MDSC-derived nitric oxide supports the development of Th17 cells in ovarian cancer dependent on the induction of endogenous NOS2 and the cGMP–cGK pathway in Th17 cells.
Abstract
Nitric oxide (NO) is a ubiquitous mediator of inflammation and immunity, involved in the pathogenesis and control of infectious diseases, autoimmunity, and cancer. We observed that the expression of nitric oxide synthase-2 (NOS2/iNOS) positively correlates with Th17 responses in patients with ovarian cancer (OvCa). Although high concentrations of exogenous NO indiscriminately suppress the proliferation and differentiation of Th1, Th2, and Th17 cells, the physiological NO concentrations produced by patients’ myeloid-derived suppressor cells (MDSCs) support the development of RORγt(Rorc)+IL-23R+IL-17+ Th17 cells. Moreover, the development of Th17 cells from naive-, memory-, or tumor-infiltrating CD4+ T cells, driven by IL-1β/IL-6/IL-23/NO-producing MDSCs or by recombinant cytokines (IL-1β/IL-6/IL-23), is associated with the induction of endogenous NOS2 and NO production, and critically depends on NOS2 activity and the canonical cyclic guanosine monophosphate (cGMP)–cGMP-dependent protein kinase (cGK) pathway of NO signaling within CD4+ T cells. Inhibition of NOS2 or cGMP–cGK signaling abolishes the de novo induction of Th17 cells and selectively suppresses IL-17 production by established Th17 cells isolated from OvCa patients. Our data indicate that, apart from its previously recognized role as an effector mediator of Th17-associated inflammation, NO is also critically required for the induction and stability of human Th17 responses, providing new targets to manipulate Th17 responses in cancer, autoimmunity, and inflammatory diseases.
Nitric oxide (NO; a product of nitrite reduction or the NO synthases NOS1, NOS2, and NOS3; Culotta and Koshland, 1992), is a pleiotropic regulator of neurotransmission, inflammation, and autoimmunity (Culotta and Koshland, 1992; Bogdan, 1998, 2001; Kolb and Kolb-Bachofen, 1998) implicated both in cancer progression and its immune-mediated elimination (Culotta and Koshland, 1992; Coussens and Werb, 2002; Hussain et al., 2003; Mantovani et al., 2008). In different mouse models, NO has been paradoxically shown to both promote inflammation (Farrell et al., 1992; Boughton-Smith et al., 1993; McCartney-Francis et al., 1993; Weinberg et al., 1994; Hooper et al., 1997) and to suppress autoimmune tissue damage through nonselective suppression of immune cell activation (Bogdan, 2001; Bogdan, 2011), especially at high concentrations (Mahidhara et al., 2003; Thomas et al., 2004; Niedbala et al., 2011). Although previous studies demonstrated a positive impact of NO on the induction of Th1 cells (Niedbala et al., 2002) and forkhead box P3–positive (FoxP3+) regulatory T (T reg) cells (Feng et al., 2008) in murine models, the regulation and function of the NO synthase (NOS)–NO system have shown profound differences between mice and humans (Schneemann and Schoedon, 2002, Schneemann and Schoedon, 2007; Fang, 2004), complicating the translation of these findings from mouse models to human disease.
In cancer, NOS2-derived NO plays both cytotoxic and immunoregulatory functions (Bogdan, 2001). It can exert distinct effects on different subsets of tumor-infiltrating T cells (TILs), capable of blocking the development of cytotoxic T lymphocytes (CTLs; Bronte et al., 2003), suppressing Th1 and Th2 cytokine production, and modulating the development of FoxP3+ T reg cells (Brahmachari and Pahan, 2010; Lee et al., 2011). NOS2-driven NO production is a prominent feature of cancer-associated myeloid-derived suppressor cells (MDSCs; Mazzoni et al., 2002; Kusmartsev et al., 2004; Vuk-Pavlović et al., 2010; Bronte and Zanovello, 2005), which in the human system are characterized by a CD11b+CD33+HLA-DRlow/neg phenotype consisting of CD14+ monocytic (Serafini et al., 2006; Filipazzi et al., 2007; Hoechst et al., 2008; Obermajer et al., 2011) and CD15+ granulocytic (Zea et al., 2005; Mandruzzato et al., 2009; Rodriguez et al., 2009) subsets (Dolcetti et al., 2010; Nagaraj and Gabrilovich, 2010).
Production of NO in chronic inflammation is supported by IFN-γ and IL-17 (Mazzoni et al., 2002; Miljkovic and Trajkovic, 2004), the cytokines produced by human Th17 cells (Veldhoen et al., 2006; Acosta-Rodriguez et al., 2007a,b; van Beelen et al., 2007; Wilson et al., 2007). Human Th17 cells secrete varying levels of IFN-γ (Acosta-Rodriguez et al., 2007a; Acosta-Rodriguez et al., 2007b; Kryczek et al., 2009; Miyahara et al., 2008; van Beelen et al., 2007; Wilson et al., 2007) and have been implicated both in tumor surveillance and tumor progression (Miyahara et al., 2008; Kryczek et al., 2009; Martin-Orozco and Dong, 2009). Induction of Th17 cells typically involves IL-1β, IL-6, and IL-23 (Bettelli et al., 2006; Acosta-Rodriguez et al., 2007a,b; Ivanov et al., 2006; van Beelen et al., 2007; Veldhoen et al., 2006; Wilson et al., 2007; Zhou et al., 2007), with the additional involvement of TGF-β in most mouse models (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006; Zhou et al., 2007; Ghoreschi et al., 2010), but not in the human system (Acosta-Rodriguez et al., 2007a; Wilson et al., 2007). IL-1β1, IL-6, and IL-23 production by monocytes and DCs, and the resulting development of human Th17 cells, can be induced by bacterial products, such as LPS or peptidoglycan (Acosta-Rodriguez et al., 2007a; Acosta-Rodriguez et al., 2007b; van Beelen et al., 2007). However, the mechanisms driving Th17 responses in noninfectious settings, such as autoimmunity or cancer, remain unclear.
Here, we report that the development of human Th17 cells from naive, effector, and memory CD4+ T cell precursors induced by the previously identified Th17-driving cytokines (IL-1β, IL-6, and IL-23) or by IL-1β/IL-6/IL-23-producing MDSCs, is promoted by exogenous NO (or NO produced by human MDSCs) and critically depends on the induction of endogenous NOS2 in differentiating CD4+ T cells.
RESULTS
MDSC-associated NOS2 and MDSC-produced exogenous NO promote the Th17 phenotype in OvCa patient TILs and naive and memory CD4+ T cells
High frequencies of Th17 cells observed in ovarian cancer (OvCa; Kryczek et al., 2009; Miyahara et al., 2008) and our observations that the OvCa environment is a potent inducer of Th17 responses (Miyahara et al., 2008), prompted us to test the role of cancer-associated MDSCs and MDSC-produced factors in the development of human Th17 cells.
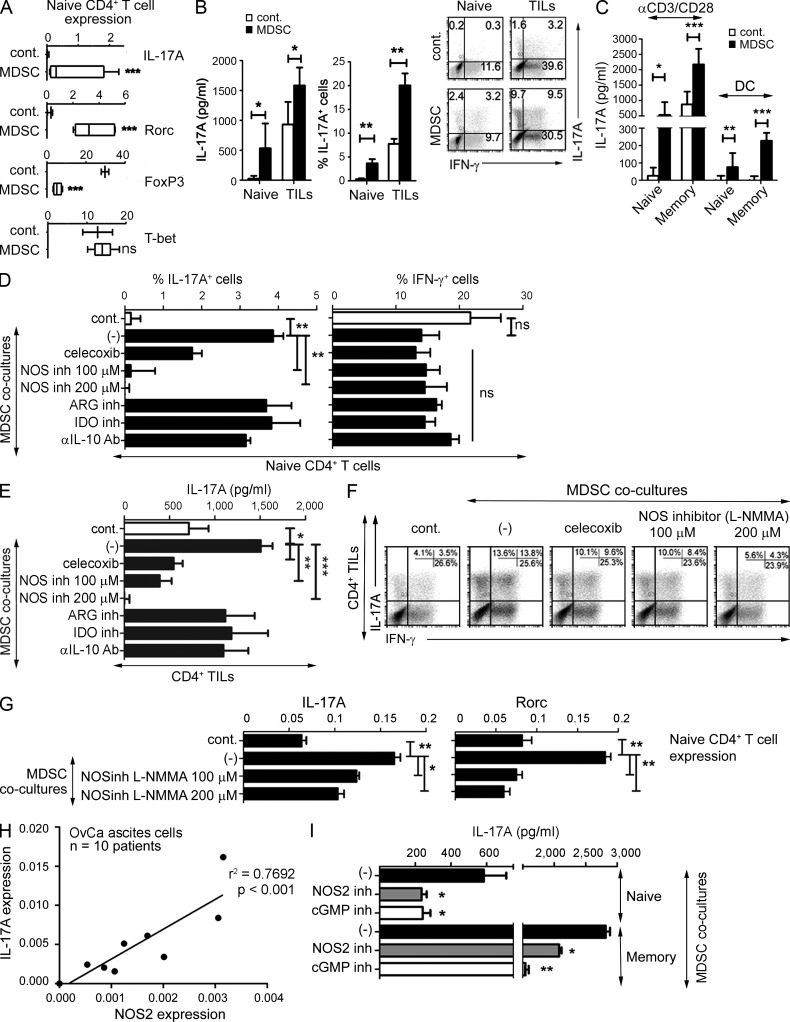
As shown in Fig. 1, OvCa patient-isolated MDSCs, expressing the typical CD11b+CD33+HLA-DR−/lowCD80− phenotype (Gabrilovich and Nagaraj, 2009; Nagaraj and Gabrilovich, 2010; see Obermajer et al., 2011 for complete MDSC phenotype), selectively enhanced the expression of Rorc (encoding RORγt) and IL-17A (Fig. 1 A) and the production of IL-17A protein (Fig. 1, B and C) by CD4+ T cells, activated by anti-CD3/CD28 antibodies or allogeneic DCs (Fig. 1 C), showing no impact on the expression of the Th1 marker, T-bet, and an inhibitory impact on the T reg cell marker, FoxP3 (Fig. 1 A). Unexpectedly, although inhibition of other MDSC products, including IL-10, indoleamine 2,3-dioxygenase (IDO), and arginase, did not impact the induction of Th17 cells (Fig. 1, D and E), the inhibition of NOS, the key enzyme in the synthesis of the nominally suppressive MDSC product NO (Huang et al., 2006; Movahedi et al., 2008), dose-dependently inhibited the ability of MDSCs to enhance IL-17A production (Fig. 1, D–F) and Rorc expression (Fig. 1 G) in activated CD4+ T cells. In addition, the Th17-promoting effect of MDSCs was also reduced by the inhibition of cyclooxygenase 2 (COX2; Fig. 1, D–F), the factor needed for the optimal MDSC expression of NOS2 (Obermajer et al., 2011).
