Abstract
Aims/hypothesis
Epidemiological studies that have examined associations between long-term exposure to traffic-related air pollution and type 2 diabetes mellitus in adults are inconsistent, and studies on insulin resistance are scarce. We aimed to assess the association between traffic-related air pollution and insulin resistance in children.
Methods
Fasting blood samples were collected from 397 10-year-old children in two prospective German birth cohort studies. Individual-level exposures to traffic-related air pollutants at the birth address were estimated by land use regression models. The association between air pollution and HOMA of insulin resistance (HOMA-IR) was analysed using a linear model adjusted for several covariates including birthweight, pubertal status and BMI. Models were also further adjusted for second-hand smoke exposure at home. Sensitivity analyses that assessed the impact of relocating, study design and sex were performed.
Results
In all crude and adjusted models, levels of insulin resistance were greater in children with higher exposure to air pollution. Insulin resistance increased by 17.0% (95% CI 5.0, 30.3) and 18.7% (95% CI 2.9, 36.9) for every 2SDs increase in ambient NO2 and particulate matter ≤10 μm in diameter, respectively. Proximity to the nearest major road increased insulin resistance by 7.2% (95% CI 0.8, 14.0) per 500 m.
Conclusions/interpretation
Traffic-related air pollution may increase the risk of insulin resistance. Given the ubiquitous nature of air pollution and the high incidence of insulin resistance in the general population, the associations examined here may have potentially important public health effects despite the small/moderate effect sizes observed.
Keywords: Air pollution, Children, Cohort study, HOMA-IR, Insulin resistance, Traffic
Introduction
Air pollution exposure increases the risk of cardiopulmonary morbidity, acute events [1] and mortality [2]. Recent studies also suggest that exposure to chronic traffic and air pollution influences the development and progression of atherosclerosis [3, 4], possibly via systemic oxidative stress and low-grade inflammation in endothelial cells and macrophages [5]. These underlying biological mechanisms are also involved in the pathogenesis of type 2 diabetes mellitus, particularly in the progression of insulin resistance [6]. It has also been shown in animal models that exposure to particulate matter 2.5 μm or less in diameter (PM2.5) for 24 weeks exaggerates insulin resistance, visceral inflammation and adiposity in diet-induced obese mice [7]. Further exposure for a duration of 10 months led to oxidative stress, decreased mitochondrial count in visceral adipose depots and decreased mitochondrial size in interscapular adipose depots [8]. Adverse effects have been found to be associated with indoor air pollution exposure, for example between environmental tobacco smoke (ETS) exposure and type 2 diabetes mellitus incidence and susceptibility among adults [9] and adolescents [10]. It thus seems biologically plausible that air pollution may be a risk factor for insulin resistance and type 2 diabetes mellitus.
The few cross-sectional and longitudinal studies that have examined associations between exposure to air pollution and the development of type 2 diabetes mellitus in different adult age groups [11–17] have yielded inconsistent findings. As insulin resistance is considered to be a strong risk factor for the development of diabetes in adults, studying the effects of air pollution on insulin resistance in healthy children could lead to new insights into this complex disease.
To the best of our knowledge, no study has yet investigated the association between traffic-related air pollution and insulin resistance in school-aged children. The present study aimed to investigate the relation between long-term exposure to traffic-related air pollution (NO2, PM2.5, PM10, PM2.5 absorbance, and proximity to nearest major road) at the birth address and the HOMA of insulin resistance index (HOMA-IR) in 10-year-old children in two German birth cohorts.
Methods
Study area and study population
The study population consists of a subsample of children living in Munich, South Germany, and Wesel, West Germany, who participated in the 10-year follow-up of two birth cohorts. In both cohorts, only healthy full-term neonates were recruited. The German Infant Study on the Influence of Nutrition Intervention plus Environmental and Genetic Influences on Allergy (GINIplus) is a multicentre, two-armed study consisting of 5,991 newborns. One study arm is a prospective, double-blind, randomised intervention trial with hypoallergenic formulae, while the second arm is observational and does not include an intervention. The study design has been previously described in detail [18]. The Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood (LISAplus) Study consists of 3,097 healthy neonates, recruited at birth, who have a birthweight >2,500 g. LISAplus was designed as a population-based observational study, and children have been followed-up at the age of 6, 12 and 18 months and 2, 4, 6 and 10 years [19]. The regional ethics committees approved both studies, and parents gave written informed consent.
At the age of 10 years, children from both cohorts were randomly assigned to one of two groups based on the last digit of their identification number. The first group did not undergo any further selection and will hereafter be referred to as the population-based sample. This population-based sample was enriched with a potentially sensitive subgroup consisting of children with a BMI at age 10 years above the 85th percentile selected from the second group (enrichment sample). A random subset of children from both the population-based sample and the enrichment sample were invited for fasting blood sampling at the age of 10 years.
The present study population consists of 397 children without diabetes (type 1 or 2) for whom air pollution exposure data at birth and insulin and glucose measurements are available.
Laboratory analyses
Glucose measurements in blood were performed by standard laboratory methods by the individual hospitals. Fasting insulin in serum was measured centrally by the fully mechanised system, LIAISON (DiaSorin, Saluggia, Italy). The lower limit of detection for this method was 3.5 pmol/l. Quality control samples showed intra- and inter-assay coefficients of variation below 5.8%. HOMA-IR was calculated as described by Matthews et al [20].
