Abstract
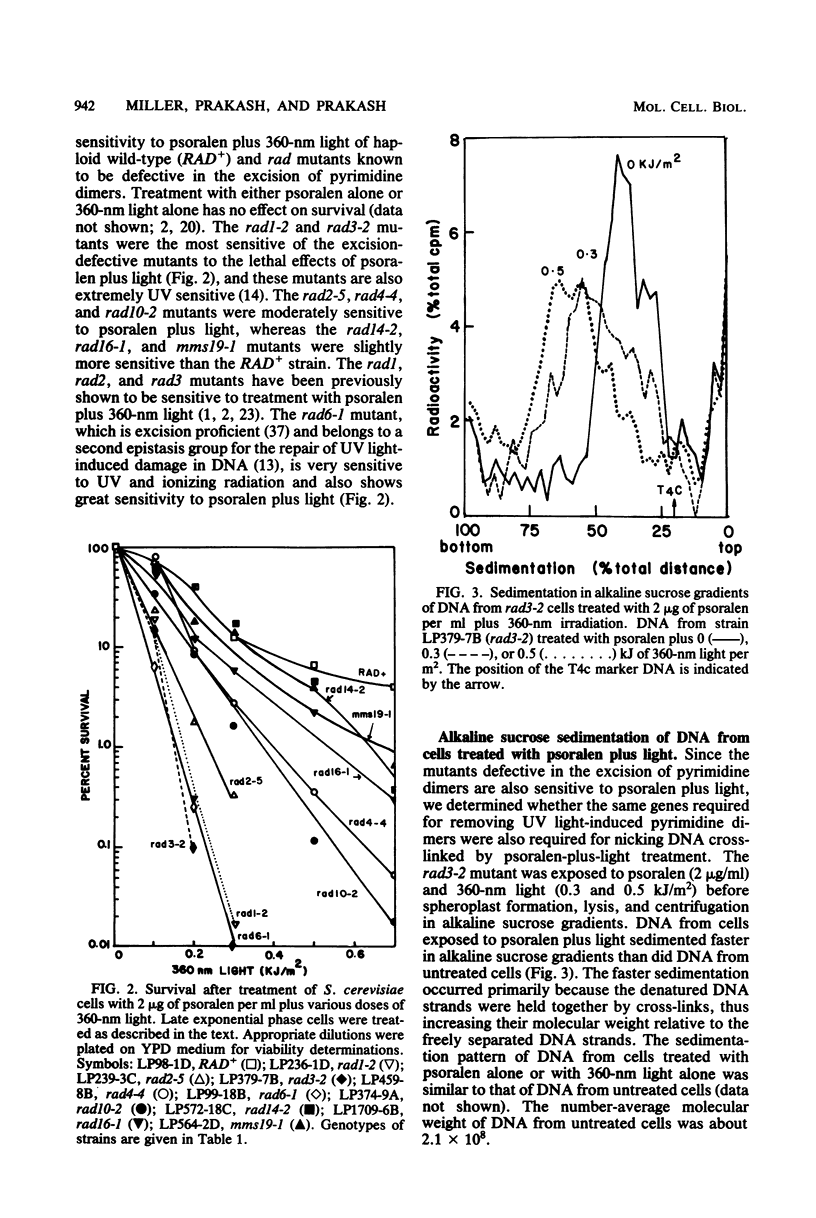
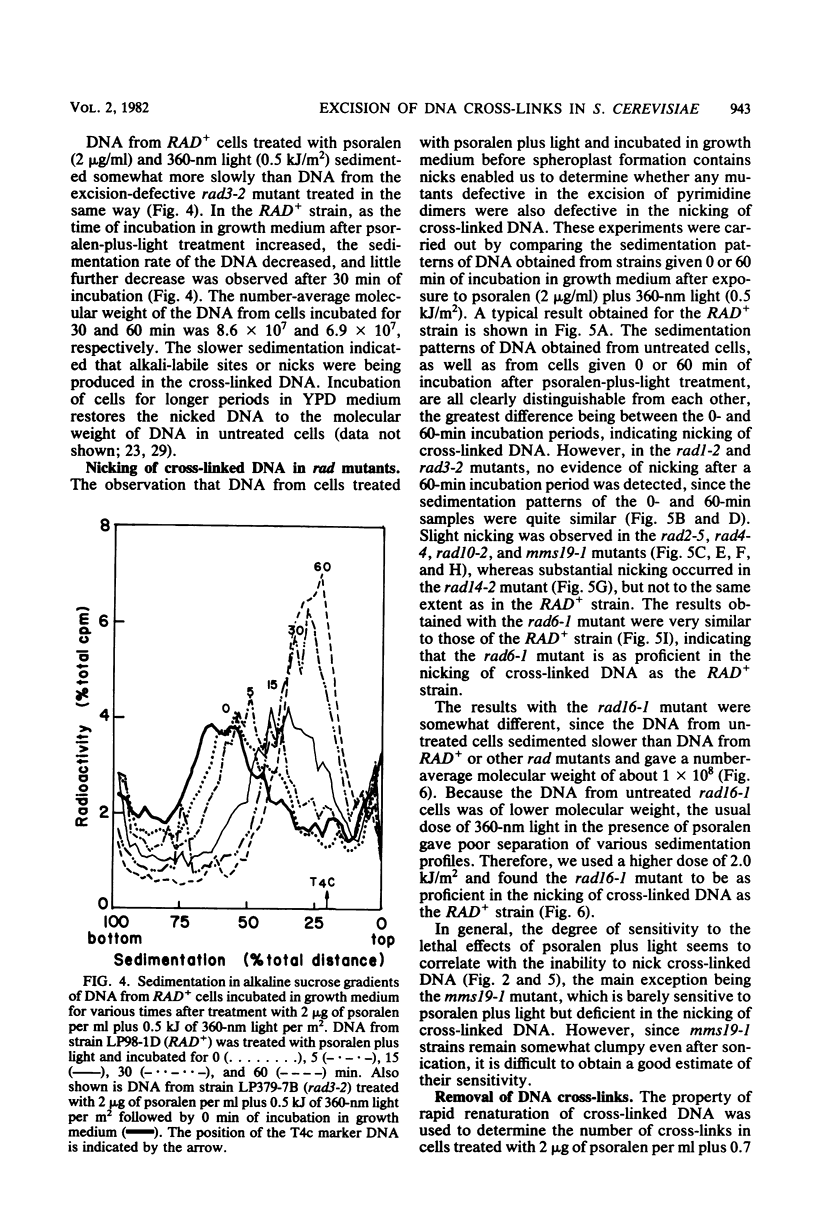
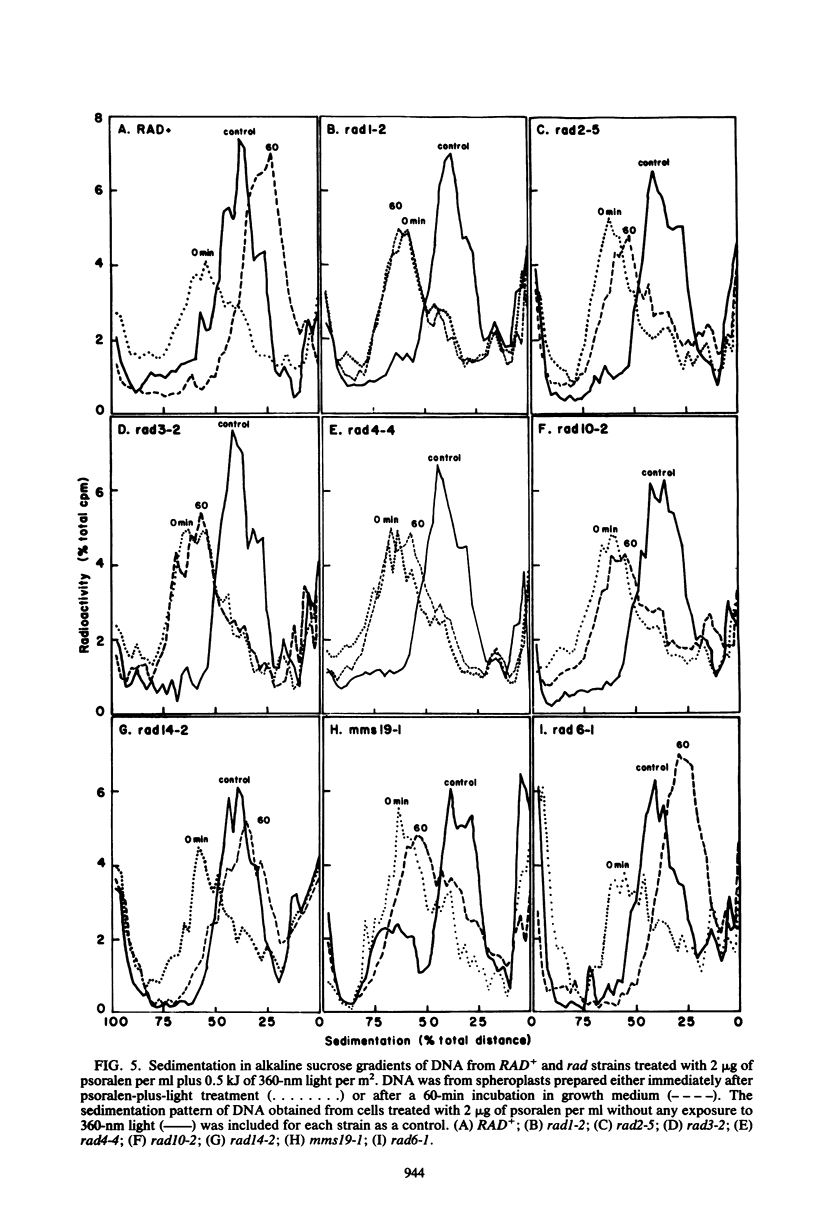
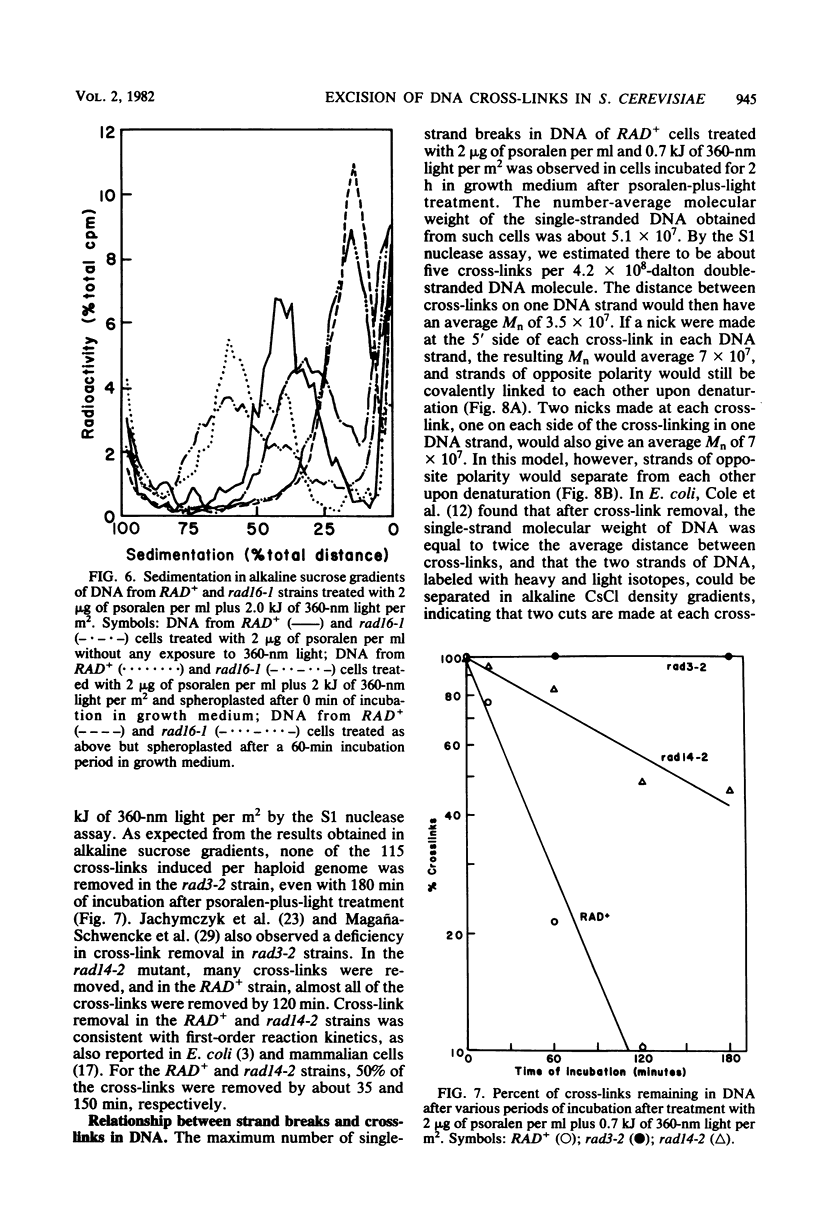
Excision of interstrand DNA cross-links induced by 4,5',8-trimethyl psoralen plus 360-nm light was examined in wild type (RAD+) and various radiation-sensitive (rad) mutants of Saccharomyces cerevisiae known to be defective in the excision of UV light-induced pyrimidine dimers. Alkaline sucrose sedimentation of DNA after incubation of psoralen-plus-light-treated cells indicated little or no nicking of cross-linked DNA in rad1-2, rad2-5, rad3-2, rad4-4, rad10-2, and mms19-1 mutants. In the rad14-2 mutant, substantial nicking was observed but to a much lesser extent than in the RAD+ strains, whereas the rad16-1 mutant was as proficient in nicking as the RAD+ strain. Removal of cross-links was also examined in RAD+, rad3-2, and rad14-2 strains by determining the sensitivity of alkali-denatured and -neutralized DNA to hydrolysis by S1 nuclease. No cross-link removal was observed in the rad3-2 mutants, and the rad14-2 mutant was much less efficient than the RAD+ strain in removing cross-links.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Averbeck D., Averbeck S. Dose-rate effects of 8-methoxypsoralen plus 365-NM irradiation on cell killing in Saccharomyces cerevisiae. Mutat Res. 1978 May;50(2):195–206. doi: 10.1016/0027-5107(78)90024-6. [DOI] [PubMed] [Google Scholar]
- Averbeck D., Moustacchi E. 8-Methoxypsoralen plus 365 nm light effects and repair in yeast. Biochim Biophys Acta. 1975 Jul 23;395(4):393–404. doi: 10.1016/0005-2787(75)90063-5. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hur E., Prager A., Riklis E. Measurement of DNA crosslinks by S1 nuclease: induction and repair in psoralen-plus-360 nm light treated Escherichia coli. Photochem Photobiol. 1979 May;29(5):921–924. doi: 10.1111/j.1751-1097.1979.tb07792.x. [DOI] [PubMed] [Google Scholar]
