoleone is the stable product of auto-oxidation of dihydrosorgoleone, an unstable Striga seed germination stimulant present in the sorghum rhizosphere. Sorgoleone is highly phytotoxic as it disrupts photosystem II electron transfer, is thought to be responsible for the well-known allelopathy of sorghum, and has been the subject of considerable studies due to its potential as a bio-herbicide. Several genes controlling the biosynthetic pathway of sorgoleone are known (Baerson et al., 2007; Pan et al., 2007) as well as their positions on the aligned genomic sequences of sorghum chromosomes SBI-04, SBI-05, SBI-06 and SBI-08 (Ramu et al., 2010; Satish et al., 2011). Genomic regions associated with production of sorgolactone may also be involved in regulating sorgoleone production (Haussmann et al., 2004; Ejeta, 2007). The immediate precursor of sorgoleone, dihydrosorgoleone, is now thought to be a minor component of the germination stimulant for seeds of parasitic weeds (Striga sp.) that is exuded by sorghum root hairs (Rich and Ejeta, 2007). Sorgoleone itself has no Striga germination stimulant activity (Rich and Ejeta, 2007), but was once thought to be a key factor in the mechanistic basis for resistance to Striga infection (Chang et al., 1986; Netzly et al., 1988). However, Hess et al. (1992) found very little variation in levels of sorgoleone between many high and low Striga germination stimulant sorghum accessions.
Genomic regions associated with production of strigalactones might also be involved in regulating sorgoleone production (Haussmann, et al., 2004; Ejeta, 2007). Sorghum produces at least five different strigalactones, 5-deoxystrigol, sorgolactone, strigol, strigyl acetate and sorgomol (Cook et al., 1972; Hauck et al., 1992; Siame et al., 1993; Awad et al., 2006; Xie et al., 2008), and these are chemically distinct from sorgoleone. Subsequently it was discovered that the strigolactone 5-deoxystrigol, that is released from sorghum roots in response to N and phosphorus deficiency stress, apparently attracts vascular arbuscular mycorrhizae symbionts – largely responsible for sorghum stimulation of Striga seed germination (Bouwmeester et al., 2007; Yoneyama et al., 2007). More recent studies support this important role for strigalactones as germination stimulants for Striga, e.g. the discovery of a major recessive gene for low Striga germination capacity lgs that causes a 35-fold reduction in the level of 5-deoxystrigol in the Striga-resistant sorghum line SRN39 compared with the Striga-susceptible sorghum line Tabat (Yoneyama et al., 2010), and is associated with Striga resistance in sorghum. This gene maps to a distal region of the long arm of sorghum chromosome SBI-05 that may be associated with one or more genes controlling strigalactone biosynthesis (Satish et al., 2011), and not with those previously identified as controlling the final steps of sorgoleone biosynthesis (Baerson et al., 2007; Pan et al., 2007; Cook et al., 2010). Future research will hopefully unravel the interconnectivity in the biosynthetic pathways and regulation of sorgoleone and strigalactone exudation (Akiyama and Hayashi, 2006; Gomez-Roldan et al., 2008) and their functional relationships to both Striga germination stimulation and BNI capacity in sorghum. The discovery of sorgoleone's BNI function adds a new dimension to the functional significance of its release from sorghum roots (Subbarao et al., 2012b). The International Crop Research Institute for the Semi-Arid Tropics (ICRISAT) has recently developed several mapping populations of random inbred lines based on crosses of sorghum parental lines that differ in sorgoleone exudation (G. V. Subbarao and C. T. Hash, unpubl. data), and these are being used to map additional sorghum genomic regions contributing to genetic variation in sorgoleone exudation. As these populations are generally based on elite germplasm, this approach has the advantage of facilitating deployment of BNI traits in relevant high-yielding cultivars of sorghum. Association mapping approaches for sorgoleone could be explored by evaluating the mini-core sub-set (10 % of the core collection and 1 % of the entire collection, which amounts to 242 accessions) of ICRISAT'S global sorghum germplasm collection, a large portion of which were included in the recently developed Generation Challenge Programme's sorghum reference germplasm set of 384 wild and cultivated accessions. The association panel is being subjected to genotyping-by-sequencing (Elshire et al., 2011) to provide the very high density single nucleotide polymorphism marker fingerprints necessary for whole-genome scan approaches to association mapping (C. T. Hash, pers. comm.). This in turn will facilitate allele mining of traits linked to sorgoleone exudation (Brown et al., 2008; Casa et al., 2008). The basic tools for the identification of alleles that accelerate sorgoleone exudation as a strategy to improve BNI capacity in sorghum are thus available. Once superior alleles that control sorgoleone exudation are identified, they can be rapidly transferred to genetic backgrounds of elite sorghum hybrid parental lines and/or open-pollinated varieties by marker-assisted backcrossing. With the introgression of favourable alleles of one or two major genes (to accelerate the exudation of sorgoleone) into elite genetic backgrounds, it should be possible to improve the BNI capacity in sorghum.
Wheat and barley
Wild progenitors of crop species and traditional varieties/landraces often possess traits that do not exist in the elite germplasm (Manske et al., 2000). Wild progenitors and wild relatives have been used extensively as the source of traits for disease resistance and tolerance to abiotic stresses in wheat breeding (Friebe et al., 1996; Munns et al., 2000). The discrepancy between wild relatives and elite germplasm is often attributed to the impact of decades of breeding and selection under favourable agronomic conditions (Buso and Bliss, 1988). Preliminary results suggested a lack of significant BNI capacity in cultivated wheat (Subbarao et al., 2006b). However, subsequent evaluation of wild wheats indicated that roots of L. racemosus possess high BNI capacity (Fig. 13; Subbarao et al., 2007c). The BNI activity released from L. racemosus effectively suppressed soil nitrification for >60 d (Subbarao et al., 2007c). Using chromosome addition lines derived from the hybridization of L. racemosus with cultivated wheat (Kishii et al., 2004), it was shown that the genes conferring high BNI capacity were located on chromosomes Lr#n, Lr#I and Lr#J, and could be successfully introduced into and expressed in cultivated wheat (Fig. 14; Subbarao et al., 2007c). These results indicate that there exists a potential for developing future wheat cultivars with sufficient BNI capacity to suppress soil nitrification in wheat production systems (Subbarao et al., 2007c; Zahn, 2007).
Fig. 13.
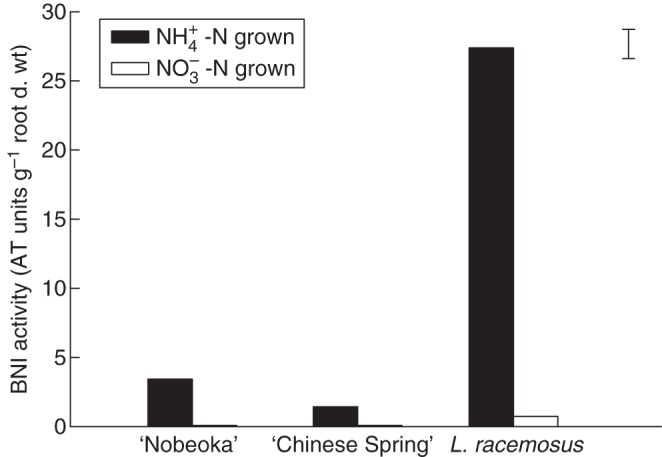
BNI activity released from roots of two cultivars of cultivated wheat and its wild relative L. racemosus. Plants were grown with either NH4+ or NO3− as their N source. Root exudate was collected from intact roots in aerated distilled water with 200 µm Ca over a 24 h period. The vertical bar represents Fisher's l.s.d. (P < 0·001) for the interaction term (N source × species; adapted from Subbarao et al., 2007c).
Fig. 14.
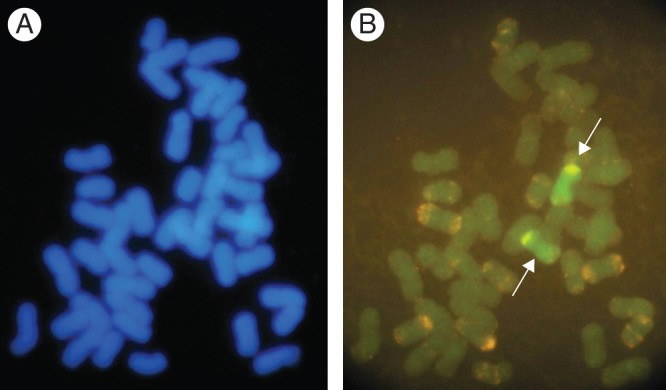
Karyotype analysis of DALr#n, a chromosome addition line derived from L. racemosus × T. aestivum. (A) DAPI (4′,6-diamidino-2-phenylindole) staining revealed 44 chromosomes. (B) The probe of L. racemosus genomic DNA (green) and TaiI and Afa family repetitive sequences showed the presence of two Lr#n chromosomes. The arrows indicate Lr#n chromosomes (adapted from Subbarao et al., 2007c).