Exposure assessment
We used land use regression (LUR) models to estimate long-term spatial variability of NO2, PM10, PM2.5 and PM2.5 absorbance at the birth address of each individual. The LUR models were developed in the framework of an ongoing European Study of Cohorts for Air Pollution Effects (ESCAPE; (15/10/2012): www.escapeproject.eu). The development of the LUR models has been described in previous publications [21–24]. Briefly, concentrations of NO2 were measured at 40 monitoring sites, and concentrations of PM2.5 and filter absorbance of PM2.5 at 20 monitoring sites in Munich-Augsburg and the Ruhr area. The measurement period in Munich was between October 2008 and November 2009, and measurements at all selected sites were carried out three times for 14 consecutive days, in different seasons. All air pollution measurements were performed according to standard operating procedures available on the ESCAPE project website (www.escapeproject.eu/manuals/), accessed 15 October 2012.
Europe-wide geographic information system (GIS) data (such as digital road network, land use data CORINE [COordination and INformation on the Environmental program, initiated by the European Commission] and population density data), centrally provided within the framework of the ESCAPE study, was used to generate potential predictor variables that describe each monitoring site in terms of location, surrounding land use, population density and traffic patterns.
The measured air pollutant concentrations and the GIS-generated predictor variables were used to develop local LUR models for each study centre. A detailed description of the LUR model development process and predictors are provided in Beelen et al [21] and Eeftens et al [22]. The final LUR models were used to estimate outdoor air pollution concentrations at the addresses of study participants.
In addition, local GIS data for the Munich and Wesel study areas were collected and used to estimate the distance between the child’s birth address and the centre of the nearest major road defined as a road with traffic intensity >5,000 vehicles/day.
Covariate assessment
All adjustment covariates were selected a priori. Socioeconomic status was defined as the largest number of years of education of either parent (low, <10 years of school; medium, 10–11 years of school; high, ≥12 years of school). Onset (yes/no) of puberty was assessed via a questionnaire (signs of puberty, e.g. pubic hair or menarche). Height and weight at age 10 years were measured by a physician during a clinical examination in the study centres by standard procedures. Exposure to second-hand smoke (ETS) was ascertained at several time points between birth and the age of 10 years (details provided previously [25]), and the percentage of years during which the parents or another person smoked in the home was classified into three mutually exclusive categories: no ETS, ≤50% or >50% of years recorded.
Statistical analysis
Children were included in the analysis if they had valid measurements of both fasting blood insulin and glucose as well as information on at least one exposure variable. The HOMA-IR was log-transformed to base e, as its distribution was skewed. For the exposure variables, extreme observations exceeding mean ± 4SD were excluded (n = 1 for NO2 and PM2.5 absorbance, and n = 7 for distance to the nearest major road). Generalised additive models (gam), as implemented in R [26] (library mgcv, gam procedure), were used to assess the linearity of associations between insulin resistance and air pollutants. These models allow the use of smooth functions for covariates or exposures that may have a non-linear relationship with the outcome. Subsequently, linear regression models were used to analyse the relationship between insulin resistance quantified by HOMA-IR and exposure to traffic-related air pollution. Models were adjusted for sex, age, study (including GINIplus intervention status), parental education, study centre, study design, BMI, puberty status, birthweight and ETS at home during childhood. In addition, analyses were performed separately in the population-based sample and the enrichment sample, as well as stratified by sex and relocations (never moved, moved before the age of 3, or moved after the age of 3). Results are presented as percentage differences with 95% CI, which correspond to the back-transformed β-coefficients from the linear regression analyses on log-transformed HOMA-IR multiplied by 100 [27]. Effect sizes correspond to a 2SD increase in traffic-related air pollutant or a 500 m decrease in distance to the nearest major road. Statistical significance was defined by a two-sided α level of 5%. All statistical analyses were performed using R 2.14 [26].
Results
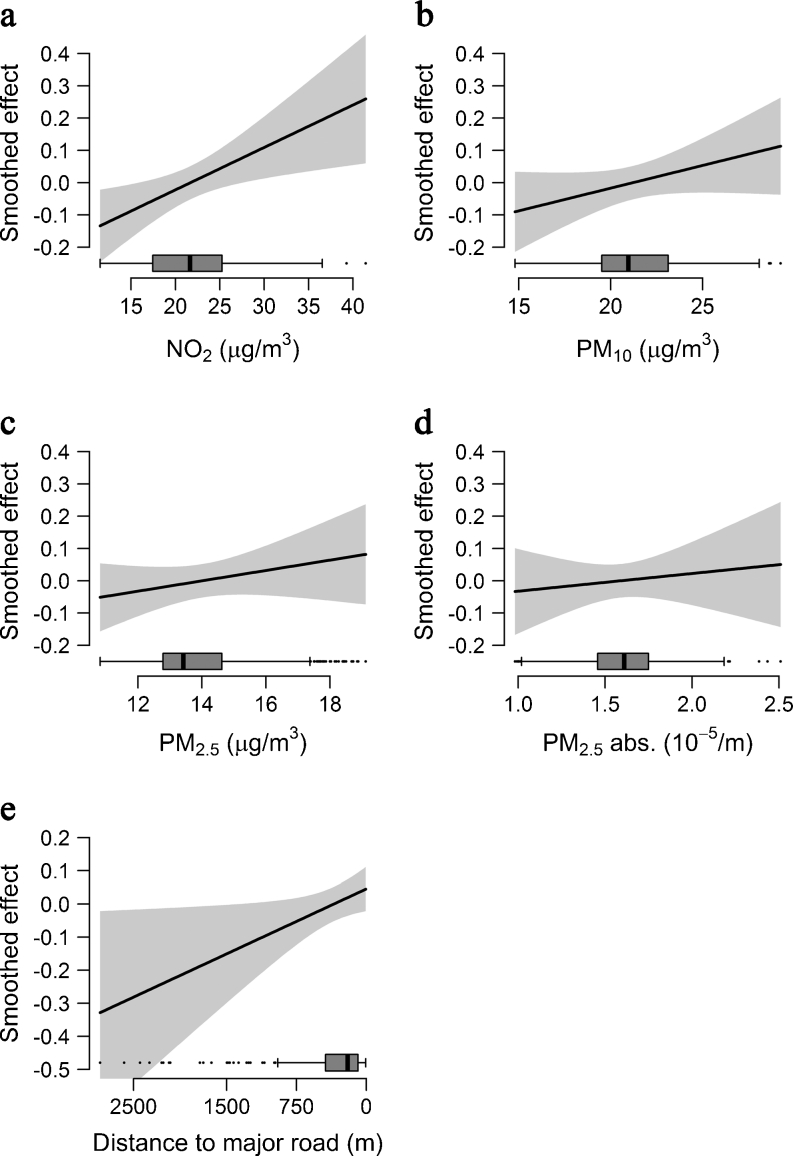
The characteristics of the study population are summarised in Table 1. Measured fasting blood insulin and glucose levels and geocoded residential address information at birth were available for 397 children. The majority (82.1%) were recruited in Munich. Children living in Wesel were more likely to have been exposed to ETS at home between birth and 10 years of age, and their families were more likely to be of lower socioeconomic status. However, there were no differences in age, BMI and HOMA-IR between children living in Munich and Wesel. All pollutant concentrations, except for PM2.5 absorbance, were higher in Wesel than in Munich. Children from Wesel tended to live further away from a major road. All exposure measures were linearly associated with insulin resistance by generalised additive modelling (Fig. 1).
