Abstract
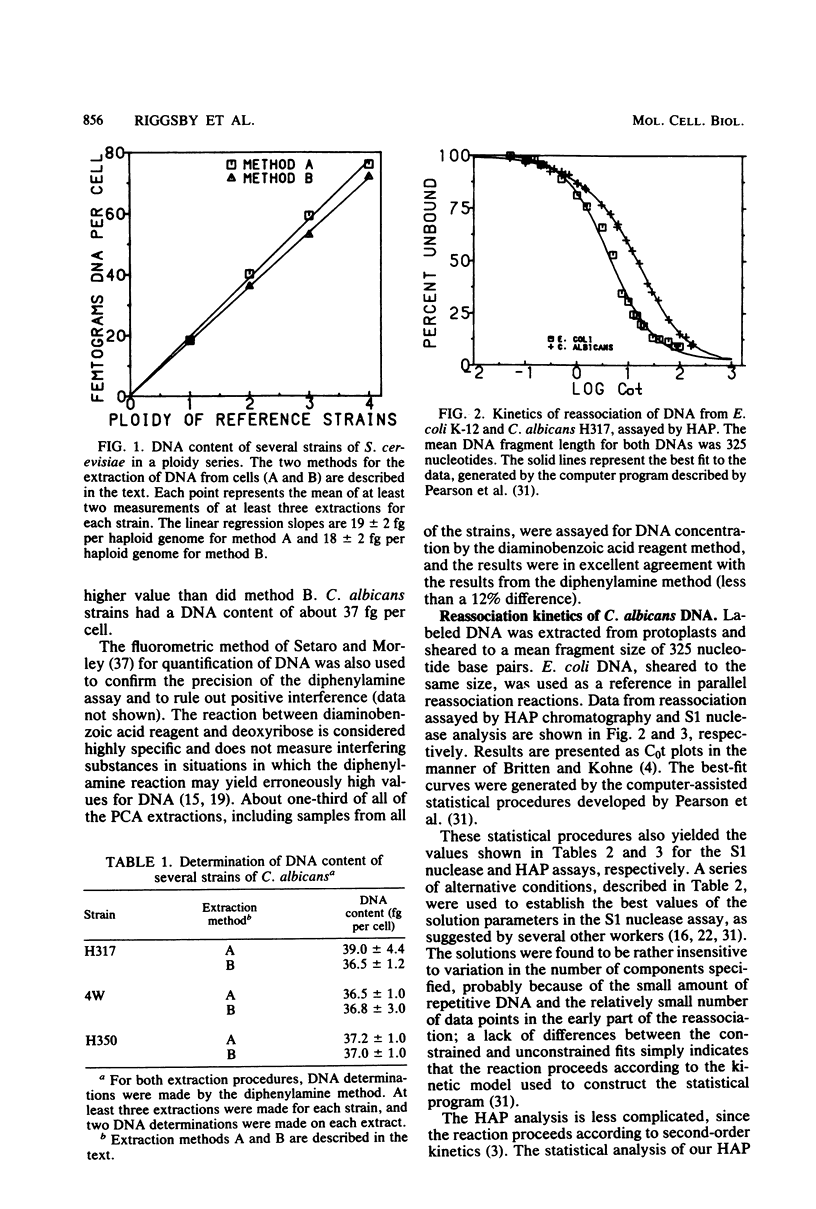
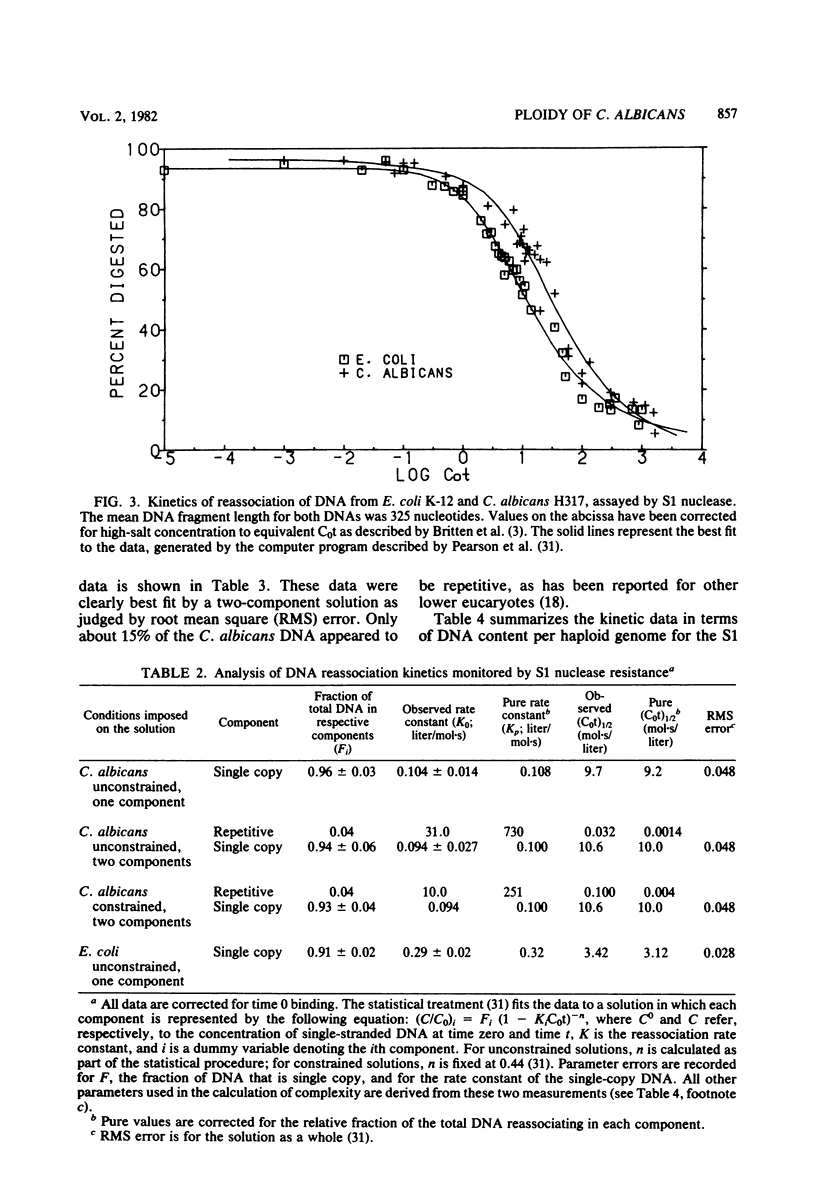
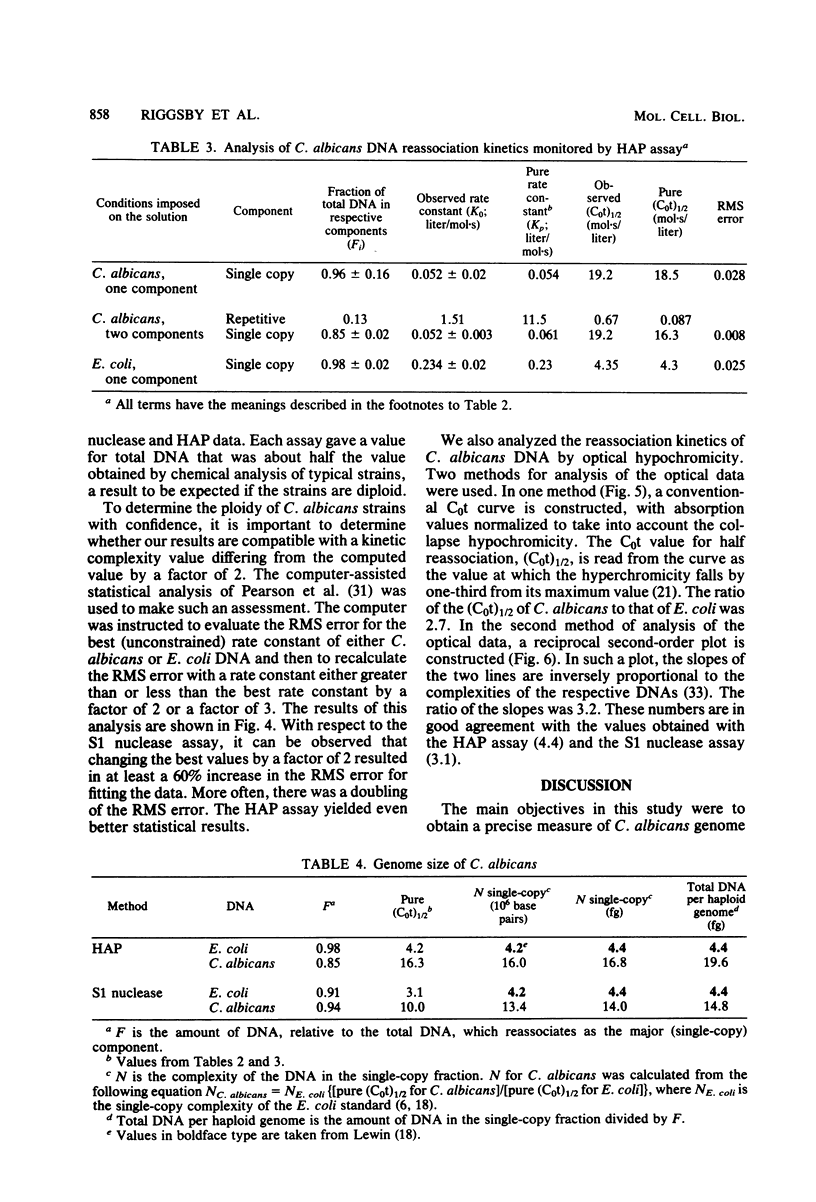
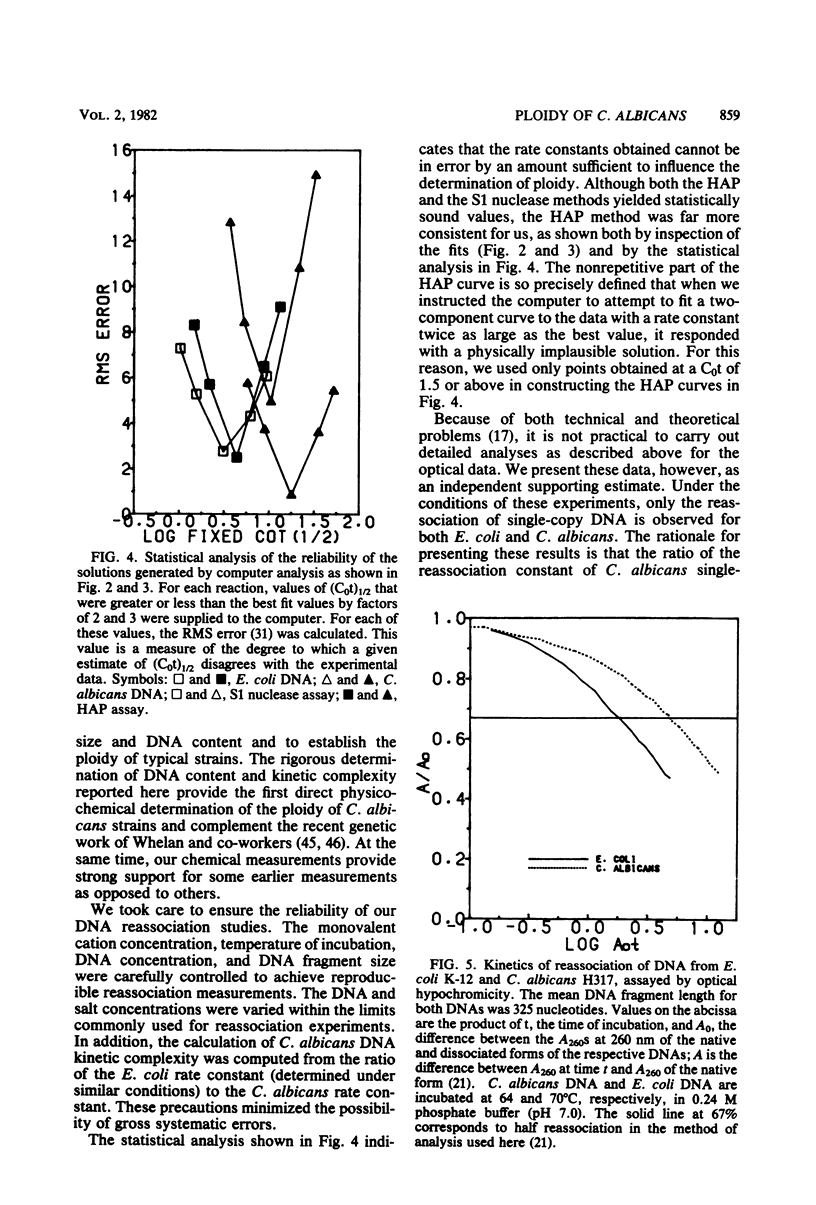
Candida albicans is a dimorphic fungus that is pathogenic for humans. No sexual cycle has been reported for this fungus, and earlier reports have differed on whether typical strains of C. albicans are haploid or diploid. Previous estimates of the DNA content of C. albicans varied by one order of magnitude. We used three independent methods to measure the kinetic complexity of the single-copy DNA from a typical strain of C. albicans (strain H317) to determine the DNA content per haploid genote; we obtained values of 15 and 20 fg per cell by using S1 nuclease and hydroxyapatite assays, respectively. Optical assays for DNA reassociation kinetics, although not definitive in themselves, yielded values in this range. Chemical measurements of the DNA content of several typical strains, including strain H317, yielded values clustered about a mean of 37 fg per cell. We concluded that these strains are diploid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahearn D. G. Medically important yeasts. Annu Rev Microbiol. 1978;32:59–68. doi: 10.1146/annurev.mi.32.100178.000423. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Graham D. E., Neufeld B. R. Analysis of repeating DNA sequences by reassociation. Methods Enzymol. 