Summary
Human embryonic (hESCs) and induced pluripotent stem cells (hiPSCs) have been shown to differentiate into primordial germ cells (PGCs) but not into spermatogonia nor haploid spermatocytes or spermatids. Here we show that hESCs and hiPSCs differentiate directly into advanced male germ cell lineages including post-meiotic, spermatid-like cells in vitro without genetic manipulation. Furthermore, our procedure mirrors spermatogenesis in vivo by differentiating pluripotent stem cells into UTF1, PLZF and CDH1-positive spermatogonia-like cells, HIWI and HILI-positive spermatocyte-like cells, and haploid cells expressing acrosin, transition protein 1 and protamine 1, proteins found uniquely in either spermatids and/or sperm. These spermatids show uniparental genomic imprints similar to human sperm on two loci: H19 and IGF2. These results demonstrate that male pluripotent stem cells have the capability to directly differentiate into advanced germ cell lineages and may represent a novel strategy for studying spermatogenesis in vitro.
Keywords: germ cell differentiation, haploid, spermatogenesis, spermatogonia, stem cells
Introduction
Infertility affects perhaps 15% of couples, with male factors responsible for 40–60% (Schlegel, 2009). In men without a genetic cause for infertility, stem cell transplantation represents a possible treatment option (Marques-Mari et al., 2009; Mathews et al., 2009; Yao et al., 2011). Protocols to preserve future fertility in boys undergoing cancer therapies who cannot yet bank their own sperm are under development(Hermann et al., 2007; Sadri-Ardekani et al., 2011; Schlatt et al., 2009). However for adult and prepubescent patients rendered sterile prior to sperm collection, there are no current treatments to restore fertility.
Mouse ESCs(Geijsen et al., 2004; Hayashi et al., 2011), non-human primate (NHP) and hESCs(Bucay et al., 2009; Fukunaga et al., 2010; Kee et al., 2009; Panula et al., 2011; Park et al., 2009; Teramura et al., 2007; Tilgner et al., 2008; Yamauchi et al., 2009) have been differentiated into primordial germ cells (PGCs), fetal precursors of the spermatogenic lineage. In addition, recent studies suggest that human pluripotent stem cells (hPSCs) can enter meiosis, and in some cases produce haploid products(Eguizabal et al., 2011; Kee et al., 2009; Panula et al., 2011). In this study, we developed an in vitro method which achieves two significant endpoints. First, male hESCs and hiPSCs differentiate directly into adult-type spermatogonia. Secondly, differentiating stem cells give rise to cells which are phenotypically like post-meiotic round spermatids. These results highlight the full plasticity of hPSCs by showing their ability to undergo spermatogenesis in vitro culminating with hapoloid round spermatid-like cells. These results also contribute to the overall goal of both understanding germ cell development in vitro, as well as ultimately generating gametes that may prove invaluable for understanding infertility mechanisms.
Results
Mouse SSC Conditions Elevate Expression of Germ Cell Markers
Human testis cells have been shown to directly de-differentiate into PSCs by culturing cells in PSC conditions(Conrad et al., 2008; Ko et al., 2009; Kossack et al., 2009). Thus, we examined whether ESCs could directly differentiate into germline stem cells. Our goal was to differentiate PSCs into SSC-like cells because this spermatogenic lineage has shown an exceptional ability to re-colonize sterilized testes and thus restore fertility in certain species including mice and NHPs (Brinster and Avarbock, 1994; Jahnukainen et al., 2011). One advantage of this strategy is that there are established protocols for culturing and expanding rodent SSCs in vitro(Kanatsu-Shinohara et al., 2003). We cultured H1 (WA01) hESCs and HFF1 (human foreskin fibroblast 1) iPSCs directly in standardized mouse SSC culture conditions. After 10-day culture, we observed significant increases in VASA+ cells, with ~60% of H1 cells and ~40% HFF1 cells expressing VASA (Fig. 1A, 1B and enlarged insets). These VASA+, germ-like cells showed typical, VASA staining patterns as seen in human testis sections (Suppl. Fig. 1S, A). 10 day culture was an optimum time point as day 7 cultures yielded lower numbers of VASA+ cells and day 15 did not yield an appreciable increase (Suppl. Fig. 1S, B). In comparison to previous protocols, we see a 3–4 fold increase in VASA+ cells derived from hPSCs, all within 10 days post differentiation.