Table 1.
Characteristics of the total and city-specific populations
| Characteristic | Total (n = 397) | Munich (n = 326) | Wesel (n = 71) |
|---|---|---|---|
| Design | |||
| Enrichment samplea | 167 (42.1) | 117 (35.9) | 50 (70.4) |
| Population-based | 230 (57.9) | 209 (64.1) | 21 (29.6) |
| Study | |||
| GINI observation | 125 (31.5) | 84 (25.8) | 41 (57.7) |
| GINI intervention | 131 (33.0) | 114 (35.0) | 17 (23.9) |
| LISA | 141 (35.5) | 128 (39.3) | 13 (18.3) |
| Socioeconomic statusb,c | |||
| Low | 26 (6.6) | 19 (5.9) | 7 (9.9) |
| Medium | 83 (21.1) | 51 (15.8) | 32 (45.1) |
| High | 285 (72.3) | 253 (78.3) | 32 (45.1) |
| Sex | |||
| Male | 175 (44.1) | 147 (45.1) | 28 (39.4) |
| Female | 222 (55.9) | 179 (54.9) | 43 (60.6) |
| Birthweight (kg) | 3.5 ± 0.5 | 3.4 ± 0.5 | 3.6 ± 0.5 |
| % of years with ETS at home | |||
| No ETS | 256 (64.5) | 224 (68.7) | 32 (45.1) |
| ≤50% | 66 (16.6) | 51 (15.6) | 15 (21.1) |
| >50% | 75 (18.9) | 51 (15.6) | 24 (33.8) |
| Puberty startedd,e | |||
| No | 252 (64.6) | 202 (63.3) | 50 (70.4) |
| Yes | 138 (35.4) | 117 (36.7) | 21 (29.6) |
| Age of the child (years) | 10.2 ± 0.2 | 10.2 ± 0.3 | 10.3 ± 0.2 |
| BMI at 10 years (kg/m2) | 18.3 ± 2.9 | 18.3 ± 2.9 | 18.2 ± 2.9 |
| HOMA-IR (GM) | 1.5 ± 1.8 | 1.5 ± 1.8 | 1.5 ± 1.8 |
| Distance to major road (m) (GM) | 190.2 ± 3.3 | 175.0 ± 3.1 | 280.8 ± 3.7 |
| NO2 (μg/m3) | 21.7 ± 5.3 | 21.2 ± 5.5 | 24.0 ± 3.1 |
| PM10 (μg/m3) | 21.2 ± 3.0 | 20.3 ± 2.4 | 25.5 ± 1.2 |
| PM2.5 (μg/m3) | 14 ± 1.9 | 13.3 ± 1.0 | 17.5 ± 0.7 |
| PM2.5 absorbance (10−5/m) | 1.6 ± 0.3 | 1.7 ± 0.2 | 1.2 ± 0.2 |
Values are n (%) or mean ± SD
aBMI at 10 years >85th percentile
bHighest number of years of education of either parent
cInformation was missing for n=3 children
dDefined as a parental report of signs of puberty (e.g. pubic hair, menarche)
eInformation was missing for n=10 children
GM, geometric mean
Fig. 1.
Smooth associations between insulin resistance and exposure variables assessed using generalised additive models adjusted for sex, age and BMI. Box plots on the x-axis show the distribution of the exposure variables NO2 (a), PM10 (b), PM2.5 (c), PM2.5 absorbance (d) and distance to the nearest major road (e); abs., absorbance
Table 2 shows the percentage differences in HOMA-IR per 2SD increase for NO2, PM10, PM2.5 and PM2.5 absorbance concentrations, as well as a 500 m decrease in distance to the nearest major road after adjustment for different sets of covariates. All estimates for the association between air pollutants and insulin resistance were greater than 0.0%. Insulin resistance was statistically significantly increased by 17.0% (95% CI 5.0, 30.3) per 2SD increase in NO2 after adjustment for sex, birthweight, study centre, parental education, study, study design, age, puberty status and BMI (adjustment 1). The association of insulin resistance and PM10 was also significant (increase of 18.7% [95% CI 2.9, 36.9] per 2SD increase in PM10). Effect estimates for PM2.5 (22.5%, 95% CI −0.9, 51.5) and PM2.5 absorbance (8.6%, 95% CI −5.7, 25.0) were elevated, but the difference did not reach statistical significance.