1974;29:363–418. doi: 10.1016/0076-6879(74)29033-5. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Ciferri O., Sora S., Tiboni O. Effect of gene dosage on tryptophan synthetase activity in Saccharomyces cerevisiae. Genetics. 1969 Mar;61(3):567–576. doi: 10.1093/genetics/61.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G., Hirschman S. Z. A neighbor-interaction analysis of the hypochromism and spectra of DNA. J Mol Biol. 1965 Sep;13(2):407–427. doi: 10.1016/s0022-2836(65)80106-1. [DOI] [PubMed] [Google Scholar]
- Fisher M. P., Dingman C. W. Role of molecular conformation in determining the electrophoretic properties of polynucleotides in agarose-acrylamide composite gels. Biochemistry. 1971 May 11;10(10):1895–1899. doi: 10.1021/bi00786a026. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Hatzfeld J. DNA labelling and its assay in yeast. Biochim Biophys Acta. 1973 Feb 23;299(1):34–42. doi: 10.1016/0005-2787(73)90395-x. [DOI] [PubMed] [Google Scholar]
- Hirschman S. Z., Felsenfeld G. Determination of DNA composition and concentration by spectral analysis. J Mol Biol. 1966 Apr;16(2):347–358. doi: 10.1016/s0022-2836(66)80178-x. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Krumlauf R., Marzluf G. A. Characterization of the sequence complexity and organization of the Neurospora crassa genome. Biochemistry. 