Currently wheat production uses a third of the global N-fertilizer production (Raun and Johnson, 1999). Introducing a sufficient level of BNI capacity into cultivated wheat thus would have a large impact on reducing N leakage. However, the alien chromosome of this chromosome addition line may also carry many undesirable traits that could reduce yield. For example, preliminary field evaluations indicate that the introduction of the Lr#n chromosome into Chinese Spring (i.e. DAL#r) made them susceptible to rust (M. Kishii, unpubl. res.). It will be necessary therefore, to transfer to wheat only small segments of this L. racemosus chromosome containing favourable alleles of genes linked to the BNI trait to minimize the negative linkage drag that is normally associated with introgressions from wild relatives of wheat.
Various chromosomal manipulations can be deployed to induce a translocation between wheat and alien chromosomes, including the use of a gametocidal chromosome system (Endo, 2007), irradiation and mutants such as ph1b that reduce the stringency of pairing control mechanisms to allow pairing of homeologous chromosomes (Sears, 1977, 1993). Reciprocal exchange of alien chromosome segments with the corresponding wheat chromosomes without disrupting the genetic balance would be desirable. Centromeric or Robertsonian translocations could provide reciprocal or near-reciprocal translocations in which half of the target L. racemosus chromosome (short or long arm) replaces the corresponding wheat chromosome arms. Since the Lr#n chromosome of L. racemosus that controls BNI function has homoeology to both of wheat homoeologous groups 3 and 7 (Kishii et al., 2004) and the fact that we have obtained a naturally occurring substitution line with the group 3 chromosome of wheat, it will be desirable to generate translocations with wheat chromosomes of the corresponding groups. The production of such translocations has been achieved by crossing the Lr#n chromosome addition line with the 3B and 7B chromosome monosomic lines of wheat, in which one of the 3B or 7B chromosomes is missing, to produce an F1 hybrid where chromosome breakage and re-fusion at centromeric regions could be induced between Lr#n and 3B or 7B chromosomes during meiosis (Kishii, 2011).
Crosses of Lr#n addition and translocation lines with the Chinese Spring ph1b mutant have also been made by the International Center for the Improvement of Maize and Wheat (CIMMYT) in an effort to generate additional translocations incorporating smaller segments of Lr#n that carry the BNI trait but with a reduced risk of problems associated with linkage drag. Homozygous translocation lines are currently available in the Chinese Spring background but, due to the poor agronomic background of this line, these translocations are being transferred into elite CIMMYT bread wheat. This should allow realistic evaluation of BNI potential to reduce N leakage from wheat systems and increase grain yields at lower N-fertilizer applications. While a range of translocations including some with smaller segments of Lr#n are being produced, small segments are not always needed, as history has shown that one good centromeric translocation can have a large impact on wheat breeding (Lukaszewski, 2000; Singh et al., 2006). The best example of this is the 1BL.IRS translocation involving the short arm of chromosome 1R from rye (Secale cerale), which is present in most wheat cultivars in the Middle East and West Asia (most of these are CIMMYT derived), and in a significant proportion of cultivars in China, the USA and East Europe (Stokstad, 2007). The wide distribution of these wheats can be attributed to their high yield in diverse environments, despite all of the known disease resistance genes in the 1RS segment no longer being completely effective. However, if the original translocation is accompanied by many undesirable traits, it will be necessary to perform further reduction of the introgressed L. racemosus chromosome segment, a process currently underway through use of the ph1b mutant that permits homoeologous recombination between wheat and alien chromosomes (Sears, 1977; Lukaszewski, 2000). As N is an increasingly expensive input in agricultural systems, both yield at low N and responsiveness to added N are important in simultaneously reducing environmental pollution, increasing food production and reducing input costs. Field evaluations of the response of chromosome addition lines of Lr#n, Lr#I and Lr#J lines to N fertilization (250 kg N ha−1) at two locations at CIMMYT indicate that the Lr#I line did not respond to N fertilization, i.e. there was no difference in grain yield between N-fertilizer and no-N field plots. Also, TA7646, which is carrying a homologous chromosome to Lr#I (Qi et al., 1997; Kishii et al., 2004), did not respond to N fertilization during these yield evaluation trials at two field locations at CIMMYT (M. Kishii and I. Ortiz-Monasterio, CIMMYT, unpubl. res.). Currently, efforts are underway at CIMMYT to introduce Lr#n, Lr#I and Lr#J into various high-yielding backgrounds to assess the value of the BNI trait for yield maintenance under sub-optimum N fertilization levels. The material under development in elite backgrounds could be utilized to develop new cultivars rapidly if field trials indicate that translocations show high BNI capacity.
Introduction of the BNI trait from L. racemosus to barley would be more challenging as diploid barley is sensitive to chromosome manipulation [compared with tetraploid durum wheat (Triticum turgidum) or hexaploid bread wheat]. Also, a gene to induce homeologous recombination like that found in wheat has not been reported for barley. One possible method to introduce an L. racemosus chromosome into barley could be through the use of a tetraploid barley line, which has its chromosome number doubled with colchicine, as this would allow a better tolerance to the addition of alien chromosomes. The utilization of barley chromosome addition lines of wheat is an alternative. A set of these addition lines has been produced (Islam et al., 1975), and it may be possible to manipulate the homoeologous barley and L. racemosus chromosomes in wheat; first, by crossing the corresponding barley and L. racemosus chromosome addition lines to generate the required centromeric translocation, and then transferring the translocation into barley by crossing the tetraploid barley chromosome substitution line with cultivated diploid barley.
Deploying BNI function in production agriculture
Soil physical, chemical and biological properties influence the rhizosphere environment and thus the release of BNIs from roots; in addition, these factors also may determine the effectiveness and stability of the released BNIs in suppressing soil nitrification. For example, alkaline pH may limit the expression and stability of BNI function, thus heavy clay soils such as Vertisols that are generally alkaline in pH may not be suitable for the expression of BNI function. Also high bacterial activity in soils with relatively high OM content (such as organic soils) may enhance the degradation of BNIs, thus making the BNI function less effective. There is little information on how soil temperature and moisture status (linked to inter- and intraseasonal variability or to stresses due to excess or insufficient moisture) modulate the BNI function. For instance, when modelling the rhizosphere and its associated gradients with exudates from roots, it was shown that adsorption properties, solute life time and soil water contents are the key determinants of both the extent of the rhizosphere and the time to reach a steady state, indicating their fundamental roles in the interactions between roots and soil organisms (Raynaud, 2010). Also, the impact of H. diplandra ecotypes (stimulation or suppression) on nitrification in tropical savannas has been shown to be highly heterogeneous at both inter- and intra-annual scales, but could be partially explained by a climatic parameter such as average T° or total precipitations in the months prior the date of measurement (Lata, 1999).
For annual crops the crop duration, often ≤120 d, may not be adequate (given the BNI activity release rates observed for sorghum and other major food crops; Subbarao et al., 2007b, c, 2009c, 2012b, Zakir et al., 2008; Zhu et al., 2012) to reach the critical threshold levels needed to reduce the bulk soil nitrification potential. It is likely that the impact of BNI may be confined to the rhizosphere–soil environment. Tropical pastures with high BNI capacity and extensive root systems coupled with their perennial habits (e.g. Brachiaria spp.) can significantly reduce the soil nitrification potential and nitrifier population (i.e. low-nitrifying production environments). This could be exploited for the benefit of annual crops, such as maize, wheat and rice which receive most of the fertilization, but at present have little inherent BNI capacity, by integrating pastures with crop production using agro-pastoral systems or mixed crop–livestock systems. The pasture component could provide the required BNI activity to suppress soil nitrifier activity and thus the nitrification potential to improve the N economy of the annual crops (a weak contributor of BNIs) that follow the pasture phase. The stability of the residual BNI effects, determined as the soil NH4+ oxidation rate, where an annual crop such as maize or soybean is grown after Brachiaria pasture is not yet known, but this could determine the interval between pasture phases in such agro-pastoral systems (Subbarao et al., 2012a).