Figure 1.
Key role of NOS2 and the canonical cGMP-mediated NO signaling pathway in the MDSC-promoted Th17 differentiation of TILs from OvCa patients and human naive and memory CD4+ Th cells. (A) Induction of IL-17A, Rorc (encoding RORγt), FoxP3 (indicating de novo differentiation of FoxP3+ T reg cells from naive precursors), and T-bet gene expression in anti-CD3/CD28–expanded naive CD4+ T cells by tumor–isolated MDSCs (mean ± SD from six patients), as compared with control CD11b+ cells (mean ± SD from three healthy donors). (B) IL-17A production levels and percentages of IL-17A+ cells (mean ± SD from n donors), and representative intracellular staining (IL-17A vs. IFN-γ, right) in naive CD4+ T cells (n = 6 healthy donors) or OvCa-infiltrating CD4+ TILs (n = 3 patients) stimulated with anti-CD3/CD28 antibodies in the presence of cancer-isolated MDSCs or control CD11b+ cells. (C) IL-17A production by naive versus memory CD4+ T cells (mean ± SD from n = 4 healthy donors) stimulated with either anti-CD3/CD28 antibodies or TNF-matured allogeneic DCs in the presence of MDSCs or control CD11b+ cells. (D and E) Percentage of IL-17A+ or IFN-γ+ CD4+ T cells (D) and IL-17A secretion (E) in anti-CD3/CD28/MDSC–expanded naive CD4+ T cells (D) and CD4+ TILs (E) in the presence of specific inhibitors of NOS (L-NMMA), COX2 (celecoxib), IDO-, ARG-, or IL-10. The data (mean ± SD) from one representative experiment (performed in replicates: D, triplicate cultures; E, quadruplicate cultures). The results were confirmed in three independent experiments using different patients/healthy donors. (F) Representative staining of IL-17A+ or IFN-γ+ CD4+ T cells in co-cultures of anti-CD3/CD28–expanded CD4+ TILs and MDSCs in the presence of specific inhibitors of COX2 (celecoxib) and NOS (L-NMMA). The results were confirmed in three independent experiments using different patients. (G) Relative gene expression of IL-17A and Rorc, induced in anti-CD3/CD28–expanded naive CD4+ T cells cultured in the presence of MDSCs in the presence of a specific inhibitor of NOS (L-NMMA). The graphs present the mean ± SD from one representative experiment (triplicate cultures) of two (using different patients/healthy donors). (H) Relative gene expression of NOS2 and IL-17A in 7 d ex vivo anti-CD3/CD28–expanded cultures of OvCa ascites cells from 10 OvCa patients (n = 10; r2 = 0.7692; P < 0.001). (I) IL-17A production in anti-CD3/CD28–expanded cultures of naive or memory CD4+ T cells in the presence of MDSCs, with or without specific inhibitors of NOS2 (1400W) or cGMP (ODQ). Data (mean ± SD) from one representative experiment (triplicate cultures). The results were confirmed in three independent experiments using different patients/healthy donors. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
In accordance with the key role of MDSC-associated NOS2 in the induction of Th17 responses in cancer patients in vivo, we observed that the levels of NOS2 expression in the ascites cells from OvCa patients positively correlated with the ability of these cells to produce IL-17A after short-term ex vivo stimulation (Fig. 1 H). Moreover, we observed that the Th17-promoting effects of MDSCs could be prevented both by the selective inhibition of NOS2 activity as well as the inhibition of cyclic guanosine monophosphate function (cGMP; Fig. 1 I), further demonstrating that human Th17 responses critically depend on NOS2 and the canonical cGMP– cGMP-dependent protein kinase (cGK)–mediated signaling pathway associated with the physiological NO concentrations produced by human cancer-isolated MDSCs (Fig. 2 A).
Figure 2.
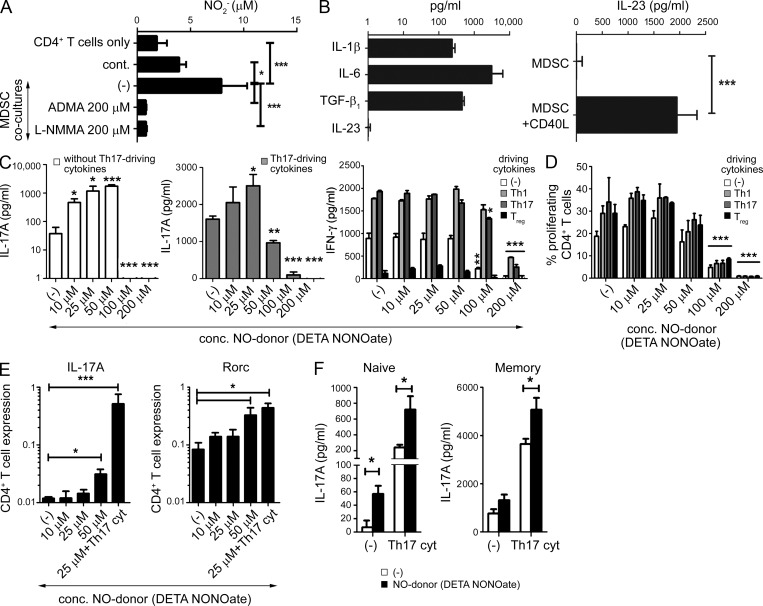
Exogenous NO supports the cytokine-driven induction of Th17 function in memory Th cells and promotes the de novo induction of Th17 cells from naive precursors. (A) NO2− levels (mean ± SD from four patients) in co-cultures of CD4+ TILs with tumor-isolated MDSCs (as compared with blood CD11b+ cells) and in the presence of NOS inhibitors ADMA and l-NMMA. (B) Expression of IL-1β, IL-6, TGF-β1 (spontaneous expression, left), and IL-23 (stimulation with CD40L, right) in MDSCs. Data (mean ± SD) from three experiments involving MDSCs from three different patients. (C) Induction of IL-17A or IFN-γ production by anti-CD3/CD28–stimulated bulk CD4+ T cells from healthy donors, cultured in the absence or presence of Th1 (200 U/ml rhIL-12, 200 ng/ml αIL-4-Ab), Th17 (20 ng/ml rhIL-1β, 50 ng/ml rhIL-6, 10 ng/ml rhIL-23), and T reg cell (5 ng/ml TGF-β1, 10 nM 9-cis retinoic acid) -driving cytokines, and physiological concentrations of exogenous NO donor (DETA-NONOate). IL-17A was undetectable in Th1- and T reg cell–driving conditions. Percentage of FoxP3+ cells in control cultures were as follows: (−), 10.4%; Th1, 12.7%; Th17, 5.2%; and T reg cell, 41.2%. The graphs present the mean ± SD from one representative experiment (triplicate cultures) of two (healthy donors). (D) Suppression of CD4+ T cells differentiating in Th1-, Treg-, and Th17-driving conditions by high concentrations (>100 µM) of DETA-NONOate. Proliferation of CFSE-labeled anti-CD3/CD28–stimulated bulk CD4+ T cells cultured in the absence or presence of Th1, Th17, and T reg cell–driving cytokines and supplemented with increasing concentrations of DETA-NONOate. The graph presents the mean ± SD from one representative experiment (triplicate cultures) of two (different healthy donors). (E and F) Relative gene expression of IL-17A (log scale) and Rorc (log scale), and secretion of IL-17A by naive (E) or naive and memory (F) CD4+ T cells, expanded with anti-CD3/CD28 antibodies in the absence or presence of Th17-driving cytokines and NO donor (DETA-NONOate). The graphs present the mean ± SD from one representative experiment (E, triplicate cultures; F, quadruplicate cultures). The results were confirmed in three independent experiments using cells of different healthy donors. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Although MDSCs produced all the previously identified Th17-driving cytokines (Fig. 2 B), either spontaneously (IL-1β, IL-6, and TGF-β1) or after their stimulation (IL-23) with CD40L (the CD4+ T cell–expressed APC-activating factor [Lane et al., 1992], similar data were obtained with CD40L-expressing J558 cells or CD4+ T cells; not depicted), the advantage of MDSCs in driving the Th17 phenotype was particularly pronounced in the case of naive, as compared with memory, Th cells (Fig. 1 C). Human naive Th cells, compared with their memory counterparts, were previously shown to be less sensitive to the induction of the Th17 phenotype by recombinant cytokines (van Beelen et al., 2007), indicating that such an additional signal, which can be promoted by MDSCs, is essential for the de novo induction of Th17 cells.
Whereas, consistent with previous studies (Mahidhara et al., 2003; Niedbala et al., 2011), high doses of exogenous NO (>100 µM; known to have cytostatic function; Bogdan, 2001) nonselectively blocked CD4+ T cell differentiation (Fig. 2 C) and their proliferation (Fig. 2 D) in all conditions tested (Th17-, Th1-, or T reg cell–driving conditions), the application of lower, standard cell-signaling doses (Kolb and Kolb-Bachofen, 1998; Karpuzoglu and Ahmed, 2006) of NO donor (10–25 µM; comparable to the MDSC-produced NO levels; Fig. 2 A) did not affect CD4+ T cell proliferation. Instead, these lower doses of NO selectively enhanced IL-17A production (Fig. 2 C and not depicted) without affecting IFN-γ production (Fig. 2 C), further confirming the ability of physiological NO concentrations to selectively support human Th17 development.
