Abstract
Objective
Polycystic kidney disease (PKD) is a common cause of end stage renal failure and many of these patients suffer vascular dysfunction and hypertension. It remains unclear whether PKD is associated with abnormal microvascular structure. Thus, this study examined the renovascular structure in PKD.
Methods
PKD rats (PCK model) and controls were studied at 10 weeks of age, and mean arterial pressure (MAP), renal blood flow and creatinine clearance were measured. Microvascular architecture and cyst number and volume were assessed using micro-computed tomography, and angiogenic pathways evaluated.
Results
Compared to controls, PKD animals had an increase in MAP (126.4±4.0 vs. 126.2±2.7mmHg) and decreased clearance of creatinine (0.39±0.09 vs. 0.30±0.05ml/min), associated with a decrease in microvascular density, both in the cortex (256±22 vs. 136±20 vessels per cm2) and medullar (114±14 vs. 50±9 vessels/cm2) and an increase in the average diameter of glomeruli (104.14±2.94 vs. 125.76±9.06 mm). PKD animals had increased fibrosis (2.2±0.2 fold vs. control) and a decrease in the cortical expression in hypoxia inducible factor 1-α and vascular endothelial growth factor.
Conclusion
PKD animals have impaired renal vascular architecture, which can have significant functional consequences. The PKD microvasculature could represent a therapeutic target to decrease the impact of this disease.
Keywords: polycystic kidney disease, vasculature, cysts, kidney, micro-computed tomography, angiogenesis, renal function
Introduction
Hereditary PKD is the most common lethal monogenic genetic diseases of man, affecting ~1/1,000 to 1:20,000 individuals and constitutes a common cause of end-stage renal disease.1, 2 Autosomal dominant PKD has two disease loci that encode ciliary glycoproteins (polycystin-1 and -2), while the autosomal recessive form of the disease (ARPKD) has a single locus and encodes fibrocystin-1. These proteins are thought to regulate renal tubular and vascular development.3, 4 PKD is considered to originate from the tubular cells in the collecting duct, leading to formation of parenchymal cysts with subsequent tubular and renal dysfunction, and ultimately renal failure.
However, the abnormalities in PKD extend beyond cyst development and renal tubular dysfunction, as around 50% of patients with ADPKD develop hypertension5, 6 even in the presence of unchanged global renal function, with the majority of children with ARPKD being hypertensive as well. Furthermore, previous studies have shown in PKD a decrease in VEGF expression7 and abnormal vascular function.8, 9 It has also been suggested that renal cysts can compress the vascular tree, activating the renal angiotensin system10 leading to hypertension,11, 12, further underscoring a possible link between PKD and vascular disease.2 Whether the functional renal vascular impairment8, 9, 13, 14 in PKD is accompanied by alterations in renovascular architecture remains unknown, largely due to the lack of accurate and high-resolution methods to study the intact 3D renal microvascular architecture.
Micro-CT is a powerful technique that permits assessment of the 3D pattern of microvascular structure and provides a useful means for studying the spatial distribution of vessels within an organ.15–17 This technique may therefore provide a unique insight into the early structural changes of the renal vascular tree in PKD, what may serve to identify novel therapeutic targets in the treatment of this disease.
The PCK rat is an animal model commonly used in the study of PKD.18, 19 It is characterized by a naturally occurring splicing mutation of the ARPKD gene (Pkhd1)19, 20 and shares many features of human ADPKD, with the kidneys appearing normal at birth and having progressive cystic development.19 This model has been extensively used for the evaluation of novel therapies in PKD17, 21 mostly focusing on its renal tubular functional abnormalities. However, whether this model also has abnormal vascular architecture, as described in adult forms of PKD, remains unclear.
Thus, the present study will test the hypothesis that the PCK rats will have decrease expression of angiogenic factors that will lead to decreased vascular density in the renal microvasculature.