Table 2.
Percentage difference (95% CI) in insulin resistance (HOMA-IR) per 2SD increase in traffic-related air pollutants and per 500 m decrease in distance to the nearest major road
| Exposure | Adjustment 1a | Adjustment 2b | ||
|---|---|---|---|---|
| % (95% CI) | p value | % (95% CI) | p value | |
| Per 2SD increase in | ||||
| NO2 (μg/m3) | 17.0 (5.0, 30.3) | 0.005 | 15.8 (3.8, 29.1) | 0.009 |
| PM10 (μg/m3) | 18.7 (2.9, 36.9) | 0.019 | 17.5 (1.9, 35.6) | 0.027 |
| PM2.5 (μg/m3) | 22.5 (−0.9, 51.5) | 0.062 | 20.5 (−2.6, 49.0) | 0.087 |
| PM2.5 absorbance (10−5/m) | 8.6 (−5.7, 25.0) | 0.254 | 7.1 (−7.1, 23.5) | 0.343 |
| Per 500 m decrease in distance to major road (m) | 7.2 (0.8, 14.0) | 0.028 | 6.7 (0.3, 13.5) | 0.040 |
2SDs correspond to 10.6 μg/m3 for NO2, 6 μg/m3 for PM10, 3.7 μg/m3 for PM2.5 and 0.5 × 10−5/m for PM2.5 absorbance
aAdjustment 1: sex, birthweight, study centre, parental education, study, study design, puberty status, age and BMI
bAdjustment 2: adjustment 1 plus ETS
Log-transformed (to the base e) distance to the nearest major road correlated with modelled NO2 (r = −0.50, p < 0.001), PM10 (r = −0.17, p < 0.001), PM2.5 (r = −0.10, p = 0.04) and PM2.5 absorbance (r = −0.56, p < 0.001). Insulin resistance was significantly increased by 7.2% (95% CI 0.8, 14.0) per 500 m decrease in distance to a major road. All associations were slightly attenuated, but remained elevated, after additional adjustment for ETS (Table 2, adjustment 2).
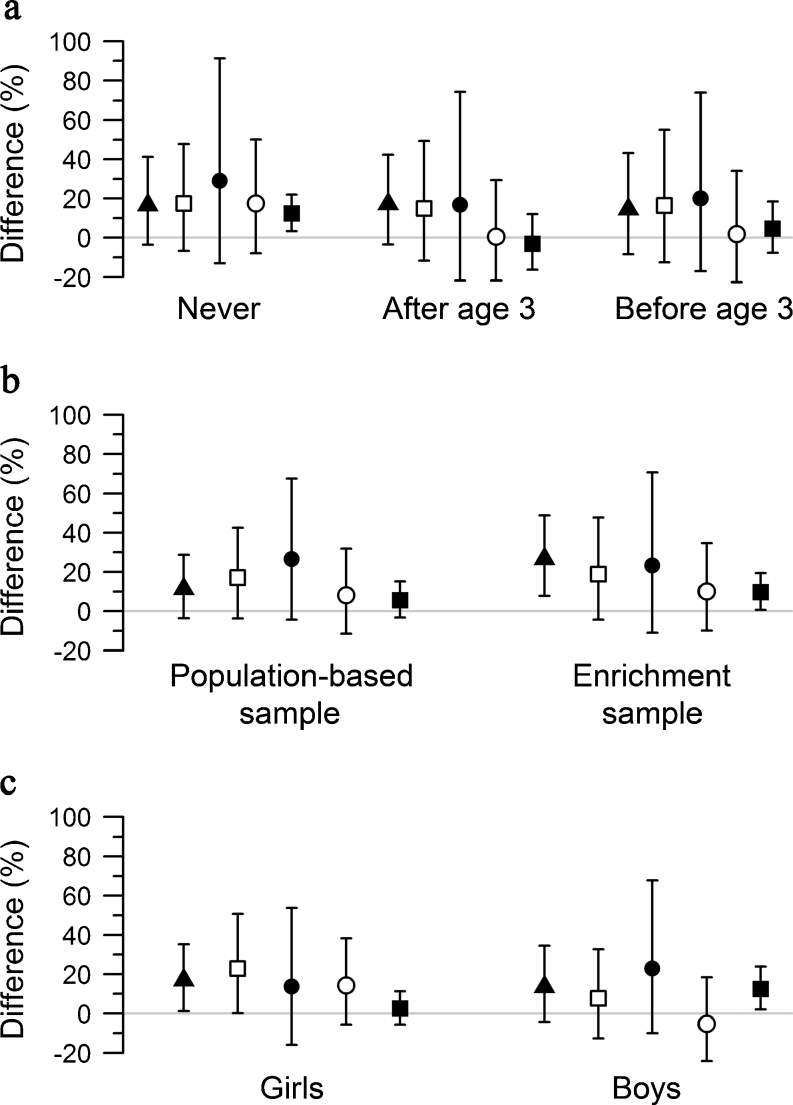
Figure 2 shows the results from the stratified analyses. Effect estimates tended to be higher in children who had never moved between birth and 10 years than in children who had moved (Fig. 2a). Specifically, the point estimates for proximity to a major road and PM2.5 absorbance were only elevated in ‘never movers’. Less heterogeneity was observed between moving strata for NO2, PM10 and PM2.5 exposure.
Fig. 2.