For crops that produce BNIs in their plant tissue, but do not release them from their root systems, e.g. crucifers (Bending and Lincoln, 2000), the incorporation of plant residues into the soil could be an alternative way to control soil nitrification. In addition, Brachiaria pastures could also be used as cover crops to use their biomass as a mulch after 3–4 months of growth, followed by direct sowing of maize or soybean into the mulch. This is an emerging agronomic practice that is being developed by progressive farmers for controlling nitrification in maize and soybean cultivation in Llanos, Colombia. Such novel agronomic practices supplement the addition of BNIs by Brachiaria's shoot tissues (Subbarao et al., 2008), in addition to that added by the root systems. Thus multidisciplinary efforts through crop genetic improvements of BNI capacity, proper agronomic practices in appropriate cropping systems could be combined to utilize the BNI function to design and promote low-nitrifying production systems in agriculture. In addition, the boundaries of the agro-ecosystems where BNI function can be effectively deployed will have to be defined with the help of agro-ecologists and agronomists. This will help breeders and molecular biologists targeting the desired BNI traits in crops and pastures from a genetic improvement perspective for an entire agro-ecosystem.
PERSPECTIVES
As we race to satisfy the growing global demand for food through massive injections of reactive N (i.e. N-fertilizer) into agricultural systems, the unintended (eutrophication of lakes and NO3− contamination of ground water) and unknown (N2O and NO emissions and their impact on global warming) consequences on the environment have become of great concern (Canfield et al., 2010). Never in the recent history of planet Earth (i.e. during the last 10 000 years) have such major perturbations in the N cycle occurred similar to those that the Earth has experienced since the 1930s (Dansgaard et al., 1993; Petit et al., 1999; Rioual et al., 2001; Rockstrom et al., 2009; Canfield et al., 2010). Current annual N-fertilizer inputs into agricultural systems have reached close to 150 Tg, a level one and a half times greater than Earth's N-fixing capacity (Vitousek et al., 1997; Tilman et al., 2001). This suggests that humans have nearly doubled the reactive N load of Earth in just over 50 years (1960–2010); most of this additional reactive N is routed through just 11 % of the Earth's surface (Newbould, 1989). This has created serious environmental problems, and there is great concern as to how we can protect our environment, while meeting the food demand of the growing world population (Rockstrom et al., 2009). This is a major challenge and requires a new paradigm of approaches on how to manage N in agricultural systems.
The Green Revolution quadrupled the world food production largely through the development of high-yielding, fertilizer-responsive wheat, rice and maize cultivars along with increased N-fertilizer inputs and major changes in agronomic practices (Matson et al., 1999; Tilman et al., 2001; Dinnes et al., 2002; Hungate et al., 2003). In our quest for enhancing food production, we rather failed to consider the flow of industrially produced reactive N through the multiple pathways of soil N cycling. The consequence is the emergence of nitrification as the major N flow pathway, acting as a powerful drawing force, largely responsible for an inefficient use of N, and for the resulting N pollution associated with agricultural systems.
As discussed in this Viewpoint paper, it is not necessary and prudent that most N be cycled through the nitrification pathway to achieve higher productivity. Nature has shown us that by routing the reactive N through multiple pathways and restricting the flow through the nitrification path, N can be cycled more effectively with limited leakage into the environment (Schimel and Bennett, 2004; Weigelt et al., 2005; Barot et al., 2007; Harrison et al., 2007; Houlton et al., 2007; Hewins and Hyatt, 2009; Ashton et al., 2010). As nitrification and denitrification are the two major biological drivers for the production of NO3−, N2O and NO (i.e. reactive N forms largely responsible for environmental pollution), suppressing nitrification is critical for the development of low N2O-emitting and low NO3−-producing agricultural systems.
The BNI capacity in field crops and pastures can be genetically enhanced using both conventional and molecular genetics tools to develop the next-generation cultivars that have the ability to suppress nitrification (Philippot and Hallin, 2011; Subbarao et al., 2012a). Wild relatives of wheat seem a promising source of the BNI trait needed to improve the BNI capacity of cultivated wheat (Subbarao et al., 2007c; Zahn, 2007). Efforts are under way to transfer the high BNI capacity trait located on Lr#n, Lr#I and Lr#J to cultivated wheat. Molecular breeding approaches such as marker-assisted breeding and metabolic engineering can be deployed to introduce the biosynthetic pathway for brachialactone (a powerful BNI) synthesis and release from root systems of major food crops – wheat, maize and rice. We have demonstrated the effectiveness of BNI function in tropical Brachiaria grasses in suppressing soil nitrification and N2O emissions. Also, potential exists to select and breed for the high BNI trait in other tropical pasture grasses such as P. maximum (that have high productivity and forage quality but lack BNI capacity in their root systems) using both conventional and molecular breeding tools.
For an effective deployment of BNI function as a strategy to control nitrification, a multidisciplinary effort using a systems approach is necessary to understand the interactions among crops/pastures, the relative contributions of BNIs from root exudation, and residue incorporation on N cycling in production systems. Exploiting the BNI function using both genetic and crop/system management approaches is the first step towards designing a low-nitrifying agronomic environment in agricultural systems. A paradigm shift thus is needed to steer N management from the current high-nitrifying environments to low-nitrifying production systems, that are sustainable from both an ecological and an economic perspective, without jeopardizing the ability to meet the global food demand.
ACKNOWLEDGEMENTS
The authors from CIAT acknowledge financial support from the Ministry of Foreign Affairs (MOFA), Japan; the Japan International Research Center for Agricultural Sciences (JIRCAS), Japan; the Ministry of Agriculture and Rural Development (MADR), Colombia; and the Federal Ministry for Economic Cooperation (BMZ-GTZ), Germany.
LITERATURE CITED
- Aanderud ZT, Bledsoe CS. Preferences for 15N-ammonium, 15N-glycine differ among dominant exotic and subordinate native grasses from a California oak wood-land. Environmental and Experimental Botany. 2009;65:205–209. [Google Scholar]
- Akiyama K, Hayashi H. Strigolatones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Annals of Botany. 2006;97:925–931. doi: 10.1093/aob/mcl063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton I, Miller WAE, Bowman WD, Suding KN. Niche complementarity due to plasticity in resource use: plant partitioning of chemical N forms. Ecology. 2010;91:3252–3260. doi: 10.1890/09-1849.1. [DOI] [PubMed] [Google Scholar]
- Awad A, Sato D, Kusumoto D, Kamioka H, Takeuchi Y, Yoneyama K. Characterization of strigolactones, germination stimulants for the root parasitic plants Striga and Orobanche produced by maize, millet and sorghum. Plant Growth Regulation. 2006;48:221–227. [Google Scholar]
- Baerson SR, Dayan FE, Rimando AM, et al. A functional genomics investigation of allelochemical biosynthesis in Sorghum bicolor root hairs. Journal of Biological Chemistry. 2007;283:3231–3247. doi: 10.1074/jbc.M706587200. [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Olson RK, Reiners WA. Protein binding phenolics and the inhibition of nitrification in subalpine balsam-fir soils. Soil Biology Biochemistry. 1983;15:419–423. [Google Scholar]
- Barker AV, Mills HA. Ammonium and nitrate nutrition of horticultural crops. Horticultural Review. 1980;2:395–423. [Google Scholar]