Exogenous NO strongly enhanced the induction of Th17 cells driven by recombinant cytokines (Fig. 2, C, E, and F), indicating its direct impact on T cells rather than its modulation of MDSC functions. NO induced IL-17A expression (Fig. 2 E) and secretion (Fig. 2 F) by both naive and memory CD4+ T cells and enhanced their expression of Rorc (but not GATA3, FoxP3, or T-bet; Fig. 2 E and not depicted). The distinct Th17-promoting effect of exogenous NO was evident even in the absence of Th17-driving cytokines (IL-1β, IL-6, and IL-23; Fig. 2, C, E, and F), indicating that NO is a direct inducer of Th17 differentiation rather than a mere enhancer of the effects of Th17-inducing cytokines.
Cytokine- or MDSC-driven Th17 differentiation depends on the induction of endogenous NOS2 in naive CD4+ T cells and elevation of endogenous NOS2 in memory CD4+ T cells
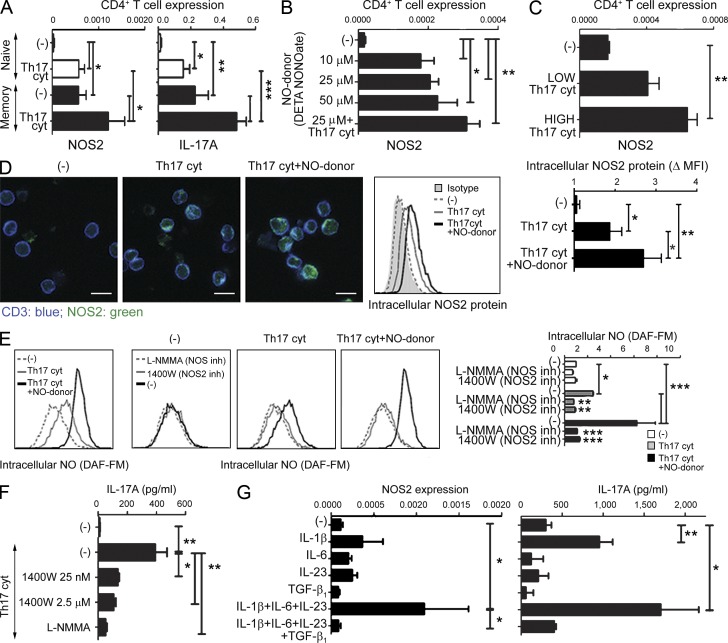
Unexpectedly, we observed that Th17-driving cytokines, as well as exogenous NO, all induce the expression of endogenous NOS2 in the expanding population of naive CD4+ T cells (>99% pure at the day of analysis) and further enhance its expression in memory Th cells (Fig. 3, A–C). A similar induction of endogenous NOS2 was also observed in blood-isolated naive and memory CD4+ T cells and tumor-isolated TILs differentiated in the presence of cancer-isolated MDSCs (not depicted).
Figure 3.
Endogenous T cell–expressed NOS2 and T cell-produced NO are required for de novo Th17 differentiation from naive precursors and induction of the Th17 phenotype in memory cells. (A) Comparative induction of NOS2 (left) and IL-17A (right) gene expression in naive and memory CD4+ T cells (mean ± SD from three healthy donors) stimulated with anti-CD3/CD28 antibodies in the absence or presence of Th17-driving cytokines. (B) Dose-dependent induction of NOS2 gene expression in naive CD4+ T cells stimulated with anti-CD3/CD28 antibodies in the presence of increasing concentrations of NO donor (DETA-NONOate) and Th17-driving cytokines. The graph presents the mean ± SD from one representative experiment (performed with triplicate cultures). The results were confirmed in three independent experiments using different healthy donors. (C) Dose-dependent induction of NOS2 gene expression in bulk CD4+ T cells, stimulated with anti-CD3/CD28 antibodies and Th17-driving cytokines (high: 20 ng/ml IL-1β, 50 ng/ml IL-6, 10 ng/ml IL-23; low: 25× dilution). The graph presents the mean ± SD from one representative experiment (triplicate cultures). The results were confirmed in three independent experiments using different healthy donors. (D) Induction of intracellular NOS2 protein (left and middle, representative data; right, mean ± SD from n = 3 healthy donors) in CD3+-gated bulk CD4+ T cells stimulated with anti-CD3/CD28 antibodies in the presence of Th17-driving cytokines and NO donor (DETA-NONOate). Bar, 10 µm. Data in the right panel is represented as the fold change of the mean fluorescence intensity (MFI) over the isotype control. (E) Induction of intracellular NO (DAF-FM staining; representative experiment, left; mean ± SD from n = 3 healthy donors, right) in CD3+-gated bulk CD4+ T cells stimulated with anti-CD3/CD28 antibodies in the presence of Th17-driving cytokines and NO donor (DETA-NONOate), or the presence of general NOS inhibitor (L-NMMA) or NOS2-specific inhibitor (1400W). Data in the right panel is expressed as the fold increase of DAF-FM MFI over CD4+ T cells cultured in the absence of Th17-driving cytokines and NO donor. When not otherwise indicated, statistically significant differences compared with the absence of NO inhibitors are shown. (F) IL-17A secretion by naive CD4+ T cells stimulated with Th17-driving cytokines in the presence of a general NOS inhibitor (L-NMMA) or NOS2-specific inhibitor (1400W). The graph presents the mean ± SD from one representative experiment (quadruplicate cultures). The results were confirmed in three independent experiments using different healthy donors. (G) Induction of NOS2 (left, mean ± SD from four healthy donors) gene expression correlated with the IL-17A production (right, mean ± SD from three healthy donors) in bulk CD4+ T cells by the individual Th17-inducing factors IL-1β, IL-6, IL-23, and/or TGF-β1. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
To eliminate the possibility that such enhanced NOS2 levels in expanding T cell cultures result from their contamination with rare non–T cells expressing very high levels of NOS2, we evaluated the presence of intracellular NOS2 protein in individual CD4+ T cells. As shown in Fig. 3 D, the addition of Th17-driving cytokines uniformly induced distinct elevation of NOS2 in the individual differentiating T cells, with further enhancement of intracellular NOS2 protein observed in the T cells that developed in the added presence of NO donor.
In accordance with the enzymatic activity of the CD4+ T cell-expressed NOS2, we observed intracellular accumulation of NO in the individual Th17-differentiated cells by flow cytometry (Fig. 3 E). The intracellular NO accumulation resulted from endogenous NOS2 activity, as it was completely blocked using two different small molecule inhibitors of NOS2 activity (pan-NOS-inhibitor and NOS2-selective inhibitor; Fig. 3 E). In line with the requirement for endogenous NOS2–NO-signaling in the effective induction of human Th17 cells, blockade of the endogenous NOS2 in CD4+ T cells differentiating in the presence of Th17-driving cytokines suppressed their ability to secrete IL-17A (Fig. 3 F).
The induction of NOS2 in CD4+ T cells was closely correlated with the activity of the individual Th17-driving factors (and their combinations) in inducing Th17 differentiation (Fig. 3 G). IL-1β, recently shown to be the critical component of the Th17-promoting cytokine cocktail (Chung et al., 2009; Guo et al., 2009), was sufficient to induce significant expression of NOS2 in CD4+ T cells, but its combination with the additional Th17-driving cytokines (IL-6 and IL-23) was needed for the optimal induction of NOS2 (Fig. 3 G). This effect was further amplified by exogenous NO (Fig. 3, B, D and E). In contrast to IL1β, IL-6, and IL-23, which together promoted the elevation of endogenous NOS2 in human CD4+ T cells (and associated IL-17A production), human TGF-β1 proved to be a suppressor of both IL-17A and NOS2 (Fig. 3 G). Unlike the potent induction of endogenous NOS2 in CD4+ T cells driven toward Th17 development, neither NOS1 (undetectable) nor NOS3 expression were induced during this process (not depicted).
Interestingly, the induced levels of endogenous NOS2 were much higher in human memory than in naive CD4+ T cells (Fig. 3 A), which is consistent with the observed differences in the effectiveness of IL-17 induction in these two populations (van Beelen et al., 2007; Fig. 1, C and I, Fig. 2 F, and Fig. 3 A). However, very high differences in baseline NOS2 levels between human memory and naive CD4+ T cells (mean ± SD: naive, 0.00003 ± 0.00002 vs. memory, 0.0006 ± 0.0002; P = 0.0053) could not be seen in their murine counterparts, where both memory and naive CD4+ T cells expressed similarly high baseline levels of NOS2 (naïve, 0.0034 ± 0.001 vs. memory, 0.0032 ± 0.001; P = not significant), and were not significantly modulated in the presence of Th17-promoting cytokines (mouse naive, 0.0048 ± 0.001 vs. memory, 0.005 ± 0.001; P = not significant). These observations are consistent with the previously reported differences in the regulation of mouse and human immune system by NO (Fang, 2004; Schneemann and Schoeden, 2007; Schneemann and Schoedon, 2002), different susceptibility of human and mouse naive CD4+ T cells to Th17-inducing factors (Acosta-Rodriguez et al., 2007a; Acosta-Rodriguez et al., 2007b; van Beelen et al., 2007), and the recently reported lack of positive impact of exogenous NO on mouse CD4+ T cells at any concentrations of NO donor (Niedbala et al., 2011; and out current mouse data [not depicted]) .