Percentage difference in HOMA-IR per 2SD increase in NO2 (black triangle), PM10 (white square), PM2.5 (black circle), PM2.5 absorbance (white circle), and per 500 m decrease in distance to the nearest major road (black square) stratified by moving house (a), study design (b) and sex (c)
Effect estimates for NO2, PM2.5 absorbance and distance to the next major road also tended to be higher in the enrichment sample with children of higher BMI compared with the population-based sample (Fig. 2b). We did not observe any evidence of effect modification by sex (Fig. 2c).
Discussion
We assessed associations between long-term exposure to traffic-related air pollutants and insulin resistance in school-aged children. We found consistent linear effects for NO2 and PM10, as well as suggestive evidence for other pollutants and distance to a major road. Effect estimates for exposure to NO2 and PM10, as well as living close to a major road, were statistically significant. Associations were robust to additional adjustment for ETS.
To the best of our knowledge, no study has yet investigated the long-term effects of air pollution on insulin resistance in children.
In order to understand the role of traffic-related air pollution exposure in the pathogenesis of insulin resistance, we also review here the evidence for an association between traffic-related air pollution and type 2 diabetes mellitus in adults, which has been investigated in several studies [11–17]. However, it remains difficult to completely reconcile our results with those of these other studies, as diabetes is a complex disease caused by multiple factors, not only insulin resistance.
Recently, Andersen et al [11] reported borderline significant associations between confirmed diabetes cases and NO2 exposure in participants of the Danish Diet, Cancer and Health Cohort Study. Larger effects were observed in never smokers and physically active people, but not for all diabetes cases. Furthermore, in the same cohort, NO2 and traffic load within a 200 m buffer were associated with diabetes-associated mortality [12]. An analysis of two large prospective cohorts [13] did not provide conclusive evidence for an association between exposure to PM in the previous 12 months and incident diabetes, but an association with distance to road was found in women. In our study, we also observed an association with distance to major road, especially in children who had not moved since birth.
While a study in a semirural area in the Netherlands [14] did not find any consistent associations between type 2 diabetes mellitus prevalence and exposure to traffic-related air pollution, despite some indications for an association with traffic within a 250 m buffer, a study conducted in the highly industrialised Ruhr district [15] found a significantly increased hazard for diabetes associated with increasing PM2.5 absorbance and NO2, as well as with proximity to a major road, but only among women with a low educational level. Moreover, Krämer et al [15] reported interaction effects between traffic-related air pollution exposure and the complement factor C3c, a surrogate marker for subclinical inflammation.
In summary, results in adults are inconsistent, especially for PM exposure. Observed effects were higher for NO2 exposure than for PM10 exposure [15], and risk estimates for NOx were only marginally attenuated when modelled together with PM2.5 [16]. The observations that home proximity to a major road is associated with adverse effects in women [13, 15], and that there are indications of a relationship between type 2 diabetes mellitus and traffic within a 250 m buffer [14], imply that traffic-related air pollution could be responsible for associations of air pollution with type 2 diabetes mellitus in adults. This hypothesis is consistent with the associations between traffic-related air pollution and insulin resistance reported in the present study.
The short-term effects of traffic-related air pollution in youth age 10–18 years have been studied by Kelishadi et al [28]. Air pollution (Pollutant Standard Index, CO and PM10) in the 7 days before blood sampling was significantly associated with HOMA-IR in their study, but NO2 was not. We stratified our analyses on the basis of the time a child had lived at the birth address after birth. Effect estimates tended to be larger in children who lived longer at the birth address and who had not moved. Unfortunately, the low sample size did not allow an examination of whether children moved to a less or more polluted location. Nevertheless, we observed a slight increase in insulin resistance for children who were exposed to higher levels of NO2, PM10 and PM2.5 at birth, but moved before the age of 3 years.
Studies on air pollution and insulin resistance in humans are scarce, but associations between air pollution and metabolic outcomes have been studied using several animal models. For example, PM2.5 increased HOMA-IR in rats fed a high-fat diet for 6 weeks but not in rats fed a normal diet [29]. Furthermore, ambient air pollution exaggerated adipose inflammation and insulin resistance in diet-induced obese mice [7]. In our study, we found a tendency for larger effect estimates in the subset of children with higher BMIs (enrichment sample) than in the population-based sample.
A previous study including both men and women [17] found significant associations between type 2 diabetes mellitus and air pollutants only among women. Other studies, in which only a female population was recruited a priori, found indications for an association between air pollution and type 2 diabetes mellitus [15, 16]. In our study, which is restricted to children, we did not find any indications for a risk-modifying effect of sex. However, the speculation that the air pollution exposure assessment may be more accurate for adult women, who are more likely to spend a greater proportion of time at home, than for adult men is not applicable to our study population.
There is some evidence that air pollution is associated with lower birthweight and growth restrictions [30, 31]—also shown previously in one of the cohorts of the present study [32]—which are known risk factors for type 2 diabetes mellitus [33]. Thus, one may speculate that lower birthweight is an intermediate phenotype between air pollution and insulin resistance. However, we found no evidence to suggest that this may be true in our cohort of children with birthweights >2,500 g.
Biological mechanisms
Measured NO2 concentration is considered an indicator for the complex mixture of various gaseous and particulate components originating from both traffic combustion and wear of road and vehicles. PM may also originate from these sources, but also industry. In particular, the Wesel study area is located in proximity to the highly industrialised Ruhr area in Germany, and the air pollution estimates for this city probably reflect pollution originating from a mixture of industrial sources, long-range transportation, atmospheric chemistry and local mobile traffic. Sources of air pollution in Munich are more likely to be dominated by local traffic exhaust (for detailed information on the local LUR models, see Beelen et al [21] and Eeftens et al [22]). Although toxicity differs between air pollutants, they are all considered potent oxidisers that act either directly on lipids and proteins or indirectly through the activation of intracellular oxidant pathways [34, 35]. The oxidative potential of PM from traffic sources on protein and lipid oxidation in young adults has been shown previously [36], as have NO2 effects on levels of oxidised LDL in adolescents [28]. Oxidative stress caused by exposure to air pollutants may therefore play a major role in the development of insulin resistance.
