Abstract
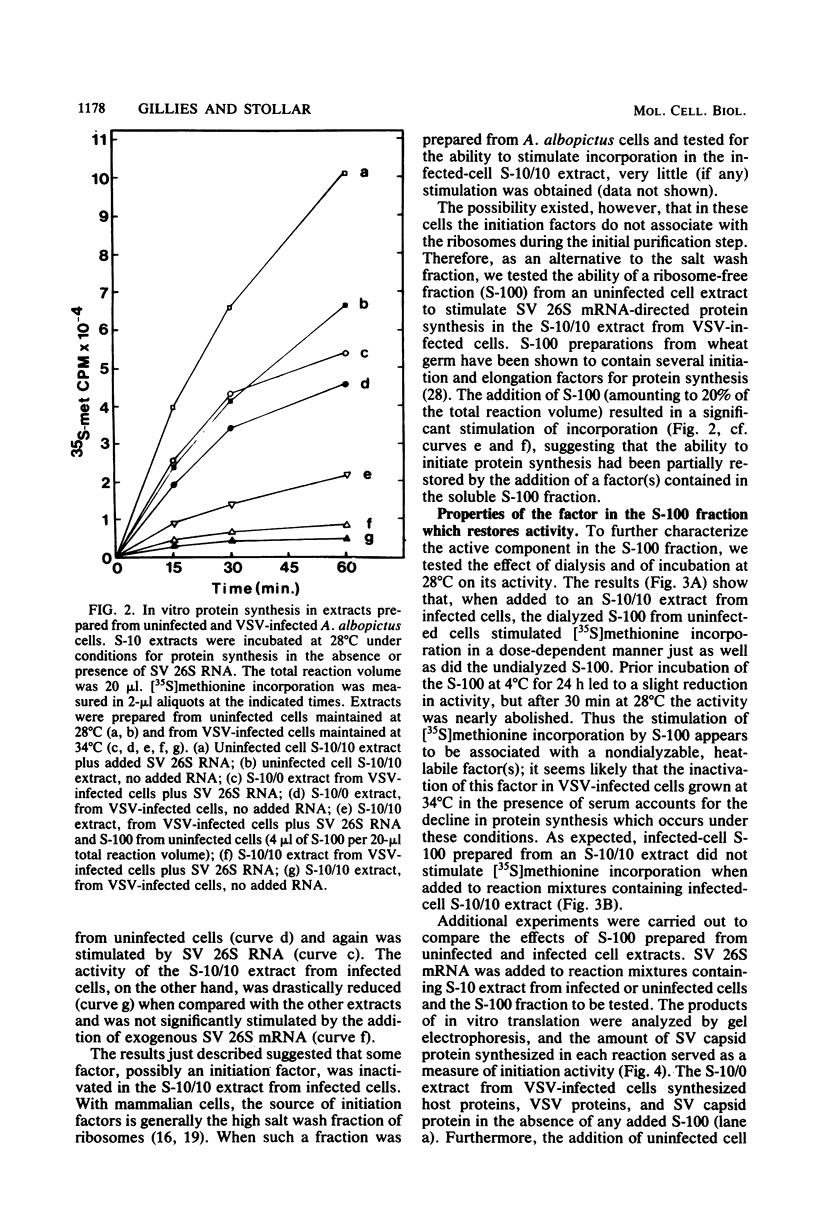
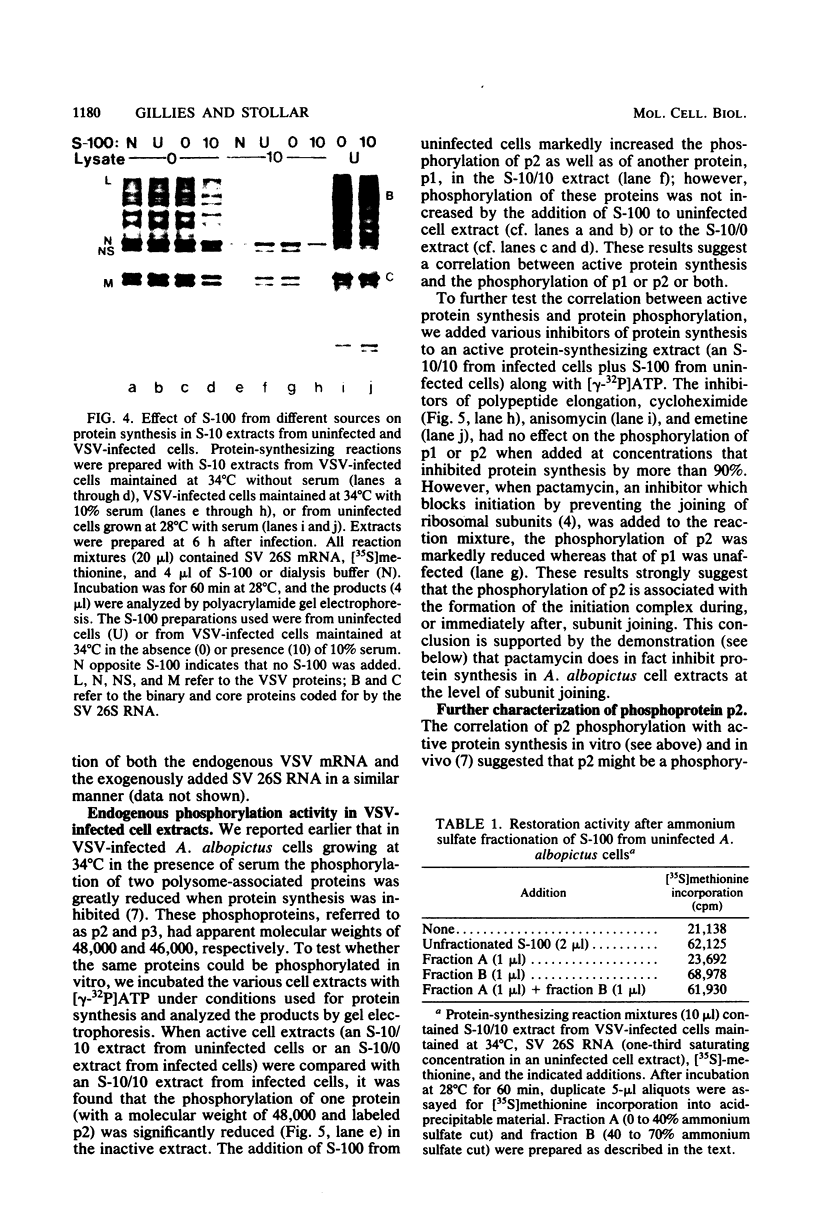
Aedes albopictus cells (clone LT-C7) showed a marked cytopathic effect and inhibition of protein synthesis (both host and viral) after infection with vesicular stomatitis virus (VSV), but only if (i) cultures were incubated at 34 degrees C rather than 28 degrees C and (ii) serum was present in the medium (S. Gillies and V. Stollar, Mol. Cell. Biol. 2:66-75, 1982). To learn more about how protein synthesis is shut off in VSV-infected A. albopictus cells, we have compared cell-free protein synthesis in extracts prepared from VSV-infected cells and control cells. Extracts prepared 6 h after infection from VSV-infected cells maintained at 34 degrees C in the presence of serum reflected what was observed with intact cells in at least two respects: (i) they showed a markedly diminished capacity to carry out protein synthesis (whether directed by endogenous or exogenously added mRNA), and (ii) there was decreased phosphorylation in vitro by [gamma-32P]ATP of a specific ribosomal protein (Gillies and Stollar, Mol. Cell. Biol. 2:66-75, 1982). In addition, and consistent with a block at the level of initiation, the formation of 80S initiation complexes, as measured by binding of VSV 12 to 18S mRNA, was reduced in the inactive extracts. Addition of an S-100 fraction from uninfected cells to the inactive extract reversed each of the aforementioned changes; i.e., it restored protein synthetic activity, it stimulated the formation of 80S initiation complexes, and it increased phosphorylation of the specific ribosomal protein referred to above. The active component in the S-100 fraction was heat labile and non-dialyzable and, upon ammonium sulfate fractionation of the S-100 fraction, was found in the 40 to 70% saturation fraction. Our findings suggest that VSV infection of A. albopictus cells inhibits protein synthesis by inactivating a macromolecular component, probably a protein, in the S-100 fraction which may be involved in the initiation of protein synthesis. More specifically, we suggest that this component is involved in the joining of the ribosomal subunits to form 80S initiation complexes.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carrasco L. The inhibition of cell functions after viral infection. A proposed general mechanism. FEBS Lett. 1977 Apr 1;76(1):11–15. doi: 10.1016/0014-5793(77)80110-5. [DOI] [PubMed] [Google Scholar]
