Abstract
We have examined the possible role of adenosine 3′,5′-phosphate (cAMP) in functions associated with the plasma membranes of Saccharomyces cerevisiae. Purified membranes from this source contained an adenylate cyclase which was insensitive to activation by fluoride or guanine nucleotides, only weakly responsive to changes of carbon source in the growth medium, and strongly stimulated by vanadate. They also contained at least two classes of receptor proteins for guanine nucleotides (as measured by binding of labeled 5′-guanylyl methylene diphosphate) with apparent dissociation constants equal to 1.0 × 10−7 and 3 × 10−6 M, a protein kinase capable of phosphorylating added histones, the activity of which was stimulated by cAMP, and cAMP receptors that may function as regulatory subunits for this kinase. Membrane proteins were also susceptible to phosphorylation by endogenous kinase(s), with polypeptides of apparent molecular weights equal to 160 × 103, 135 × 103, 114 × 103, and 58 × 103 as the major targets. Of these, the 114,000-molecular-weight polypeptide was probably identical to the proton-translocating ATPase of the membranes. However, the cAMP-dependent protein kinase did not appear to be involved in these reactions. Intact (rho+ or rho0) cells responded to dissipation of the proton electrochemical gradient across their plasma membranes by rapid and transient changes in their intracellular level of cAMP, as suggested earlier (J. M. Trevillyan and M. L. Pall, J. Bacteriol., 138:397-403, 1979). Thus, although yeast plasma membranes contain all the essential components of a stimulus-responsive adenylate cyclase system, the precise nature of the coupling device and the targets involved remain to be established.
Full text
PDF


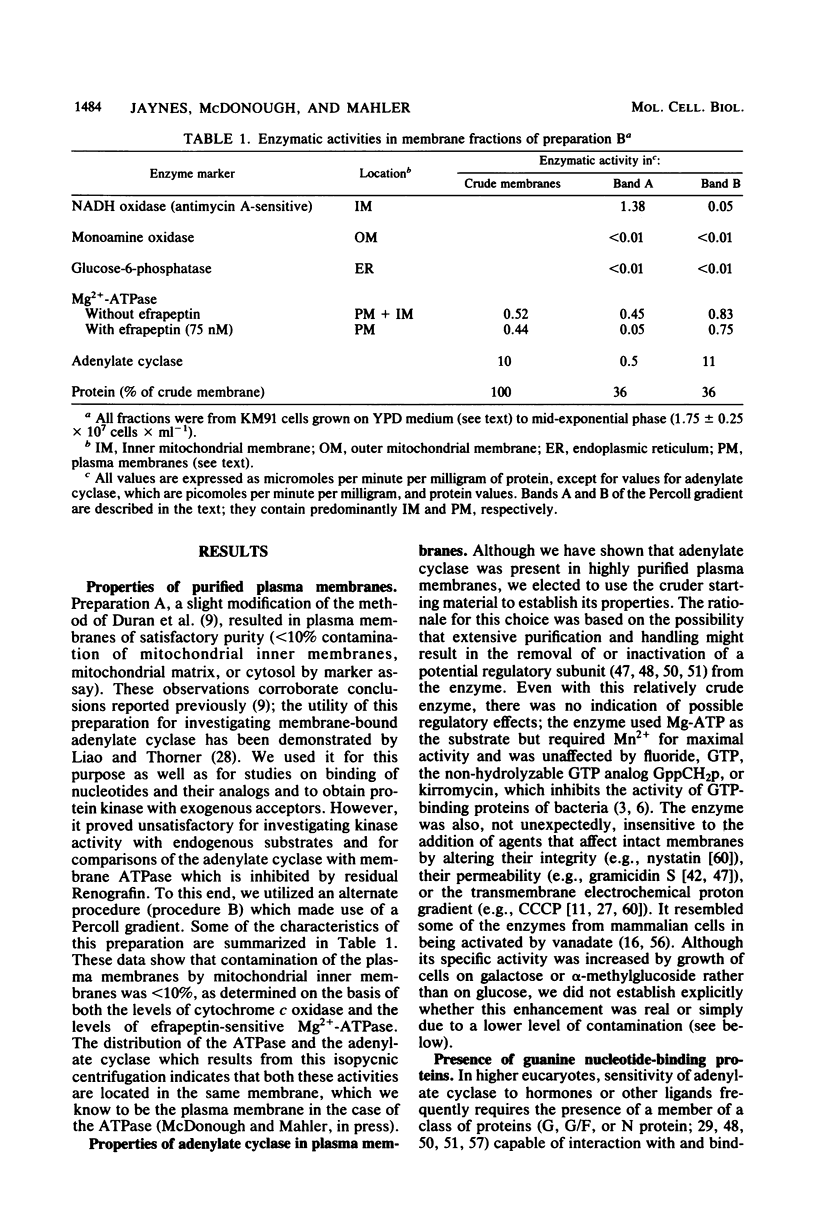
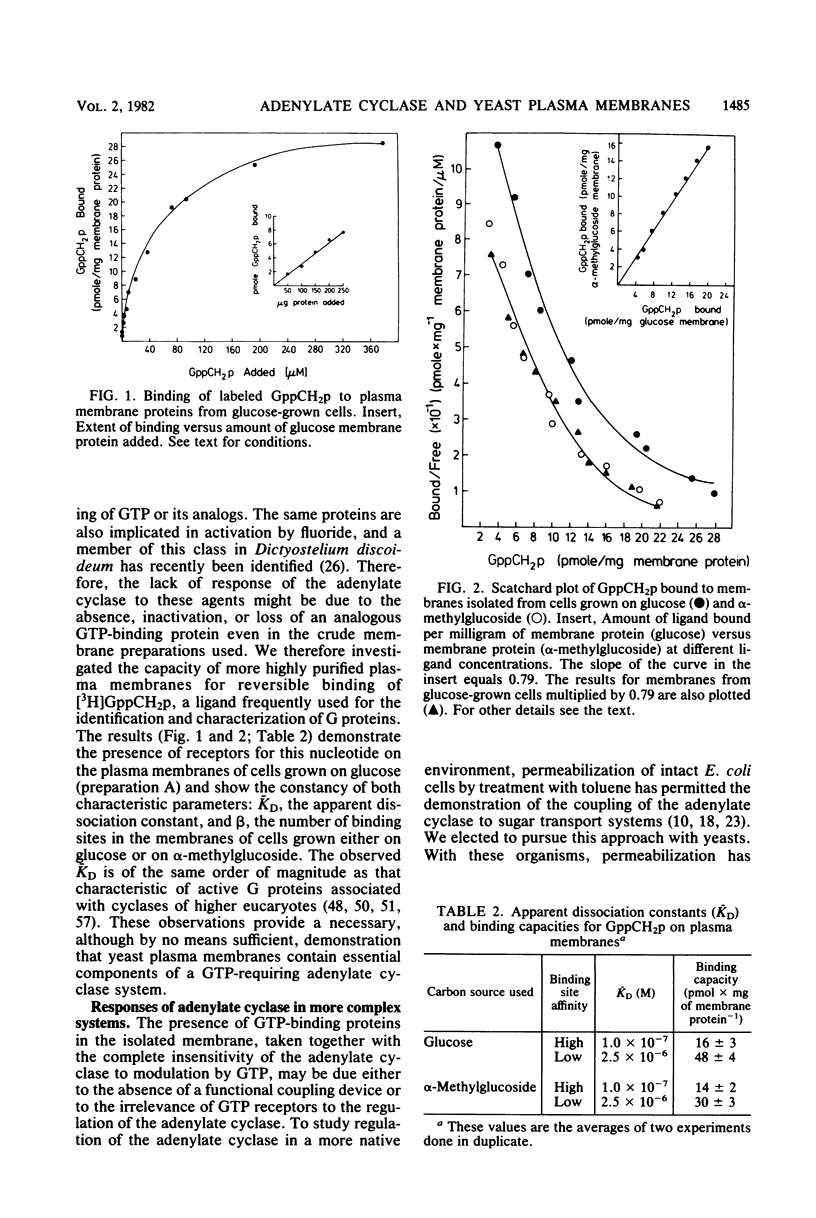

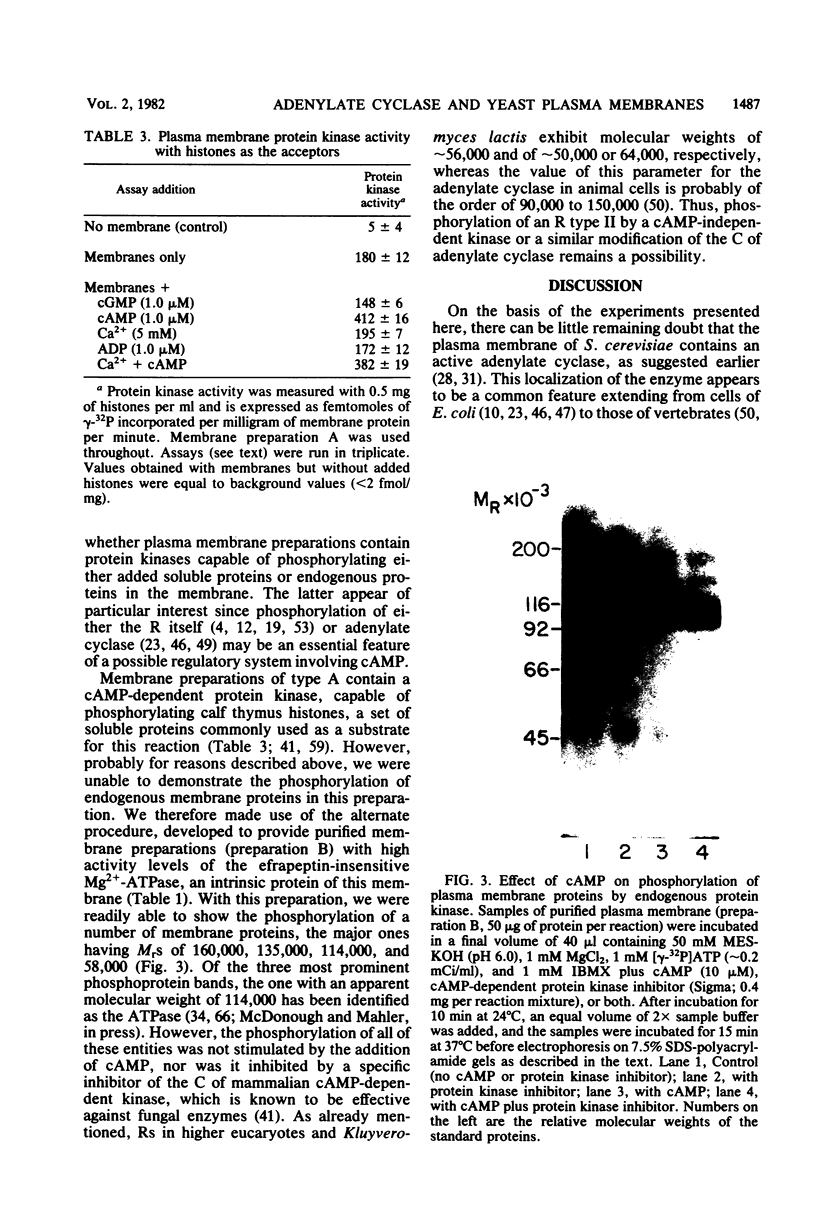



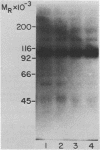
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams B. G. Method for decryptification of -glucosidase in yeast with dimethyl sulfoxide. Anal Biochem. 1972 Jan;45(1):137–146. doi: 10.1016/0003-2697(72)90014-0. [DOI] [PubMed] [Google Scholar]