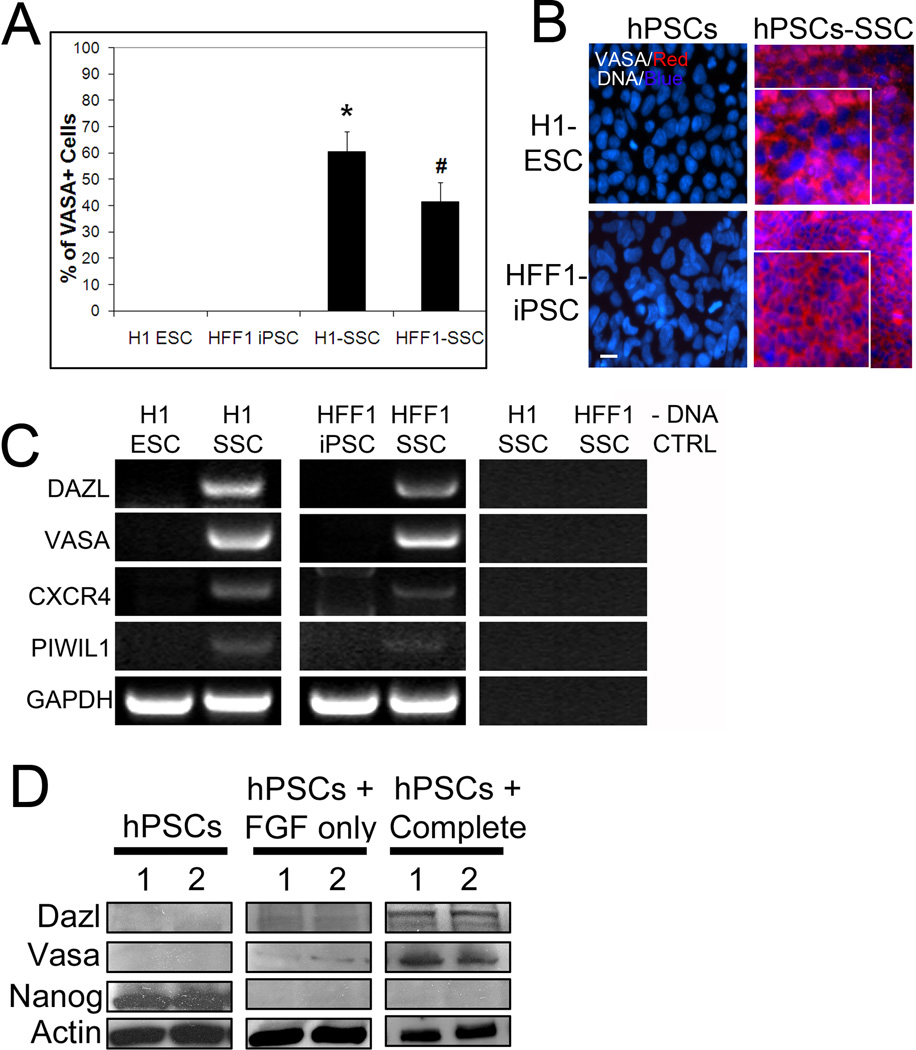
Figure 1. Differentiation of hPSCs in spermatogonial stem cell culture yields significant percentages of VASA+ cells.
(A) H1 ESCs and HFF-1 iPSCs cultured in mouse SSC conditions for 10 days and then stained for VASA. % VASA expression was quantified in the parent PSC lines and the differentiated lines. Representative graphical analysis from 5 separate trials, > 5000 cells counted for each condition, is shown. * signifies p < 0.01 comparing H1 ESC to H1 SSC. # signifies p < 0.01 comparing HFF1 iPSC to HFF1 SSC. (B) Representative images of PSCs and PSCs differentiated in SSC culture conditions for 10 days and stained for VASA. DNA labeled with Hoechst. Scale, 50 µm. Enlarged insets show typical, perinuclear localization of VASA. (C) Reverse transcription (RT) PCR for germ cell markers DAZL, VASA, CXCR4 and PIWIL1 in PSCs and their differentiated counterparts. GADPH is shown as a loading control. No DNA (-DNA) is also shown as a negative control. (D) Representative western blot analyses showing upregulation of germ cell marker expression and a concomitant loss of the pluripotent marker Nanog in complete SSC culture conditions (with GDNF and FGF). Despite loss of Nanog in FGF only SSC medium (i.e. without GDNF), germ cell markers were not expressed. Actin is a loading control.
We further analyzed H1 and HFF1 cells cultured in mouse SSC conditions (termed H1 SSC and HFF1 SSC) for expression of additional germ cell markers. Deleted-in-Azoospermia-like (DAZL) and VASA are two germline specific, RNA binding proteins that are important in germ cell development and normal spermatogenesis(Castrillon et al., 2000; Kee et al., 2009). Recently, Kee et. al. showed that some hESCs, mainly female lines, express low levels of VASA mRNA(Kee et al., 2009). Here, both male hESC and hiPSC lines do not exhibit expression of VASA mRNA (Fig. 1C). H1 SSC and HFF1 SSC cells showed an increase in all germ cell markers tested, including CXCR4 and PIWIL1, by RT-PCR, suggesting that this is an efficient way to generate germ cell lineages (Fig. 1C). VASA and DAZL protein expression was also elevated in H1 SSCs and HFF1 SSCs compared to the undifferentiated, parent PSC lines (Fig. 1D). We also observed that germ cell differentiation was dependent on the growth factor GDNF (glial-derived neurotrophic factor, hPSCs + Complete). Cells differentiated without GDNF (hPSCs + FGF only) demonstrated no increase in VASA or DAZL protein expression but did show a loss of the pluripotent marker Nanog, suggesting that both H1 hESCs and HFF1 hiPSCs differentiated (Fig. 1D). These results suggest that GDNF containing SSC medium efficiently and rapidly differentiates hPSCs into germ cell lineages.