Methods
Experimental Design
All procedures were approved by the Institutional Animal Care and Use Committee. Animals (female gender, n=11) were divided into PKD (PCK rat model) and wild type controls (Sprague-Dawley). At 10 weeks of age, MAP was assessed non-invasively using the tail cuff method11 and RBF in-vivo using high-frequency ultrasound.22 Blood and urine samples were obtained at time of euthanasia, achieved by cervical dislocation, for assessment of creatinine clearance (CrCl) and tissue prepared for ex-vivo analysis of renal vascular structure using micro-CT. Pathways involved in the development and progression of renal vascular changes in PKD were investigated.
Systemic hemodynamics, renal cysts and renal function
Blood pressure measurement
Conscious animals were trained to the tail cuff for 2 weeks prior to blood pressure (BP) measurement. On the day of the studies, a cuff was placed around the animal’s tail to obtain a mean of systolic BP readings obtained, using the CODA blood pressure measurement system.11
Renal Blood Flow
Animals were anesthetized with isoflurane 2% and imaged with a High-Resolution Ultrasound (Vevo770, Visualsonics, Toronto, CA23, 30MHz frequency probe) for the assessment of RBF.22 RBF was calculated as renal artery area x velocity time integral x heart rate, where renal artery area was calculated as πxr2. Global renal perfusion was the calculated as RBF/renal volume (mL/min/cc).
Glomerular function
Creatinine in blood and urine were measured using an autoanalyzer (Hitachi 717; Roche, Mannheim, Germany). CrCl = U Cr x U Vol/P Cr, where U Cr is urinary creatinine, U Vol is urinary volume and P Cr is plasma creatinine. Furthermore, we estimated proteinuria, as an estimation of glomerular function, using the Bradford assay (absorbance at 595nm) with appropriate standards and standard curve, and express it as protein/creatinine ratio.
Micro-computed tomography
At time of euthanasia, animals were anesthetized with isoflurane 5% (in 2L O2), blood was drawn by cardiac puncture and a catheter (20G) inserted in the descending aorta below the level of the renal arteries. Subsequently, a solution of normal saline and heparin were infused using a Harvard pump at a flow rate of 0.9 mL/min (physiological perfusion pressure of 100 mm Hg).16 When the perfusate draining through the veins was free of blood, radiopaque Microfil silicone rubber (MV-122, Flow Tech, Inc) was perfused through the descending aorta at the same flow rate and physiological perfusion pressure. This contrast agent essentially remains in the intravascular compartment.16 Filling was deemed complete when the Microfil flowed freely from the superior vena cava. Kidneys were then removed, kept at 4°C for 1 day, and then prepared as previously described.24
Kidney samples were the scanned with micro-CT as previously described.15, 16 Briefly, samples were scanned at 0.49° angular increments, providing 721 views around 360°, images recorded, digitized, and transferred to a controlling computer. The 3D volume images, reconstructed with a modified Feldkamp’s filtered backprojection algorithm were displayed with a cubic voxel of 17 μm and the radiopacity of each voxel was represented by a 16-bit gray-scale value. Image analysis was then performed with the Analyze™ software package (Biomedical Imaging Resource, Mayo Foundation).
Renal cyst and volume
After creating 3D volume renderings of the cysts, intensity thresholding was used to automatically produce binary images to identify renal cysts, and then calculate the cross sectional areas of cysts, and the entire kidney cyst number. Furthermore, we calculated the total volumes of the cortex and the medulla (cc) and their fraction of the total kidney volume.
Renal microvasculature
For analysis of the cortex, the three-dimensional tomographic images were oriented so that the z-axis was perpendicular to the radial vessels, and then tomographically analyzed. The cortex was divided into outer and inner regions, and the outer medulla divided into an OSOM and ISOM, as previously described.25 The spatial density of cortical microvessels (diameters <500 μm) was calculated in each cross-section. Microvessels were further classified as small (diameters between 18 and 99 μm), medium (diameters between 100 to 199 μm), and large microvessels (>200 μm). The VVF in each renal region was measured from their cross-sectional slices (Otissue), from an interlobar artery (Oartery), and in the background matrix outside the kidney (Obg). The VVF in each tissue was then calculated as previously described:25 (Otissue-Obkg)/(Oartery-Obkg).