- Adhya S., Garges S. How cyclic AMP and its receptor protein act in Escherichia coli. Cell. 1982 Jun;29(2):287–289. doi: 10.1016/0092-8674(82)90145-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Douglass J., Smith D. Conformational alteration of protein synthesis elongation factor EF-Tu by EF-Ts and by kirromycin. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3264–3267. doi: 10.1073/pnas.74.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinali G., Wolf H., Parmeggiani A. Effect of kirromycin on elongation factor Tu. Location of the catalytic center for ribosome-elongation-factor-Tu GTPase activity on the elongation factor. Eur J Biochem. 1977 May 2;75(1):55–65. doi: 10.1111/j.1432-1033.1977.tb11503.x. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán A., Bowers B., Cabib E. Chitin synthetase zymogen is attached to the yeast plasma membrane. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3952–3955. doi: 10.1073/pnas.72.10.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feucht B. U., Saier M. H., Jr Fine control of adenylate cyclase by the phosphoenolpyruvate:sugar phosphotransferase systems in Escherichia coli and Salmonella typhimurium. J Bacteriol. 1980 Feb;141(2):603–610. doi: 10.1128/jb.141.2.603-610.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. The proton-translocating pumps of oxidative phosphorylation. Annu Rev Biochem. 1980;49:1079–1113. doi: 10.1146/annurev.bi.49.070180.005243. [DOI] [PubMed] [Google Scholar]
- Geahlen R. L., Carmichael D. F., Hashimoto E., Krebs E. G. Phosphorylation of cAMP-dependent protein kinase subunits. Adv Enzyme Regul. 1982;20:195–209. doi: 10.1016/0065-2571(82)90016-4. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein A., Lampen J. O. Beta-D-fructofuranoside fructohydrolase from yeast. Methods Enzymol. 1975;42:504–511. doi: 10.1016/0076-6879(75)42159-0. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M. Genetic approaches to cyclic AMP effects in cultured mammalian cells. Cell. 1980 Nov;22(2 Pt 2):329–330. doi: 10.1016/0092-8674(80)90342-6. [DOI] [PubMed] [Google Scholar]
- Hall R. M., Trembath M. K., Linnane A. W., Wheelis L., Criddle R. S. Factors affecting petite induction and the recovery of respiratory competence in yeast cells exposed to ethidium bromide. Mol Gen Genet. 1976 Mar 30;144(3):253–262. doi: 10.1007/BF00341723. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Peterkofsky A. Glucose-sensitive adenylate cyclase in toluene-treated cells of Escherichia coli B. J Biol Chem. 1975 Jun 25;250(12):4656–4662. [PubMed] [Google Scholar]
