Summary
p53 is a transcription factor that mediates tumor suppressor responses. Correct folding of the p53 protein is essential for these activities, and point mutations that induce conformational instability of p53 are frequently found in cancers. These mutant p53s not only lose wild-type activity but can also acquire the ability to promote invasion and metastasis. We show that folding of wild-type p53 is promoted by an interaction with the chaperonin CCT. Depletion of this chaperone in cells results in the accumulation of misfolded p53, leading to a reduction in p53-dependent gene expression. Intriguingly, p53 proteins mutated to prevent the interaction with CCT show conformational instability and acquire an ability to promote invasion and random motility that is similar to the activity of tumor-derived p53 mutants. Our data therefore suggest that both growth suppression and cell invasion may be differentially regulated functions of wild-type p53.
Highlights
-
•
p53 is a client protein for CCT
-
•
Inhibition of CCT binding leads to misfolding of p53 in cells
-
•
CCT binding can regulate whether p53 shows growth suppressive or invasive behavior
-
•
CCT binding is a mechanism through which the conformation of p53 can be controlled
Introduction
The p53 tumor suppressor protein plays an important role in preventing malignant development (Vousden and Prives, 2009), functioning primarily as a transcription factor to regulate the expression of a large number of genes that induce cellular responses such as cell-cycle arrest and apoptosis (Beckerman and Prives, 2010). While effective in preventing cancer development, these activities of p53 must also be tightly controlled to allow normal growth and development. Numerous mechanisms through which p53 is regulated have been described, including the control of translation, protein stability, subcellular localization, and interaction with other components of the transcriptional machinery (Hollstein and Hainaut, 2010). In many cancers, the function of p53 is ablated through point mutations that lead to the expression of a mutant p53 protein (Joerger and Fersht, 2007). These tumor-associated point mutations occur predominantly in the central DNA binding domain of p53 and result in a diminished ability of p53 to bind consensus sites in the promoters of p53-regulated genes. While some of these mutations result in amino-acid substitutions of residues within p53 that directly contact the DNA (contact mutants), other mutations result in the misfolding of the p53 protein. The p53 DNA binding domain shows a low thermodynamic stability in vitro, and mutations in this region can lead to further instability, causing the protein to become denatured at 37°C (Joerger and Fersht, 2007) and a potential to form p53 protein aggregates within the cell (Xu et al., 2011). The net effect of these tumor-associated point mutants is both the loss of wild-type p53 activity and a gain of function that contributes to the invasive behavior of cancers (Muller et al., 2011). The mechanisms underlying this gain of function are still under investigation but at least partially reflect the ability of the mutant p53 proteins to modulate the activity of other transcription factors such as p63, p73, and SREBP (Freed-Pastor and Prives, 2012).
Molecular chaperones are a group of proteins that assist in protein folding (Hartl et al., 2011). They not only prevent misfolding and aggregation of proteins but can also target misfolded proteins for degradation. Probably the best-understood chaperones are the heat shock proteins Hsp70 and Hsp90, which play a role in conformational maturation and help to target improperly folded proteins for ubiquitination and proteolysis, and the ring complex chaperonins, which enclose proteins within their structure for folding of newly synthesized peptides (Mayer, 2010). Chaperonins are double-ring oligomers, each ring enclosing a cavity where protein folding takes place through an energy-consuming process (Douglas et al., 2011; Valpuesta et al., 2002). In eukaryotes, the cytosolic group II chaperonin CCT (also known as TRiC) consists of a double ring, each one containing eight subunits (CCTα−θ in mammals and CCT1−8 in yeast). Like other chaperonins, CCT has two main conformations that are controlled by ATP hydrolysis. The open conformation recognizes unfolded peptides, and ATP binding and hydrolysis induce the closed conformation, which results in the folding of the protein (Douglas et al., 2011; Yébenes et al., 2011). Although the mechanism of substrate-CCT recognition and binding remains under investigation, each of the subunits can recognize different polar and hydrophobic motifs within substrate proteins (Yam et al., 2008a). Potentially up to 15% of all newly synthesized polypeptides can associate with the CCT complex, although only a few proteins have so far been shown to depend on this chaperonin for folding and function (Thulasiraman et al., 1999; Valpuesta et al., 2002). CCT plays an important role in the folding of newly synthesized proteins (Frydman et al., 1994; Yam et al., 2008a) but can also prevent the aggregation of proteins with polyglutamine regions (Kitamura et al., 2006; Tam et al., 2006) and so potentially contributes to the suppression of misfolding diseases such as Huntington, Parkinson, and Alzheimer. These activities are executed in conjunction with other chaperones or cochaperones (Siegers et al., 1999).