Glomeruli
For glomerular quantification, the entire renovascular tree was tomographically connected. Then the glomeruli were tomographically isolated and using an intensity threshold that converted the image into a binary image and the glomeruli were counted using Matlab®, and expressed as number of glomeruli per mm3. The average glomerular diameter was also found by analyzing the binary image of the glomeruli. The central 20 cross-sections of the binary image were used in order to get a representative number of glomeruli for the average.
Tissue analysis
Renal tissue was obtained at time of harvest and histological samples (5μm in thickness) were stained with hematoxylin & eosin for gross morphology and assessment of cysts.
For the assessment of fibrosis, slides were stained with Picrosirius Red, and imaged using a Nikon Eclipse 50i clinical microscope (Nikon Instruments Inc., Melville, NY) and Spot Advanced modular image software (Spot™ Imaging Solutions, Sterling Heights, MI). The percentage area of fibrosis was quantified using MetaMorph microscopy automation and image analysis software (Molecular Devices, LLC, Sunnyvale, CA) and averaged from four random fields of view (x20 magnification) for each of the cortex and medulla per slide.
Furthermore, we performed Western blotting on renal tissue following standard protocols,26–28 with β-actin (1:1000; Sigma, St. Louis, MO) as the loading control. Primary antibodies included HIF-1α (1:1000, Novus Biologicals, Littleton, CO), VEGF 1 (1:10000, Abcam, Cambridge, MA), VEGF A (Abcam, 1μg/ml), VEGF C (1:1000, Novus Biologicals), and NFKβ p65 (1:2000, Cell Signaling, Beverly, MA).
Statistical Analysis
Data are expressed as mean±SEM. Comparison between the groups was performed using unpaired Student’s t-test. Statistical significance was accepted for a value of p≤0.05. Furthermore, we performed a Pearsons test to establish correlations between cyst size, volume and number with changes in microvascular density.
Results
Compared to controls, 10 week-old PKD animals had an increase in body weight (191±3.6 and 211±8.0 grams, respectively, p=0.004) and the kidneys of the affected animals were significantly larger (Figure 1A). The volume of both the cortex and the medulla of PKD animals (1081±139 and 920±66 cc, respectively) were significantly larger than controls (594±38 and 640±25 cc, respectively, both p<0.05). The increase in kidney volume in PKD resulted mostly from expansion of the medulla (control medulla: 47.98±0.8, and PKD medulla: 53.58±2.18%, p=0.038), while the cortex slightly shrunk (control cortex: 52.01±0.8 and PKD cortex: 46.41±2.1%, p=0.038). At 10 weeks of age, cysts occupied 10.2±2.03% of the kidney volume and were mainly restricted to the medullary region (Figure 1B) with an average of 36.2±8.1 cysts and total cyst volume of 207.2±23.1cc. PKD animals had significantly higher MAP (Figure 1C), while renal perfusion and clearance of creatinine were significantly decreased compared to controls (Figure 1D and 1E). The protein/creatinine ratio was not different between PCK animals and controls (Figure 1F).
Figure 1.