Persistent expression of endogenous NOS2 and persistent cGMP-signaling are required for the functional stability of Th17 cells: Reversal of established Th17 cells from cancer patients by NOS- and cGMP-inhibitors
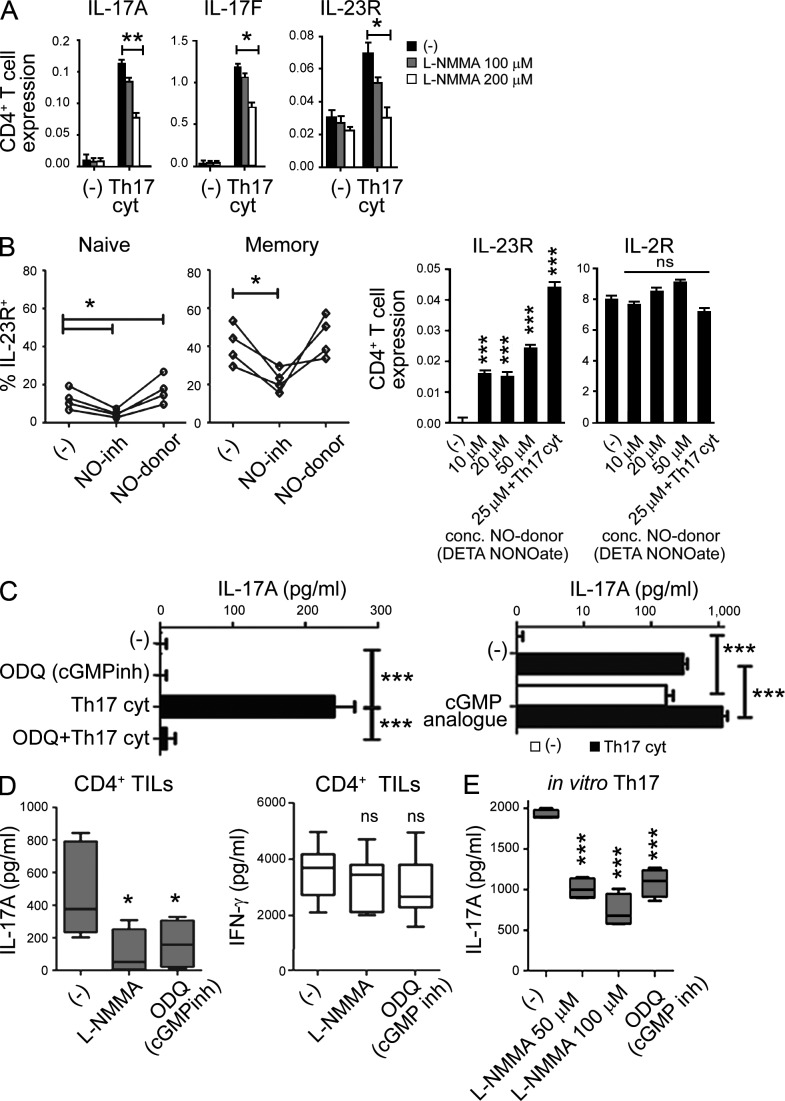
Interestingly, NOS2 blockade in CD4+ T cell cultures activated in the presence of Th17-driving cytokines revealed that endogenous NO is not only required for the induction of IL-17A and IL-17F expression, but also for the optimal expression of IL-23 receptor (Fig. 4, A and B), known to be essential for the maintenance of Th17 function (Stritesky et al., 2008). Indeed, NO induced IL-23R expression on naive CD4+ T cells, which express significantly less IL-23R than memory CD4+ T cells (Fig. 4 B, left). In contrast to IL-23R, no impact of NO on IL-2R expression was observed (Fig. 4 B, right). Furthermore, NOS inhibition reduced IL-23R expression by memory CD4+ T cells (Fig. 4 B), suggesting the requirement for NO in the optimal delivery of IL-23–mediated signals, which may contribute to the persistence of the Th17 phenotype.
Figure 4.
Endogenous NOS2 and persistent cGMP signaling are required for the NO-assisted de novo induction of Th17 cells and for the stability of human in vivo-developed Th17 cells from cancer patients. (A) Relative gene expression of IL-17A, IL-17F, and IL-23R in bulk CD4+ T cells, expanded with anti-CD3/CD28 in the absence or presence of Th17-driving cytokines and general NOS inhibitor (L-NMMA). The graphs present the mean ± SD from a representative experiment (triplicate cultures) of two (using different patients/healthy donors), that both yielded similar results. (B) Regulation of (left) surface IL-23R expression on naive and memory CD4+ T cells (mean ± SD from four healthy donors) activated with anti-CD3/CD28 in the presence of NOS inhibitor (L-NMMA) or NO donor (DETA-NONOate). (right) Relative gene expression of IL-23R and IL-2R (right) in naive CD4+ T cells in the presence of increasing concentrations of NO donor and Th17-driving cytokines. Statistically significant differences compared with the absence of DETA-NONOate and Th17-driving cytokines are indicated. The graphs present the mean ± SD from a representative experiment (performed with triplicate cultures). The results were confirmed in three independent experiments using different healthy donors. (C) IL-17A production by naive CD4+ T cells stimulated with anti-CD3/CD28 antibodies in the absence or presence of cGMP inhibitor (ODQ, left) or supplemented with cGMP analogue (Br-cGMP, right) in the absence or presence of Th17-driving cytokines. The graphs present the mean ± SD from a representative experiment (triplicate cultures). The results were confirmed in three independent experiments using different healthy donors. (D) IL-17A (left) or IFN-γ (right) production by OvCa-isolated CD4+ TILs (mean ± SD from five patients), expanded with anti-CD3/CD28 antibodies and restimulated in the absence or presence of NOS inhibitor (L-NMMA) or cGMP inhibitor (ODQ) for 48 h (statistically significant differences compared with the absence of inhibitors are indicated). (E) IL-17A production by in vitro–generated Th17 cells (generated in 8 d cultures of CD4+ T cells stimulated with anti-CD3/CD28 in the presence of Th17-driving cytokines), pretreated or not for 48 h with NOS inhibitor (L-NMMA) or cGMP inhibitor (ODQ). The data are shown as mean ± SD from 4 healthy donors. Statistically significant differences compared with condition in the absence of inhibitors are indicated. ns, P > 0.05; *, P < 0.05; **, P < 0.01; ***, P < 0.001.
NO has been shown to signal predominantly via the cGMP–cGK signaling cascade (Fischer et al., 2001), whereas high concentrations of NO involve additional NO-dependent modification of a wider spectrum of endogenous proteins and nonspecific cytotoxic effects (de Vera et al., 1996; Thomas et al., 2004). To define the pathway of NO signaling relevant to Th17 induction, and to identify new targets for therapeutic modulation of Th17 responses and Th17-dependent pathologies, we evaluated the role of cGMP–cGK signaling in these phenomena. Our data show that the cGMP-specific inhibitor ODQ blocks IL-17A production in naive CD4+ T cells activated in the presence of Th17-driving cytokines, whereas the addition of a membrane-permeable cGMP analogue alone can induce IL-17A production and further increase the production of IL-17A induced by Th17-driving cytokines (Fig. 4 C).
Both NO and the cGMP signaling cascade proved to be required for the stability of established Th17 cells that developed in cancer patients (OvCa TILs) in vivo or were generated from healthy donors in vitro, because inhibition of either NOS or cGMP selectively suppressed IL-17A but not IFN-γ production by these cells (Fig. 4, D and E). These data indicate that targeting of NO-activated cGMP–cGK signaling can be evaluated for therapeutic intervention in Th17-mediated disorders.
DISCUSSION
Prompted by the correlation between the local expression of IL-17A and NOS2 observed in the tumor environment of patients with OvCa, we tested the role of the NOS2–NO–cGMP–cGK pathway in the development of Th17 cells from human naive, memory, and tumor-infiltrating CD4+ T cells. We observed that endogenous NOS2 activity and intracellular NO production induced in CD4+ T cells by previously identified Th17 inducers (IL-1β, IL-6, and IL-23) or by cancer-infiltrating MDSCs are critically required for the de novo induction of Th17 cells in vitro and for the stability of in vivo–arising Th17 cells from cancer patients. Although, in accordance with previous studies (Kolb and Kolb-Bachofen, 1998; Bogdan, 2001; Mahidhara et al., 2003; Niedbala et al., 2011), high concentrations of exogenous NO nonselectively inhibited immune activation, including the proliferation and differentiation of Th17 cell precursors, the levels of NO produced by myeloid cells, and, endogenously, by CD4+ T cells supported the induction of Th17 responses and were essential for the functional stability of Th17 cells. These results help to explain the heterogeneous and often paradoxical effects of NO and Th17 cells in the regulation of inflammation, autoimmunity, and cancer (Bogdan, 2001).
The current data and the previously reported importance of COX2 and prostaglandin E2 (PGE2) in the induction and stability of NOS2 production by MDSCs (Obermajer et al., 2011) and the ability of NO to enhance COX2 activation (Kim et al., 2005) indicate a close interplay between these two inflammatory systems, which may provide new insights to the mechanism of the COX2–PGE2-driven development of Th17 responses to different pathogens (Chizzolini et al., 2008; Boniface et al., 2009; Gopal et al., 2012) and potential new therapeutic targets.
Interestingly, although MDSCs suppress naive CD8+ T cell proliferation and acquisition of cytolytic functions (Kusmartsev et al., 2004; Gabrilovich and Nagaraj, 2009; Nagaraj and Gabrilovich, 2010; Obermajer et al., 2011), they do not impair naive CD40L-expressing CD4+ T cell proliferation, but instead promote the de novo induction of Th17 cells, an effect that may explain the paradoxic generation and presence of inflammatory Th17 cells in the immunosuppressive cancer-associated environment (Coussens and Werb, 2002; Mantovani et al., 2008; Kryczek et al., 2009; Martin-Orozco and Dong, 2009).
Th17 cells have been shown to have differential impact on cancer progression in different cancers and in different mouse models (Zou and Restifo, 2010; Wilke et al., 2011; Grivennikov et al., 2010; Lança and Silva-Santos, 2012; Coussens et al., 2013). Their preferential ability to be attracted to tumor sites and subsequently promote local recruitment of other inflammatory cells or convert to Th1 cells can lead to the enhancement of local antitumor immunity in particularly advanced tumors (Benchetrit et al., 2002; Muranski et al., 2008; Hinrichs et al., 2009; Kryczek et al., 2009; Martin-Orozco et al., 2009), which may explain the positive correlation of intratumoral Th17 numbers and local production of IL-17 with survival in OvCa patients (Kryczek et al., 2009; Wilke et al., 2011). However, in several models, Th17 cells have also been shown to drive early tumorigenesis by promoting chronic inflammation, DNA damage, and tumor-associated angiogenesis, as well as by exhibiting potent suppressive activity (Numasaki et al., 2003; Langowski et al., 2006; Wang et al., 2009; Wu et al., 2009; Charles et al., 2009; Grivennikov et al., 2012). Further studies are needed to understand the regulation and functional relevance of these different Th17 activities in the tumor environment.
