Abstract
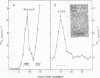
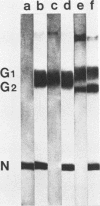
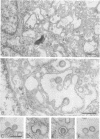
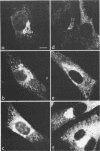
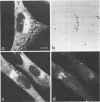
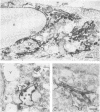
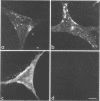
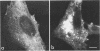
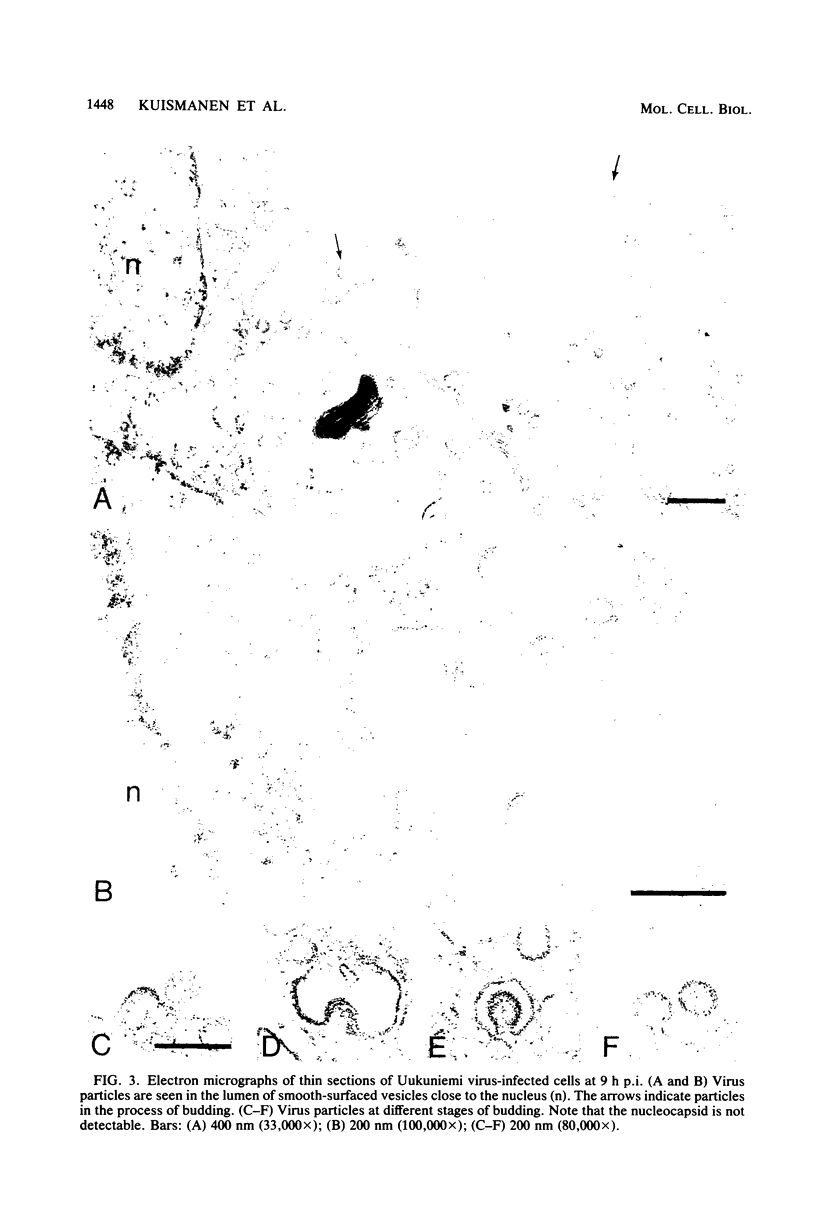
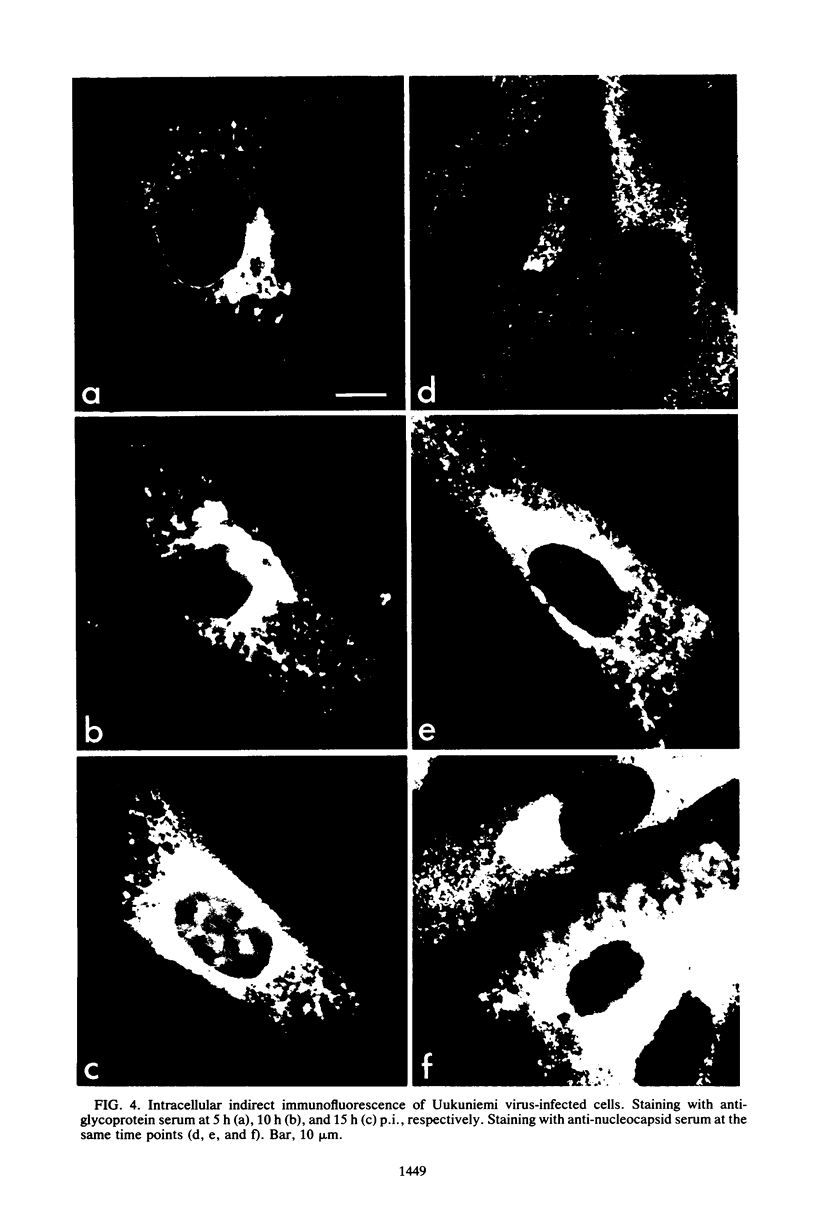
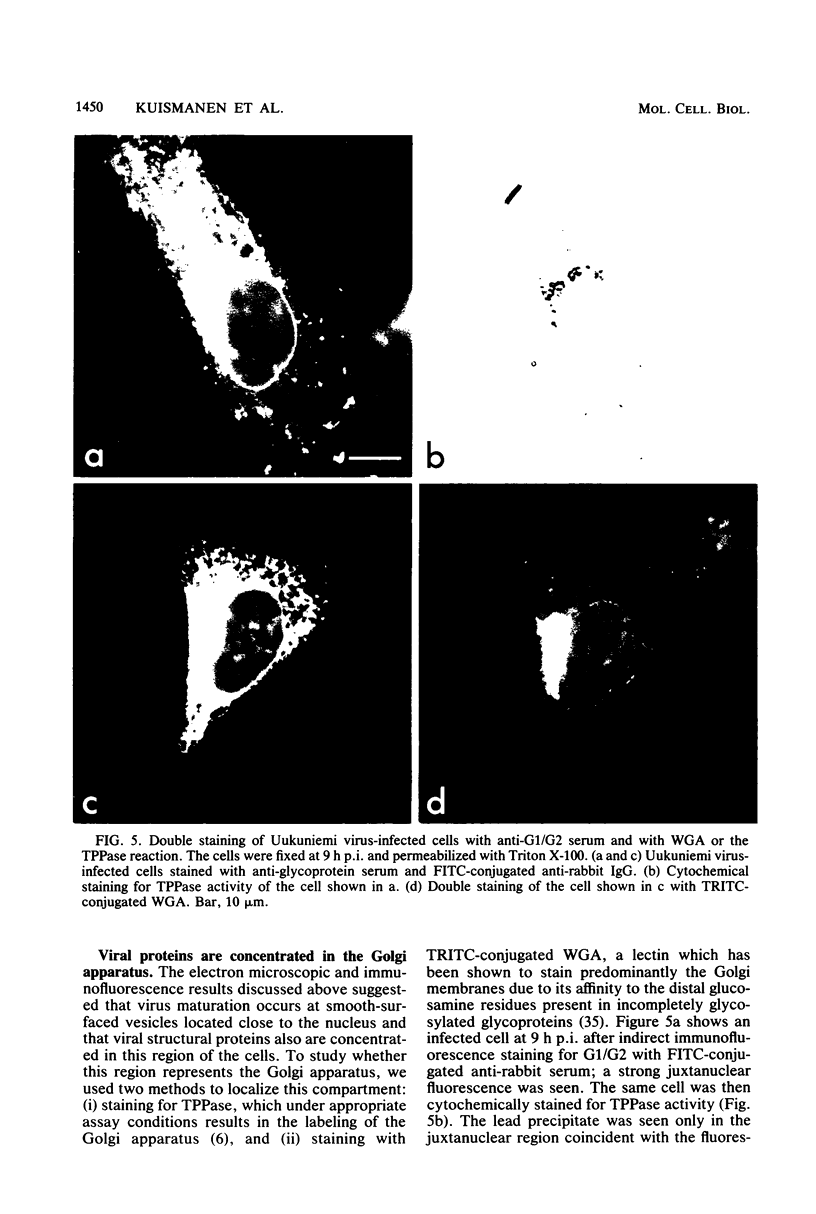
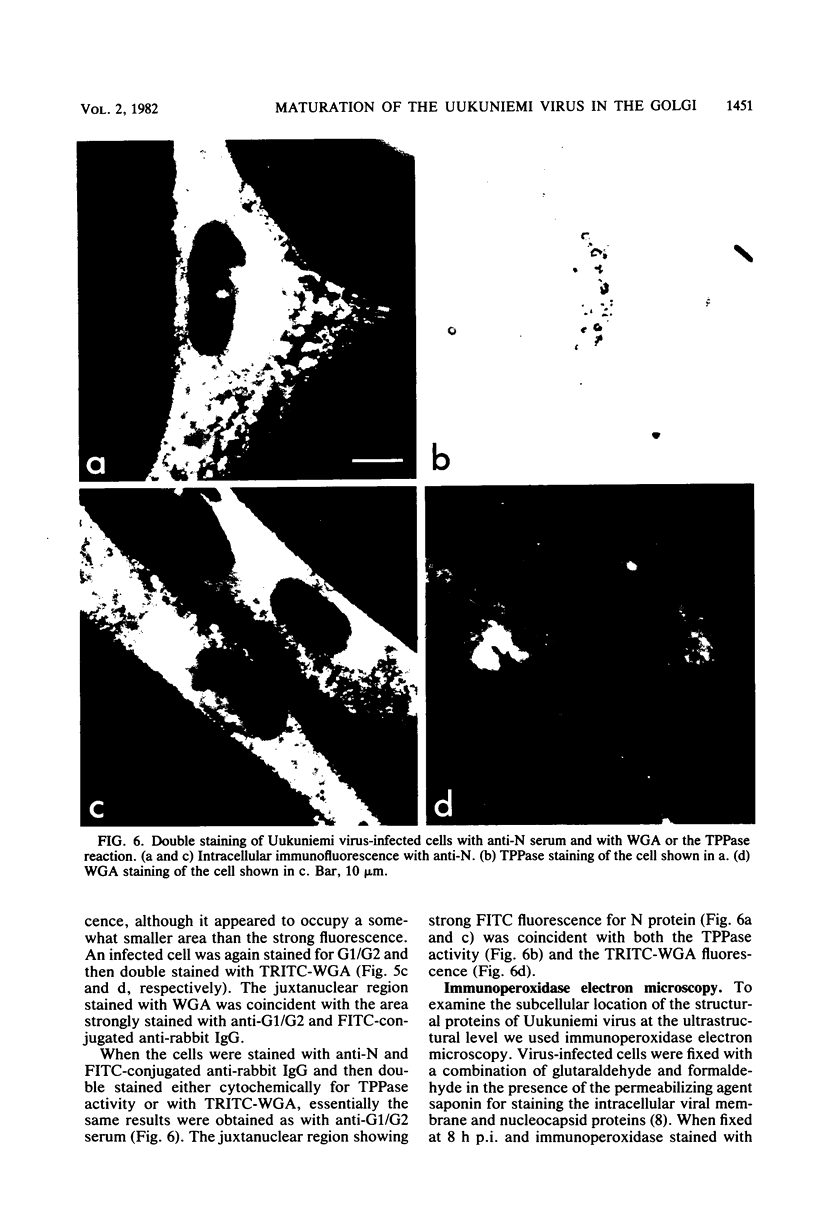
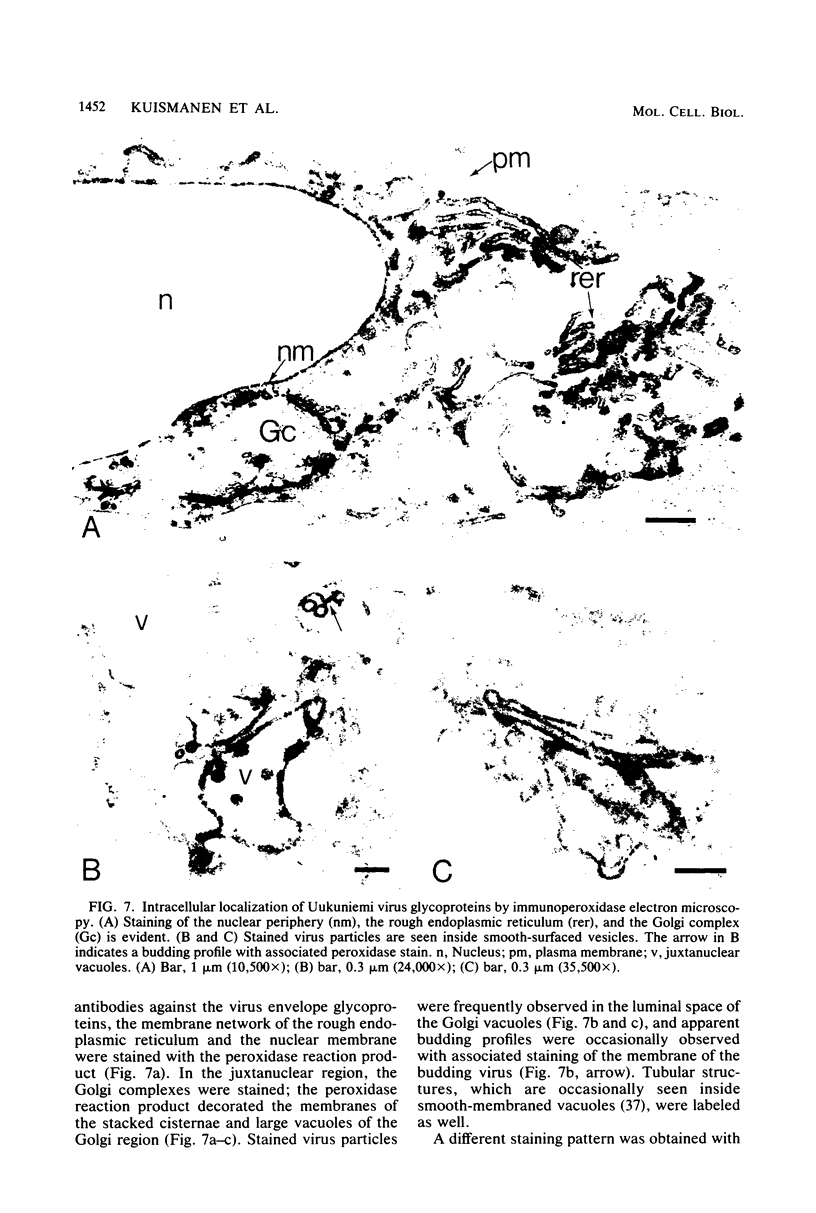
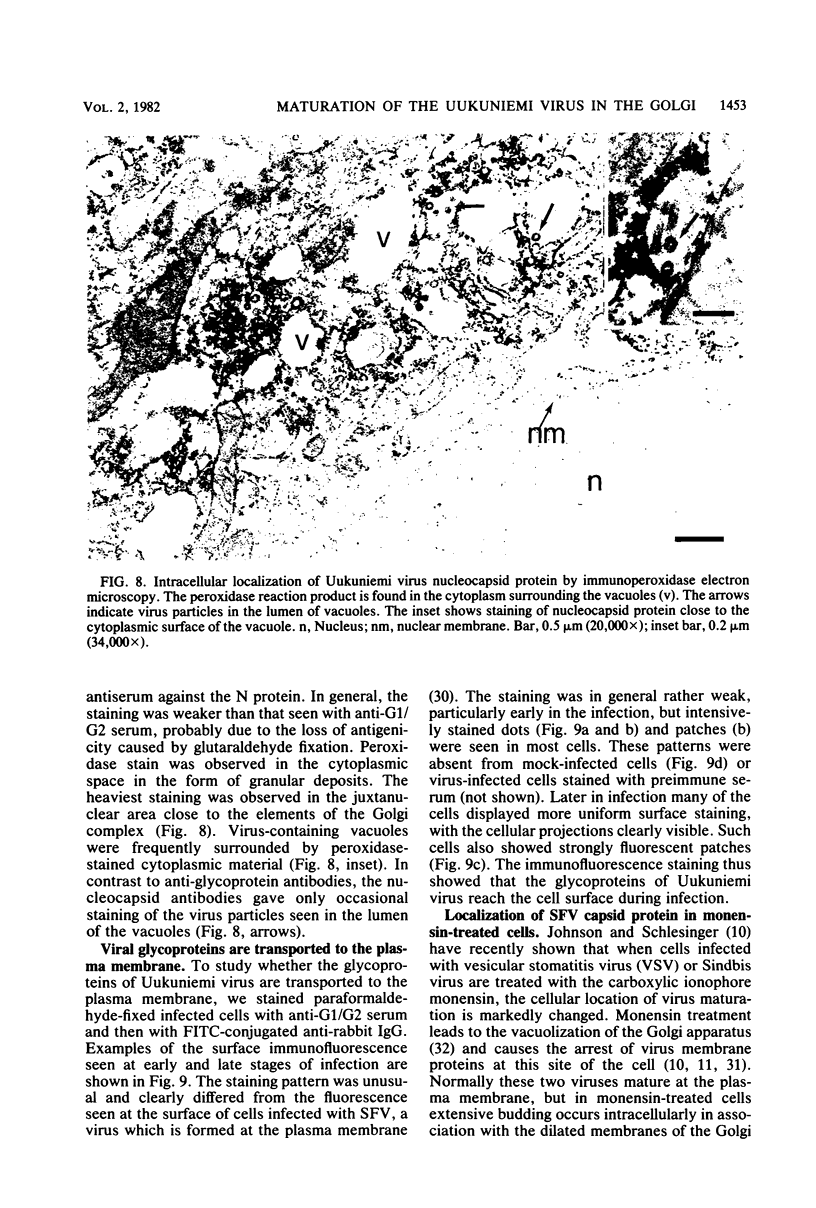
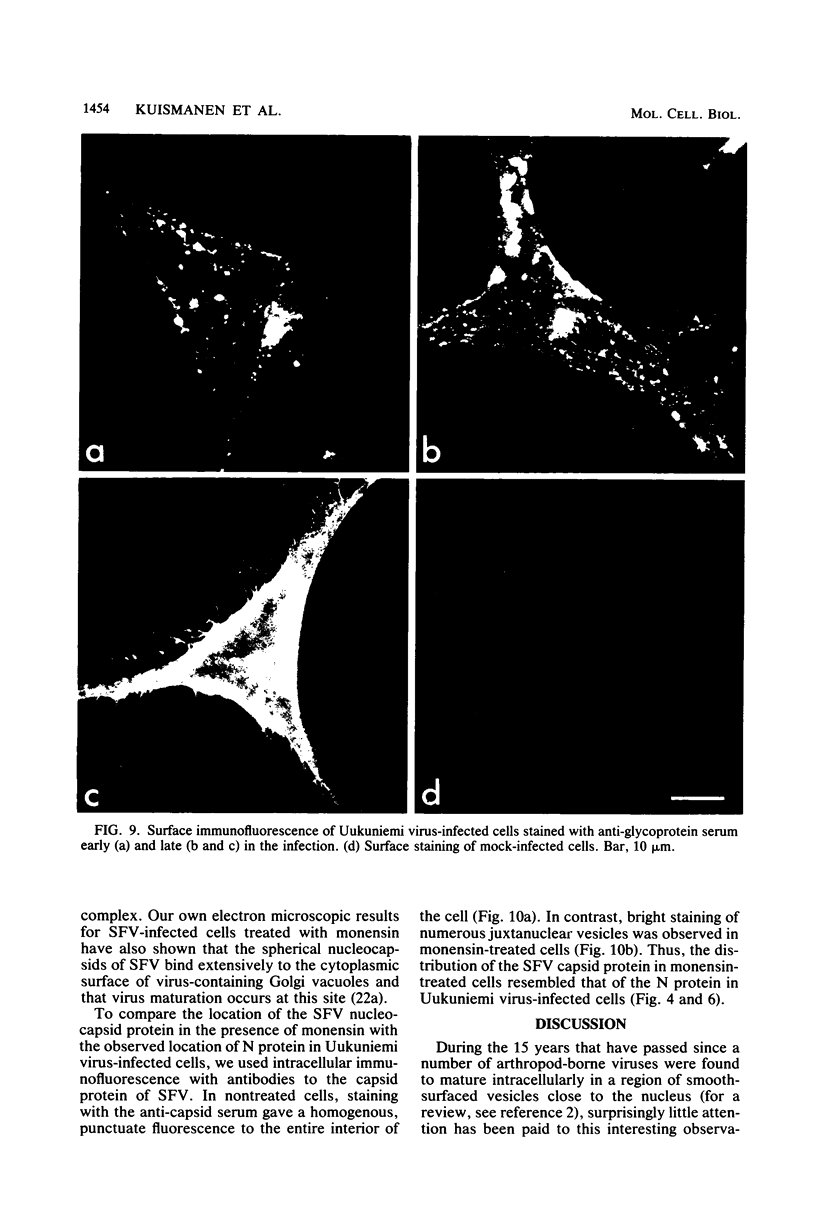
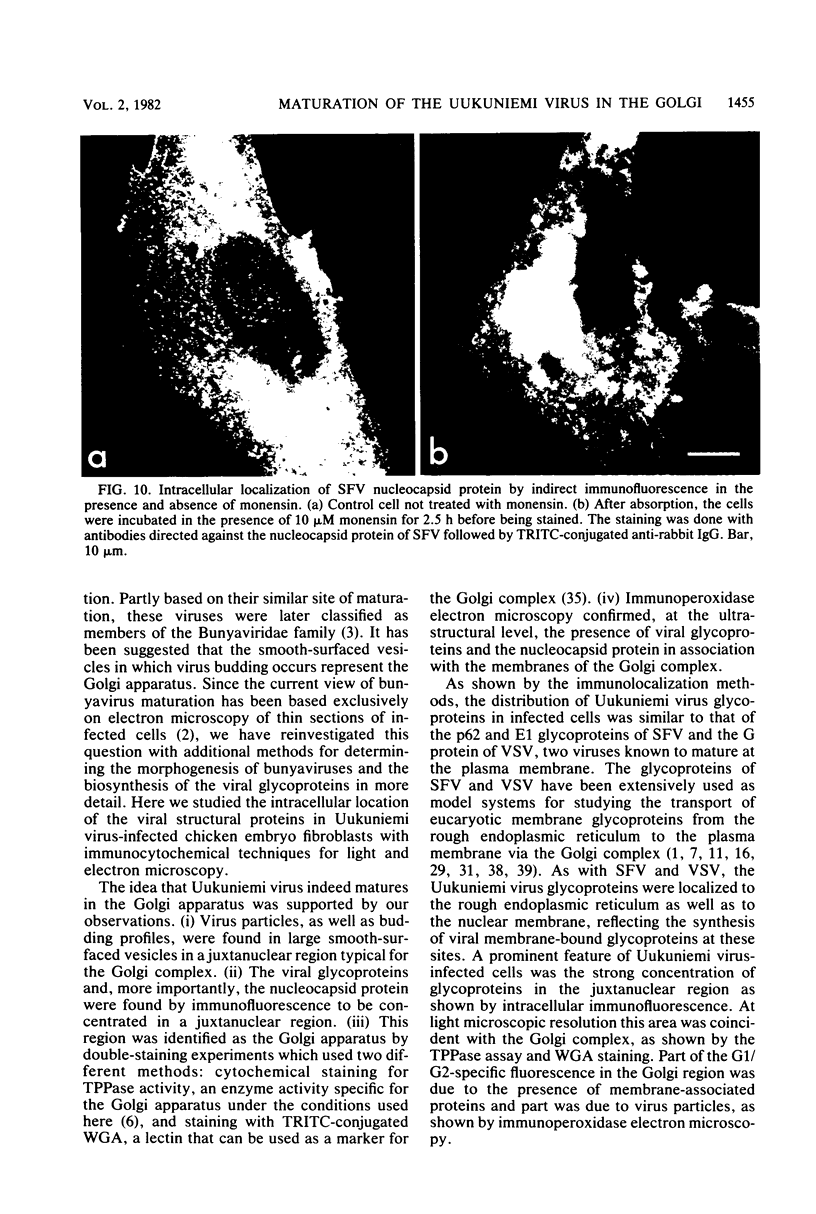
We studied the maturation of Uukuniemi virus and the localization of the viral surface glycoproteins and nucleocapsid protein in infected cells by electron microscopy, indirect immunofluorescence, and immunoelectron microscopy with specific antisera prepared in rabbits against the two glycoproteins G1 and G2 and the nucleocapsid protein N. Electron microscopy of thin sections from infected cells showed virus particles maturing at smooth-surfaced membranes close to the