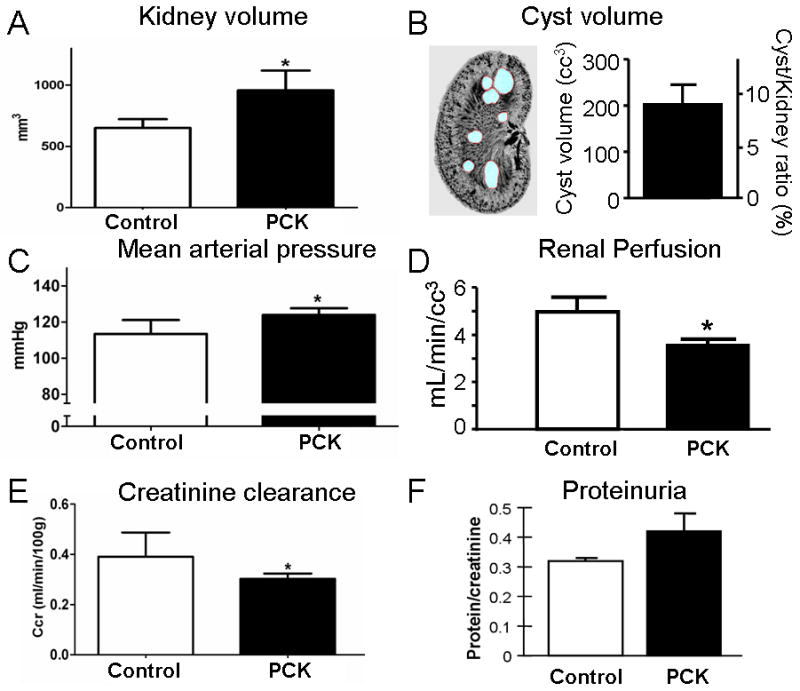
General characteristics of control and PCK animals. Compared to controls, 10 weeks old PCK rats had increased renal volume (A), with 9.7±1.6% of the entire kidney occupied by cysts (B). PCK animals had a small but significant increase in mean arterial pressure (C) and a decrease in renal perfusion (D), with impaired glomerular function, as assessed by clearance of creatinine (E) and proteinuria (F). *p<0.05 vs. control.
Representative 3D micro-CT images of the renal microvasculature of control and PKD rats (Figures 2A and 2B, respectively), demonstrating a decrease in microvascular density in PKD animals. Furthermore, tomographically isolated vessels show irregular luminal changes (arrows) seen in PKD (D), and not in control (C). The decrease in microvessels was observed both in the cortex (control: 256±22 and PKD: 136±20 vessels per cm2, p<0.05) and the medulla (control: 114±14, PKD: 50±9 vessels per cm2, p<0.05). The decreased density of cortical microvessels in PKD animals was observed both in the outer and inner cortex (Table), with a comparable decrease in VVF suggesting that this did not result from expansion of the extravascular compartment. Importantly, the decrease in microvessels corresponded to vessels under 100 microns in diameter, both in the outer (control: 389±42 and PKD: 169±58 per cm2, p=0.01) and inner cortex (control: 620±72 and PKD: 262±73 per cm2, p<0.05), while the distribution of microvessels over 100 microns was only lower in the inner cortex (control: 351±55 and PKD: 129±43 per cm2, p=0.006), but not in the outer cortex (control: 115±33 and PKD: 70±34 per cm2, p=0.18). Furthermore, in both the groups of animals, the inner cortex had more vessels with a diameter >200 microns, compared to the outer cortex (51±12 vs 1±1 per cm2, respectively, p=0.004). In PKD only the inner, but not the outer, cortical vessel density decrease was accompanied by a lower VVF compared to controls. We did not find a correlation between the degree of cyst number or size and the changes in overall vessel density/distribution.
Figure 2.
Top, representative 3D images of kidneys from control (A) and PCK (B) animals, showing a decreased density of cortical microvessels in PCK animals. Samples were scanned with micro-CT, reconstructed and displayed at a resolution of 17 μm cubic voxel resolution. Bottom, representative 3D images of cortical renal vasculature of control (C) and PCK (D) animals displayed at 17 μm resolution, demonstrating segmental irregularities in PKD vasculature.
Table.