- Centrella M., Lucas-Lenard J. Regulation of protein synthesis in vesicular stomatitis virus-infected mouse L-929 cells by decreased protein synthesis initiation factor 2 activity. J Virol. 1982 Mar;41(3):781–791. doi: 10.1128/jvi.41.3.781-791.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnbrough C., Hunt T., Jackson R. J. A complex between met-tRNA F and native 40S subunits in reticulocyte lysates and its disappearance during incubation with double-stranded RNA. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1556–1564. doi: 10.1016/0006-291x(72)90891-1. [DOI] [PubMed] [Google Scholar]
- Fresno M., Carrasco L., Vazquez D. Initiation of the polypeptide chain by reticulocyte cell-free systems. Survey of different inhibitors of translation. Eur J Biochem. 1976 Sep 15;68(2):355–364. doi: 10.1111/j.1432-1033.1976.tb10822.x. [DOI] [PubMed] [Google Scholar]
- Gillies S., Stollar V. Conditions necessary for inhibition of protein synthesis and production of cytopathic effect in Aedes albopictus cells infected with vesicular stomatitis virus. Mol Cell Biol. 1982 Jan;2(1):66–75. doi: 10.1128/mcb.2.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S., Stollar V. The production of high yields of infectious vesicular stomatitis virus in A. albopictus cells and comparisons with replication in BHK-21 cells. Virology. 1980 Dec;107(2):509–513. doi: 10.1016/0042-6822(80)90317-7. [DOI] [PubMed] [Google Scholar]
- Gillies S., Stollar V. Translation of vesicular stomatitis and Sindbis virus mRNAs in cell-free extracts of Aedes albopictus cells. J Biol Chem. 1981 Dec 25;256(24):13188–13192. [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. Effect of experimental diabetes and insulin on phosphorylation of rat liver ribosomal protein S6. Nature. 1976 Jan 15;259(5539):148–150. doi: 10.1038/259148a0. [DOI] [PubMed] [Google Scholar]
- Jaye M. C., Godchaux W., 3rd, Lucas-Lenard J. Further studies on the inhibition of cellular protein synthesis by vesicular stomatitis virus. Virology. 1982 Jan 15;116(1):148–162. doi: 10.1016/0042-6822(82)90410-x. [DOI] [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Lebleu B., Marbaix G., Wérenne J., Burny A., Huez G. Effect of aurintricarboxylic acid and of NaF on the binding of globin messenger RNA to reticulocyte 40S ribosomal subunits. Biochem Biophys Res Commun. 1970 Aug 11;40(3):731–739. doi: 10.1016/0006-291x(70)90964-2. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Porter M. Translational control of protein synthesis after infection by vesicular stomatitis virus. J Virol. 1980 Dec;36(3):719–733. doi: 10.1128/jvi.36.3.719-733.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvaldi J., Lucas-Lenard J. Differences in the ribosomal protein gel profile after infection of L cells with wild type or temperature-sensitive mutants of vesicular stomatitis virus. Biochemistry. 1977 Sep 20;16(19):4320–4327. doi: 10.1021/bi00638a030. [DOI] [PubMed] [Google Scholar]