nucleus. Localization of the G1/G2 and N proteins by indirect immunofluorescence at different stages after infection showed the antigens to be present throughout the cell interior but concentrated in the juxtanuclear region. The G1/G2 antiserum also appeared to stain the nuclear and plasma membranes. Double staining with tetramethylrhodamine isothiocyanate-conjugated wheat germ agglutinin, which preferentially stains the Golgi complex, and fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G, which stained the G1/G2 or N proteins, showed that the staining of the juxtanuclear region coincided. Similarly, double staining for thiamine pyrophosphatase, an enzyme activity specific for the Golgi complex, showed the fluorescence and the cytochemical stain to coincide in the juxtanuclear region. Immunoperoxidase electron microscopy of cells permeabilized with saponin revealed that the viral glycoproteins were present in the rough endoplasmic reticulum and the nuclear and Golgi membranes; the latter was heavily stained. With this method, the N protein was localized to the cytoplasm, especially around smooth-surfaced vesicles in the Golgi region. Taken together, the results indicate that Uukuniemi virus and its structural proteins accumulate in the Golgi complex, supporting the idea that this compartment rather than the plasma membrane is the site of virus maturation. This raises the interesting possibility that deficient transport of the glycoproteins to the plasma membrane and hence their accumulation in the Golgi complex determines the site of virus maturation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Calisher C. H., Casals J., Chumakov M. P., Gaidamovich S. Y., Hannoun C., Lvov D. K., Marshall I. D., Oker-Blom N., Pettersson R. F. Bunyaviridae. Intervirology. 1980;14(3-4):125–143. doi: 10.1159/000149174. [DOI] [PubMed] [Google Scholar]
- Cok M. L., Stevens J. G. Replication of varicella-zoste virus in cell culture: an ultrastructural study. J Ultrastruct Res. 1970 Aug;32(3):334–350. doi: 10.1016/s0022-5320(70)80014-4. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Essner E., Schiller B. Nucleoside diphosphatase and thiamine pyrophosphatase activities in the endoplasmic reticulum and golgi apparatus. J Histochem Cytochem. 1971 Jun;19(6):349–360. doi: 10.1177/19.6.349. [DOI] [PubMed] [Google Scholar]