Quantitation of renal vasculature assessed by micro-CT in control and PCK rats.
| control | PCK | |
|---|---|---|
| Vascular density (per cc2) | ||
| Outer cortex | 163±22 | 79±18* |
| Inner cortex | 350±21 | 194±23* |
| Outer strip outer medulla | 184±18 | 90±15* |
| Inner strip outer medulla | 44±10 | 9±3* |
| Glomeruli | ||
| Total number | 28,124±2464 | 34,044±7585 |
| Glomerular diameter (μm) | 104.14±2.94 | 125.76±9.06* |
| Vascular Volume Fraction (%) | ||
| Outer cortex | 22.27±0.94 | 21.03±1.67 |
| Inner cortex | 25.15±1.27 | 20.83±1.32* |
| Outer strip outer medulla | 21.25±0.83 | 19.22±2.92 |
| Inner strip outer medulla | 24.54±1.53 | 18.01±0.52* |
p<0.05 compared to control
PCK animals also had an increased in mean glomerular diameter, suggesting hypertrophy of the glomeruli in PKD, while their total number was not different (Table). We did observe an inverse correlation between cyst size and glomerular density (r2=0.9812, p=0.001). A decrease in vascular density with a change in VVF and vascular/tissue ratio was also observed in the outer and inner strips of the outer medullary region (Table).
By 10 weeks of age, PKD animals showed the development of cysts (Figure 3A–B) and increased fibrosis, both in the cortex (Figures 3C and 3D) and the medulla (Figures 3E and 3F), as measured by Picrosirius red (Figure 3G). Furthermore, PKD animals had a decreased expression of HIF 1-α, VEGFR-1 receptor, VEGF A and C compared to controls (Figures 3H–K).
Figure 3.
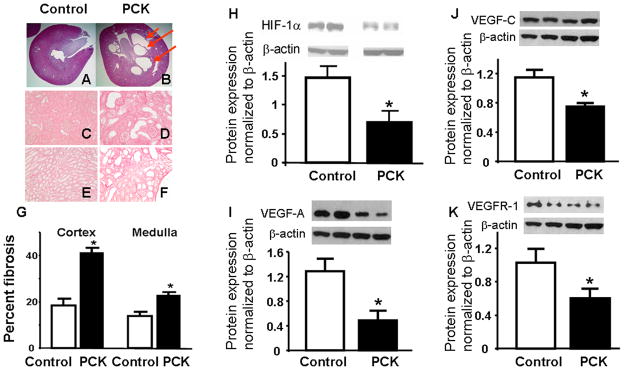
Histological and protein expression analysis of kidneys from controls and PCK animals. A and B showed Hematoxolin & Eosin staining of kidneys from control and PCK rats showing cystic structures in PCK (3B) and not in control (3A). Furthermore, Picrosirius red staining for fibrosis demonstrates increased fibrosis in both the cortex and medulla in PCK rats (3D and 3F, respectively) compared to control (3C and 3E, respectively), quantified in 3G. H–K shows cortical protein expression, densitometry and quantitation normalized to β-actin, of angiogenic factors, showing decreased expression of hypoxia inducible factor (HIF) 1-α and vascular endothelial growth factor (VEGF) pathways. * p<0.05 compared to control. VEGFR-1: VEGF receptor-1.
Discussion
This study demonstrates that PKD is associated with decreased microvascular density, which may play a role in the vascular dysfunction and decreased renal function observed in PKD. Importantly, it shows that the PCK rat model of PKD developed vascular abnormalities, as seen in the adult form of PKD,29 suggesting a potential utility of this model to test novel therapeutic strategies in this disease.
The majority of PKD patients develop hypertension and vascular changes.5, 6, 8 In fact, previous studies have shown that kidneys of PKD patients have “rarefaction” of the renal vascular tree, characterized by a decrease in vascular density,17 and associated with vascular dysfunction,8, 9 that in combination could be responsible for the hypertension seen in these patients.5 In the present study we extend these findings to show that the decrease in vessel density in PKD involves both the outer and inner cortex, and mainly on the small microvessels, which are largely responsible for vascular resistance. Furthermore, we also found that the inner cortical vasculature had a larger mean diameter than the outer cortex, and any given change in their vascular density of those vessels, as seen in PKD, will have a larger impact on VVF. The vessel size and location specificity of the changes observed in our study may also partly explain the changes in renal blood flow and renal function observed in PKD.