1979 Aug 21;18(17):3705–3713. doi: 10.1021/bi00584a011. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Lohr D., Kovacic R. T., Van Holde K. E. Quantitative analysis of the digestion of yeast chromatin by staphylococcal nuclease. Biochemistry. 1977 Feb 8;16(3):463–471. doi: 10.1021/bi00622a020. [DOI] [PubMed] [Google Scholar]
- Mitchell R. M., Loeblich L. A., Klotz L. C., Loeblich A. R., 3rd DNA organization of Methanobacterium thermoautotrophicum. Science. 1979 Jun 8;204(4397):1082–1084. doi: 10.1126/science.377486. [DOI] [PubMed] [Google Scholar]
- Murray M. G., Palmer J. D., Cuellar R. E., Thompson W. F. Deoxyribonucleic acid sequence organization in the mung bean genome. Biochemistry. 1979 Nov 13;18(23):5259–5266. doi: 10.1021/bi00590a034. [DOI] [PubMed] [Google Scholar]
- OGUR M., MINCKLER S., LINDEGREN G., LINDEGREN C. C. The nucleic acids in a polyploid series of Saccharomyces. Arch Biochem Biophys. 1952 Sep;40(1):175–184. doi: 10.1016/0003-9861(52)90085-4. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Ogletree F. F., Abdelal A. T., Ahearn D. G. Germ-tube formation by atypical strains of Candida albicans. Antonie Van Leeuwenhoek. 1978;44(1):15–24. doi: 10.1007/BF00400073. [DOI] [PubMed] [Google Scholar]