Our data also indicate that in analogy to the positive feedback loop between Th1 cells and IFN-γ–producing NK and CD8+ T cells (Kalinski and Moser, 2005), Th17 cells not only promote NO-dependent effector mechanisms of immunity (Miljkovic and Trajkovic, 2004), but also benefit from interaction with NO-producing cells (neutrophils, macrophages, and MDSCs), leading to the establishment of a positive feedback between such NO- and IL-17–producing effector and helper cells.
Our observations demonstrating that the stability of Th17 function requires endogenous NOS2 and that the induction of endogenous NOS2 in CD4+ T cells benefits from the synergy between the previously identified Th17-driving cytokines (IL-1β, IL-6, and IL-23; Fig. 3 G) help to explain the paradox that whereas the synergy between IL-1β and IL-6 (or other Stat3 inducers) is sufficient for the effective induction of Th17 cells (Bettelli et al., 2007; Guo et al., 2009), IL-23 signaling is an important component of the development of the Th17 phenotype and Th17 functions in most models (Veldhoen et al., 2006; Bettelli et al., 2006; Ivanov et al., 2006; Acosta-Rodriguez et al., 2007a,b; van Beelen et al., 2007; Wilson et al., 2007; Zhou et al., 2007). Similar to previous studies (Acosta-Rodriguez et al., 2007a; Wilson et al., 2007), we observed that TGF-β1 suppresses the development of human Th17 cells (Fig. 3 G), an effect that is mirrored by the ability of TGF-β1 to suppress endogenous NOS2 in CD4+ T cells. These data suggest that the differences in the relative importance of TGF-β1 in the development of mouse Th17 cells in different models (Bettelli et al., 2006; Mangan et al., 2006; Veldhoen et al., 2006; Zhou et al., 2007; Ghoreschi et al., 2010) may need to be evaluated in the context of its impact on the production of endogenous (T cell–derived) and exogenous (produced by MDSCs and other myeloid or stromal cells) NO, which may potentially vary in different settings. Likewise, fundamental differences between mice and humans regarding NOS activity, NO production, and NO regulation (Schneemann and Schoedon, 2002; Fang, 2004; Schneemann and Schoeden, 2007; this study) may explain different requirements for TGF-β1 in the development of Th17 cells in mice versus humans. Interestingly, mouse Th17 cells induced by TGF-β1 and IL-6 have been shown to suppress T cell effector functions, whereas mouse Th17 cells differentiated with IL-1β, IL-6, and IL-23 are not immunosuppressive (Chalmin et al., 2012), highlighting the difficulties in cross-interpreting the results of mouse and human studies involving the interplay of Th17 cells and cancer.
Similarly, it remains to be seen if such TLR ligands as LPS or peptidoglycan, shown to be particularly effective in promoting the DC-mediated induction of human Th17 cells (Acosta-Rodriguez et al., 2007a; van Beelen et al., 2007), are particularly effective in inducing DC production of NO or alternative activators of the cGMP/cGK-mediated signaling pathway. Whether potential differences in this regard may contribute to the different efficiency of induction of Th17 cells from naive versus memory and effector precursors (van Beelen et al., 2007; this study) deserves further exploration.
Our data suggest that the previously observed differences in the ability of human memory and naive CD4+ T cells to develop into Th17 cells (van Beelen et al., 2007; Fig. 1 C and Fig. 2 F) may, at least partially, result from much higher baseline levels of endogenous NOS2 in human memory CD4+ T cells, compared with naive CD4+ T cells (Fig. 3 A). Our preliminary data indicate that a similar difference does not exist in the mouse system, where both naive and memory cells express very high baseline levels of NOS2, and do not further elevate its levels during Th17 differentiation (unpublished data). These differences in the baseline levels of NOS2 between human and mouse cells and the requirement for inflammatory factors in the expression of NOS2 by human cells are consistent with the significant delay in the demonstration of the presence of NOS2 in human cells (Hibbs et al., 1992; Ochoa et al., 1992; Geller et al., 1993) and its cloning (Geller et al., 1993).
The current identification of NO, NOS/NOS2, and the cGMP–cGK-signaling pathway as critical requirements for the induction and stability of human Th17 cells, both arising in vivo in cancer-bearing patients and induction in vitro from naive and memory precursor cells from healthy donors, suggests several potential therapeutic strategies. These strategies include inhibition of NO production or signaling in Th17-dependent malignant tumors or in Th17-mediated inflammatory/autoimmune processes, or the activation of these pathways to boost desirable Th17 immunity in Th17-susceptible tumors or chronic infections, such as M. tuberculosis. Because Th17 cells have a high propensity to migrate to and accumulate in tumor lesions, the current demonstration that NOS2 blockade can revert Th17 cells into cells preferentially producing IFN-γ suggests that the sequential application of NO donors or NO-increasing factors in vivo (or ex vivo to prepare tumor-homing T cells for adoptive immunotherapy) followed by systemic NOS2 inhibition (to promote their transition from Th17 to Th1 cells) may result in particularly high therapeutic effectiveness, promoting T cell accumulation at tumor sites and their subsequent conversion to type-1 effector cells. The feasibility of such approaches is enhanced by the availability of large numbers of NO donors and inhibitors that have been evaluated for the treatment of autoimmune and inflammatory diseases, as well as for vasodilation in hypertensive coronary disease, erectile dysfunction, and pulmonary hypertension.
MATERIALS AND METHODS
Media and reagents.
Cells were cultured in IMDM medium (Invitrogen) with 10% FCS (Gemini). DETA-NONOate was purchased from Cayman Chemical and used at the concentration of 25 µM, unless otherwise specified. General NOS inhibitors l-NMMA (Sigma-Aldrich) and ADMA (Sigma-Aldrich) and the NOS2-specific inhibitor 1400W (Sigma-Aldrich) were used at the concentrations of, respectively, 100 µM, 200 µM, and 200 nM, unless indicated otherwise. Arginase inhibitor nor-NOHA (Cayman Chemical) was used at 200 µM, IDO inhibitor 1-Methyl-D-tryptophan (Sigma-Aldrich) was used at 1 mM, neutralizing α-IL-10 mAb (R&D Systems; clone 25209) was used at 1.0 µg/ml, COX2 inhibitor celecoxib (Biovision) was used at 20 µM, c-GMP analogue Br-cGMP (Sigma-Aldrich) was used at 100 µM, and cGMP inhibitor ODQ was used at 10 µM. Th1, Th17, and T reg cell–driving cytokines were used at the following concentrations (unless stated otherwise): 20 ng/ml IL-1β (Miltenyi Biotec), 50 ng/ml IL-6 (Thermo Fisher Scientific), 10 ng/ml IL-23 (R&D Systems), 5 ng/ml TGF-β1 (R&D Systems), and 10 nM 9-cis retinoic acid (Sigma-Aldrich). CFSE (Invitrogen) labeling kit to monitor cell proliferation was used according to the manufacturer’s protocol. CD3/CD28 stimulation was accomplished with anti-CD3/CD28 human or mouse T cell-activator Dynabeads (at 2 µl/ml; Invitrogen). Soluble (s)CD40L was used at 1 µg/ml in combination with 1 µg/ml of Enhancer for Ligands (Enzo Life Sciences). Nitrite formed by the spontaneous oxidation of NO under physiological conditions in cell culture supernatants was detected with the Griess reagent kit (Invitrogen) according to the manufacturer’s protocol.
Isolation of peripheral blood naive and memory human CD4+ T cells and mouse splenic CD4+ T cells.
Human PBMCs were isolated from buffy coats provided by the Central Blood Bank of Pittsburgh, PA. T cells were isolated from PBMCs by negative selection using the CD4+ T cell enrichment cocktail (STEMCELL Technologies) in combination with either anti-CD45RO or CD45RA depletion antibodies, resulting in a >95% pure CD3+ population of uniform CD4+CD45RA+/−CD45RO+/− cells. Allogeneic combinations of T cells and MDSCs were used to allow testing of the impact of tumor-derived MDSCs (or control blood-isolated CD11b+ cells) on the differentiation of healthy donor naive or memory blood-isolated T cells.
Mouse naive and memory CD4+ T cells were isolated from the spleens of C57BL/6 (B6) mice using naive and memory CD4+ T cell isolation kits (Miltenyi Biotec). 6–8-wk-old wild-type B6 mice were purchased from Taconic. Specific pathogen–free mice were used in all experiments and housed in pathogen-free conditions at Children’s Hospital of Pittsburgh, Pennsylvania. All of the animal studies were conducted with the approval of the University of Pittsburgh Institutional Animal Care and Use Committee.
Isolation of MDSCs and OvCa-infiltrating CD4+ T cells (TILs).
Human OvCa ascites cells were obtained intraoperatively from previously untreated patients with primarily advanced stage III or IV epithelial OvCa, after obtaining written informed consent. The nature and possible consequences of the studies were explained. All specimens were provided under protocols approved by the University of Pittsburgh or Roswell Park Cancer Institute Institutional Review Boards. Human OvCa ascites obtained from the University of Pittsburgh (IRB0406147) were used in the isolation of cancer-associated CD11b+ cells (MDSCs) and subsequent isolation of CD4+ TILs. The median age of patients was 56 yr of age (range 39–69 yr). 12 patients were Caucasian, and 1 patient was African-American. The majority of patients were FIGO Stage IIIC, one patient was Stage IIIA, and one patient was Stage IIA. Tumor histology was serous in 9 cases (69.2%), clear cell in 2 cases (15.4%), mucinous in 1 case (7.7%), and mixed histology in 1 case (7.7%). Human OvCa ascites obtained from the Roswell Park Cancer Institute (CIC02-15) were used in the isolation of bulk OvCa primary cells and their CD3/CD28-driven expansion for 7 d in culture. The median age of patients was 64 (range 50–85). Nine patients were Caucasian and one was Hispanic. The majority of patients were FIGO Stage IIIC, and three patients were Stage IV. Tumor histology was serous in 7 cases (70%), papillary serous in 2 cases (20.0%), and mixed histology in 1 case (10%).