- Brendel M., Haynes R. H. Kinetics and genetic control of the incorporation of thymidine monophosphate in yeast DNA. Mol Gen Genet. 1972;117(1):39–44. doi: 10.1007/BF00268835. [DOI] [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Inactivation of Escherichia coli, F' episomes at transfer, and bacteriophage lambda by psoralen plus 360-nm light: significance of deoxyribonucleic acid cross-links. J Bacteriol. 1971 Sep;107(3):846–852. doi: 10.1128/jb.107.3.846-852.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S., Levitan D., Sinden R. R. Removal of psoralen interstrand cross-links from DNA of Escherichia coli: mechanism and genetic control. J Mol Biol. 1976 May 5;103(1):39–59. doi: 10.1016/0022-2836(76)90051-6. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Repair of DNA containing interstrand crosslinks in Escherichia coli: sequential excision and recombination. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1064–1068. doi: 10.1073/pnas.70.4.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B. S., Parry J. M. The isolation, genetics and survival characteristics of ultraviolet light-sensitive mutants in yeast. Mutat Res. 1968 Jul-Aug;6(1):37–55. doi: 10.1016/0027-5107(68)90101-2. [DOI] [PubMed] [Google Scholar]
- Cox B., Game J. Repair systems in Saccharomyces. Mutat Res. 1974 Aug;26(4):257–264. doi: 10.1016/s0027-5107(74)80023-0. [DOI] [PubMed] [Google Scholar]
- Doty P., McGill B. B., Rice S. A. THE PROPERTIES OF SONIC FRAGMENTS OF DEOXYRIBOSE NUCLEIC ACID. Proc Natl Acad Sci U S A. 1958 May;44(5):432–438. doi: 10.1073/pnas.44.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eigner J., Doty P. The native, denatured and renatured states of deoxyribonucleic acid. J Mol Biol. 1965 Jul;12(3):549–580. doi: 10.1016/s0022-2836(65)80312-6. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Tatsumi M. Cross-link repair in human cells and its possible defect in Fanconi's anemia cells. J Mol Biol. 1977 Jul 15;113(4):635–649. doi: 10.1016/0022-2836(77)90227-3. [DOI] [PubMed] [Google Scholar]
- Game J. C., Zamb T. J., Braun R. J., Resnick M., Roth R. M. The Role of Radiation (rad) Genes in Meiotic Recombination in Yeast. Genetics. 1980 Jan;94(1):51–68. doi: 10.1093/genetics/94.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring E. S., Grossman L. I., Krupnick D., Cryer D. R., Marmur J. The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol. 1970 Sep 14;52(2):323–335. doi: 10.1016/0022-2836(70)90033-1. [DOI] [PubMed] [Google Scholar]
- Henriques J. A., Moustacchi E. Isolation and characterization of pso mutants sensitive to photo-addition of psoralen derivatives in Saccharomyces cerevisiae. Genetics. 1980 Jun;95(2):273–288. doi: 10.1093/genetics/95.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jachymczyk W. J., Chlebowicz E., Swietlinska Z., Zuk J. Alkaline sucrose sedimentation studies of MMS-induced DNA single-strand breakage and rejoining in the wild type and in UV-sensitive mutants of Saccharomyces cerevisiae. Mutat Res. 1977 Apr;43(1):1–10. doi: 10.1016/0027-5107(77)90126-9. [DOI] [PubMed] [Google Scholar]
- Jachymczyk W. J., von Borstel R. C., Mowat M. R., Hastings P. J. Repair of interstrand cross-links in DNA of Saccharomyces cerevisiae requires two systems for DNA repair: the RAD3 system and the RAD51 system. Mol Gen Genet. 1981;182(2):196–205. doi: 10.1007/BF00269658. [DOI] [PubMed] [Google Scholar]
- Jackson J. A., Fink G. R. Gene conversion between duplicated genetic elements in yeast. Nature. 1981 Jul 23;292(5821):306–311. doi: 10.1038/292306a0. [DOI] [PubMed] [Google Scholar]