- Barot S, Ugolini A, Brikci B. When do soil decomposers and ecosystem engineers enhance plant production? Functional Biology. 2007;21:1–10. [Google Scholar]
- Basaraba J. Influence of vegetable tannins on nitrification in soil. Plant Soil. 1964;21:8–16. [Google Scholar]
- Bate GC. Nitrogen cycling in savanna ecosystems. Ecological Bulletin. 1981;33:463–475. [Google Scholar]
- Bellamy PH, Loveland PJ, Bradley RI, Murray R, Lark RM, Kirk GJD. Carbon losses from all soils across England and Wales 1978–2003. Nature. 2005;437:245–248. doi: 10.1038/nature04038. [DOI] [PubMed] [Google Scholar]
- Bending GD, Lincoln SD. Inhibition of soil nitrifying bacteria communities and their activities by glucosinolate hydrolysis products. Soil Biology and Biochemistry. 2000;32:1261–1269. [Google Scholar]
- Berner RA. GEOCARBSULF: a combined model for phanerozoic atmospheric O2 and CO2. Geochimica et Cosmochimica Acta. 2006;70:5653–5664. [Google Scholar]
- Bertin C, Yang X, Weston LA. The role of root exudates and allelochemicals in the rhizosphere. Plant and Soil. 2003;256:67–83. [Google Scholar]
- Boudsocq S, Lata JC, Mathieu J, Abbadie L, Barot S. Modelling approach to analyse the effects of nitrification inhibition on primary production. Functional Ecology. 2009;23:220–230. [Google Scholar]
- Boudsocq S, Barot S, Loeuille N. Evolution of nutrient acquisition: when adaptation fills the gap between contrasting ecological theories. Proceedings of the Royal Society B: Biological Sciences. 2011;278:449–457. doi: 10.1098/rspb.2010.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudsocq S, Niboyet A, Lata JC, et al. Plant preference for ammonium versus nitrate: A neglected determinant of ecosystem functioning? American Naturalist. 2012;180:60–69. doi: 10.1086/665997. [DOI] [PubMed] [Google Scholar]
- Bouwmeester HJ, Roux C, Lopez-Raez JA, Bécard G. Rhizosphere communication of plants, parasitic plants and AM fungi. Trends in Plant Science. 2007;12:224–230. doi: 10.1016/j.tplants.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Bremner JM, Blackmer AM. Nitrous oxide: emission from soils during nitrification and denitrification of fertilizer nitrogen. Science. 1978;199:295–296. doi: 10.1126/science.199.4326.295. [DOI] [PubMed] [Google Scholar]
- Bremner JM, McCarty GW. Effects of terpenoids on nitrification in soil. Soil Science Society of America Journal. 1988;52:1630–1633. [Google Scholar]
- Bremner JM, Breitenbeck GA, Blackmer AM. Effect of nitrapyrin on emission of nitrous oxide from soils fertilized with anhydrous ammonia. Geophysical Research Letters. 1981;8:353–356. [Google Scholar]
- Britto DT, Siddiqi MY, Glass ADM, Kronzucker HJ. Futile transmembrane NH4+ cycling: a cellular hypothesis to explain ammonium toxicity in plants. Proceedings of the National Academy of Sciences, USA. 2001;98:4255–4258. doi: 10.1073/pnas.061034698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent FE, Rauschkolb RS. Nitrogen fertilization and water pollution. California Agriculture. 1977;31:24–25. [Google Scholar]
- Brown PJ, Rooney WL, Franks C, Kresovich S. Efficient mapping of plant height quantitative trait loci in a sorghum association population with introgressed dwarfing genes. Genetics. 2008;180:629–637. doi: 10.1534/genetics.108.092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burford JR, Sahrawat KL. Management of Vertisols for improved agricultural production: Proceedings of an IBSRAM inaugural workshop. 1989 18–22 February 1985, ICRISAT Center, Patancheru, India, ICRISAT, Patancheru 502 324, Andhra Pradesh, India. [Google Scholar]
- Burney JA, Davis SJ, Lobell DB. Greenhouse gas mitigation by agricultural intensification. Proceedings of National Academy of Sciences, USA. 2010;107:12052–12057. doi: 10.1073/pnas.0914216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buso GSC, Bliss FA. Variability among lettuce cultivars grown at two levels of available phosphorus. Plant and Soil. 1988;111:67–73. [Google Scholar]
- Canfield DE, Glazer AN, Falkowski PG. The evolution and future of earth's nitrogen cycle. Science. 2010;330:192–196. doi: 10.1126/science.1186120. [DOI] [PubMed] [Google Scholar]
- Casa AM, Pressoir G, Brown PJ, et al. Community resources and strategies for association mapping in sorghum. Crop Science. 2008;48:30–40. [Google Scholar]
- Castaldi S, Carfora A, Fiorentino A, et al. Inhibition of net nitrification activity in a Mediterranean woodland: possible role of chemicals produced by Arbutus unedo. Plant and Soil. 2009;315:273–283. [Google Scholar]
- Celik I. Land-use effects on organic matter and physical properties of soil in a southern Mediterranean highland of Turkey. Soil Tillage Research. 2005;83:270–277. [Google Scholar]
- Chang M, Netzly DH, Butler LG, Lynn DG. Chemical regulation of distance: characterization of the first natural host germination stimulant for Striga asiatica. Journal of the American Chemical Society. 1986;108:7858–7860. doi: 10.1021/ja00284a074. [DOI] [PubMed] [Google Scholar]
- Clark FE. Losses of nitrogen accompanying nitrification. Transactions of the International Society of Soil Science. 1962;IV and V:173–176. [Google Scholar]
- Clark FE, Beard WE, Smith DH. Dissimilar nitrifying capacities of soils in relation to losses of applied nitrogen. Proceedings of the Soil Science Society of America. 1960;24:50–54. [Google Scholar]
- Cook CE, Whichard LP, Wall ME, et al. Germination stimulants II. The structure of strigol, a potent seed germination stimulant for witchweed (Striga lutea Lour.) Journal of the American Chemistry Society. 1972;94:6198–6199. [Google Scholar]
- Cook D, Rimado AM, Clemente TE, et al. Alkylresorcinol synthases expressed in Sorghum bicolor root hairs play an essential role in the biosynthesis of the allelopathic benzoquinone sorgoleone. The Plant Cell. 2010;22:867–887. doi: 10.1105/tpc.109.072397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AB. Suppression of nitrate formation with an exotic conifer plantation. Plant and Soil. 1986;93:383–394. [Google Scholar]
- Crutzen PJ, Ehhalt DH. Effects of nitrogen fertilizers and combustion on the stratospheric ozone layer. Ambio. 1977;6:112–116. [Google Scholar]
- Dansgaard W, Johnsen SJ, Clausen HB, et al. Evidence for general instability of past climate from a 250-kyr ice-core record. Nature. 1993;364:218–220. [Google Scholar]
- Davis J, Haglund C. Life cycle inventory (LCI) of fertilizer production. Fertilizer products used in Sweden and Western Europe. Chalmers University of Technology: Sweden; 1999. SIK-Report No. 654. Master's Thesis. [Google Scholar]
- DeCicco J, Fung F. Global warming on the road: the climate impact of America's automobiles. 2006 www.environmentaldefense.org . [Google Scholar]
- Dinnes DL, Karlen DL, Jaynes DB, et al. Nitrogen management strategies to reduce nitrate leaching in tile drained Mid-Western soils. Agronomy Journal. 2002;94:153–171. [Google Scholar]
- Donaldson JM, Henderson GS. Nitrification potential of secondary succession upland oak forests: 1. Mineralization and nitrification during laboratory incubations. Soil Science Society of America Journal. 1990a;54:892–897. [Google Scholar]
- Donaldson JM, Henderson GS. Nitrification potential of secondary succession upland oak forests II. Regulation of ammonium-oxidizing bacteria populations. Soil Science Society of America Journal. 1990b;54:898–902. [Google Scholar]
- Ejeta G. Breeding for Striga resistance in sorghum: exploitation of an intricate host–parasite biology. Crop Science. 2007;47(suppl 3):S216–S227. [Google Scholar]
- Elliott ET. Aggregate structure and carbon, nitrogen and phosphorus in native and cultivated soils. Soil Science Society of America Journal. 1986;50:627–633. [Google Scholar]
- Elshire RJ, Glaubitz JC, Sun Q, et al. A robust, simple genotyping-by-sequencing (GBS) approach for high diversity species. PLoS One. 2011;6:e19379.. doi: 10.1371/journal.pone.0019379. http://dx.doi.org/10.1371/journal.pone.0019379 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo TR. The gametocidal chromosome as a tool for chromosome manipulation in wheat. Chromosome Research. 2007;15:67–75. doi: 10.1007/s10577-006-1100-3. [DOI] [PubMed] [Google Scholar]