hPSCs Cultured in Mouse SSC Conditions Express PLZF
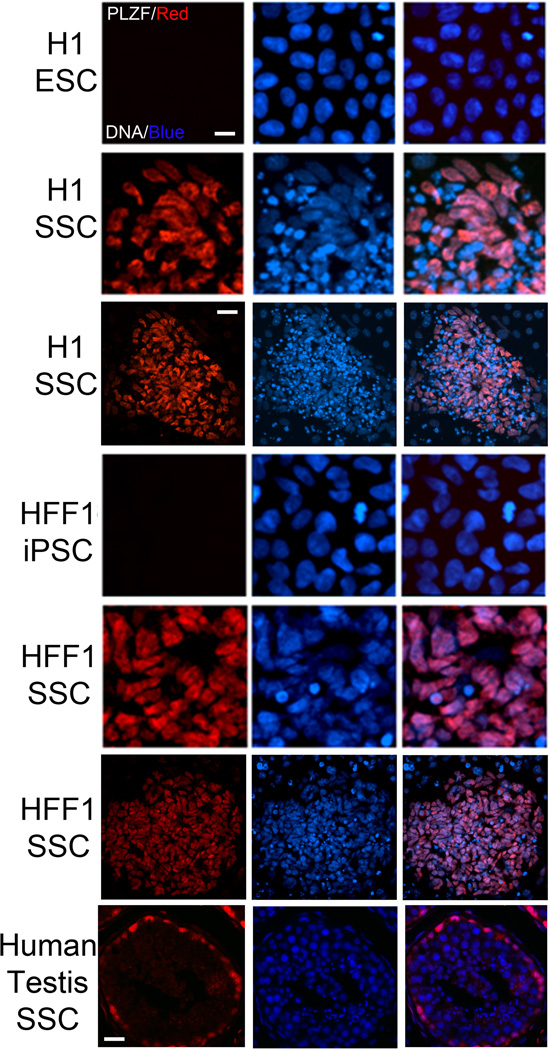
CXCR4 is a chemokine receptor expressed by spermatogonia that plays a role in SSC maintenance(Payne et al., 2010). Because we detected elevations in CXCR4 in both H1 SSCs and HFF1 SSCs (Fig. 1C), we next evaluated whether H1 SSCs and HFF1 SSCs expressed PLZF, a zinc-finger transcription factor that is a consensus marker of stem and progenitor spermatogonia. PLZF, or ZBTB16, plays a critical role in SSC self-renewal and growth(Buaas et al., 2004; Costoya et al., 2004; Hobbs et al., 2010). 10 day culture in mouse SSC conditions induced expression of PLZF, localized to the nucleus, in both H1 and HFF1 SSCs (Fig. 2). This nuclear expression of PLZF mirrors that observed in human testes (Fig. 2, 7th row). Futhermore our protocol generates a high percentage of PLZF-positive cells within differentiating colonies (Fig. 2, low magnification views, 3rd and 6th rows), with ~82% of H1 SSCs and ~78% HFF1 SSCs expressing PLZF (Suppl. Fig. 2S, A). Unlike other method, our protocol induces PLZF expression (Suppl. Fig. 2S, B). This suggests that we are more closely mirroring the early events of in vivo spermatogenesis.
Figure 2. Differentiation of hPSCs in SSC conditions results in the expression of the SSC marker PLZF.
While the parent PSC lines do not express detectable levels of PLZF, 10 day culture in SSC conditions upregulates PLZF (red) expression in both lines. Hoechst (blue): DNA. Scale, 40 µm. Global view (3rd and 6th rows) of differentiated colonies shows a large portion of cells expressing PLZF. Scale, 100 µm. 7th row panel depicts PLZF staining in human testis sections.
SSC Conditions Yield Post-meiotic, Acrosin-positive Cells
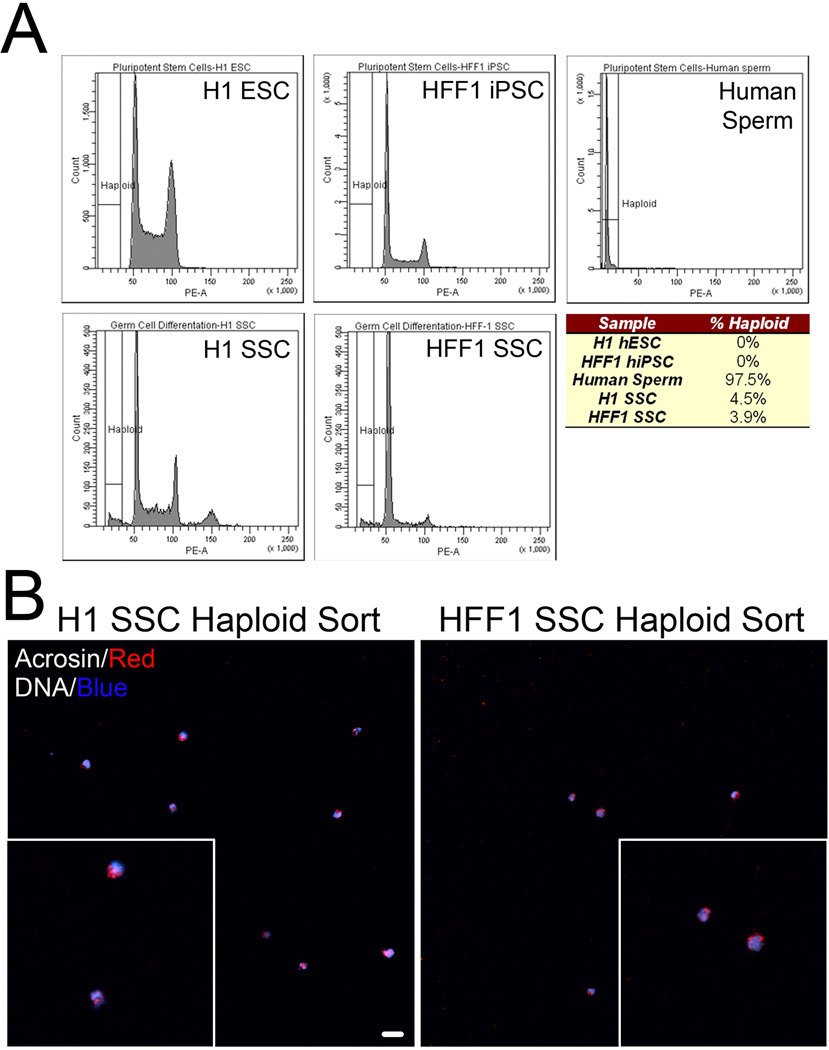
SSCs are defined in part by their ability to produce gametes through a complex combination of division and differentiation. Mouse SSCs can differentiate into haploid cells in vitro(Feng et al., 2002; Geijsen et al., 2004; Nayernia et al., 2006), so we next quantified whether haploid cells were produced in H1 SSCs and HFF1 SSCs. Flow cytometry analyses indicated that a haploid population exists in H1 SSCs (4.5%) and HFF1 SSCs (3.9%) corresponding to haploid peaks observed with human sperm (Fig. 3A; Suppl. Fig. 3S, A). We further confirmed haploidy of isolated cells by fluorescence in situ hybridization (FISH) with an LNA probe to satellite DNA found on chromosomes 1, 9, 16 and Y (Suppl. Fig. 3S, C) After FACS , the majority of haploid cells isolated from both H1 SSCs and HFF1 SSCs exhibit polar acrosin localization (Fig.3B, enlarged insets, Suppl. Fig. 3S, B). These results suggest that we are able to generate a small percentage of acrosin-positive, haploid cells in vitro from hPSCs within 10 days of SSC culture. Ten days proved optimal since haploid cells were lost after 20 days (Suppl. Fig. 3S, D).