- Hashimoto E., Takio K., Krebs E. G. Studies on the site in the regulatory subunit of type I cAMP-dependent protein kinase phosphorylated by cGMP-dependent protein kinase. J Biol Chem. 1981 Jun 10;256(11):5604–5607. [PubMed] [Google Scholar]
- Hixson C. S., Krebs E. G. Characterization of a cyclic AMP-binding protein from bakers' yeast. Identification as a regulatory subunit of cyclic AMP-dependent protein kinase. J Biol Chem. 1980 Mar 10;255(5):2137–2145. [PubMed] [Google Scholar]
- Jaspers H. T., Christianse K., van Steveninck J. An improved method for the preparation of yeast enzymes in situ. Biochem Biophys Res Commun. 1975 Aug 18;65(4):1434–1439. doi: 10.1016/s0006-291x(75)80389-5. [DOI] [PubMed] [Google Scholar]
- Jaynes P. K., McDonough J. P., Mahler H. R. Identification of cAMP binding proteins associated with the plasma membrane of the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1980 May 14;94(1):16–22. doi: 10.1016/s0006-291x(80)80180-x. [DOI] [PubMed] [Google Scholar]
- Kornberg H. L. Formation and utilization of PEP in microbial carbohydrate transport. Curr Top Cell Regul. 1981;18:313–327. doi: 10.1016/b978-0-12-152818-8.50024-4. [DOI] [PubMed] [Google Scholar]
- Krebs E. G., Beavo J. A. Phosphorylation-dephosphorylation of enzymes. Annu Rev Biochem. 1979;48:923–959. doi: 10.1146/annurev.bi.48.070179.004423. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leichtling B. H., Coffman D. S., Yaeger E. S., Rickenberg H. V., al-Jumaliy W., Haley B. E. Occurrence of the adenylate cyclase "G protein" in membranes of Dictyostelium discoideum. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1187–1195. doi: 10.1016/s0006-291x(81)80137-4. [DOI] [PubMed] [Google Scholar]
- Liao H., Thorner J. Yeast mating pheromone alpha factor inhibits adenylate cyclase. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1898–1902. doi: 10.1073/pnas.77.4.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limbird L. E. Activation and attenuation of adenylate cyclase. The role of GTP-binding proteins as macromolecular messengers in receptor--cyclase coupling. Biochem J. 1981 Apr 1;195(1):1–13. doi: 10.1042/bj1950001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Assimos K., Lin C. C. Derepression in Saccharomyces cerevisiae can be dissociated from cellular proliferation and deoxyribonucleic acid synthesis. J Bacteriol. 1975 Aug;123(2):637–641. doi: 10.1128/jb.123.2.637-641.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler H. R., Jaynes P. K., McDonough J. P., Hanson D. K. Catabolite repression in yeast: mediation by cAMP. Curr Top Cell Regul. 1981;18:455–474. doi: 10.1016/b978-0-12-152818-8.50033-5. [DOI] [PubMed] [Google Scholar]