Both wild-type and mutant p53 have been shown to be regulated by binding to Hsp70 and Hsp90 (Blagosklonny et al., 1996; Walerych et al., 2009). However, a role for the chaperonins in the control of p53 has not been investigated. We show here that p53 is a client of the CCT complex and that failure to interact with this molecular chaperone can promote oncogenic functions of p53 in the absence of classic tumor-derived DNA binding domain mutations.
Results
p53 Binds to CCT
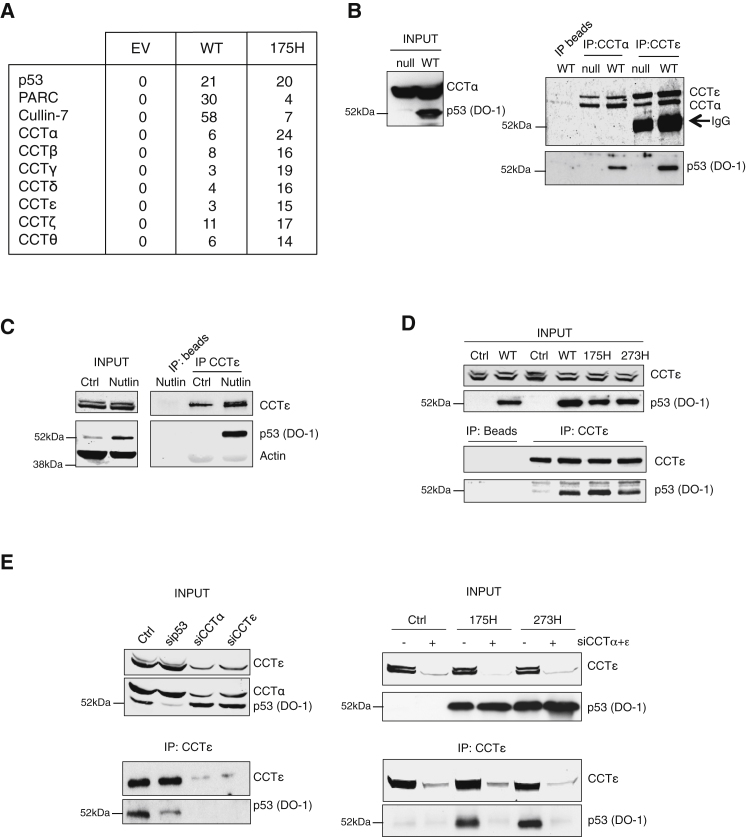
We carried out a proteomic analysis to identify proteins that interact with wild-type p53 and a tumor-derived point mutant, 175H. Peptides from three known p53-interacting proteins—p53 itself, PARC, and Cullin-7 (Andrews et al., 2006; Nikolaev et al., 2003) —were most frequently identified in immunoprecipitation with wild-type p53 (Figure 1A), validating this approach. Interestingly, peptides from seven of the eight CCT subunits were also found in complex with p53 (Figure 1A), consistent with our recent study identifying CCT subunits as part of a p53 interactome (Coffill et al., 2012). To further study the consequences of the interaction of p53 with CCT subunits in cells, we utilized several cell lines that each expressed broadly equal endogenous levels of various CCT subunits (Figure S1A available online). Coprecipitation of endogenous wild-type p53 with endogenous CCTα and CCTε from HCT116 cells (Figure 1B) or U2OS cells (where p53 was stabilized by treatment of cells with the Mdm2 inhibitor Nutlin 3; Figure 1C) demonstrated the ability of these proteins to interact in cells. To compare the binding of wild-type and mutant p53 directly in the same cells, we turned to H1299, a p53 null tumor cell line transiently transfected with p53-expression constructs (Figure 1D). These results indicated that wild-type p53, 175H, and 273H all efficiently interacted with endogenous CCT. Similarly, wild-type p53 and 248W were coprecipitated with CCTα in HCT116 cells (Figure S1B). The specificity of the binding was confirmed by showing that the coprecipitation of p53 was dependent on CCT expression (Figure 1E).
Figure 1.