In addition, some studies have reported that short-term and long-term increases in PM and NO2 exposure lead to elevated inflammatory biomarkers [28, 37–39]. For children, adverse effects were also shown for C-reactive protein, TNF-α and IL-1 β [40]. However, there are also large studies that have not found evidence for a relationship between PM and inflammation [41, 42]. A possible explanation for these diverging findings may be variation in the particulate size and the structure and chemistry of the pollutant, which may lead to differences in the inflammatory response depending on the susceptibility of the exposed individual.
Strength and limitations
The primary strengths of this study are the prospective design and the availability of data on lifestyle and multiple risk factors such as exposure to ETS, which allowed us to adjust for many factors that may confound the association between insulin resistance and traffic-related air pollution. However, an important limitation of this study is the potential for exposure misclassification, as the modelling of air pollution estimates at the birth address was based on air pollution concentrations that were measured some years after the birth of the children. Changes in infrastructure—for example, related to road transport, such as noise protection or bypasses, or related to housing, such as additional housing blocks—may have hampered the correct estimation of air pollution concentrations at the birth address. Changes in the residential address may also be a source of exposure misclassification.
However, in a birth cohort study in Ohio, USA, differences in the results for exposure to diesel exhaust particles estimated via LUR models at the birth address and the time-weighted average of exposure at all addresses (including day care and moving) until the age of 3 years were found to be small for the total population [43], and in a birth cohort study in Stockholm, Sweden, strong correlations between early and later air pollution exposure, modelled at residential, day care and school addresses, up to the age of 8 years (r = 0.70–0.89) were observed [44]. Recent studies have also shown that LUR models are temporally stable over at least an 8-year period [45, 46].
These results suggest that exposure assessment at the residential address at birth may be a good approximation of perinatal and early childhood exposure. We were particularly interested in investigating associations with exposures at the birth address, as this corresponds to a developmental period when children are probably more vulnerable and a time when children spend the majority of their time at the home address.
The degree of exposure misclassification resulting from changes in the residential address after birth depends on the age of the child at the first move. We addressed this issue in a sensitivity analysis (see Fig. 2a) and observed a tendency for lower effect estimates in movers than non-movers. We assume that the reported estimates for the total population may thus be biased towards the null because of potential exposure misclassification.
A further limitation of this study is related to the enrichment of the population-based sample with a susceptible group of children, which may have overexaggerated the true effect sizes. However, the stratified sensitivity analysis also shows elevated estimates in the population-based sample, although the effect sizes were larger in the enrichment sample.
To our knowledge, this is the first prospective study that investigated the relationship of long-term traffic-related air pollution and insulin resistance in children. Insulin resistance levels tended to increase with increasing traffic-related air pollution exposure, and this observation remained robust after adjustment for several confounding factors, including BMI and passive smoking.
Conclusions
In conclusion, our data suggest that air pollution exposure at the birth address may increase the risk of insulin resistance at the age of 10 years. Given the ubiquitous nature of air pollution and the high incidence of insulin resistance in the general population, the associations examined here may have potentially important public health effects despite the small/moderate effect sizes observed.
Acknowledgements
We thank the families for their participation in the studies, the obstetric units for allowing recruitment, the GINI and LISA study teams for their excellent work, and E. Fuertes (School of Population and Public Health, University of British Columbia, Canada and Helmholtz Zentrum München, Germany) for editorial assistance.
The GINIplus birth cohort study was designed and/or conducted by: J. Heinrich, H.E. Wichmann, S. Sausenthaler, M. Chen, M. Schnappinger, P. Rzehak (Helmholtz Zentrum München); D. Berdel, A. von Berg, C. Beckmann, I. Groß (Department of Pediatrics, Marien-Hospital, Wesel, Germany); S. Koletzko, D. Reinhard, S. Krauss-Etschmann (Department of Pediatrics, Ludwig Maximilians University, Munich, Germany); C.P. Bauer, I. Brockow, A. Grübl, U. Hoffmann (Department of Pediatrics, Technical University, Munich, Germany); U. Krämer, E. Link, C. Cramer (IUF-Institut für Umweltmedizinische Forschung at the Heinrich Heine University, Düsseldorf, Germany); H. Behrendt (Centre for Allergy and Environment, Technical University, Munich, Germany)
The LISAplus birth cohort study was designed and/or conducted by: J. Heinrich, H.E. Wichmann, S. Sausenthaler, C.M. Chen, M. Schnappinger (Helmholtz Zentrum München-German Research Center for Environmental Health, Institute of Epidemiology, Munich, Germany); M. Borte, U. Diez (Department of Pediatrics, Municipal Hospital ‘St Georg’, Leipzig, Germany): A. von Berg, C. Beckmann, I. Groß (Marien-Hospital Wesel, Department of Pediatrics, Wesel, Germany); B. Schaaf (Pediatric Practice, Bad Honnef, Germany); I. Lehmann, M. Bauer, S. Röder (Helmholtz Centre for Environmental Research–UFZ, Department of Environmental Immunology/Core Facility Studies, Leipzig, Germany); O. Herbarth, C. Dick, J. Magnus (University of Leipzig, Institute of Hygiene and Environmental Medicine, Leipzig, Germany); U. Krämer, E. Link, C. Cramer (IUF-Institut für Umweltmedizinische Forschung, Düsseldorf, Germany); C.P. Bauer, U. Hoffmann (Technical University Munich, Department of Pediatrics, Munich, Germany); H. Behrendt (ZAUM-Center for Allergy and Environment, Technical University, Munich, Germany)
Funding
This study was supported in part by a grant from the German Federal Ministry of Education and Research (BMBF) to the German Center for Diabetes Research (DZD e.V.), and the research leading to these results has received funding from the European Community’s Seventh Framework Program (FP7/2007-2011) under grant agreement number: 211250.