Figure 3. hPSCs differentiated in SSC culture exhibit haploid features.
(A) FACS ploidy analysis reveals a small haploid peak in hPSCs cultured in SSC culture conditions for 10 days. This peak corresponds to the haploid peak observed in human sperm. Chart below represents % of haploid cells in undifferentiated and SSC-mediated differentiated hPSCs. Data is representative of five cell sorts with 500,000 cells sorted per experiment. (B) FACS isolated haploid cells from H1 SSC (left) and HFF1 SSC (right) were seeded coverslips and stained with acrosin (red) and Hoechst (DNA, blue). Global view shows several isolated cells with polar acrosin localization. Scale, 50 µm. Insets show zoomed view of acrosin-positive, haploid cells.
hPSC Differentiation in SSC Conditions Generates Cells Which Express Markers For Spermatogonia, Pre-meiotic Spermatocytes, Post-meiotic Spermatocytes and Round Spermatids
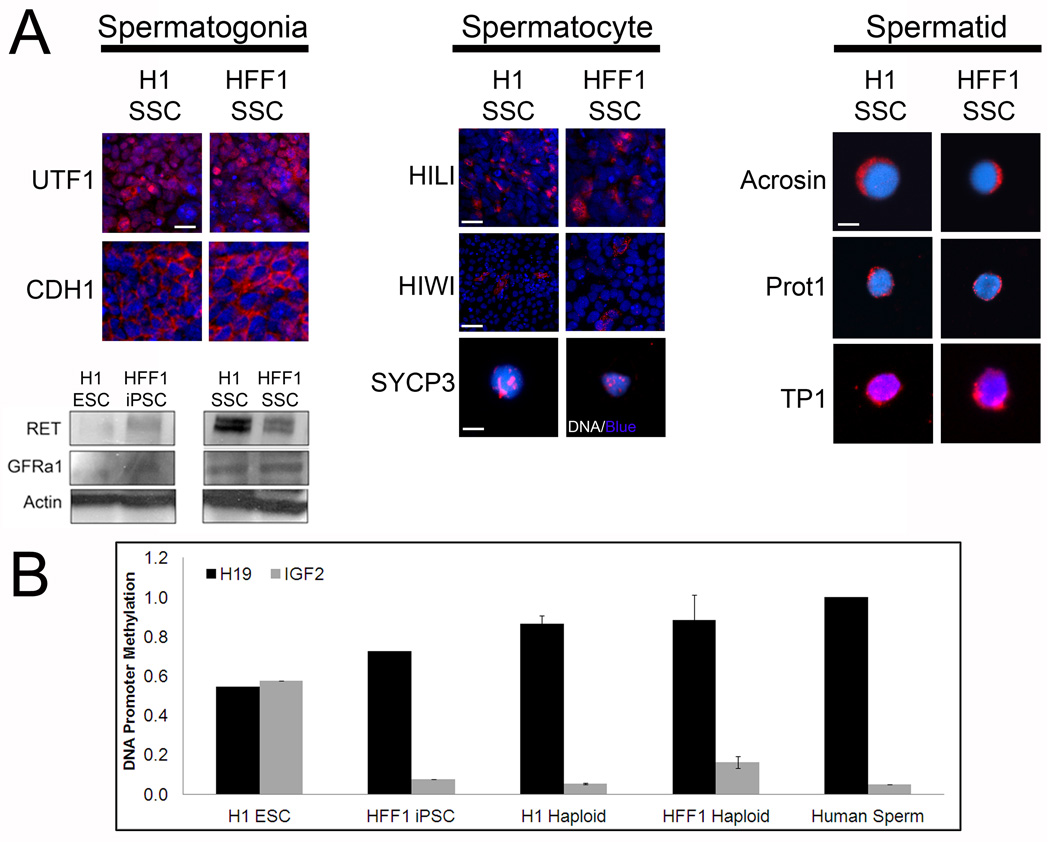
Because differentiation in SSC conditions altered cell cycle profiles (Suppl. Fig. 4S, A-B) and yielded a small percentage of haploid cells in addition to a large population of PLZF-positive spermatogonia, we next evaluated whether H1 ESCs and HFF1 iPSCs differentiated into intermediate cell types observed in in vivo spermatogenesis. In addition to PLZF, we observed expression of UTF1 and CDH1 (Fig. 4A, left column), proteins expressed both in spermatogonia and PSCs. Unlike PSCs, we observed an increase in protein expression of RET and GFRα1 (Fig. 4A, western blots), receptors for GDNF found on spermatogonia.
Figure 4. Differentiation of hPSCs in SSC culture yields cells that express markers for spermatogonia, spermatocytes and spermatids.