Wild-Type and Mutant p53 Interact with CCTα and CCTε Subunits
(A) Protein complexes from H1299 cells transfected with empty vector (EV), FLAG-wild-type p53 (WT), or FLAG-175H (175H) constructs were immunoprecipitated with a FLAG antibody and analyzed by mass spectrometry. Frequency of peptide detection of each p53-interacting protein is shown.
(B and C) Immunoprecipitation of endogenous CCTα (crosslinked) or CCTε subunits in p53 null and wild-type p53 (WT) HCT116 cells (B) and U2OS cells (C) treated with 5 μM Nutlin. Total expression and immunoprecipitated proteins were detected by western blot with antibodies specific to p53, CCTα, and CCTε, as indicated.
(D) Immunoprecipitation of endogenous CCTε from H1299 cells transfected with p53 (WT, 273H, and 175H). Input and immunoprecipitated proteins were detected by western blot with antibodies specific to p53 and CCTε.
(E) HCT116 cells were transfected with nontargeting siRNA control (Ctrl), siRNA targeting p53 (sip53), CCTα (siCCTα) and CCTε (siCCTε) (left), and H1299 null for p53 or stably expressing 175H or 273H were transfected with a mixture of siRNA against CCTα and CCTε (right). Following immunoprecipitation of CCTε, expression of p53 and CCTε was analyzed by western blot. See also Figure S1.
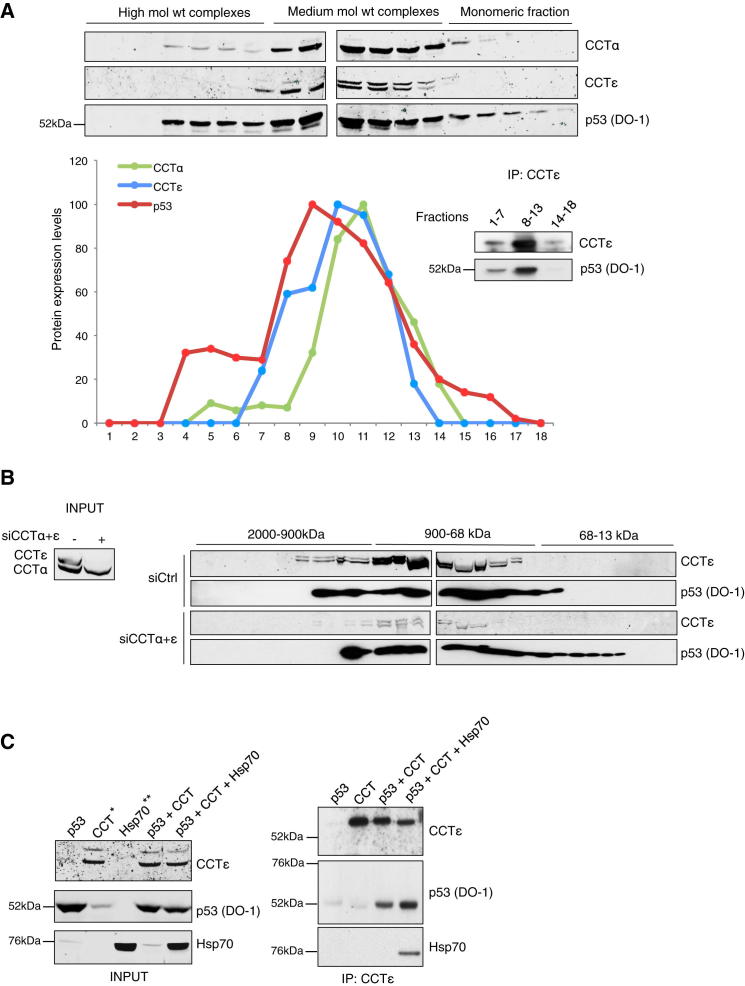
CCT is a large, multisubunit structure (∼960 kDa), and we used gel filtration to determine whether p53 could associate with the whole CCT complex (Figure 2A). Endogenous wild-type p53 largely comigrated with CCTα and CCTε in high-molecular-weight complexes, consistent with an interaction with the whole CCT complex (Figure 2A). Immunoprecipitation of pooled fractions confirmed that the majority of the CCTε was present in fractions 8–13 and coprecipitated with p53 (Figure 2A). Similar results were observed in lysates from H1299 cells transiently transfected with wild-type p53 (Figure S2). Depletion of CCTα and CCTε resulted in the release of some p53 from the larger complexes, supporting the interaction of p53 with CCT (Figure 2B), although the retention of some p53 in larger complexes following CCT depletion likely reflects p53’s interaction with other proteins (Collavin et al., 2010). Quantification of p53 levels in each fraction indicated that approximately 12% of the total p53 was released from the larger complexes, suggesting this proportion of p53 is bound to CCT under these conditions. Cooperation between various chaperone complexes in protein folding has been described, and p53 is known to associate with several other chaperones, including Hsp70 (Blagosklonny et al., 1996; Walerych et al., 2009). Hsp70 contributes to the interaction between VHL and CCT (Melville et al., 2003), and we also found that Hsp70 slightly increased the binding of p53 to CCTε (Figure 2C).