- Kaye J., Smith C. A., Hanawalt P. C. DNA repair in human cells containing photoadducts of 8-methoxypsoralen or angelicin. Cancer Res. 1980 Mar;40(3):696–702. [PubMed] [Google Scholar]
- Kutter E. M., Wiberg J. S. Degradation of cytosin-containing bacterial and bacteriophage DNA after infection of Escherichia coli B with bacteriophage T4D wild type and with mutants defective in genes 46, 47 and 56. J Mol Biol. 1968 Dec;38(3):395–411. doi: 10.1016/0022-2836(68)90394-x. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. UV mutagenesis in radiation-sensitive strains of yeast. Genetics. 1976 Feb;82(2):207–232. doi: 10.1093/genetics/82.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUSAJO L., RODIGHIERO G., COLOMBO G., TORLONE V., DALLACQUA F. PHOTOSENSITIZING FUROCOUMARINS: INTERACTION WITH DNA AND PHOTO-INACTIVATION OF DNA CONTAINING VIRUSES. Experientia. 1965 Jan 15;21:22–24. doi: 10.1007/BF02136362. [DOI] [PubMed] [Google Scholar]
- MUSAJO L., RODIGHIERO G., DALLACQUA F. EVIDENCES OF A PHOTOREACTION OF THE PHOTOSENSITIZING FUROCOUMARINS WITH DNA AND WITH PYRIMIDINE NUCLEOSIDES AND NUCLEOTIDES. Experientia. 1965 Jan 15;21:24–26. doi: 10.1007/BF02136363. [DOI] [PubMed] [Google Scholar]
- Magaña-Schwencke N., Henriques J. A., Chanet R., Moustacchi E. The fate of 8-methoxypsoralen photoinduced crosslinks in nuclear and mitochondrial yeast DNA: comparison of wild-type and repair-deficient strains. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1722–1726. doi: 10.1073/pnas.79.6.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone R. E., Esposito R. E. The RAD52 gene is required for homothallic interconversion of mating types and spontaneous mitotic recombination in yeast. Proc Natl Acad Sci U S A. 1980 Jan;77(1):503–507. doi: 10.1073/pnas.77.1.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee R. H., Lawrence C. W. Genetic analysis of gamma-ray mutagenesis in yeast. I. Reversion in radiation-sensitive strains. Genetics. 