- Green J., Griffiths G., Louvard D., Quinn P., Warren G. Passage of viral membrane proteins through the Golgi complex. J Mol Biol. 1981 Nov 15;152(4):663–698. doi: 10.1016/0022-2836(81)90122-4. [DOI] [PubMed] [Google Scholar]
- Hedman K. Intracellular localization of fibronectin using immunoperoxidase cytochemistry in light and electron microscopy. J Histochem Cytochem. 1980 Nov;28(11):1233–1241. doi: 10.1177/28.11.7000891. [DOI] [PubMed] [Google Scholar]
- Helenius A., von Bonsdorff C. H. Semlike Forest virus membrane proteins. Preparation and characterization of spike complexes soluble in detergent-free medium. Biochim Biophys Acta. 1976 Jul 15;436(4):895–899. doi: 10.1016/0005-2736(76)90421-1. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Knudson D. L. Rhabdoviruses. J Gen Virol. 1973 Jun;20(Suppl):105–130. doi: 10.1099/0022-1317-20-Supplement-105. [DOI] [PubMed] [Google Scholar]
- Käriäinen L., Hashimoto K., Saraste J., Virtanen I., Penttinen K. Monensin and FCCP inhibit the intracellular transport of alphavirus membrane glycoproteins. J Cell Biol. 1980 Dec;87(3 Pt 1):783–791. doi: 10.1083/jcb.87.