- Olaiya A. F., Sogin S. J. Ploidy determination of Canadida albicans. J Bacteriol. 1979 Dec;140(3):1043–1049. doi: 10.1128/jb.140.3.1043-1049.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaiya A. F., Steed J. R., Sogin S. J. Deoxyribonucleic acid-deficient strains of Candida albicans. J Bacteriol. 1980 Mar;141(3):1284–1290. doi: 10.1128/jb.141.3.1284-1290.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Davidson E. H., Britten R. J. A program for least squares analysis of reassociation and hybridization data. Nucleic Acids Res. 1977 Jun;4(6):1727–1737. doi: 10.1093/nar/4.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulter R., Jeffery K., Hubbard M. J., Shepherd M. G., Sullivan P. A. Parasexual genetic analysis of Candida albicans by spheroplast fusion. J Bacteriol. 1981 Jun;146(3):833–840. doi: 10.1128/jb.146.3.833-840.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rau D. C., Klotz L. C. A unified theory of nucleation-rate-limited DNA renaturation kinetics. Biophys Chem. 1978 Mar;8(1):41–51. doi: 10.1016/0301-4622(78)85021-2. [DOI] [PubMed] [Google Scholar]
- Sarachek A., Rhoads D. D., Schwarzhoff R. H. Hybridization of Candida albicans through fusion of protoplasts. Arch Microbiol. 1981 Mar;129(1):1–8. doi: 10.1007/BF00417169. [DOI] [PubMed] [Google Scholar]