The GINI Intervention study was funded for 3 years by grants from the Federal Ministry for Education, Science, Research and Technology (Grant no. 01 EE 9401–4), and the 6 and 10 year follow-up of the GINIplus study was partly funded by the Federal Ministry of Environment (IUF, FKZ 20462296). The LISAplus study was funded by Helmholtz Zentrum München and partly by grants from the Federal Ministry of Environment (BMU) (for IUF, FKZ 20462296), and Federal Ministry for Education, Science, Research and Technology (nos 01 EG 9705/2 and 01EG9732).
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
ET carried out the statistical analysis and drafted the manuscript. JC wrote the exposure assessment section of the manuscript. JK measured insulin levels. JH and CM were involved in the conception and design of the insulin resistance substudy. JH, BH, DB, AvB, SK and C-PB designed and/or conducted the birth cohort studies. All authors contributed substantially to the interpretation of the data and its discussion, revised the manuscript critically for important intellectual content, and approved the final version.
Abbreviations
- ESCAPE
European Study of Cohorts for Air Pollution Effects
- ETS
Environmental tobacco smoke
- GINIplus
German Infant Study on the Influence of Nutrition Intervention plus Environmental and Genetic Influences on Allergy
- GIS
Geographic information system
- HOMA-IR
HOMA of insulin resistance
- LISAplus
Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood
- LUR
Land use regression
- PM
Particulate matter
- PM2.5
Particulate matter ≤2.5 μm in diameter
- PM10
Particulate matter ≤10 μm in diameter
References
- 1.Rückerl R, Schneider A, Breitner S, Cyrys J, Peters A. Health effects of particulate air pollution: a review of epidemiological evidence. Inhal Toxicol. 2011;23:555–592. doi: 10.3109/08958378.2011.593587. [DOI] [PubMed] [Google Scholar]
- 2.Gan WQ, Koehoorn M, Davies HW, et al. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect. 2011;119:501–507. doi: 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer M, Moebus S, Möhlenkamp S, et al. Urban particulate matter air pollution is associated with subclinical atherosclerosis: results from the HNR (Heinz Nixdorf Recall) study. J Am Coll Cardiol. 2010;56:1803–1808. doi: 10.1016/j.jacc.2010.04.065. [DOI] [PubMed] [Google Scholar]
- 4.Künzli N, Jerrett M, Garcia-Esteban R, et al. Ambient air pollution and the progression of atherosclerosis in adults. PLoS One. 2010;5:e9096. doi: 10.1371/journal.pone.0009096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Q, Yue P, Deiuliis JA, et al. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Liu C, Xu Z, et al. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol Sci. 2011;124:88–98. doi: 10.1093/toxsci/kfr211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care. 2011;34:892–897. doi: 10.2337/dc10-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weitzman M, Cook S, Auinger P, et al. Tobacco smoke exposure is associated with the metabolic syndrome in adolescents. Circulation. 2005;112:862–869. doi: 10.1161/CIRCULATIONAHA.104.520650. [DOI] [PubMed] [Google Scholar]
- 11.Andersen ZJ, Raaschou-Nielsen O, Ketzel M, et al. Diabetes incidence and long-term exposure to air pollution: a cohort study. Diabetes Care. 2012;35:92–98. doi: 10.2337/dc11-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raaschou-Nielsen O, Sørensen M, Ketzel M, et al. Long-term exposure to traffic-related air pollution and diabetes-associated mortality: a cohort study. Diabetologia. 2013;56:36–46. doi: 10.1007/s00125-012-2698-7. [DOI] [PubMed] [Google Scholar]
- 13.Puett RC, Hart JE, Schwartz J, et al. Are particulate matter exposures associated with risk of type 2 diabetes? Environ Health Perspect. 2011;119:384–389. doi: 10.1289/ehp.1002344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dijkema MB, Mallant SF, Gehring U, et al. Long-term exposure to traffic-related air pollution and type 2 diabetes prevalence in a cross-sectional screening-study in the Netherlands. Environ Health. 2011;10:76. doi: 10.1186/1476-069X-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krämer U, Herder C, Sugiri D, et al. Traffic-related air pollution and incident type 2 diabetes: results from the SALIA cohort study. Environ Health Perspect. 2010;118:1273–1279. doi: 10.1289/ehp.0901689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coogan PF, White LF, Jerrett M, et al. Air pollution and incidence of hypertension and diabetes in African American women living in Los Angeles. Circulation. 2012;125:767–772. doi: 10.1161/CIRCULATIONAHA.111.052753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brook RD, Jerrett M, Brook JR, Bard RL, Finkelstein MM. The relationship between diabetes mellitus and traffic-related air pollution. J Occup Environ Med. 2008;50:32–38. doi: 10.1097/JOM.0b013e31815dba70. [DOI] [PubMed] [Google Scholar]
- 18.Berg A, Krämer U, Link E, et al. Impact of early feeding on childhood eczema: development after nutritional intervention compared with the natural course: the GINIplus study up to the age of 6 years. Clin Exp Allergy. 2010;40:627–636. doi: 10.1111/j.1365-2222.2009.03444.x. [DOI] [PubMed] [Google Scholar]
- 19.Zutavern A, Brockow I, Schaaf B, et al. Timing of solid food introduction in relation to atopic dermatitis and atopic sensitization: results from a prospective birth cohort study. Pediatrics. 2006;117:401–411. doi: 10.1542/peds.2004-2521. [DOI] [PubMed] [Google Scholar]