Figure 2.
p53 Interacts with the Whole CCT Complex
(A) Lysate from wild-type p53 HCT116 cells was separated by gel chromatography and p53, CCTα, and CCTε expression assessed in the fractions 1–18 by western blotting and quantified. CCTε was immunoprecipitated from fractions 1–7 (high-molecular-weight complexes), 8–13 (medium/low-molecular-weight complexes), and 14–18 (monomeric fraction) and analyzed for p53 and CCTε expression by western blotting.
(B) Wild-type p53 HCT116 cells were transfected with control or CCTα and CCTε siRNA. Knockdown of CCTα and CCTε was verified by western blot using 2% of the lysates (left). The remaining lysates were separated by gel chromatography and p53 and CCTε proteins were visualized by immunoblot.
(C) In vitro-translated p53 was incubated with purified CCT complex and/or Hsp70 as indicated. Immunoprecipitation of Hsp70 and p53 with CCTε (right) and total proteins in the lysate used for the immunoprecipitation (left) were assessed by western blotting with Hsp70, CCTε, and p53 antibodies. ∗CCT input lane contaminated with spillover from the adjacent p53 lane. ∗∗Hsp70 alone lysate was not used for the immunoprecipitation. IP, immunoprecipitation. See also Figure S2.
Visualization of p53/CCT Complex
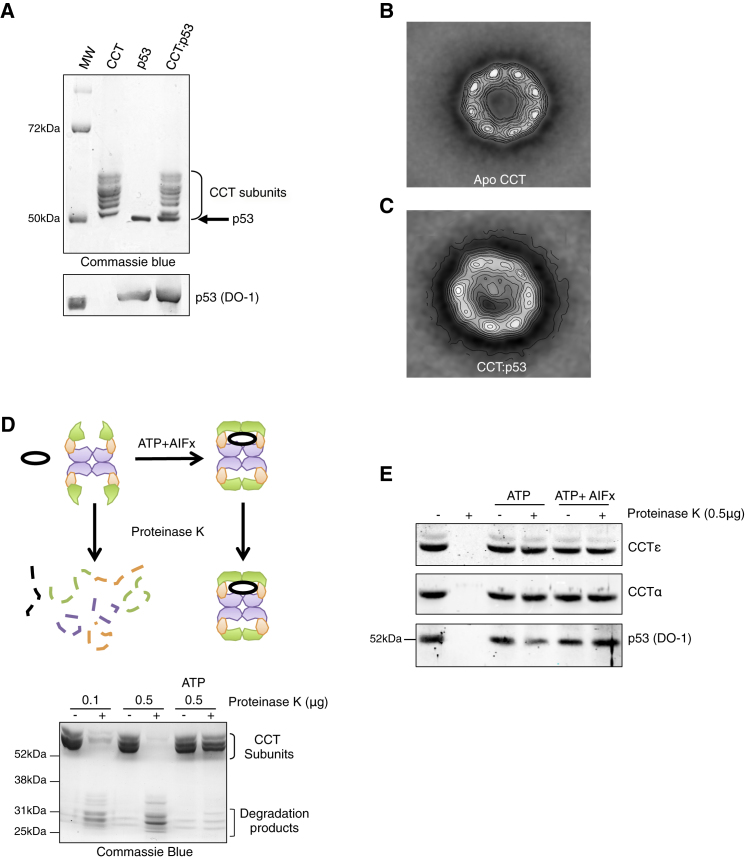
To test directly whether p53 interacts with the CCT complex, we mixed bacterially expressed wild-type p53 with purified bovine CCT. Following separation by native electrophoresis, the high-molecular-weight band corresponding to CCT was excised and subjected to SDS electrophoresis, which revealed the presence of CCT subunits and p53 (Figure 3A), confirming the presence of a stable CCT:p53 complex. The complex was then subjected to electron microscopy of negatively stained specimens. First, over 1,000 end-on views of the isolated apo-CCT particles were processed, and the average image revealed the typical presence of the doughnut-shaped structure with an empty cavity surrounded by eight similar masses (Figure 3B). The averaging of almost 2,000 CCT:p53 particles revealed a similar structure except for the presence of mass inside the cavity that interacted mainly with one CCT subunit (Figure 3C; Figures S3A–S3D). Previous studies have shown that the ATP-dependent conformational change leading to closure of the CCT complex also results in its protection against proteinase K digestion (Meyer et al., 2003). We were also able to confirm a dose-dependent degradation of the CCT complex following incubation with proteinase K, which was prevented by the addition of ATP (Figure 3D). Importantly, this protection from degradation also applied to the p53 protein bound to CCT, as the closed conformation of the chaperonin was able to protect the trapped p53 from protease attack (Figure 3E). Taken together, these data demonstrate that p53 interacts with the CCT complex and can be trapped in the interior of the chaperonin cavity in an ATP-hydrolysis-dependent manner.