- Schweizer E., Halvorson H. O. On the regulation of ribosomal RNA synthesis in yeast. Exp Cell Res. 1969 Aug;56(2):239–244. doi: 10.1016/0014-4827(69)90008-1. [DOI] [PubMed] [Google Scholar]
- Setaro F., Morley C. D. A rapid colorimetric assay for DNA. Anal Biochem. 1977 Aug;81(2):467–471. doi: 10.1016/0003-2697(77)90721-7. [DOI] [PubMed] [Google Scholar]
- Shepherd M. G., Yin C. Y., Ram S. P., Sullivan P. A. Germ tube induction in Candida albicans. Can J Microbiol. 1980 Jan;26(1):21–26. doi: 10.1139/m80-004. [DOI] [PubMed] [Google Scholar]
- Stewart P. R. Analytical methods for yeasts. Methods Cell Biol. 1975;12:111–147. doi: 10.1016/s0091-679x(08)60955-3. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Low repetitive DNA content in Aspergillus nidulans. Science. 1978 Dec 1;202(4371):973–975. doi: 10.1126/science.362530. [DOI] [PubMed] [Google Scholar]
- Torres-Bauzá L. J., Riggsby W. S. Protoplasts from yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1980 Aug;119(2):341–349. doi: 10.1099/00221287-119-2-341. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Magee P. T. Natural heterozygosity in Candida albicans. J Bacteriol. 1981 Feb;145(2):896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- van der Walt J. P., Pitout M. J. Ploidy differences in Sporobolomyces salmonicolor and Candida albicans. Antonie Van Leeuwenhoek. 1969;35(2):227–231. [PubMed] [Google Scholar]