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Käriäinen L., Söderlund H. Structure and replication of alpha-viruses. Curr Top Microbiol Immunol. 1978;82:15–69. doi: 10.1007/978-3-642-46388-4_2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurila P., Virtanen I., Wartiovaara J., Stenman S. Fluorescent antibodies and lectins stain intracellular structures in fixed cells treated with nonionic detergent. J Histochem Cytochem. 1978 Apr;26(4):251–257. doi: 10.1177/26.4.207770. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Braell W. A., Schwartz A. L., Strous G. J., Zilberstein A. Synthesis and assembly of membrane and organelle proteins. Int Rev Cytol Suppl. 1981;12:247–307. doi: 10.1016/b978-0-12-364373-5.50016-0. [DOI] [PubMed] [Google Scholar]
- Lyons M. J., Heyduk J. Aspects of the developmental morphology of California encephalitis virus in cultured vertebrae and arthropod cells and in mouse brain. Virology. 1973 Jul;54(1):37–52. doi: 10.1016/0042-6822(73)90112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madoff D. H., Lenard J. A membrane glycoprotein that accumulates intracellularly: cellular processing of the large glycoprotein of LaCrosse virus. Cell. 1982 Apr;28(4):821–829. doi: 10.1016/0092-8674(82)90061-7. [DOI] [PubMed] [Google Scholar]