In this study we also observed changes in the medullary microvasculature. The decrease in VVF was not seen in the OSOM, but only in the ISOM, where the larger cysts were seen. Furthermore, the fact that cysts were observed mainly in the ISOM, suggests that the changes in vascular density can not be solely explained by direct effects of cyst compression, and may involve an abnormal regulation of angiogenic factors. In fact, in this study we showed that the decrease in microvessels is associated with downregulation in the expression of the angiogenic factor HIF 1-α as well as VEGF and its receptors. The lack of association between cyst number or size and the changes in overall vessel density/distribution suggests that other factors beyond cyst size/number/volume play a role in this response. However, it is important to keep in mind the sample size and time of assessment used in this study. Future studies focusing on arresting cyst development while maintaining other conditions of the disease (e.g. crossing PCK with Brattleboro rats) could provide further input on the effect of cyst development in PKD.
One of the main roles of the renal vasculature is regulation of blood pressure and the control of the filtering of blood by the renal glomeruli. Here we confirmed previous reports on the hypertrophy of glomeruli,29 evidenced as an increase in the mean diameter of glomeruli observed in this study. Interestingly, we observed a similar number of glomeruli between control and PCK, potentially suggesting that the decrease may not be on vessels connected to glomeruli, but those that serve other functions in the cortex. That being said, we can not exclude the possibility that some of the microvessels in PCK may have decreased in luminal area and were below the detection threshold of the micro-CT. Nevertheless, these changes emphasize the abnormal structure and to some extent function, e.g., vasoconstriction of the PCK vasculature. Further studies are needed to determine the functionality of these vessels.
Polycystic kidney disease is considered to originate from the distal tubules and collecting ducts, leading to the tubular dilation and/or formation of cysts that compress both the tubular and vascular compartments. In this study, we showed the feasibility to measure not only the percent area occupied by cysts, but also the number of cysts in the entire kidney and total cyst volume. This information can be used to more accurately assess disease progression, and examine the impact of novel therapeutic interventions in the future. The use of micro-CT in this study implies the use of post-mortem samples, what may constitute a limitation of this study, and limit the monitoring of disease progression. Our group is currently performing studies geared to obtain this information non-invasively.
In this study we chose to analyze the renal vasculature using 3D micro-CT, a tool that has been extensively utilized to assess the microvasculature of different organs,12, 16, 17, 24, 30 providing a more complete and inclusive analysis than possible using traditional histology. Utilization of technologies like micro-CT will allow the study of the patterning that govern the development or regression of microvasculature. In this study the importance of this analysis was evidenced by the irregular and segmental variation in vessel diameter in PKD (Figure 2B). Furthermore, it permits the calculation of the whole kidney measurement of glomeruli in a specific disease, a variable that when integrated with the vascular structural information will be critical to provide overall information on the renovascular architecture.15, 24 Lastly, use of techniques like micro-CT provides the unique opportunity to assess the vascular tree not only in disease, but also the impact of different therapeutic interventions. Most importantly, it allows study the 3D spatial interaction between the microvasculature and PKD cysts, thereby providing information that could be used as experimental endpoints to determine the efficacy of treatments. However, a number of considerations should be made regarding the significance of the observations here reported. Sample cooling (4°C) during preparation can decrease vascular tone and relax the vasculature, thus potentially attenuating the effect of vascular dysfunction on the observed changes. Therefore, our micro-CT findings describe vascular structure, but not the functionality of these structures. Combining these findings with functional data will provide a more complete assessment of the vasculature in PKD.
Here we used a PCK rat model of disease, a model that was originally described as an example of the adult form of PKD,19 but subsequent found to be a model of ARPKD. Despite the difference in inheritance and some milder progression rate (compared to ADPKD), it shares many characteristics with ADPKD, leading to its use in previous studies to test different therapies for the tubular aspect of the ADPKD.21, 31 The vascular changes seen in this study share many of the vascular abnormalities seen in adult PKD, and as such can serve as a platform to study the development of disease as well as allowing their use to test novel therapies.