(A) Left: 10 days post differentiation cultures of H1 and HFF1 SSCs express pre-meiotic spermatogonial markers UTF1 and CDH1. Scale, 50 µm. Differentiation also induces expression of two membrane receptors: RET and GFRa1. Actin is a loading control. Center: expression of spermatogonia-to-spermatocyte marker HILI, spermatocyte-to-spermatid marker HIWI and meiotic marker SYCP3. Scale for HILI 200 µm, scale for HIWI, 500 µm and scale for SYCP3, 10 µm. Right: expression of post-meiotic spermatid markers Acrosin, Protamine 1 (Prot1) and Transition Protein 1 (TP1). Haploid cells were sorted by FACS and immunostained with antibodies directed at the indicated protein. Scale, 10 µm. (B) H1 ESCs, HFF1 iPSCs, fertile human sperm, and haploid cells obtained by FACS from H1 and HFF1 SSC cultures were examined for methylation on imprinting control regions (ICRs) for H19 (paternally imprinted) and IGF2 (maternally imprinted). Methylation statuses were examined using Qiagen Epitect Methyl II PCR Array. Graph shows average % methylation with error bars.
Differentiation of hPSCs in SSC conditions showed an increase in PIWIL1 RNA expression (Fig. 1C). PIWIL1, also known as HIWI, is essential in spermatogenic progression from SSCs to round spermatids(Deng and Lin, 2002). We examined expression of three spermatocyte markers for pre-meiotic spermatocytes/differentiating spermatogonia, meiotic spermatocytes and post-meiotic spermatocytes. We identified cells in both differentiating H1 ESCs and HFF1 iPSCs expressing pre-meiotic HILI protein, meiotic marker SYCP3 (synaptonemal complex 3), involved in recombination and segregation of meiotic chromosomes; and post-meiotic HIWI (Fig. 4A, center column). While there were a large number of HILI-positive cells, very few cells expressed SYCP3 or HIWI, suggesting that there is bottleneck prior to meiosis.
We next isolated the haploid peaks from FACS and immunostained isolated cells for spermatid markers. During spermiogenesis, acrosin expression is turned on and histones are replaced by protamines via transition proteins(Carrell et al., 2007). Haploid cells isolated from H1 and HFF1 SSC cultures express post-meiotic, sperm markers: acrosin, protamine 1 and transition protein 1 (Fig. 4, right column). In particular, acrosin staining exhibits polar localization in both cell lines (Fig. 4A, 1st row). These haploid cells resemble round spermatids by acrosin localization, the nuclear/perinuclear localization of transition protein 1 (TP1) and the perinuclear localization of protamine 1 (Prot1) (Fig. 4A, right column), which localizes to the perinuclear region of haploid cells and enters the nucleus at the elongated spermatid stage(Carrell et al., 2007). All acrosin positive cells were also positive for TP1 (Suppl. Fig. 4S, C). These haploid cells also resemble round spermatids observed in human and NHPs (Carrell et al., 2007; Moreno et al., 2006; Ramalho-Santos et al., 2002) (Also see Suppl. Fig. 4S, D). These results coupled with the preceding PIWIL1 expression data suggest that PSCs are able to directly differentiate into postmeiotic, round-spermatid like cells in vitro.
During in vivo germ cell specification, genomic imprints are removed at the primordial germ cell stage and then re-established during spermatogenesis(Lucifero et al., 2002). In mice, differentiating PSCs into functional germ cells results in progeny that exhibit epigenetic disease phenotypes(Nayernia et al., 2006; Nolte et al., 2010). One explanation was improper imprinting during gametogenesis(Lucifero and Reik, 2006). To evaluate imprinting statuses on haploid spermatids differentiated here, we isolated haploid cells by FACS and examined the methylation status of the imprinting control region (ICR) for paternally imprinted (H19) and maternally imprinted genes (IGF2). As previously reported, iPSCs showed aberrant imprinting(Pick et al., 2009), but ESCs showed typical somatic cell imprinting on ICRs for H19 and IGF2 (Fig.4B). Isolation of haploid cells from H1 SSC cultures showed imprinting patterns similar to those observed in human sperm with H19 ICR methylation around 90% and IGF2 ICR methylation around 5% (Fig. 4B). Haploid cells from HFF1 SSCs showed similar levels of H19 ICR methylation to human sperm (~90%), but IGF2 methylation (~14%) was slightly elevated above methylation observed in human sperm (Fig. 4B). These results suggest that haploid products obtained show similar DNA methylation patterns on at least 2 parent-of-origin genomic imprints.
Discussion
Several studies have shown that human PSCs differentiate in vitro and in vivo into the 3 germ layers: endoderm, mesoderm, and ectoderm. However, only recently have studies show that human PSCs exhibit greater plasticity by differentiating into germ cell lineages. Our study shows that male diploid PSCs differentiate into advanced haploid lineages, including round spermatids. While female PSCs do not differentiate and undergo cell death in our protocol (data not shown), methods have been developed to generate haploid oocyte-like cells from female lines (for review, (Virant-Klun et al., 2011)), furthering demonstrating that both male and female PSCs possess the potential to differentiate into any adult cell type, including gametes.