- Murphy F. A., Harrison A. K., Whitfield S. G. Bunyaviridae: morphologic and morphogenetic similarities of Bunyamwera serologic supergroup viruses and several other arthropod-borne viruses. Intervirology. 1973;1(4):297–316. doi: 10.1159/000148858. [DOI] [PubMed] [Google Scholar]
- NOVIKOFF A. B., GOLDFISCHER S. Nucleosidediphosphatase activity in the Golgi apparatus and its usefulness for cytological studies. Proc Natl Acad Sci U S A. 1961 Jun 15;47:802–810. doi: 10.1073/pnas.47.6.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Kuismanen E., Pettersson R. F. Monosaccharide sequence of protein-bound glycans of Uukuniemi virus. J Virol. 1982 Feb;41(2):390–400. doi: 10.1128/jvi.41.2.390-400.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesonen M., Käriäinen L. Incomplete complex oligosaccharides in semliki forest virus envelope proteins arrested within the cell in the presence of monensin. J Mol Biol. 1982 Jun 25;158(2):213–230. doi: 10.1016/0022-2836(82)90430-2. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F. Effect of Uukuniemi virus infection on host cell macromolecule synthesis. Med Biol. 1974 Apr;52(2):90–97. [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., von Bonsdorff C. H. Ribonucleoproteins of Uukuniemi virus are circular. J Virol. 1975 Feb;15(2):386–392. doi: 10.1128/jvi.15.2.386-392.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L. The ribonucleic acids of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1973 Dec;56(2):608–619. doi: 10.1016/0042-6822(73)90062-7. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Rothman J. E., Bursztyn-Pettegrew H., Fine R. E. Transport of the membrane glycoprotein of vesicular stomatitis virus to the cell surface in two stages by clathrin-coated vesicles. J Cell Biol. 1980 Jul;86(1):162–171. doi: 10.1083/jcb.86.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., von Bonsdorff C. H., Hashimoto K., Käriäinen L., Keränen S. Semliki forest virus mutants with temperature-sensitive transport defect of envelope proteins. Virology. 1980 Jan 30;100(2):229–245. doi: 10.1016/0042-6822(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Plasma cell immunoglobulin secretion: arrest is accompanied by alterations of the golgi complex. J Exp Med. 1977 Nov 1;146(5):1332–1345. doi: 10.1084/jem.146.5.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmanen I., Seppälä P., Pettersson R. F. In vitro translation of Uukuniemi virus-specific RNAs: identification of a nonstructural protein and a precursor to the membrane glycoproteins. J Virol. 1981 Jan;37(1):72–79. doi: 10.1128/jvi.37.1.72-79.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bonsdorff C. H., Saikku P., Oker-Blom N. Electron microscope study on the development of Uukuniemi virus. Acta Virol. 1970 Mar;14(2):109–114. [PubMed] [Google Scholar]
- Wehland J., Willingham M. C., Gallo M. G., Pastan I. The morphologic pathway of exocytosis of the vesicular stomatitis virus G protein in cultured fibroblasts. Cell. 1982 Apr;28(4):831–841. doi: 10.1016/0092-8674(82)90062-9. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Pettersson R. Surface structure of Uukuniemi virus. J Virol. 1975 Nov;16(5):1296–1307. doi: 10.1128/jvi.16.5.1296-1307.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]