1979 Oct;93(2):361–373. doi: 10.1093/genetics/93.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Rodighiero G., Breccia A., Dall'Acqua F., Malesani G. Skin-photosensitizing furocoumarins: photochemical interaction between DNA and -O14CH3 bergapten (5-methoxy-psoralen). Photochem Photobiol. 1966 Sep;5(9):739–745. doi: 10.1111/j.1751-1097.1966.tb05823.x. [DOI] [PubMed] [Google Scholar]
- Prakash L. Characterization of postreplication repair in Saccharomyces cerevisiae and effects of rad6, rad18, rev3 and rad52 mutations. Mol Gen Genet. 1981;184(3):471–478. doi: 10.1007/BF00352525. [DOI] [PubMed] [Google Scholar]
- Prakash L. Defective thymine dimer excision in radiation-sensitive mutants rad10 and rad16 of Saccharomyces cerevisiae. Mol Gen Genet. 1977 Apr 29;152(3):125–128. doi: 10.1007/BF00268808. [DOI] [PubMed] [Google Scholar]
- Prakash L. Lack of chemically induced mutation in repair-deficient mutants of yeast. Genetics. 1974 Dec;78(4):1101–1118. doi: 10.1093/genetics/78.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L., Prakash S. Three additional genes involved in pyrimidine dimer removal in Saccharomyces cerevisiae: RAD7, RAD14 and MMS19. Mol Gen Genet. 1979 Nov;176(3):351–359. doi: 10.1007/BF00333097. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975 Nov 15;98(4):781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in radiation-sensitive mutants rad3, rad4, rad6 and rad9 of Saccharomyces cerevisiae. Mutat Res. 1977 Oct;45(1):13–20. doi: 10.1016/0027-5107(77)90038-0. [DOI] [PubMed] [Google Scholar]