The generation of viable sperm and spermatids in vitro from PSCs and even somatic cells in humans and other primates has many biomedical justifications even though the endeavor is fraught with experimental and bioethical challenges(Daley, 2007). Furthermore the stringencies which with these in vitro sperm are evaluated vary according the necessary endpoint. The greatest stringency is for the generation of fully functional sperm or spermatids useful and safe for reproduction in ART (Assisted Reproductive Technology) clinics. This objective is justified by the Oncofertility Consortium, which seeks the benevolent objective of preserving fertility in male cancer survivors who were rendered infertile during their therapies but were also too young or fragile to produce a sperm specimen for cryobanking(Woodruff, 2010). It is also justified by ART practitioners for the potential treatment of infertile men suffering from either diagnosed or idiopathic male infertility in cases in which neither sperm nor elongated spermatids can be obtained. Discovering of the stages during spermatogenesis at which various forms of idiopathic male infertility arrest would greatly aid in the diagnoses, and perhaps eventual treatments, of these still mysterious processes. Learning of these spermatogenic arrest sites might also contribute to the design of novel contraceptives. Additionally the epigenetic modifications enabling the properly imprinted sperm chromatin and the replacement of nuclear proteins to form the sperm nucleus could be better investigated in these types of cell cultures versus in intact tissues, especially since our protocol seems to generate haploid products with similar parent-of-origin imprints observed in fertile human sperm. Anticipated improvements in the efficiency of in vitro spermatogenesis may also help understanding how mitochondria are modified to create the sperm mitochondria as well as how the somatic centrosome is reduced during male meiosis to form the sperm tail’s basal body and the sperm centrosome(Schatten, 1994).
Differentiating hPSCs into SSCs is an important step in evaluating the possibly use of transplanting patient-specific PSCs or SSCs to restore fertility. The ability to directly differentiate hPSCs into SSCs without having to go through a PGC differentiation protocol is likewise important for decreasing differentiation strategy time-frames to increase utilization within a clinical setting. Furthermore, differentiation in SSC conditions yielded several cell types observed during spermatogenesis to show that ultimately, patient-specific spermatogenesis could be studied in vitro with only PSCs. We observed the expression of putative markers for spermatogenesis, including markers for undifferentiated spermatogonia, differentiating spermatogonia, pre-meiotic spermatocytes, meiotic and post-meiotic spermatocytes, and spermatids. Differing from published results, our study not only outlines an approach for generating large numbers of VASA+ cells but also describes a methodology for rapidly and directly differentiating human PSCs into advanced germ cell lineages, including round spermatids, using only extrinsic factors with no genetic manipulation. Thus the differentiation method presented here may contribute to the overall objective of using patient-specific hiPSCs to generate germ cell lineages capable of restoring fertility in sterile male patients.
Complete spermatogenesis in vitro has not yet been accomplished in humans. Because this strategy attempts to mimic in vivo spermatogenesis by generating undifferentiated spermatogonia as well as haploid round spermatids, it provides evidence that studying spermatogenesis through the round spermatid stage in vitro is feasible, though additional work is needed to confirm that an individual cell progresses through all pre-meiotic, meiotic and postmeiotic stages. While techniques for utilizing round spermatids to fertilize oocytes have not been proven in human and NHPs, this protocol may allow researchers to study cytoplasmic events during early spermatogenesis from hPSCs to SSCs as well as characterize events associated with spermiogenesis to the round spermatid stage.
Our differentiation scheme may also represent a tool for exploring root causes of male factor infertility. By deriving hiPSCs from infertile men, such as from patients with Sertoli-cell-only (SCO) syndrome, followed by direct differentiation with our protocol, we can examine where spermatogenesis arrests, and in the case of SCO patients, identify whether hiPSCs can differentiate into SSCs and whether viability of SSCs is a major concern. Advances such as these would undoubtedly shed new light on root causes for male factor infertility as well as examine whether multiple disorders contribute to a patient’s infertility. These potential clinical applications thus underscore the importance for improving our differentiation protocol to increase efficiency of haploid cell generation as well as progressing further through spermiogenesis. Whether these in vitro generated gamete precursors have reproductive capabilities in vivo, helpful for infertility patients, will be important to evaluate pre-clinically, though they will be of keen biological importance regardless.
Experimental Procedures
Mouse spermatogonial stem cell differentiation medium and FACS
H1 (WA01, WiCell) hESCs and HFF1 (parent fibroblastsfrom ATCC, iPS derived internally(Easley et al., 2012) hiPSCs were cultured for 10 days in mouse spermatogonial stem cell (SSC) medium containing the following (all from Sigma, unless noted): MEMalpha (Invitrogen), 0.2% Bovine Serum Albumin, 5 µg/ml insulin, 10 µg/ml transferrin, 60 µM putrescine, 2 mM L-glutamine (Invitrogen), 50 µM β-mercaptoethanol, 1 ng/ml hbFGF (human basic fibroblast growth factor, BD Biosciences), 20 ng/ml GDNF (glial-derived neurotrophic factor, R&D Systems), 30 nM sodium selenite, 2.36 µM palmitic acid, 0.21 µM palmitoleic acid, 0.88 µM stearic acid, 1.02 µM oleic acid, 2.71 µM linoleic acid, 0.43 µM linolenic acid, 10 mM HEPES, and 0.5X penicillin/streptomycin (Invitrogen). To isolate haploid cells by FACS, H1 SSCs and HFF1 SSCs were stained with the Vybrant DyeCycle Violet Live-cell stain (Invitrogen) in the SSC medium listed above but substituting OptiMeM with no phenol red and run on a FACS Aria sorter (BD Biosciences). Haploid cells were then cultured on poly-d-lysine-coated coverslips and fixed with 4% para-formaldehyde prior to immunostaining.
Supplementary Material
Highlights.
-
-
In vitro culture induces germ cell differentiation of hPSCs.
-
-
hPSCs differentiate into spermatogonia, spermatocytes and haploid spermatids.
-
-
Haploid spermatids have uniparental imprints similar to fertile human sperm.