In summary, this study describes the 3D microvascular structure in PKD animals. We observed that a decrease in vessel density in PKD involves both the outer and inner cortex, and mainly vessels under 100μm in diameter. Use of technologies like the one used here could provide novel information regarding the vascular and cyst structure in PKD.
Perspectives.
This study describes the microvasculature abnormalities observed in PKD, what may account for many of the vascular derangements in PKD, providing a potential new paradigm for the treatment of this disease. Furthermore, it provides further evidence that the PCK rat model of PKD shares many vascular features with ADPKD, suggesting that this model may be used to test novel interventions for ARPKD.
Acknowledgments
Funding: National Institutes of Health grants DK 90728 and HL88048, Novartis, and the Mayo Foundation.
The authors want to acknowledge Mrs. Xiafang Wang for her assistance with the PCK model and Mr. Bruce Knudsen for the assistance with the measurement of blood pressure.
Abbreviations
- PKD
polycystic kidney disease
- ADPKD
autosomal dominant polycystic kidney disease
- ARPKD
autosomal recessive polycystic kidney disease
- VEGF
vascular endothelial growth factor
- Micro-CT
micro-computed tomography
- MAP
mean arterial pressure
- RBF
renal blood flow
- CrCl
creatinine clearance
- OSOM
outer strip outer medulla
- ISOM
inner strip outer medulla
- VVF
vascular volume fraction
- HIF 1-α
hypoxia inducible factor 1-α
- NFκ-β
nuclear factor kappa beta
Bibliography
- 1.Grantham JJ. Clinical practice. Autosomal dominant polycystic kidney disease. N Engl J Med. 2008;359(14):1477–1485. doi: 10.1056/NEJMcp0804458. [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76(2):149–168. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 4.Hughes J, Ward CJ, Peral B, Aspinwall R, Clark K, San Millan JL, Gamble V, Harris PC. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10(2):151–160. doi: 10.1038/ng0695-151. [DOI] [PubMed] [Google Scholar]
- 5.Kelleher CL, McFann KK, Johnson AM, Schrier RW. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S population. Am J Hypertens. 2004;17(11 Pt 1):1029–1034. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74(9):1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tao Y, Kim J, Yin Y, Zafar I, Falk S, He Z, Faubel S, Schrier RW, Edelstein CL. VEGF receptor inhibition slows the progression of polycystic kidney disease. Kidney Int. 2007;72(11):1358–1366. doi: 10.1038/sj.ki.5002550. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Iversen J, Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11(8):1371–1376. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- 9.Wang D, Iversen J, Wilcox CS, Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64(4):1381–1388. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 10.Doulton TW, Saggar-Malik AK, He FJ, Carney C, Markandu ND, Sagnella GA, MacGregor GA. The effect of sodium and angiotensin-converting enzyme inhibition on the classic circulating renin-angiotensin system in autosomal-dominant polycystic kidney disease patients. J Hypertens. 2006;24(5):939–945. doi: 10.1097/01.hjh.0000222765.30348.0d. [DOI] [PubMed] [Google Scholar]
- 11.Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol. 2012 doi: 10.1152/ajprenal.00645.2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stroope A, Radtke B, Huang B, Masyuk T, Torres V, Ritman E, LaRusso N. Hepato-renal pathology in pkd2ws25/- mice, an animal model of autosomal dominant polycystic kidney disease. Am J Pathol. 2010;176(3):1282–1291. doi: 10.2353/ajpath.2010.090658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Nimri MA, Komers R, Oyama TT, Subramanya AR, Lindsley JN, Anderson S. Endothelial-derived vasoactive mediators in polycystic kidney disease. Kidney Int. 2003;63(5):1776–1784. doi: 10.1046/j.1523-1755.2003.00913.x. [DOI] [PubMed] [Google Scholar]