- Mahler H. R., Lin C. C. Exogenous adenosine 3': 5'-monophosphate can release yeast from catabolite repression. Biochem Biophys Res Commun. 1978 Aug 14;83(3):1039–1047. doi: 10.1016/0006-291x(78)91500-0. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Serrano R. Phosphorylated intermediate of the ATPase from the plasma membrane of yeast. Eur J Biochem. 1981 May 15;116(2):413–417. doi: 10.1111/j.1432-1033.1981.tb05350.x. [DOI] [PubMed] [Google Scholar]
- Malpartida F., Serrano R. Reconstitution of the proton-translocating adenosine triphosphatase of yeast plasma membranes. J Biol Chem. 1981 May 10;256(9):4175–4177. [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Oshima Y., Ishikawa T. Isolation and characterization of yeast mutants deficient in adenylate cyclase and cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2355–2359. doi: 10.1073/pnas.79.7.2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K., Uno I., Toh-E A., Ishikawa T., Oshima Y. Cyclic AMP may not be involved in catabolite repression in Saccharomyes cerevisiae: evidence from mutants capable of utilizing it as an adenine source. J Bacteriol. 1982 Apr;150(1):277–285. doi: 10.1128/jb.150.1.277-285.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough J. P., Jaynes P. K., Mahler H. R. Partial characterization of the plasma membrane ATPase from a rho0 petite strain of Saccharomyces cerevisiae. J Bioenerg Biomembr. 1980 Aug;12(3-4):249–264. doi: 10.1007/BF00744687. [DOI] [PubMed] [Google Scholar]
- Nimmo H. G., Cohen P. Hormonal control of protein phosphorylation. Adv Cyclic Nucleotide Res. 1977;8:145–266. [PubMed] [Google Scholar]
- Ovchinnikov Y. A. Physico-chemical basis of ion transport through biological membranes: ionophores and ion channels. Eur J Biochem. 1979 Mar;94(2):321–336. doi: 10.1111/j.1432-1033.1979.tb12898.x. [DOI] [PubMed] [Google Scholar]
- Pall M. L. Adenosine 3',5'-phosphate in fungi. Microbiol Rev. 1981 Sep;45(3):462–480. doi: 10.1128/mr.45.3.462-480.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall M. L. Cyclic AMP and the plasma membrane potential in Neurospora crassa. J Biol Chem. 1977 Oct 25;252(20):7146–7150. [PubMed] [Google Scholar]