OvCa primary cells were harvested by centrifugation. CD11b+ cells (i.e., MDSCs) were obtained after centrifugation of OvCa ascites, followed by red blood cell lysis and positive magnetic selection of CD11b+ cells (CD11b EasySep Isolation kit; STEMCELL Technologies). The isolated cells were >95% CD11b+ and uniformly expressed the CD11b+CD33+CD34+ MDSC phenotype (Obermajer et al., 2011). CD4+ T cells (TILs) were obtained after positive magnetic selection of CD11b+ cells followed by negative selection using the CD4+ T cell enrichment cocktail (STEMCELL Technologies). Control CD11b+ cells were isolated from healthy donor buffy coats, using the same method.
Th17 cell generation.
T cells were stimulated with anti-CD3/CD28 Dynabeads (2.0 µl/ml; Invitrogen) in the presence or absence of allogeneic OvCa-isolated MDSCs or control CD11b+ cells, pretreated or not with inhibitors, and/or in the presence of the Th17-inducing cytokine cocktail: 20 ng/ml IL-1β, 10 ng/ml IL-23, 50 ng/ml IL-6, and/or 10 ng/ml TGF-β1. All experiments used 105 T cells per well at a concentration of 5 × 105 cells/ml. All experiments in this study used the 1:4 ratio of MDSC (or control CD11b+ cells) to T cells determined to be optimal based on our preliminary experiments (which tested the MDSC:T cell ratios of 1:1, 1:2, 1:4, and 1:8). As an alternative to stimulation with anti-CD3/CD28 beads, T cells were stimulated with mature DCs (mDCs; monocytes were isolated by positive magnetic selection using the EasySep CD14+ isolation kit [STEMCELL Technologies] and cultured for 6 d in 24-well plates [BD] in the presence of rhuGM-CSF and IL-4 [both 1,000 U/ml; gifts from Schering Plough] and afterward matured for 48 h with TNF), with a DC/T cell ratio of 1:10. On days 4–6, expanded T cells were analyzed for the expression of Th17-associated factors (mRNA expression) and cytokine secretion (ELISA). On day 7–8, expanded T cells were assayed for intracellular NOS2 and NO, and were further restimulated for intracellular cytokine staining. The purity of anti-CD3/CD28–activated T cell cultures increased from an initial >95% to >99% by the time of analysis, as determined by flow cytometry. Note that, consistent with the key role of IL-1β and IL-6, and the negative role of TGF-β1, in the induction of human Th17 cells (Acosta-Rodriguez et al., 2007a; Wilson et al., 2007), and a similar recently reported synergy between IL-1β and other Stat3 activators (Guo et al., 2009), the combination of IL-1β with IL-6 and/or IL-23 was sufficient for the optimal induction of IL-17A production, with TGF-β1 having a negative effect (Fig. 3 G).
ELISA.
ELISA analysis was performed for IL-17A (R&D) and IFN-γ secretion by day 5 expanded T cells in culture [or by day 7–8 expanded T cells washed and replated at 106 cells/ml and restimulated with anti-CD3/CD28 beads for 24–48 h]. ELISA analysis of IL-23 production by OvCa ascites-isolated MDSCs or control blood-isolated CD11b+ cells was performed after 24 h stimulation with CD40L-expressing J558 cells [or sCD40L (Enzo Life Sciences)] or CD4+ T cells. 24 h-conditioned medium from OvCa ascites-isolated MDSCs or control blood-isolated CD11b+ cells were analyzed for IL-6, IL-10, IL-23, IL-1β, and TGF-β1 by sandwich ELISA (R&D).
Flow cytometry.
Two- and three-color cell surface and intracellular immunostaining analyses were performed using an Accuri C6 flow cytometer. OvCa ascites-isolated cells were stained with the antibodies CD11b-FITC, CD33-APC, CD34-PE/Cy7, HLA-DR-FITC, CD14-PE, CD80-FITC, CD83-PE, CD15-PE, and CD8-FITC (BD and eBioscience; Obermajer et al., 2011). IL-23R was detected with IL-23R-FITC mAb (R&D Systems). Rat IgG2α-PE, IgG1-FITC, IgG1-APC, and IgG1-PE/Cy7 isotype controls, and the rat IgG2α-FITC isotype control, were from BD.
Intracellular staining.
Cells were harvested, fixed, and permeabilized using the Foxp3 Fix/Perm Buffer Set solution (eBioscience). For intracellular cytokine production only, T cells were stimulated with PMA (50 ng/ml; Sigma-Aldrich) and ionomycin (1 µg/ml; Sigma-Aldrich), and after 4 h, brefeldin A (10 µg/ml) was added for an additional 4–10 h before staining. The following antibodies were used: IFN-γ-FITC and IL-17A-PE (eBio64DEC17; eBioscience), Foxp3-Alexa Fluor 488 (BioLegend), CD3 (unlabeled monoclonal mouse anti–human CD3 [eBioscience] followed by secondary goat anti–mouse IgG F[ab′]2-Alexa Fluor 647 [Cell Signaling Technology]), and NOS2 (unlabeled polyclonal rabbit anti–human NOS2 [Millipore] followed by secondary goat anti–rabbit IgG F[ab′]2-Alexa Fluor 488 [Cell Signaling Technology]). Stainings (including for both primary and secondary antibodies, where appropriate) were performed at room temperature for 30 min, and then washed and resuspended in FACS buffer before analysis. For intracellular NO detection, DAF-FM diacetate (Molecular Probes) was used, which passively diffuses across cell membranes and is deacetylated by intracellular esterases to membrane-impermeant DAF-FM, which then reacts with NO to form a fluorescent benzotriazole with excitation/emission maxima of 495/515 nm (Nagy et al., 2003). DAF-FM diacetate was loaded at 10 µM for 2 h at 37°C.
Confocal microscopy.
T cells were harvested and directly centrifuged onto 12-mm-diam circular glass coverslips (Propper) coated for 1 h at 37°C with 0.005% human fibronectin (Sigma-Aldrich) in PBS in 24-well plates. The coverslips were then incubated in 4% paraformaldehyde for 15 min, washed with PBS, and incubated for 1 h at room temperature in staining buffer containing 0.3% Triton X-100 (Sigma-Aldrich), 5% goat serum (Life Technologies), and 1% BSA (Thermo Fisher Scientific) in PBS. The slides were then incubated for 3 h at room temperature with staining buffer containing unlabeled primary antibodies for NOS2 (Millipore) and CD3 (eBioscience), washed with PBS, and incubated for 30 min at room temperature with staining buffer containing the secondary antibodies anti–rabbit Alexa Fluor 488 and anti–mouse Alexa Fluor 647 (Cell Signaling Technology). Coverslips were washed with PBS and mounted on SuperFrost Plus Slides (Thermo Fisher Scientific) using ProLong Gold antifade reagent (Invitrogen). Confocal analyses were conducted using a Leica TCS SL DMRE Microsystem.
TaqMan analysis of mRNA expression.
mRNA expression was analyzed in day 7–8 anti-CD3/CD28–expanded OvCa primary cell cultures, and in day 4–6–expanded CD4+ TILs and naive and memory CD4+ T cells. TaqMan analysis was performed on the StepOne Plus System (Applied Biosystems) using TaqMan-recommended inventoried or made-to-order assays (Gene IDs: il17a:Hs00174383, il17f:Hs01028648, il2rα:Hs00907779, il23r:Hs00332759, nos2:Hs01075527, rorc:Hs01076112, tbet:Hs00203436, gata3:NM_002051, and foxp3:Hs00203958). The expression of each gene was normalized to HPRT1 and expressed as relative expression, i.e., fold increase (2−ΔCT), where ΔCT = CT(target gene) − CT(HPRT1).
Statistical analysis.
The figures demonstrating key phenomena and critical mechanisms involve aggregate data from multiple patients and healthy donors (expressed as means ± SD; the donor numbers are provided in the individual figure panels and the corresponding legends). Data from representative experiments (typically used in the studies comparing different reagents or different concentrations) were obtained from replicate cultures (means ± SD; numbers of replicates provided in figure legends) with each experiment confirmed in additional independent experiments with cells from different donors, as indicated in the figure legends. All data were evaluated using GraphPad Prism Version 5 software and analyzed using Student’s t test (two-tailed) and 1-way and 2-way analysis of variance, where appropriate, with P < 0.05 considered as significant (*, P < 0.05; **, P < 0.01; ***, P < 0.001). A linear correlation between two continuous variables was tested with the r2 coefficient of determination.
Acknowledgments
The authors thank Drs. Olja Finn, Yoram Vodovotz, and Grant Gallagher for their critical reading of the manuscript and comments.
This work was supported by grants from the National Institutes of Health (1P01 CA132714; 1F30 CA165410; 5T32 CA082084), GYNCOE Funds, and by a UICC American Cancer Society Beginning Investigators Fellowship funded by the American Cancer Society.The authors have no conflicting financial interests.
N. Obermajer and P. Kalinski designed the study, analyzed the data, and prepared the manuscript; N. Obermajer performed the experiments; J.L. Wong participated in designing the study, performing the experiments, analyzing the data, and preparing the manuscript; K. Chen participated in performing the experiments; M. Scott and T.R. Billiar provided reagents and expertise and participated in study design and manuscript preparation; S. Khader and J.K. Kolls provided expertise and participated in manuscript preparation; K. Odunsi and R.P. Eedwards provided critical expertise and clinical material and participated in manuscript preparation.