- 14.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5(4):221–228. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bentley MD, Jorgensen SM, Lerman LO, Ritman EL, Romero JC. Visualization of three-dimensional nephron structure with microcomputed tomography. Anat Rec (Hoboken) 2007;290(3):277–283. doi: 10.1002/ar.20422. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Porcel M, Lerman A, Ritman EL, Wilson SW, Best PJM, Lerman LO. Altered microvascular structure in experimental hypercholesterolemia. Circulation. 2000;102(17):2028–2030. doi: 10.1161/01.cir.102.17.2028. [DOI] [PubMed] [Google Scholar]
- 17.Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, Harris PC, Larusso NF. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol. 2004;165(5):1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261(1):17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- 19.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE. The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int. 2001;59(1):126–136. doi: 10.1046/j.1523-1755.2001.00473.x. [DOI] [PubMed] [Google Scholar]
- 20.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nature genetics. 2002;30(3):259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Harris PC, Somlo S, Batlle D, Torres VE. Effect of calcium-sensing receptor activation in models of autosomal recessive or dominant polycystic kidney disease. Nephrol Dial Transplant. 2009;24(2):526–534. doi: 10.1093/ndt/gfn527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan JC, Wang B, Boesen EI, D’Angelo G, Pollock JS, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol. 2009;297(1):F228–235. doi: 10.1152/ajprenal.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Porcel M, Gheysens O, Chen IY, Wu JC, Gambhir SS. Image-guided cardiac cell delivery using high-resolution small-animal ultrasound. Mol Ther. 2005;12(6):1142–1147. doi: 10.1016/j.ymthe.2005.07.532. [DOI] [PubMed] [Google Scholar]
- 24.Zhu XY, Chade AR, Rodriguez-Porcel M, Bentley MD, Ritman EL, Lerman A, Lerman LO. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24(10):1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 25.Ortiz MC, Garcia-Sanz A, Bentley MD, Fortepiani LA, Garcia-Estan J, Ritman EL, Romero JC, Juncos LA. Microcomputed tomography of kidneys following chronic bile duct ligation. Kidney Int. 2000;58(4):1632–1640. doi: 10.1111/j.1523-1755.2000.00324.x. [DOI] [PubMed] [Google Scholar]
- 26.Chade AR, Mushin OP, Zhu X, Rodriguez-Porcel M, Grande JP, Textor SC, Lerman A, Lerman LO. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46(4):772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Porcel M, Gheysens O, Paulmurugan R, Chen IY, Peterson KM, Willmann JK, Wu JC, Zhu X, Lerman LO, Gambhir SS. Antioxidants improve early survival of cardiomyoblasts after transplantation to the myocardium. Mol Imaging Biol. 2010;12(3):325–334. doi: 10.1007/s11307-009-0274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu XY, Rodriguez-Porcel M, Bentley MD, Chade AR, Sica V, Napoli C, Caplice N, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109(17):2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 29.Wei W, Popov V, Walocha JA, Wen J, Bello-Reuss E. Evidence of angiogenesis and microvascular regression in autosomal-dominant polycystic kidney disease kidneys: a corrosion cast study. Kidney Int. 2006;70(7):1261–1268. doi: 10.1038/sj.ki.5001725. [DOI] [PubMed] [Google Scholar]
- 30.Zhu XY, Daghini E, Chade AR, Rodriguez-Porcel M, Napoli C, Lerman A, Lerman LO. Role of oxidative stress in remodeling of the myocardial microcirculation in hypertension. Arterioscler Thromb Vasc Biol. 2006;26(8):1746–1752. doi: 10.1161/01.ATV.0000227469.40826.01. [DOI] [PubMed] [Google Scholar]
- 31.Qian Q, Hunter LW, Li M, Marin-Padilla M, Prakash YS, Somlo S, Harris PC, Torres VE, Sieck GC. Pkd2 haploinsufficiency alters intracellular calcium regulation in vascular smooth muscle cells. Hum Mol Genet. 2003;12(15):1875–1880. doi: 10.1093/hmg/ddg190. [DOI] [PubMed] [Google Scholar]