- Erickson A, Ramsewak RS, Smucker AJ, Nair MGJ. Nitrification inhibitors from the roots of Leucaena leucocephala. Journal of Agricultural and Food Chemistry. 2000;48:6174–6177. doi: 10.1021/jf991382z. [DOI] [PubMed] [Google Scholar]
- FA O. FAOSTAT Database – Agriculture production. Rome: Food and Agriculture Organization of the United Nations. 2011 [Google Scholar]
- Fenwick GR, Heaney RK, Mullin WJ. Glucosinolates and their breakdown products in food and food plants. In: Furia TE, editor. Critical reviews in food science and nutrition. Boca Raon, FL: CRC Press; 1983. pp. 123–201. [DOI] [PubMed] [Google Scholar]
- Fillery IRP. Plant-based manipulation of nitrification in soil: a new approach to managing N loss? Plant and Soil. 2007;294:1–4. [Google Scholar]
- Finzi AC, Norby RJ, Calfapietra C, et al. Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proceedings of National Academy of Sciences, USA. 2007;104:14014–14019. doi: 10.1073/pnas.0706518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher MJ, Rao IM, Ayarza MA, et al. Carbon storage by introduced deep-rooted grasses in the South American savannas. Nature. 1994;371:236–238. [Google Scholar]
- Foley JA, Defries R, Asner GP, et al. Global consequences of land use. Science. 2005;309:570–574. doi: 10.1126/science.1111772. [DOI] [PubMed] [Google Scholar]
- Frank DA, Groffman PM. Plant rhizosphere N processes: what we don't know and why we should care. Ecology. 2009;90:1512–1519. doi: 10.1890/08-0789.1. [DOI] [PubMed] [Google Scholar]
- Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS. Characterization of wheat-alien translocations conferring resistance to diseases and pest: current status. Euphytica. 1996;91:59–87. [Google Scholar]
- Galloway JN, Townsend AR, Erisman JW, et al. Transformation of the nitrogen cycle: recent trends, questions and potential solutions. Science. 2008;320:889–892. doi: 10.1126/science.1136674. [DOI] [PubMed] [Google Scholar]
- Glass ADM. Nitrogen use efficiency of crop plants: physiological constraints upon nitrogen absorption. Critical Reviews in Plant Sciences. 2003;22:453–470. [Google Scholar]
- Gomez-Roldan V, Fermas S, Brewer PB, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Subbarao GV, Nakahara K, et al. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. Journal of Agricultural and Food Chemistry. 2007;55:1385–1388. doi: 10.1021/jf062593o. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Watanabe T, Pearse SJ, Ito O, Hossain ZAKM, Subbarao GV. Biological nitrification inhibition (BNI) by Brachiaria humidicola roots varies with soil type and inhibits nitrifying bacteria, but not other major soil microorganisms. Soil Science and Plant Nutrition. 2009;55:725–733. [Google Scholar]
- Guthrie TT, Bomke AA. Nitrification inhibition by N-Serve and ATC in soils with varying texture. Soil Science Society of America Journal. 1980;44:314–320. [Google Scholar]
- Hari P, Kulmala L. Boreal forest and climate change. New York: Springer; 2008. [Google Scholar]
- Harrison K, Bol AR, Bardgett RD. Preferences for different nitrogen forms by coexisting plant species and soil microbes: comment. Ecology. 2007;88:989–999. doi: 10.1890/06-1018. [DOI] [PubMed] [Google Scholar]
- Hart SC, Stark JM, Davidson EA, Firestone MK. Nitrogen mineralization, immobilization and nitrification. In: Weaver RW, Angle JS, Bottomley BS, editors. Methods of soil analysis, Part 2. Microbiological and biochemical properties. Madison, WI: Soil Science Society of America; 1994. pp. 985–1018. [Google Scholar]
- Hauck C, Muller S, Schilknecht H. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. Journal of Plant Physiology. 1992;139:474–478. [Google Scholar]
- Haussmann BIG, Hess DE, Omanya GO, et al. Genomic regions influencing resistance to the parasitic weed Striga hermonthica in two recombinant inbred populations of sorghum. Theoretical and Applied Genetics. 2004;109:1005–1016. doi: 10.1007/s00122-004-1706-9. [DOI] [PubMed] [Google Scholar]
- Hawkes CV, Wren IF, Herman DJ, Firestone MK. Plant invasion alters nitrogen cycling by modifying the soil nitrifying community. Ecology Letters. 2005;8:976–985. doi: 10.1111/j.1461-0248.2005.00802.x. [DOI] [PubMed] [Google Scholar]
- Haynes RJ, Goh KM. Ammonium and nitrate nutrition of plants. Biological Reviews. 1978;53:465–510. [Google Scholar]
- Hendrickson LL, Keeney DR, Walsh LM, Liegel EA. Evaluation of nitrapyrin as a means of improving N use efficiency in irrigated sands. Agronomy Journal. 1978;70:699–708. [Google Scholar]
- Hess DE, Ejeta G, Butler LG. Selecting sorghum genotypes expressing a quantitative biosynthetic trait that confers resistance to Striga. Phytochemistry. 1992;31:493–497. [Google Scholar]
- Hewins DB, Hyatt LA. Flexible N uptake and assimilation mechanisms may assist biological invasion by Alliaria petiolata. Biological Invasions. 2009;12:2639–2647. [Google Scholar]
- Hodge A, Robinson D, Fitter AH. Are microorganisms more effective than plants at competing for nitrogen? Trends in Plant Science. 2000;5:304–308. doi: 10.1016/s1360-1385(00)01656-3. [DOI] [PubMed] [Google Scholar]
- Hofstra N, Bouwman AF. Denitrification in agricultural soils: summarizing published data and estimating global annual rates. Nutrient Cycling Agroecosystems. 2005;72:267–278. [Google Scholar]
- Houlton BZ, Sigman DM, Schuur EAG, Hedin LO. A climate-driven switch in plant nitrogen acquisition within tropical forest communities. Proceedings of National Academy of Sciences, USA. 2007;104:8902–8906. doi: 10.1073/pnas.0609935104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber DM, Warren HL, Nelson DW, Tsai CY. Nitrification inhibitors – new tools for food production. Bioscience. 1977;27:523–529. [Google Scholar]
- Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB. Nitrogen and climate change. Science. 2003;302:1512–1513. doi: 10.1126/science.1091390. [DOI] [PubMed] [Google Scholar]
- Iizumi T, Mizumoto M, Nakamura K. A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Applied and Environmental Microbiology. 1998;64:3656–3662. doi: 10.1128/aem.64.10.3656-3662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Revised IPCC guidelines for national greenhouse gas inventories: reference manual. London: IPCC; 1996. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Climate change: the physical science basis – summary for policy makers. Paris: World Meteorological Organization/United Nations Environmental Program; 2007. [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change) Fifth assessment report (AR5) Geneva, Switzerland: 2012. XXXV/Doc. 7. [Google Scholar]
- Ishikawa T, Subbarao GV, Ito O, Okada K. Suppression of nitrification and nitrous oxide emission by the tropical grass Brachiaria humidicola. Plant and Soil. 2003;255:413–419. [Google Scholar]
- Islam AKMR, Shepherd KW, Sparrow DHB. Addition of individual barley chromosomes to wheat. In: Gaul H, Thiemig VK, editors. Proceedings of 3rd international barley genetics symposium. Munich, Germany: 1975. pp. 260–270. [Google Scholar]
- Jarvis SC. Future trends in nitrogen research. Plant and Soil. 1996;181:47–56. [Google Scholar]
- Jordan CF, Todd RL, Escalante G. Nitrogen conservation in a tropical rain forest. Oecologia. 1979;39:123–128. doi: 10.1007/BF00346002. [DOI] [PubMed] [Google Scholar]
- Ju XT, Xing GX, Chen XP, et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proceedings of National Academy of Sciences, USA. 2009;106:3041–3046. doi: 10.1073/pnas.0813417106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahrl E, Li Y, Su Y, Tenngkeit T, Walkes A, Xu J. Greenhouse gas emissions from nitrogen use in China. Environmental Science Policy. 2010;13:688–694. [Google Scholar]
- Kramer KJ, Moll HC, Nonhebel S. Total greenhouse gas emissions related to the Dutch crop production system. Agriculture, Ecosystems and Environment. 1999;72:9–16. [Google Scholar]
- Kongshaug G. Energy consumption and greenhouse gas emissions in fertilizer production. Marrakech, Morocco: 1998. IFA Technical Conference. 28 September to 1 October, 1998. [Google Scholar]
- Kuesters J, Jenssen T IFA Technical Conference. Selecting the right fertilizer from an environmental life cycle perspective. Marrakech, Morocco: 1998. 28 September to 1 October, 1998. [Google Scholar]