Acknowledgments
The authors would like to thank Joan Brozick for her assistance with flow cytometry analyses and Lynda Guzick and Alison Logar for their assistance with FACS isolations of haploid cells. C.E. is supported by a postdoctoral fellowship from the Magee Womens Research Institute Postdoctoral Fellowship Program. This research was supported by funding from the NIH (PO1HD047675-G.S., R21HD061289-K.O., and RO1HD055475-K.O.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions C.E. carried out the majority of the experiments with assistance from J.B., and T.M. for immunostaining and B.P., H.V. and B.H. for immunohistochemistry. M.M., C.E., and A.R. performed the ICR methylation experiments. C.E., B.P., C.S., K.O. and G.P. designed experiments and wrote the manuscript.
References
- Brinster RL, Avarbock MR. Germline transmission of donor haplotype following spermatogonial transplantation. Proc Natl Acad Sci U S A. 1994;91:11303–11307. doi: 10.1073/pnas.91.24.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Bucay N, Yebra M, Cirulli V, Afrikanova I, Kaido T, Hayek A, Montgomery AM. A novel approach for the derivation of putative primordial germ cells and sertoli cells from human embryonic stem cells. Stem Cells. 2009;27:68–77. doi: 10.1634/stemcells.2007-1018. [DOI] [PubMed] [Google Scholar]
- Carrell DT, Emery BR, Hammoud S. Altered protamine expression and diminished spermatogenesis: what is the link? Hum Reprod Update. 2007;13:313–327. doi: 10.1093/humupd/dml057. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Quade BJ, Wang TY, Quigley C, Crum CP. The human VASA gene is specifically expressed in the germ cell lineage. Proc Natl Acad Sci U S A. 2000;97:9585–9590. doi: 10.1073/pnas.160274797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad S, Renninger M, Hennenlotter J, Wiesner T, Just L, Bonin M, Aicher W, Buhring HJ, Mattheus U, Mack A, et al. Generation of pluripotent stem cells from adult human testis. Nature. 2008;456:344–349. doi: 10.1038/nature07404. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Daley GQ. Gametes from embryonic stem cells: a cup half empty or half full? Science. 2007;316:409–410. doi: 10.1126/science.1138772. [DOI] [PubMed] [Google Scholar]
- Deng W, Lin H. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell. 2002;2:819–830. doi: 10.1016/s1534-5807(02)00165-x. [DOI] [PubMed] [Google Scholar]
- Easley CAt, Miki T, Castro CA, Ozolek JA, Minervini CF, Ben-Yehudah A, Schatten GP. Human Amniotic Epithelial Cells are Reprogrammed More Efficiently by Induced Pluripotency than Adult Fibroblasts. Cell Reprogram. 2012;14 doi: 10.1089/cell.2011.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguizabal C, Montserrat N, Vassena R, Barragan M, Garreta E, Garcia-Quevedo L, Vidal F, Giorgetti A, Veiga A, Izpisua-Belmonte JC. Complete Meiosis from Human Induced Pluripotent Stem Cells. Stem Cells. 2011 doi: 10.1002/stem.672. [DOI] [PubMed] [Google Scholar]
- Feng LX, Chen Y, Dettin L, Pera RA, Herr JC, Goldberg E, Dym M. Generation and in vitro differentiation of a spermatogonial cell line. Science. 2002;297:392–395. doi: 10.1126/science.1073162. [DOI] [PubMed] [Google Scholar]
- Fukunaga N, Teramura T, Onodera Y, Takehara T, Fukuda K, Hosoi Y. Leukemia inhibitory factor (LIF) enhances germ cell differentiation from primate embryonic stem cells. Cell Reprogram. 2010;12:369–376. doi: 10.1089/cell.2009.0097. [DOI] [PubMed] [Google Scholar]
- Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, Daley GQ. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–154. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Ohta H, Kurimoto K, Aramaki S, Saitou M. Reconstitution of the Mouse Germ Cell Specification Pathway in Culture by Pluripotent Stem Cells. Cell. 2011;146:1–14. doi: 10.1016/j.cell.2011.06.052. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs RM, Seandel M, Falciatori I, Rafii S, Pandolfi PP. Plzf regulates germline progenitor self-renewal by opposing mTORC1. Cell. 2010;142:468–479. doi: 10.1016/j.cell.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. doi: 10.1093/humrep/der160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kee K, Angeles VT, Flores M, Nguyen HN, Reijo Pera RA. Human DAZL, DAZ and BOULE genes modulate primordial germ-cell and haploid gamete formation. Nature. 2009;462:222–225. doi: 10.1038/nature08562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K, Tapia N, Wu G, Kim JB, Bravo MJ, Sasse P, Glaser T, Ruau D, Han DW, Greber B, et al. Induction of pluripotency in adult unipotent germline stem cells. Cell Stem Cell. 2009;5:87–96. doi: 10.1016/j.stem.2009.05.025. [DOI] [PubMed] [Google Scholar]
- Kossack N, Meneses J, Shefi S, Nguyen HN, Chavez S, Nicholas C, Gromoll J, Turek PJ, Reijo-Pera RA. Isolation and characterization of pluripotent human spermatogonial stem cell-derived cells. Stem Cells. 2009;27:138–149. doi: 10.1634/stemcells.2008-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucifero D, Mertineit C, Clarke HJ, Bestor TH, Trasler JM. Methylation dynamics of imprinted genes in mouse germ cells. Genomics. 2002;79:530–538. doi: 10.1006/geno.2002.6732. [DOI] [PubMed] [Google Scholar]