- Prakash S., Prakash L., Burke W., Montelone B. A. Effects of the RAD52 Gene on Recombination in SACCHAROMYCES CEREVISIAE. Genetics. 1980 Jan;94(1):31–50. doi: 10.1093/genetics/94.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Martin P. The repair of double-strand breaks in the nuclear DNA of Saccharomyces cerevisiae and its genetic control. Mol Gen Genet. 1976 Jan 16;143(2):119–129. doi: 10.1007/BF00266917. [DOI] [PubMed] [Google Scholar]
- Resnick M. A., Setlow J. K. Repair of pyrimidine dimer damage induced in yeast by ultraviolet light. J Bacteriol. 1972 Mar;109(3):979–986. doi: 10.1128/jb.109.3.979-986.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J., Friedberg E. C. Molecular mechanisms of pyrimidine dimer excision in Saccharomyces cerevisiae: incision of ultraviolet-irradiated deoxyribonucleic acid in vivo. J Bacteriol. 1981 May;146(2):692–704. doi: 10.1128/jb.146.2.692-704.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds R. J. Removal of pyrimidine dimers from Saccharomyces cerevisiae nuclear DNA under nongrowth conditions as detected by a sensitive, enzymatic assay. Mutat Res. 1978 Apr;50(1):43–56. doi: 10.1016/0027-5107(78)90059-3. [DOI] [PubMed] [Google Scholar]
- Ross P., Howard-Flanders P. Initiation of recA+-dependent recombination in Escherichia coli (lambda). I. Undamaged covalent circular lambda DNA molecules in uvrA+ recA+ lysogenic host cells are cut following superinfection with psoralen-damaged lambda phages. J Mol Biol. 1977 Nov 25;117(1):137–158. doi: 10.1016/0022-2836(77)90028-6. [DOI] [PubMed] [Google Scholar]
- Ruhland A., Haase E., Siede W., Brendel M. Isolation of yeast mutants sensitive to the bifunctional alkylating agent nitrogen mustard. Mol Gen Genet. 1981;181(3):346–351. doi: 10.1007/BF00425609. [DOI] [PubMed] [Google Scholar]