- 20.Matthews D, Hosker J, Rudenski A, et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.Beelen R, Hoek G, Vienneau D, et al. Development of NO2 and NOx land use regression models for estimating air pollution exposure in 36 study areas in Europe—the ESCAPE project. Atmos Environ. 2013;72:10–23. doi: 10.1016/j.atmosenv.2013.02.037. [DOI] [Google Scholar]
- 22.Eeftens M, Beelen R, Bellander T, et al. Development of land use regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas: results of the ESCAPE project. Environ Sci Technol. 2012;46:11195–11205. doi: 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- 23.Cyrys J, Eeftens M, Heinrich J, et al. Variation of NO2 and NO concentrations between and within 38 European study areas: results from the ESCPAE study. Atmos Environ. 2012;62:374–390. doi: 10.1016/j.atmosenv.2012.07.080. [DOI] [Google Scholar]
- 24.Eeftens M, Tsai MY, Ampe C, et al. Spatial variation of PM2.5, PM10, PM2.5 absorbance and PMcoarse concentrations between and within 20 European study areas and the relationship with NO2: results of the ESCAPE project. Atmos Environ. 2012;62:303–317. doi: 10.1016/j.atmosenv.2012.08.038. [DOI] [Google Scholar]
- 25.Thiering E, Brüske I, Kratzsch J, et al. Prenatal and postnatal tobacco smoke exposure and development of insulin resistance in 10 year old children. Int J Hyg Environ Health. 2011;214:361–368. doi: 10.1016/j.ijheh.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 26.R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available from www.R-project.org/, accessed 23 November 2012
- 27.Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::AID-SIM558>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 29.Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ. Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol. 2011;23:507–519. doi: 10.3109/08958378.2011.587472. [DOI] [PubMed] [Google Scholar]
- 30.van den Hooven EH, Pierik FH, de Kluizenaar Y, et al. Air pollution exposure during pregnancy, ultrasound measures of fetal growth, and adverse birth outcomes: a prospective cohort study. Environ Health Perspect. 2012;120:150–156. doi: 10.1289/ehp.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dadvand P, Parker J, Bell ML, et al. Maternal exposure to particulate air pollution and term birth weight: a multi-country evaluation of effect and heterogeneity. Environ Health Perspect. 2013;121:367–373. doi: 10.1289/ehp.1205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slama R, Morgenstern V, Cyrys J, et al. Traffic-related atmospheric pollutants levels during pregnancy and offspring's term birth weight: a study relying on a land-use regression exposure model. Environ Health Perspect. 2007;115:1283–1292. doi: 10.1289/ehp.10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 34.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol. 2011;2011:487074. doi: 10.1155/2011/487074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Møller P, Loft S. Oxidative damage to DNA and lipids as biomarkers of exposure to air pollution. Environ Health Perspect. 2010;118:1126–1136. doi: 10.1289/ehp.0901725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sørensen M, Daneshvar B, Hansen M, et al. Personal PM2.5 exposure and markers of oxidative stress in blood. Environ Health Perspect. 2003;111(2):161–166. doi: 10.1289/ehp.5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med. 2007;176:370–376. doi: 10.1164/rccm.200611-1627OC. [DOI] [PubMed] [Google Scholar]
- 38.Delfino RJ, Staimer N, Tjoa T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117:1232–1238. doi: 10.1289/journla.ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann B, Moebus S, Dragano N, et al. Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect. 2009;117:1302–1308. doi: 10.1289/ehp.0800362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calderón-Garcidueñas L, Villarreal-Calderon R, Valencia-Salazar G, et al. Systemic inflammation, endothelial dysfunction, and activation in clinically healthy children exposed to air pollutants. Inhal Toxicol. 2008;20:499–506. doi: 10.1080/08958370701864797. [DOI] [PubMed] [Google Scholar]
- 41.Forbes LJ, Patel MD, Rudnicka AR, et al. Chronic exposure to outdoor air pollution and markers of systemic inflammation. Epidemiology. 2009;20:245–253. doi: 10.1097/EDE.0b013e318190ea3f. [DOI] [PubMed] [Google Scholar]
- 42.Steinvil A, Kordova-Biezuner L, Shapira I, Berliner S, Rogowski O. Short-term exposure to air pollution and inflammation-sensitive biomarkers. Environ Res. 2008;106:51–61. doi: 10.1016/j.envres.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 43.Ryan PH, LeMasters GK, Levin L, et al. A land-use regression model for estimating microenvironmental diesel exposure given multiple addresses from birth through childhood. Sci Total Environ. 2008;404:139–147. doi: 10.1016/j.scitotenv.2008.05.051. [DOI] [PubMed] [Google Scholar]
- 44.Gruzieva O, Bellander T, Eneroth K, et al. Traffic-related air pollution and development of allergic sensitization in children during the first 8 years of life. J Allergy Clin Immunol. 2012;129:240–246. doi: 10.1016/j.jaci.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Eeftens M, Beelen R, Fischer P, et al. Stability of measured and modelled spatial contrasts in NO2 over time. Occup Environ Med. 2011;68:765–770. doi: 10.1136/oem.2010.061135. [DOI] [PubMed] [Google Scholar]
- 46.Wang R, Henderson SB, Sbihi H, Allen RW, Brauer M. Temporal stability of land use regression models for traffic-related air pollution. Atmos Environ. 2013;64:312–319. doi: 10.1016/j.atmosenv.2012.09.056. [DOI] [Google Scholar]