- Merrick W. C. Purification of protein synthesis initiation factors from rabbit reticulocytes. Methods Enzymol. 1979;60:101–108. doi: 10.1016/s0076-6879(79)60010-1. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryther J. H. Is the world's oxygen supply threatened? Nature. 1970 Jul 25;227(5256):374–375. doi: 10.1038/227374a0. [DOI] [PubMed] [Google Scholar]
- Sarver N., Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977 Jul 15;80(2):390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- Schreier M. H., Erni B., Staehelin T. Initiation of mammalian protein synthesis. I. Purification and characterization of seven initiation factors. J Mol Biol. 1977 Nov;116(4):727–753. doi: 10.1016/0022-2836(77)90268-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Francoeur A. M., Lam T. Analysis of VSV mutant with attenuated cytopathogenicity: mutation in viral function, P, for inhibition of protein synthesis. Cell. 1977 Jun;11(2):273–281. doi: 10.1016/0092-8674(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Stevens T. M. Arbovirus replication in mosquito cell lines (Singh) grown in monolayer or suspension culture. Proc Soc Exp Biol Med. 1970 May;134(1):356–361. doi: 10.3181/00379727-134-34793. [DOI] [PubMed] [Google Scholar]
- Thomas G., Siegmann M., Kubler A. M., Gordon J., Jimenez de Asua L. Regulation of 40S ribosomal protein S6 phosphorylation in Swiss mouse 3T3 cells. Cell. 1980 Apr;19(4):1015–1023. doi: 10.1016/0092-8674(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Tooker P., Kennedy S. I. Semliki Forest virus multiplication in clones of Aedes albopictus cells. J Virol. 1981 Feb;37(2):589–600. doi: 10.1128/jvi.37.2.589-600.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trachsel H., Sonenberg N., Shatkin A. J., Rose J. K., Leong K., Bergmann J. E., Gordon J., Baltimore D. Purification of a factor that restores translation of vesicular stomatitis virus mRNA in extracts from poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1980 Feb;77(2):770–774. doi: 10.1073/pnas.77.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorma H. O., Thomas A., Goumans H., Amesz H., van der Mast C. Isolation and purification of initiation factors of protein synthesis from rabbit reticulocyte lysate. Methods Enzymol. 1979;60:124–135. doi: 10.1016/s0076-6879(79)60012-5. [DOI] [PubMed] [Google Scholar]
- Walthall B. J., Spremulli L. L., Lax S. R., Ravel J. M. Isolation and purification of protein synthesis initiation factors from wheat germ. Methods Enzymol. 1979;60:193–204. doi: 10.1016/s0076-6879(79)60016-2. [DOI] [PubMed] [Google Scholar]