Footnotes
Abbreviations used:
- cGK
- cGMP-dependent protein kinase
- cGMP
- cyclic guanosine monophosphate
- COX
- cyclooxygenase
- CTL
- cytotoxic T lymphocyte
- FoxP3
- forkhead box P3
- IDO
- indoleamine 2,3-dioxygenase
- MDSC
- myeloid-derived suppressor cell
- NO
- nitric oxide
- NOS
- nitric oxide synthase
- OvCa
- ovarian cancer
- PGE2
- prostaglandin E2
- Rorc/RORγt
- retinoid-related orphan receptor gamma t
- ROS
- reactive oxygen species
- sCD40L
- soluble CD40L
- TIL
- tumor-infiltrating T cell
- Treg
- regulatory T cell
References
- Acosta-Rodriguez E.V., Napolitani G., Lanzavecchia A., Sallusto F. 2007a. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat. Immunol. 8:942–949 10.1038/ni1496 [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez E.V., Rivino L., Geginat J., Jarrossay D., Gattorno M., Lanzavecchia A., Sallusto F., Napolitani G. 2007b. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat. Immunol. 8:639–646 10.1038/ni1467 [DOI] [PubMed] [Google Scholar]
- Benchetrit F., Ciree A., Vives V., Warnier G., Gey A., Sautès-Fridman C., Fossiez F., Haicheur N., Fridman W.H., Tartour E. 2002. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 99:2114–2121 10.1182/blood.V99.6.2114 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. 2006. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 441:235–238 10.1038/nature04753 [DOI] [PubMed] [Google Scholar]
- Bettelli E., Oukka M., Kuchroo V.K. 2007. T(H)-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8:345–350 10.1038/ni0407-345 [DOI] [PubMed] [Google Scholar]
- Bogdan C. 1998. The multiplex function of nitric oxide in (auto)immunity. J. Exp. Med. 187:1361–1365 10.1084/jem.187.9.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C. 2001. Nitric oxide and the immune response. Nat. Immunol. 2:907–916 10.1038/ni1001-907 [DOI] [PubMed] [Google Scholar]
- Bogdan C. 2011. Regulation of lymphocytes by nitric oxide. Methods Mol. Biol. 677:375–393 10.1007/978-1-60761-869-0_24 [DOI] [PubMed] [Google Scholar]
- Boniface K., Bak-Jensen K.S., Li Y., Blumenschein W.M., McGeachy M.J., McClanahan T.K., McKenzie B.S., Kastelein R.A., Cua D.J., de Waal Malefyt R. 2009. Prostaglandin E2 regulates Th17 cell differentiation and function through cyclic AMP and EP2/EP4 receptor signaling. J. Exp. Med. 206:535–548 10.1084/jem.20082293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boughton-Smith N.K., Evans S.M., Hawkey C.J., Cole A.T., Balsitis M., Whittle B.J., Moncada S. 1993. Nitric oxide synthase activity in ulcerative colitis and Crohn’s disease. Lancet. 342:338–340 10.1016/0140-6736(93)91476-3 [DOI] [PubMed] [Google Scholar]
- Brahmachari S., Pahan K. 2010. Myelin basic protein priming reduces the expression of Foxp3 in T cells via nitric oxide. J. Immunol. 184:1799–1809 10.4049/jimmunol.0804394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronte V., Zanovello P. 2005. Regulation of immune responses by l-arginine metabolism. Nat. Rev. Immunol. 5:641–654 10.1038/nri1668 [DOI] [PubMed] [Google Scholar]
- Bronte V., Serafini P., Mazzoni A., Segal D.M., Zanovello P. 2003. l-arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends Immunol. 24:302–306 10.1016/S1471-4906(03)00132-7 [DOI] [PubMed] [Google Scholar]
- Chalmin F., Mignot G., Bruchard M., Chevriaux A., Végran F., Hichami A., Ladoire S., Derangère V., Vincent J., Masson D., et al. 2012. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 36:362–373 10.1016/j.immuni.2011.12.019 [DOI] [PubMed] [Google Scholar]
- Charles K.A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R.G., Kollias G., Smyth J.F., et al. 2009. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Invest. 119:3011–3023 10.1172/JCI39065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizzolini C., Chicheportiche R., Alvarez M., de Rham C., Roux-Lombard P., Ferrari-Lacraz S., Dayer J.M. 2008. Prostaglandin E2 synergistically with interleukin-23 favors human Th17 expansion. Blood. 112:3696–3703 10.1182/blood-2008-05-155408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., Dong C. 2009. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 30:576–587 10.1016/j.immuni.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Werb Z. 2002. Inflammation and cancer. Nature. 420:860–867 10.1038/nature01322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L.M., Zitvogel L., Palucka A.K. 2013. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 339:286–291 10.1126/science.1232227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culotta E., Koshland D.E., Jr 1992. NO news is good news. Science. 258:1862–1865 10.1126/science.1361684 [DOI] [PubMed] [Google Scholar]
- de Vera M.E., Shapiro R.A., Nussler A.K., Mudgett J.S., Simmons R.L., Morris S.M., Jr, Billiar T.R., Geller D.A. 1996. Transcriptional regulation of human inducible nitric oxide synthase (NOS2) gene by cytokines: initial analysis of the human NOS2 promoter. Proc. Natl. Acad. Sci. USA. 93:1054–1059 10.1073/pnas.93.3.1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcetti L., Peranzoni E., Ugel S., Marigo I., Fernandez Gomez A., Mesa C., Geilich M., Winkels G., Traggiai E., Casati A., et al. 2010. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur. J. Immunol. 40:22–35 10.1002/eji.200939903 [DOI] [PubMed] [Google Scholar]
- Fang F.C. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820–832 10.1038/nrmicro1004 [DOI] [PubMed] [Google Scholar]
- Farrell A.J., Blake D.R., Palmer R.M., Moncada S. 1992. Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann. Rheum. Dis. 51:1219–1222 10.1136/ard.51.11.1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng G., Gao W., Strom T.B., Oukka M., Francis R.S., Wood K.J., Bushell A. 2008. Exogenous IFN-gamma ex vivo shapes the alloreactive T-cell repertoire by inhibition of Th17 responses and generation of functional Foxp3+ regulatory T cells. Eur. J. Immunol. 38:2512–2527 10.1002/eji.200838411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipazzi P., Valenti R., Huber V., Pilla L., Canese P., Iero M., Castelli C., Mariani L., Parmiani G., Rivoltini L. 2007. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 25:2546–2553 10.1200/JCO.2006.08.5829 [DOI] [PubMed] [Google Scholar]
- Fischer T.A., Palmetshofer A., Gambaryan S., Butt E., Jassoy C., Walter U., Sopper S., Lohmann S.M. 2001. Activation of cGMP-dependent protein kinase Ibeta inhibits interleukin 2 release and proliferation of T cell receptor-stimulated human peripheral T cells. J. Biol. Chem. 276:5967–5974 10.1074/jbc.M009781200 [DOI] [PubMed] [Google Scholar]
- Gabrilovich D.I., Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9:162–174 10.1038/nri2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geller D.A., Lowenstein C.J., Shapiro R.A., Nussler A.K., Di Silvio M., Wang S.C., Nakayama D.K., Simmons R.L., Snyder S.H., Billiar T.R. 1993. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc. Natl. Acad. Sci. USA. 90:3491–3495 10.1073/pnas.90.8.3491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K., Laurence A., Yang X.P., Tato C.M., McGeachy M.J., Konkel J.E., Ramos H.L., Wei L., Davidson T.S., Bouladoux N., et al. 2010. Generation of pathogenic T(H)17 cells in the absence of TGF-β signalling. Nature. 467:967–971 10.1038/nature09447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal R., Lin Y., Obermajer N., Slight S., Nuthalapati N., Ahmed M., Kalinski P., Khader S.A. 2012. IL-23-dependent IL-17 drives Th1-cell responses following Mycobacterium bovis BCG vaccination. Eur. J. Immunol. 42:364–373 10.1002/eji.201141569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Greten F.R., Karin M. 2010. Immunity, inflammation, and cancer. Cell. 140:883–899 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov S.I., Wang K., Mucida D., Stewart C.A., Schnabl B., Jauch D., Taniguchi K., Yu G.Y., Osterreicher C.H., Hung K.E., et al. 2012. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 491:254–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Wei G., Zhu J., Liao W., Leonard W.J., Zhao K., Paul W. 2009. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc. Natl. Acad. Sci. USA. 106:13463–13468 10.1073/pnas.0906988106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J.B., Jr, Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R.L., Ward J.H., Menlove R.L., McMurry M.P., Kushner J.P., et al. 1992. Evidence for cytokine-inducible nitric oxide synthesis from l-arginine in patients receiving interleukin-2 therapy. J. Clin. Invest. 89:867–877 10.1172/JCI115666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinrichs C.S., Kaiser A., Paulos C.M., Cassard L., Sanchez-Perez L., Heemskerk B., Wrzesinski C., Borman Z.A., Muranski P., Restifo N.P. 2009. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 114:596–599 10.1182/blood-2009-02-203935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechst B., Ormandy L.A., Ballmaier M., Lehner F., Krüger C., Manns M.P., Greten T.F., Korangy F. 2008. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 135:234–243 10.1053/j.gastro.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Hooper D.C., Bagasra O., Marini J.C., Zborek A., Ohnishi S.T., Kean R., Champion J.M., Sarker A.B., Bobroski L., Farber J.L., et al. 1997. Prevention of experimental allergic encephalomyelitis by targeting nitric oxide and peroxynitrite: implications for the treatment of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 94:2528–2533 10.1073/pnas.94.6.2528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B., Pan P.Y., Li Q., Sato A.I., Levy D.E., Bromberg J., Divino C.M., Chen S.H. 2006. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66:1123–1131 10.1158/0008-5472.CAN-05-1299 [DOI] [PubMed] [Google Scholar]
- Hussain S.P., Hofseth L.J., Harris C.C. 2003. Radical causes of cancer. Nat. Rev. Cancer. 3:276–285 10.1038/nrc1046 [DOI] [PubMed] [Google Scholar]
- Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133 10.1016/j.cell.2006.07.035 [DOI] [PubMed] [Google Scholar]
- Kalinski P., Moser M. 2005. Consensual immunity: success-driven development of T-helper-1 and T-helper-2 responses. Nat. Rev. Immunol. 5:251–260 10.1038/nri1569 [DOI] [PubMed] [Google Scholar]