- Kielland K. Short-circuiting the nitrogen cycle: ecophysiological strategies of nitrogen uptake in plants from marginal environments. In: Ae N, Arihara J, Okada , Srinivasan A, editors. Plant nutrient acquisition: new perspectives. Vol. 17. Tokyo: Springer-Verlag; 2001. pp. 376–398. [Google Scholar]
- Kishii M. Production of five Leymus racemosus chromosome translocation lines. eWIS. 2011;111:11–13. [Google Scholar]
- Kishii M, Yamada T, Sasakuma T, Tsujimoto H. Production of wheat–Leymus racemosus chromosome addition lines. Theoretical and Applied Genetics. 2004;109:255–260. doi: 10.1007/s00122-004-1631-y. [DOI] [PubMed] [Google Scholar]
- Kroeze C. Nitrous oxide and global warming. Science Total Environment. 1994;143:193–209. doi: 10.1016/0048-9697(94)90310-7. [DOI] [PubMed] [Google Scholar]
- Lata JC. Interactions between microbial processes, nutrient cycle and grass cover functioning: study of soil nitrification under the Gramineae Hyparrhenia diplandra in a wet tropical savanna of Ivory Coast. University of Paris VI, Paris, France: 1999. PhD Thesis. [Google Scholar]
- Lata JC, Durand J, Lensi R, Abbadie L. Stable coexistence of contrasted nitrification statuses in a wet tropical savanna ecosystem. Functional Ecology. 1999;13:762–768. [Google Scholar]
- Lata JC, Guillaume K, Degrange V, Abbadie L, Lensi R. Relationships between root density of the African grass Hyparrhenia diplandra and nitrification at the decimetric scale: an inhibition–stimulation balance hypothesis. Proceedings of the Royal Society B: Biological Sciences. 2000;267:595–600. doi: 10.1098/rspb.2000.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lata JC, Degrange V, Raynaud X, Maron PA, Lensi R, Abbadie L. Grass populations control nitrification in savanna soils. Functional Ecology. 2004;18:605–611. [Google Scholar]
- Liao M, Fillery IRP, Palta JA. Early vigorous growth is a major factor influencing nitrogen uptake in wheat. Functional Plant Biology. 2004;31:121–129. doi: 10.1071/FP03060. [DOI] [PubMed] [Google Scholar]
- Likens GE, Bormann FH, Johnson NM. Nitrification: importance to nutrient losses from a cutover forested ecosystem. Science. 1969;163:1205–1206. doi: 10.1126/science.163.3872.1205. [DOI] [PubMed] [Google Scholar]
- Lodhi MAK. Inhibition of nitrifying bacteria, nitrification and mineralization in spoil soil as related to their successional stages. Bulletin of the Torrey Botanical Club. 1979;106:284–289. [Google Scholar]
- Lodhi MAK. Additional evidence of inhibition of nitrifiers and possible cycling of inhibitors produced by selected plants in a climax community. Bulletin of the Torrey Botanical Club. 1982;109:199–204. [Google Scholar]
- Leninger S, Urich T, Schloter M, et al. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature. 2006;442:806–809. doi: 10.1038/nature04983. [DOI] [PubMed] [Google Scholar]
- Lukaszewski AJ. Manipulation of the 1RS.1BL translocation in wheat by induced homoeologous recombination. Crop Science. 2000;40:216–225. [Google Scholar]
- Magill AH, Aber JD, Benston GM, et al. Long-term nitrogen additions and nitrogen saturation in two temperate forests. Ecosystems. 2000;3:238–253. [Google Scholar]
- Maire V, Gross N, da Silveira Pontes L, Picon-Cochard C, Soussana JF. Trade-off between root nitrogen acquisition and shoot nitrogen utilization across 13 co-occurring pasture grass species. Functional Ecology. 2009;23:668–679. [Google Scholar]
- Manske GG, Ortiz-Monasterio JI, van Grinkel M, Rajaram S, Molina E, Vlek PLG. Traits associated with improved P-uptake efficiency in CIMMYT's semi-dwarf spring bread wheat grown on an acid Andisol in Mexico. Plant and Soil. 2000;221:189–204. [Google Scholar]
- Matson PA, McDowell Townsend AR, Vitousek PM. The globalization of N deposition: ecosystem consequences in tropical environments. Biogeochemistry. 1999;46:67–83. [Google Scholar]
- McCarty GW. Modes of action of nitrification inhibitors. Biology and Fertility of Soils. 1999;29:1–9. [Google Scholar]
- Meiklejohn J. Numbers of nitrifying bacteria in some Rhodesian soils under natural grass and improved pastures. Journal of Applied Ecology. 1968;5:291–300. [Google Scholar]
- Miles JW, do Valle CB, Rao IM, Euclides VPB. Brachiaria grasses. In: Moser L, Burson B, Sollenberger LE, editors. Madison, WI: ASA-CSSA-SSA; 2004. pp. 745–783. Warm-season (C4) grasses. [Google Scholar]
- Montagnini F, Haines B, Swank W. Factors controlling nitrification in soils of early successional and oak/hickory forest in the southern Appalachians. Forest Ecology and Management. 1989;26:77–94. [Google Scholar]
- Moore DRE, Waid JS. The influence of washings of living roots on nitrification. Soil Biology and Biochemistry. 1971;3:69–83. [Google Scholar]
- Mosier AR, Duxbury JM, Freney JR, Heinmeyer O, Minami K. Nitrous oxide emissions from agricultural fields: assessment, measurement and mitigation. Plant and Soil. 1996;181:95–108. [Google Scholar]
- Mouquet N, Moore JL, Loreau M. Plant species richness and community productivity: why the mechanism that promotes coexistence matters. Ecology Letters. 2002;5:56–65. [Google Scholar]
- Munns R, Hare RA, James RA, Rebetzke GJ. Genetic variation for improving the salt tolerance of durum wheat. Australian Journal of Agricultural Research. 2000;51:69–74. [Google Scholar]
- Munro PE. Inhibition of nitrifiers by grass root extracts. Journal of Applied Ecology. 1966;3:231–238. [Google Scholar]
- Munro PE. Inhibition of nitrite-oxidizers by roots of grass. Journal of Applied Ecology. 1966;3:227–229. [Google Scholar]
- Muromtsev GS, Voblikova VD, Kobrina NS, Koreneva VM, Krasnopolskaya LM, Sadovskaya VL. Occurrence of fusicoccanes in plants and fungi. Journal of Plant Growth Regulation. 1994;13:39–49. [Google Scholar]
- Nasholm T, Ekblad A, Nordin A, Gisler R, Hosberg M, Hosberg P. Boreal forest plants take up organic nitrogen. Nature. 1998;392:914–916. [Google Scholar]
- Netzly DH, Riopel JL, Ejeta G, Butler LG. Germination stimulants of witchweed (Striga asiatica) from hydrophobic root exudates of sorghum (Sorghum bicolor) Weed Science. 1988;36:441–446. [Google Scholar]
- Newbould P. The use of nitrogen fertilizer in agriculture. Where do we go practically and ecologically? Plant and Soil. 1989;115:297–311. [Google Scholar]
- Nimbal CI, Pedersen JF, Yerkes CN, Weston LA, Weller SC. Phytotoxicity and distribution of sorgoleone in grain sorghum. Journal of Agricultural and Food Chemistry. 1996;44:1343–1347. [Google Scholar]
- Northup PR, Zengshou Y, Dahlgren RA, Vogt KA. Polyphenol control of nitrogen release from pine litter. Nature. 1995;377:227–229. [Google Scholar]
- Paavolainen L, Kitunen V, Smolander A. Inhibition of nitrification in forest soil by monoterpenes. Plant and Soil. 1998;205:147–154. [Google Scholar]
- Pan Z, Rimando AM, Baerson SR, Fishbein M, Duke SO. Functional characterization of desaturatases involved in the formation of the terminal double bond of an unusual 16:3Δ9,12,15 fatty acid isolated from Sorghum bicolor root hairs. Journal of Biological Chemistry. 2007;282:4326–4335. doi: 10.1074/jbc.M606343200. [DOI] [PubMed] [Google Scholar]
- Patyk A. Reprints from the International Conference of Life Cycle Assessment in Agriculture, Food and Non-Food Agro-Industry and Forestry: Achievements and Prospects. Brussels, Belgium: 1996. Balance of energy consumption and emissions of fertilizer production and supply; pp. 73–87. 4–5 April, 1996. [Google Scholar]
- Peterjohn WT, Schlesinger WH. Nitrogen loss from deserts in the South Western United States. Biogeochemistry. 1990;10:67–79. [Google Scholar]
- Petit JR, Jouzel J, Raynaud D, et al. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature. 1999;399:429–436. [Google Scholar]
- Philippot L, Hallin S. Towards food, feed and energy crops mitigating climate change. Trends in Plant Science. 2011;16:476–480. doi: 10.1016/j.tplants.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Poudel DD, Horwath WR, Lanini WT. Comparison of soil N availability and leaching potential, crop yields and weeds in organic, low-input and conventional farming systems in northern California. Agriculture Ecosystems and Environment. 2002;90:125–137. [Google Scholar]