Figure 3.
The CCT-p53 Interaction Is ATP Dependent
(A) CCT:p53 binding assay revealed bands corresponding to the CCT subunits and p53. Below: western blot showing p53 expression.
(B) Two-dimensional average image of the end-on view of apo-CCT (1,143 particles).
(C) Two-dimensional average image of the CCT:p53 complex (1,957 particles).
(D) Representation of the open and closed conformation of CCT (top). When the CCT conformation is closed after ATP hydrolysis, the complex and proteins inside are protected from protease treatment (proteinase K). The purified CCT complex was incubated with ATP followed by treatment with proteinase K. Lysates were separated by 10% SDS-PAGE and degradation of CCT subunits detected by Coomassie blue staining.
(E) In-vitro-translated p53 was incubated with purified CCT complex with ATP or ATP + AIFx, followed by incubation with proteinase K. p53, CCTα, and CCTε were detected by western blot. See also Figure S3.
The N Terminus of p53 Binds CCT Subunits
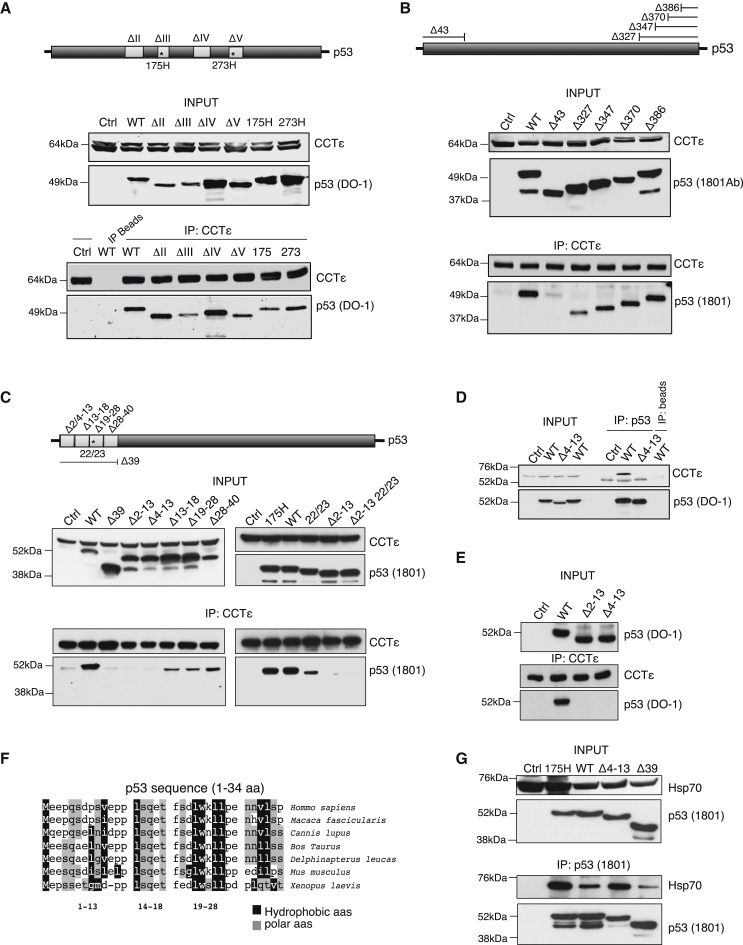
To determine which regions of p53 were important for the CCT interaction, we examined the binding of various p53 mutants to endogenous CCT. Deletions or point mutations within the DNA binding domain or the C terminus of p53 did not affect binding to CCTε (Figures 4A and 4B). Interestingly, several of the C-terminal truncations also removed the oligomerization domain and the nuclear import signals from p53, suggesting that neither of these is required for CCT binding in cells. As shown previously, CCTε localized predominantly to the cytoplasm, although some nuclear staining was also detected (Figures S4A–S4C) (Joly et al., 1994; Willison et al., 1989). Both endogenous and transfected wild-type p53 were found predominantly (although not exclusively) in the nuclear fractions (Figures S4B and S4C). However, despite detecting both p53 and CCT proteins in the nucleus, coimmunoprecipitation showed that the endogenous p53/CCT interaction occurs in the cytosol (Figure S4B).