- Pall M. L., Trevillyan J. M., Hinman N. Deficient cyclic adenosine 3',5'-monophosphate control in mutants of two genes of Neurospora crassa. Mol Cell Biol. 1981 Jan;1(1):1–8. doi: 10.1128/mcb.1.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Derepression of mitochondria and their enzymes in yeast: regulatory aspects. Arch Biochem Biophys. 1974 May;162(1):248–271. doi: 10.1016/0003-9861(74)90125-8. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Intracellular localization of enzymes in yeast. Arch Biochem Biophys. 1970 Jan;136(1):245–259. doi: 10.1016/0003-9861(70)90348-6. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A., Gazdar C. Escherichia coli adenylate cyclase complex: regulation by the proton electrochemical gradient. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1099–1103. doi: 10.1073/pnas.76.3.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Richards J. M., Tierney J. M., Swislocki N. I. ATP-dependent activation of adenylate cyclase. J Biol Chem. 1981 Sep 10;256(17):8889–8891. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Biochemical properties of hormone-sensitive adenylate cyclase. Annu Rev Biochem. 1980;49:533–564. doi: 10.1146/annurev.bi.49.070180.002533. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Erlichman J., Rosen O. M. Molecular forms and subunit composition of a cyclic adenosine 3',5'-monophosphate-dependent protein kinase purified from bovine heart muscle. J Biol Chem. 1972 Jan 10;247(1):36–44. [PubMed] [Google Scholar]
- Schamhart D. H., Ten Berge A. M., Van De Poll K. W. Isolation of a catabolite repression mutant of yeast as a revertant of a strain that is maltose negative in the respiratory-deficient state. J Bacteriol. 1975 Mar;121(3):747–752. doi: 10.1128/jb.121.3.747-752.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlanderer G., Dellweg H. Cyclid AMP and catabolite repression in yeasts, In Schizosaccharomyces pombe glucose lowers both intracellular adenosine 3':5'-monophosphate levels and the activity of catabolite-sensitive enzymes. Eur J Biochem. 1974 Nov 1;49(1):305–316. doi: 10.1111/j.1432-1033.1974.tb03835.x. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Puchstein C., Hannemann H., Söchtig E. Activation of adenylate cyclase by vanadate. Nature. 1979 Jan 11;277(5692):143–145. doi: 10.1038/277143a0. [DOI] [PubMed] [Google Scholar]
- Sternweis P. C., Northup J. K., Smigel M. D., Gilman A. G. The regulatory component of adenylate cyclase. Purification and properties. J Biol Chem. 1981 Nov 25;256(22):11517–11526. [PubMed] [Google Scholar]