- Pratt PF, Adriano DC. Nitrate concentrations in the unsaturated zone beneath irrigated fields in southern California. Soil Science Society of America Proceedings. 1973;37:321–322. [Google Scholar]
- Purchase BS. Evaluation of the claim that grass root exudates inhibit nitrification. Plant and Soil. 1974;41:527–539. [Google Scholar]
- Qi LL, Wang SL, Chen PD, Liu DJ, Friebe B, Gill BS. Molecular cytogenetic analysis of Leymus racemosus chromosomes added to wheat. Theoretical and Applied Genetics. 1997;95:1084–1091. [Google Scholar]
- Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moenne-Loccoz Y. The rhizosphere: a playground and battle-field for soil borne pathogens and beneficial microorganisms. Plant and Soil. 2009;321:341–361. [Google Scholar]
- Ramu P, Deshpande SP, Senthilvel S, et al. In-silico mapping of important genes and markers available in public domain for efficient sorghum breeding. Molecular Breeding. 2010;26:409–418. [Google Scholar]
- Rao IM, Kerridge PC, Macedo M. Adaptation to low fertility acid soils and nutritional requirements of Brachiaria. In: Miles JW, Maas BL, do Valle CB, editors. The biology, agronomy and improvement of Brachiaria. Cali, Colombia: CIAT; 1996. pp. 53–71. [Google Scholar]
- Raun WR, Johnson GV. Improving nitrogen use efficiency for cereal production. Agronomy Journal. 1999;91:357–363. [Google Scholar]
- Raynaud X. Soil properties are key determinants for the development of exudates gradients in a rhizosphere simulation model. Soil Biology and Biochemistry. 2010;42:210–219. [Google Scholar]
- Rengel Z, Marschner P. Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytologist. 2005;168:305–312. doi: 10.1111/j.1469-8137.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- Rice E, Pancholy SK. Inhibition of nitrification by climax ecosystems. American Journal of Botany. 1972;59:1033–1040. [Google Scholar]
- Rice E, Pancholy SK. Inhibition of nitrification by climax ecosystems II. Additional evidence and possible role of tannins. American Journal of Botany. 1973;60:691–702. [Google Scholar]
- Rice E, Pancholy SK. Inhibition of nitrification by climax ecosystems III. Inhibitors other than tannins. American Journal of Botany. 1974;61:1095–1103. [Google Scholar]
- Rich PJ, Ejeta G. Biology of host–parasite interactions in Striga species. In: Ejeta G, Gressl J, editors. Integrating new technologies for Striga control: towards ending the witch-hunt. Singapore: World Scientific Publishing Company PTE LTD; 2007. pp. 19–32. [Google Scholar]
- Rioual P, Andrieu-Ponel V, Rietti-Shati M, et al. High-resolution record of climate stability in France during the last interglacial period. Nature. 2001;413:293–296. doi: 10.1038/35095037. [DOI] [PubMed] [Google Scholar]
- Robinson GP. Nitrification in a New Zealand grassland soil. Plant and Soil. 1963;2:173–183. [Google Scholar]
- Robertson GP. Nitrification in forested ecosystem. Philosophical Transactions of the Royal Society B: Biological Sciences. 1982a;296:445–457. [Google Scholar]
- Robertson GP. Factors regulating nitrification in primary and secondary succession. Ecology. 1982b;63:1561–1573. [Google Scholar]
- Rodgers GA. Nitrification inhibitors in agriculture. Journal of Environmental Science and Health. 1986;A21:701–722. [Google Scholar]
- Rockstrom J, Steffen W, Noone K, et al. A safe operating space for humanity. Nature. 2009;461:472–475. doi: 10.1038/461472a. [DOI] [PubMed] [Google Scholar]
- Ross SM. Organic matter in tropical soils: current conditions, concerns and prospects for conservation. Progress in Physical Geography. 1993;17:265–305. [Google Scholar]
- Rossiter-Rachor NA, Setterfield SA, Douglas MM, Hutley LB, Cook GD, Schmidt S. Invasive Andropogon gayanus (gamba grass) is an ecosystem transformer of nitrogen relations in Australian savanna. Ecological Applications. 2009;19:1546–1560. doi: 10.1890/08-0265.1. [DOI] [PubMed] [Google Scholar]
- Sahrawat KL. Comparison of karanjin with other nitrification inhibitors for retardation of nitrification of urea N in soil. Plant and Soil. 1981;59:494–498. [Google Scholar]
- Sahrawat KL. Nitrification in some tropical soils. Plant and Soil. 1982;65:281–286. [Google Scholar]
- Sahrawat KL. Effects of nitrification inhibitors on nitrogen transformations other than nitrification in soils. Advances in Agronomy. 1989;42:279–309. [Google Scholar]
- Sahrawat KL. Nitrification inhibitors with emphasis on natural products and the persistence of fertilizer nitrogen in the soil. Nitrogen Economy in Tropical Soils. 1996;69:379–388. [Google Scholar]
- Sahrawat KL. Factors affecting nitrification in soils. Communications in Soil Sciences and Plant Analysis. 2008;39:1436–1446. [Google Scholar]
- Sahrawat KL, Keeney DR. Perspectives for research on development of nitrification inhibitors. Communications in Soil Science and Plant Analysis. 1985;16:517–524. [Google Scholar]
- Sahrawat KL, Mukerjee SK. Nitrification inhibitors 1. Studies with Karanjin, a furanoflavonoid from karanja (Pongamia glabra) seeds. Plant and Soil. 1977;47:27–36. [Google Scholar]
- Sahrawat KL, Mukerjee SK, Gulati KC. Nitrification inhibitors II. Studies with furano compounds. Plant and Soil. 1977;47:687–691. [Google Scholar]
- Salsac L, Chaillou S, Morot-Gaudry J, Lesaint C. Nitrate and ammonium nutrition in plants. Plant Physiology and Biochemistry. 1987;25:805–812. [Google Scholar]
- Satish K, Gutema Z, Grenier C, Rich PJ, Ejeta G. Molecular tagging and validation of microsatellite markers linked to the low germination stimulant gene (lgs) for Striga resistance in sorghum [Sorghum bicolor (L.) Moench] Theoretical and Applied Genetics. 2011;124:989–1003. doi: 10.1007/s00122-011-1763-9. [DOI] [PubMed] [Google Scholar]
- Schafer A, Victor DG. Global passenger travel: implications for carbon dioxide emissions. Energy. 1999;24:657–679. [Google Scholar]
- Schimel JP, Bennett J. Nitrogen mineralization: challenges of a changing paradigm. Ecology. 2004;85:591–602. [Google Scholar]
- Schlesinger WH. On the fate of anthropogenic nitrogen. Proceedings of National Academy of Sciences, USA. 2009;106:203–208. doi: 10.1073/pnas.0810193105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears ER. Induced mutant with homoeologous pairing in common wheat. Canadian Journal of Genetics and Cytology. 1977;19:585–593. [Google Scholar]
- Sears ER. Use of radiation to transfer alien chromosome segments to wheat. Crop Science. 1993;33:897–901. [Google Scholar]
- Shaviv A, Mikkelsen RL. Controlled-release fertilizers to increase efficiency of nutrient use and minimize environmental degradation – a review. Fertilizer Research. 1993;35:1–12. [Google Scholar]
- Shoji S, Kanno H. Use of polyolefin-coated fertilizers for increasing fertilizer efficiency and reducing nitrate leaching and nitrous-oxide emissions. Fertilizer Research. 1994;39:147–152. [Google Scholar]
- Siame BA, Weerasuriya Y, Wood K, Ejeta G, Buttler LG. Isolation of strigol, a germination stimulant for Striga asiatica from host plants. Journal of Agricultural and Food Chemistry. 1993;41:1486–1491. [Google Scholar]
- Singh RP, Hodson DP, Jin Y, et al. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews. 2006;54:1–13. [Google Scholar]
- Slangen J, Kerkhoff P. Nitrification inhibitors in agriculture and horticulture: a literature review. Fertilizer Research. 1984;5:1–76. [Google Scholar]
- Smart DR, Bloom AJ. Wheat leaves emit nitrous oxide during nitrate assimilation. Proceedings of National Academy of Sciences, USA. 2001;98:7875–7878. doi: 10.1073/pnas.131572798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KA, McTaggart IP, Tsuruta H. Emissions of N2O and NO associated with nitrogen fertilization in intensive agriculture, and the potential for mitigation. Soil Use Management. 1997;13:296–304. [Google Scholar]
- Smith P, Martino D, Cai Z, et al. Agriculture. In: Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA, editors. Climate change 2007: mitigation. Contribution of working group III to the fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge: Cambridge University Press; 2007. pp. 497–540. [Google Scholar]
- Smits NAC, Bobbink R, Laanbrock HJ, Paalman AJ, Hefting MM. Repression of potential nitrification activities by matgrass sward species. Plant and Soil. 2010a;337:435–445. [Google Scholar]