- Seeberg E. Reconstitution of an Escherichia coli repair endonuclease activity from the separated uvrA+ and uvrB+/uvrC+ gene products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2569–2573. doi: 10.1073/pnas.75.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg E. Strand cleavage at psoralen adducts and pyrimidine dimers in DNA caused by interaction between semi-purified uvr+ gene products from Escherichia coli. Mutat Res. 1981 Jun;82(1):11–22. doi: 10.1016/0027-5107(81)90133-0. [DOI] [PubMed] [Google Scholar]
- Sinden R. R., Cole R. S. Repair of cross-linked DNA and survival of Escherichia coli treated with psoralen and light: effects of mutations influencing genetic recombination and DNA metabolism. J Bacteriol. 1978 Nov;136(2):538–547. doi: 10.1128/jb.136.2.538-547.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unrau P., Wheatcroft R., Cox B. S. The excision of pyrimidine dimers from DNA of ultraviolet irradiated yeast. Mol Gen Genet. 1971;113(4):359–362. doi: 10.1007/BF00272336. [DOI] [PubMed] [Google Scholar]
- Waters R., Moustacchi E. The disappearance of ultraviolet-induced pyrimidine dimers from the nuclear DNA of exponential and stationary phase cells of Saccharomyces cerevisiae following various post-irradiation treatments. Biochim Biophys Acta. 1974 Jul 24;353(4):407–419. doi: 10.1016/0005-2787(74)90048-3. [DOI] [PubMed] [Google Scholar]
- Wickner R. B. Mutants of Saccharomyces cerevisiae that incorporate deoxythymidine-5'-monophosphate into deoxyribonucleic acid in vivo. J Bacteriol. 1974 Jan;117(1):252–260. doi: 10.1128/jb.117.1.252-260.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox D. R., Prakash L. Incision and postincision steps of pyrimidine dimer removal in excision-defective mutants of Saccharomyces cerevisiae. J Bacteriol. 1981 Nov;148(2):618–623. doi: 10.1128/jb.148.2.618-623.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoakum G. H., Cole R. S. Role of ATP in removal of psoralen cross-links from DNA of Escherichia coli permeabilized by treatment with toluene. J Biol Chem. 1977 Oct 25;252(20):7023–7030. [PubMed] [Google Scholar]