- Sy J., Roselle M. Cyclic AMP-dependent protein kinase of yeast. FEBS Lett. 1981 Nov 30;135(1):93–96. doi: 10.1016/0014-5793(81)80951-9. [DOI] [PubMed] [Google Scholar]
- Takai Y., Yamamura H., Nishizuka Y. Adenosine 3':5'-monophosphate-dependent protein kinase from yeast. J Biol Chem. 1974 Jan 25;249(2):530–535. [PubMed] [Google Scholar]
- Thorner J. An essential role for cyclic AMP in growth control: the case for yeast. Cell. 1982 Aug;30(1):5–6. doi: 10.1016/0092-8674(82)90004-6. [DOI] [PubMed] [Google Scholar]
- Trevillyan J. M., Pall M. L. Control of cyclic adenosine 3',5'-monophosphate levels by depolarizing agents in fungi. J Bacteriol. 1979 May;138(2):397–403. doi: 10.1128/jb.138.2.397-403.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wijk R., Konijn T. M. Cyclic 3', 5'-amp in Saccharomyces carlsbergensis under various conditions of catabolite repression. FEBS Lett. 1971 Mar 5;13(3):184–186. doi: 10.1016/0014-5793(71)80231-4. [DOI] [PubMed] [Google Scholar]
- Varimo K., Londesborough J. Adenylate cyclase activity in permeabilised yeast. FEBS Lett. 1982 Jun 7;142(2):285–288. doi: 10.1016/0014-5793(82)80153-1. [DOI] [PubMed] [Google Scholar]
- Varimo K., Londesborough J. Solubilization and other studies on adenylate cyclase of baker's yeast. Biochem J. 1976 Nov;159(2):363–370. doi: 10.1042/bj1590363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobo A., Boutry M., Goffeau A. Electrogenic proton translocation coupled to ATP hydrolysis by the plasma membrane Mg2+-dependent ATPase of yeast in reconstituted proteoliposomes. J Biol Chem. 1981 Dec 10;256(23):12081–12087. [PubMed] [Google Scholar]
- Wheeler G. E., Schibeci A., Epand R. M., Rattray J. B., Kidby D. K. Subcellular localization and some properties of the adenylate cyclase activity of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1974 Nov 4;372(1):15–22. doi: 10.1016/0304-4165(74)90068-3. [DOI] [PubMed] [Google Scholar]
- Willsky G. R. Characterization of the plasma membrane Mg2+-ATPase from the yeast, Saccharomyces cerevisiae. J Biol Chem. 1979 May 10;254(9):3326–3332. [PubMed] [Google Scholar]
- Witt J. J., Roskoski R., Jr Rapid protein kinase assay using phosphocellulose-paper absorption. Anal Biochem. 1975 May 26;66(1):253–258. doi: 10.1016/0003-2697(75)90743-5. [DOI] [PubMed] [Google Scholar]