- Karpuzoglu E., Ahmed S.A. 2006. Estrogen regulation of nitric oxide and inducible nitric oxide synthase (iNOS) in immune cells: implications for immunity, autoimmune diseases, and apoptosis. Nitric Oxide. 15:177–186 10.1016/j.niox.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Kim S.F., Huri D.A., Snyder S.H. 2005. Inducible nitric oxide synthase binds, S-nitrosylates, and activates cyclooxygenase-2. Science. 310:1966–1970 10.1126/science.1119407 [DOI] [PubMed] [Google Scholar]
- Kolb H., Kolb-Bachofen V. 1998. Nitric oxide in autoimmune disease: cytotoxic or regulatory mediator? Immunol. Today. 19:556–561 10.1016/S0167-5699(98)01366-8 [DOI] [PubMed] [Google Scholar]
- Kryczek I., Banerjee M., Cheng P., Vatan L., Szeliga W., Wei S., Huang E., Finlayson E., Simeone D., Welling T.H., et al. 2009. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 114:1141–1149 10.1182/blood-2009-03-208249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusmartsev S., Nefedova Y., Yoder D., Gabrilovich D.I. 2004. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172:989–999 [DOI] [PubMed] [Google Scholar]
- Lança T., Silva-Santos B. 2012. The split nature of tumor-infiltrating leukocytes: Implications for cancer surveillance and immunotherapy. Oncoimmunology. 1:717–725 10.4161/onci.20068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane P., Traunecker A., Hubele S., Inui S., Lanzavecchia A., Gray D. 1992. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur. J. Immunol. 22:2573–2578 10.1002/eji.1830221016 [DOI] [PubMed] [Google Scholar]
- Langowski J.L., Zhang X., Wu L., Mattson J.D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R.A., Oft M. 2006. IL-23 promotes tumour incidence and growth. Nature. 442:461–465 10.1038/nature04808 [DOI] [PubMed] [Google Scholar]
- Lee S.W., Choi H., Eun S.Y., Fukuyama S., Croft M. 2011. Nitric oxide modulates TGF-beta-directive signals to suppress Foxp3+ regulatory T cell differentiation and potentiate Th1 development. J. Immunol. 186:6972–6980 10.4049/jimmunol.1100485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahidhara R.S., Hoffman R.A., Huang S., Wolf-Johnston A., Vodovotz Y., Simmons R.L., Billiar T.R. 2003. Nitric oxide-mediated inhibition of caspase-dependent T lymphocyte proliferation. J. Leukoc. Biol. 74:403–411 10.1189/jlb.0602293 [DOI] [PubMed] [Google Scholar]
- Mandruzzato S., Solito S., Falisi E., Francescato S., Chiarion-Sileni V., Mocellin S., Zanon A., Rossi C.R., Nitti D., Bronte V., Zanovello P. 2009. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J. Immunol. 182:6562–6568 10.4049/jimmunol.0803831 [DOI] [PubMed] [Google Scholar]
- Mangan P.R., Harrington L.E., O’Quinn D.B., Helms W.S., Bullard D.C., Elson C.O., Hatton R.D., Wahl S.M., Schoeb T.R., Weaver C.T. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 441:231–234 10.1038/nature04754 [DOI] [PubMed] [Google Scholar]
- Mantovani A., Allavena P., Sica A., Balkwill F. 2008. Cancer-related inflammation. Nature. 454:436–444 10.1038/nature07205 [DOI] [PubMed] [Google Scholar]
- Martin-Orozco N., Dong C. 2009. The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr. Opin. Investig. Drugs. 10:543–549 [PubMed] [Google Scholar]
- Martin-Orozco N., Muranski P., Chung Y., Yang X.O., Yamazaki T., Lu S., Hwu P., Restifo N.P., Overwijk W.W., Dong C. 2009. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. 31:787–798 10.1016/j.immuni.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzoni A., Bronte V., Visintin A., Spitzer J.H., Apolloni E., Serafini P., Zanovello P., Segal D.M. 2002. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168:689–695 [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N., Allen J.B., Mizel D.E., Albina J.E., Xie Q.W., Nathan C.F., Wahl S.M. 1993. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 178:749–754 10.1084/jem.178.2.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miljkovic D., Trajkovic V. 2004. Inducible nitric oxide synthase activation by interleukin-17. Cytokine Growth Factor Rev. 15:21–32 10.1016/j.cytogfr.2003.10.003 [DOI] [PubMed] [Google Scholar]
- Miyahara Y., Odunsi K., Chen W., Peng G., Matsuzaki J., Wang R.F. 2008. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc. Natl. Acad. Sci. USA. 105:15505–15510 10.1073/pnas.0710686105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movahedi K., Guilliams M., Van den Bossche J., Van den Bergh R., Gysemans C., Beschin A., De Baetselier P., Van Ginderachter J.A. 2008. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 111:4233–4244 10.1182/blood-2007-07-099226 [DOI] [PubMed] [Google Scholar]
- Muranski P., Boni A., Antony P.A., Cassard L., Irvine K.R., Kaiser A., Paulos C.M., Palmer D.C., Touloukian C.E., Ptak K., et al. 2008. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 112:362–373 10.1182/blood-2007-11-120998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S., Gabrilovich D.I. 2010. Myeloid-derived suppressor cells in human cancer. Cancer J. 16:348–353 10.1097/PPO.0b013e3181eb3358 [DOI] [PubMed] [Google Scholar]
- Nagy G., Koncz A., Perl A. 2003. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J. Immunol. 171:5188–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala W., Wei X.Q., Campbell C., Thomson D., Komai-Koma M., Liew F.Y. 2002. Nitric oxide preferentially induces type 1 T cell differentiation by selectively up-regulating IL-12 receptor beta 2 expression via cGMP. Proc. Natl. Acad. Sci. USA. 99:16186–16191 10.1073/pnas.252464599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbala W., Alves-Filho J.C., Fukada S.Y., Vieira S.M., Mitani A., Sonego F., Mirchandani A., Nascimento D.C., Cunha F.Q., Liew F.Y. 2011. Regulation of type 17 helper T-cell function by nitric oxide during inflammation. Proc. Natl. Acad. Sci. USA. 108:9220–9225 10.1073/pnas.1100667108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numasaki M., Fukushi J., Ono M., Narula S.K., Zavodny P.J., Kudo T., Robbins P.D., Tahara H., Lotze M.T. 2003. Interleukin-17 promotes angiogenesis and tumor growth. Blood. 101:2620–2627 10.1182/blood-2002-05-1461 [DOI] [PubMed] [Google Scholar]
- Obermajer N., Muthuswamy R., Lesnock J., Edwards R.P., Kalinski P. 2011. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 118:5498–5505 10.1182/blood-2011-07-365825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa J.B., Curti B., Peitzman A.B., Simmons R.L., Billiar T.R., Hoffman R., Rault R., Longo D.L., Urba W.J., Ochoa A.C. 1992. Increased circulating nitrogen oxides after human tumor immunotherapy: correlation with toxic hemodynamic changes. J. Natl. Cancer Inst. 84:864–867 10.1093/jnci/84.11.864 [DOI] [PubMed] [Google Scholar]
- Rodriguez P.C., Ernstoff M.S., Hernandez C., Atkins M., Zabaleta J., Sierra R., Ochoa A.C. 2009. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69:1553–1560 10.1158/0008-5472.CAN-08-1921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneemann M., Schoeden G. 2007. Macrophage biology and immunology: man is not a mouse. J. Leukoc. Biol. 81:579, discussion :580 10.1189/jlb.1106702 [DOI] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G. 2002. Species differences in macrophage NO production are important. Nat. Immunol. 3:102 10.1038/ni0202-102a [DOI] [PubMed] [Google Scholar]
- Serafini P., Meckel K., Kelso M., Noonan K., Califano J., Koch W., Dolcetti L., Bronte V., Borrello I. 2006. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203:2691–2702 10.1084/jem.20061104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stritesky G.L., Yeh N., Kaplan M.H. 2008. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 181:5948–5955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D.D., Espey M.G., Ridnour L.A., Hofseth L.J., Mancardi D., Harris C.C., Wink D.A. 2004. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc. Natl. Acad. Sci. USA. 101:8894–8899 10.1073/pnas.0400453101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beelen A.J., Zelinkova Z., Taanman-Kueter E.W., Muller F.J., Hommes D.W., Zaat S.A., Kapsenberg M.L., de Jong E.C. 2007. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 27:660–669 10.1016/j.immuni.2007.08.013 [DOI] [PubMed] [Google Scholar]
- Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. 2006. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 24:179–189 10.1016/j.immuni.2006.01.001 [DOI] [PubMed] [Google Scholar]
- Vuk-Pavlović S., Bulur P.A., Lin Y., Qin R., Szumlanski C.L., Zhao X., Dietz A.B. 2010. Immunosuppressive CD14+HLA-DRlow/- monocytes in prostate cancer. Prostate. 70:443–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Yi T., Kortylewski M., Pardoll D.M., Zeng D., Yu H. 2009. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 206:1457–1464 10.1084/jem.20090207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg J.B., Granger D.L., Pisetsky D.S., Seldin M.F., Misukonis M.A., Mason S.N., Pippen A.M., Ruiz P., Wood E.R., Gilkeson G.S. 1994. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG-monomethyl-L-arginine. J. Exp. Med. 179:651–660 10.1084/jem.179.2.651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke C.M., Bishop K., Fox D., Zou W. 2011. Deciphering the role of Th17 cells in human disease. Trends Immunol. 32:603–611 10.1016/j.it.2011.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N.J., Boniface K., Chan J.R., McKenzie B.S., Blumenschein W.M., Mattson J.D., Basham B., Smith K., Chen T., Morel F., et al. 2007. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat. Immunol. 8:950–957 10.1038/ni1497 [DOI] [PubMed] [Google Scholar]
- Wu S.G., Rhee K.J., Albesiano E., Rabizadeh S., Wu X.Q., Yen H.R., Huso D.L., Brancati F.L., Wick E., McAllister F., et al. 2009. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15:1016–1022 10.1038/nm.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zea A.H., Rodriguez P.C., Atkins M.B., Hernandez C., Signoretti S., Zabaleta J., McDermott D., Quiceno D., Youmans A., O’Neill A., et al. 2005. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65:3044–3048 [DOI] [PubMed] [Google Scholar]
- Zhou L., Ivanov I.I., Spolski R., Min R., Shenderov K., Egawa T., Levy D.E., Leonard W.J., Littman D.R. 2007. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8:967–974 10.1038/ni1488 [DOI] [PubMed] [Google Scholar]
- Zou W., Restifo N.P. 2010. T(H)17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10:248–256 10.1038/nri2742 [DOI] [PMC free article] [PubMed] [Google Scholar]