- Lucifero D, Reik W. Artificial sperm and epigenetic reprogramming. Nat Biotechnol. 2006;24:1097–1098. doi: 10.1038/nbt0906-1097. [DOI] [PubMed] [Google Scholar]
- Marques-Mari AI, Lacham-Kaplan O, Medrano JV, Pellicer A, Simon C. Differentiation of germ cells and gametes from stem cells. Hum Reprod Update. 2009;15:379–390. doi: 10.1093/humupd/dmp001. [DOI] [PubMed] [Google Scholar]
- Mathews DJ, Donovan PJ, Harris J, Lovell-Badge R, Savulescu J, Faden R. Pluripotent stem cell-derived gametes: truth and (potential) consequences. Cell Stem Cell. 2009;5:11–14. doi: 10.1016/j.stem.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno RD, Palomino J, Schatten G. Assembly of spermatid acrosome depends on microtubule organization during mammalian spermiogenesis. Dev Biol. 2006;293:218–227. doi: 10.1016/j.ydbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Nayernia K, Nolte J, Michelmann HW, Lee JH, Rathsack K, Drusenheimer N, Dev A, Wulf G, Ehrmann IE, Elliott DJ, et al. In vitro-differentiated embryonic stem cells give rise to male gametes that can generate offspring mice. Dev Cell. 2006;11:125–132. doi: 10.1016/j.devcel.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Nolte J, Michelmann HW, Wolf M, Wulf G, Nayernia K, Meinhardt A, Zechner U, Engel W. PSCDGs of mouse multipotent adult germline stem cells can enter and progress through meiosis to form haploid male germ cells in vitro. Differentiation. 2010;80:184–194. doi: 10.1016/j.diff.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Panula S, Medrano JV, Kee K, Bergstrom R, Nguyen HN, Byers B, Wilson KD, Wu JC, Simon C, Hovatta O, et al. Human germ cell differentiation from fetal- and adult-derived induced pluripotent stem cells. Hum Mol Genet. 2011;20:752–762. doi: 10.1093/hmg/ddq520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park TS, Galic Z, Conway AE, Lindgren A, van Handel BJ, Magnusson M, Richter L, Teitell MA, Mikkola HK, Lowry WE, et al. Derivation of primordial germ cells from human embryonic and induced pluripotent stem cells is significantly improved by coculture with human fetal gonadal cells. Stem Cells. 2009;27:783–795. doi: 10.1002/stem.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne CJ, Gallagher SJ, Foreman O, Dannenberg JH, Depinho RA, Braun RE. Sin3a is required by sertoli cells to establish a niche for undifferentiated spermatogonia, germ cell tumors, and spermatid elongation. Stem Cells. 2010;28:1424–1434. doi: 10.1002/stem.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick M, Stelzer Y, Bar-Nur O, Mayshar Y, Eden A, Benvenisty N. Clone- and gene-specific aberrations of parental imprinting in human induced pluripotent stem cells. Stem Cells. 2009;27:2686–2690. doi: 10.1002/stem.205. [DOI] [PubMed] [Google Scholar]
- Ramalho-Santos J, Schatten G, Moreno RD. Control of membrane fusion during spermiogenesis and the acrosome reaction. Biol Reprod. 2002;67:1043–1051. doi: 10.1095/biolreprod67.4.1043. [DOI] [PubMed] [Google Scholar]
- Sadri-Ardekani H, Akhondi MA, van der Veen F, Repping S, van Pelt AM. In vitro propagation of human prepubertal spermatogonial stem cells. JAMA. 2011;305:2416–2418. doi: 10.1001/jama.2011.791. [DOI] [PubMed] [Google Scholar]
- Schatten G. The centrosome and its mode of inheritance: the reduction of the centrosome during gametogenesis and its restoration during fertilization. Dev Biol. 1994;165:299–335. doi: 10.1006/dbio.1994.1256. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Ehmcke J, Jahnukainen K. Testicular stem cells for fertility preservation: preclinical studies on male germ cell transplantation and testicular grafting. Pediatr Blood Cancer. 2009;53:274–280. doi: 10.1002/pbc.22002. [DOI] [PubMed] [Google Scholar]
- Schlegel PN. Evaluation of male infertility. Minerva Ginecol. 2009;61:261–283. [PubMed] [Google Scholar]
- Teramura T, Takehara T, Kawata N, Fujinami N, Mitani T, Takenoshita M, Matsumoto K, Saeki K, Iritani A, Sagawa N, et al. Primate embryonic stem cells proceed to early gametogenesis in vitro. Cloning Stem Cells. 2007;9:144–156. doi: 10.1089/clo.2006.0070. [DOI] [PubMed] [Google Scholar]
- Tilgner K, Atkinson SP, Golebiewska A, Stojkovic M, Lako M, Armstrong L. Isolation of primordial germ cells from differentiating human embryonic stem cells. Stem Cells. 2008;26:3075–3085. doi: 10.1634/stemcells.2008-0289. [DOI] [PubMed] [Google Scholar]
- Virant-Klun I, Stimpfel M, Skutella T. Ovarian pluripotent/multipotent stem cells and in vitro oogenesis in mammals. Histol Histopathol. 2011;26:1071–1082. doi: 10.14670/HH-26.1071. [DOI] [PubMed] [Google Scholar]
- Woodruff TK. The Oncofertility Consortium--addressing fertility in young people with cancer. Nat Rev Clin Oncol. 2010;7:466–475. doi: 10.1038/nrclinonc.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Hasegawa K, Chuma S, Nakatsuji N, Suemori H. In vitro germ cell differentiation from cynomolgus monkey embryonic stem cells. PLoS One. 2009;4:e5338. doi: 10.1371/journal.pone.0005338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L, Yu X, Hui N, Liu S. Application of iPS in Assisted Reproductive Technology: Sperm from Somatic Cells? Stem Cell Rev. 2011;7:714–721. doi: 10.1007/s12015-011-9236-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.