- Smits NAC, Hefting MM, Kamst-van Agterveld MP, Laanbroek HJ, Paalman AJ, Bobbink R. Nitrification along a grassland gradient: inhibition found in matgrass swards. Soil Biology and Biochemistry. 2010b;42:635–641. [Google Scholar]
- Smolander A, Priha O, Paavolainen L, Steer J, Malkonen E. Nitrogen and carbon transformation before and after clear-cutting in repeatedly N-fertilized and limed forest soils. Soil Biology and Biochemistry. 1998;30:477–490. [Google Scholar]
- Smolander A, Kanerva S, Adamezyk B, Kitunen V. Nitrogen transformations in boreal forest soils – does composition of plant secondary compounds give any explanations? Plant and Soil. 2012;350:1–26. [Google Scholar]
- Sommer K, Mertz M, Rossig K. Stickstoff zu weizen mit ammonium-nitrificiden, mitteilung 2:Proteingehalte, proteinfraktionen und backfahigkeit. Landwirtschaftliche Forschung. 1976;29:161–169. [Google Scholar]
- Steltzer H, Bowman WD. Differential influence of plant species on soil nitrogen transformations within moist meadow alpine tundra. Ecosystems. 1998;1:464–474. [Google Scholar]
- Stienstra AW, Klein Gunnewiek P, Laanbroek HJ. Repression of nitrification in soils under a climax grassland vegetation. FEMS Microbiological Ecology. 1994;14:45–52. [Google Scholar]
- Stokstad E. Deadly wheat fungus threatens world's breadbaskets. Science. 2007;315:1786–1787. doi: 10.1126/science.315.5820.1786. [DOI] [PubMed] [Google Scholar]
- Subbarao GV, Ito O, Sahrawat KL, et al. Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Critical Reviews in Plant Sciences. 2006a;25:303–335. [Google Scholar]
- Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL. A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant and Soil. 2006b;288:101–112. [Google Scholar]
- Subbarao GV, Wang HY, Ito O, Nahara K, Berry WL. NH4+ triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant and Soil. 2007a;290:245–257. [Google Scholar]
- Subbarao GV, Rondon M, Ito O, et al. Biological nitrification inhibition (BNI) – is it a widespread phenomenon? Plant and Soil. 2007b;294:5–18. [Google Scholar]
- Subbarao GV, Ban T, Masahiro K, et al. Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant and Soil. 2007c;299:55–64. [Google Scholar]
- Subbarao GV, Nakahara K, Ishikawa T, et al. Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant and Soil. 2008;313:89–99. [Google Scholar]
- Subbarao GV, Nakahara K, Hurtado MP, et al. Evidence for biological nitrification inhibition in Brachiaria pastures. Proceedings of National Academy of Sciences, USA. 2009a;106:17302–17307. doi: 10.1073/pnas.0903694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subbarao GV, Kishii M, Nakahara K, et al. Biological nitrification inhibition (BNI) – is there potential for genetic interventions in the Triticeae? Breeding Science. 2009b;59:529–545. [Google Scholar]
- Subbarao GV, Hossain AKMZ, Nakahara K, et al. Biological nitrification inhibition (BNI) potential in sorghum. The Proceedings of the International Plant Nutrition Colloquium XVI. 2009c Department of Plant Sciences, UC Davis, 2009 UC Davis, CA. (E-journal article: http://repositories.cdlib.org/cgi/viewcontent.cgi/article=1175&context=jpnc/xvi. ) [Google Scholar]
- Subbarao GV, Sahrawat KL, Nakahara K, et al. Biological nitrification inhibition – a novel strategy to regulate nitrification in agricultural systems. Advances in Agronomy. 2012a;114:249–302. [Google Scholar]
- Subbarao GV, Nakahara K, Ishikawa T, et al. Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant and Soil. 2012b http://dx.doi.org/10.1007/s11104-012-1419-9 . [Google Scholar]
- Sutton MA, Oenema O, Erisman JW, Leip A, van Grinsven H, Winiwarter W. Too much of a good thing. Nature. 2011;472:159–161. doi: 10.1038/472159a. [DOI] [PubMed] [Google Scholar]
- Sylvester-Bradley R, Mosquera D, Mendez JE. Inhibition of nitrate accumulation in tropical grassland soils: effects of nitrogen fertilization and soil disturbance. Journal of Soil Science. 1988;39:407–416. [Google Scholar]
- Taylor AE, Zeglin LH, Dooley S, Myrold DD, Bottomley PJ. Evidence for different contributions of archaea and bacteria to the ammonia-oxidizing potential of diverse Oregon soils. Applied Environmental Microbiology. 2010;76:7691–7698. doi: 10.1128/AEM.01324-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiessen H, Cuevas E, Chacon P. The role of soil organic matter in sustaining soil fertility. Nature. 1994;371:783–785. [Google Scholar]
- Tilman D, Fargione J, Wolff B, et al. Forecasting agriculturally driven global environmental change. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- Toyomasu T, Tsukahara M, Kaneko A, et al. Fusicoccins are biosynthesized by an unusual chimera diterpene synthase in fungi. Proceedings of National Academy of Sciences, USA. 2007;104:3084–3088. doi: 10.1073/pnas.0608426104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wesemael B, Paustian K, Meersmans J, Goidts E, Barancikova G, Easter M. Agricultural management explains historic changes in regional soil carbon stocks. Proceedings of National Academy of Sciences, USA. 2010;107:14926–14930. doi: 10.1073/pnas.1002592107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitousek PM, Howarath RW. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry. 1991;13:87–115. [Google Scholar]
- Vitousek PM, Matson PA. Mechanisms of nitrogen retention in forest ecosystems: a field experiment. Science. 1984;225:51–52. doi: 10.1126/science.225.4657.51. [DOI] [PubMed] [Google Scholar]
- Vitousek PM, Aber JD, Howarth W, et al. Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications. 1997;7:737–750. [Google Scholar]
- Walker TS, Bais HP, Grotewold E, Vivanco JM. Root exudation and rhizosphere biology. Plant Physiology. 2003;132:44–51. doi: 10.1104/pp.102.019661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigelt A, Bol R, Bardgett RD. Preferential uptake of soil nitrogen forms by grassland species. Oecologia. 2005;142:627–635. doi: 10.1007/s00442-004-1765-2. [DOI] [PubMed] [Google Scholar]
- Weiske A, Benckiser G, Ottow JCG. Effect of new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutrient Cycling in Agroecosystems. 2001;60:57–64. [Google Scholar]
- White C. The role of monoterpenes in soil nitrogen cycling processes in ponderosa pine. Biogeochemistry. 1991;12:43–68. [Google Scholar]
- Woldendorp JW, Laanbroek HJ. Activity of nitrifiers in relation to nitrogen nutrition of plants in natural ecosystems. Plant and Soil. 1989;115:217–228. [Google Scholar]
- Wolt JD. A meta-evaluation of nitrapyrin agronomic and environmental effectiveness with emphasis on corn production in the mid-Western USA. Nutrient Cycling in Agroecosystems. 2004;69:23–41. [Google Scholar]
- Xie X, Yoneyama K, Kusumoto D, et al. Sorgomol germination stimulant for root parasitic plants, produced by Sorghum bicolor. Tetrahedron Letters. 2008;49:2066–2068. [Google Scholar]
- Yoneyama K, Xie X, Kusumoto D, et al. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- Yoneyama K, Awad AA, Xie X, Yoneyama K, Takeuchi Y. Strigolactones as germination stimulants for root parasitic plants. Plant and Cell Physiology. 2010;51:1095–1103. doi: 10.1093/pcp/pcq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn LM. A boost from wild wheat. Science. 2007;318:171. [Google Scholar]
- Zakir HAKM, Subbarao GV, Pearse SJ, et al. Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl)propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor) New Phytologist. 2008;180:442–451. doi: 10.1111/j.1469-8137.2008.02576.x. [DOI] [PubMed] [Google Scholar]
- Zerulla W, Barth T, Dressel J, et al. 3,4-Dimethylpyrazole phosphate (DMPP) – a new nitrification inhibitor for agriculture and horticulture. Biology and Fertility of Soils. 2001;34:79–84. [Google Scholar]
- Zhu Y, Zeng H, Shen Q, Ishikawa T, Subbarao GV. Interplay among NH4+ uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant and Soil. 2012;358:131–141. [Google Scholar]
- Zvomuya F, Rosen CJ, Russelle MP, Gupta SC. Nitrate leaching and nitrogen recovery following application of polyefincoated urea to potato. Journal of Environmental Quality. 2003;32:480–489. doi: 10.2134/jeq2003.4800. [DOI] [PubMed] [Google Scholar]