Figure 4.
The First 13 Amino Acids of p53 Are Required for CCT Binding
(A–C) p53 null H1299 cells were transfected with p53 constructs, followed by immunoprecipitation of endogenous CCTε. CCT and p53 expression in the immunoprecipitates or total cell lysates were assessed by western blot. (A) CCTε binding to p53 DNA binding domain deletion/mutation constructs. The second lane shows lysates from wild-type p53 transfected cells, precipitated only with beads as a control. (B) CCTε binding to p53 C-terminal deletion constructs and N-terminal Δ43 deletion. (C) CCTε binding to p53 N-terminal deletion/mutation constructs.
(D) CCT/p53 binding in H1299 cells transfected with wild-type p53 and Δ4–13 deletion construct by immunoprecipitation with p53 (DO-1) or beads only. Protein expression was detected by western blot with CCTε and p53 antibodies.
(E) Wild-type and p53 N-terminal deletions Δ2–13 and Δ4–13 were in vitro translated. CCT/p53 binding was assessed by immunoprecipitation of CCTε present in reticulocyte lysates followed by western blot with p53 and CCTε antibodies.
(F) Alignment of p53 N-terminal 34 amino acids from different species; hydrophobic and polar amino acids are indicated in black and gray, respectively.
(G) Hsp70 binding to p53 was assessed by p53 immunoprecipitation from H1299 cells transfected with p53 constructs (wild-type, 175H, Δ4–13, and Δ39) followed by western blot with p53 and Hsp70 antibodies. See also Figure S4.
While alterations within the DNA binding and C-terminal domains of p53 did not affect the CCT interaction, deletion of the N-terminal 43 amino acids of p53 resulted in the loss of interaction with CCTε (Figure 4B). The N terminus of p53 is responsible for many protein interactions, including binding to Mdm2 and the components of the transcriptional machinery (residues 17–29; Kussie et al., 1996). While mutation in the Mdm2 binding and transactivation domains (deleted in Δ13–18 and Δ19–28, mutated in 22/23) slightly reduced but did not abolish CCTε binding, deletion within the N-terminal 13 amino acids of p53 (Δ2–13 and Δ4–13) resulted in substantial loss of interaction with CCTε (Figure 4C). Deletion of these residues also resulted in the loss of binding of p53 to CCTα (Figure S4D). The failure of these mutants to interact with CCT proteins was not a result of lack of cytoplasmic localization, since Δ2–13 was clearly detected in both nuclear and cytoplasmic fractions (Figure S4C). Importantly, this interaction was also seen in a reciprocal immunoprecipitation, where wild-type but not Δ4–13 p53 pulled down endogenous CCTε (Figure 4D). The loss of CCT binding to Δ2–13 and Δ4–13 p53 was also confirmed using in vitro translation in reticulocyte lysates, which contain endogenous CCT (Figure 4E). The N-terminal region of p53 shows moderate conservation between different species and contains polar and hydrophobic amino acids (Figure 4F), which may mediate the interaction with CCT (Gómez-Puertas et al., 2004). To assess the contribution of hydrophobic bonds to the p53-CCT interaction, we examined the effect of chaotropic salts containing cations that can weaken hydrophobic bonds (Feldman et al., 2003; Hayer-Hartl et al., 1994) (Figure S4E). The disruption of the p53-CCTε interaction following incubation with LiCl and MgSO4 suggests a possible contribution of hydrophobic interactions. Taken together, these results show that the first 13 amino acids of p53 are necessary for the p53-CCTε interaction.
To further assess whether CCT impacts Mdm2 binding by p53, we examined the effect of CCT depletion on the endogenous p53/Mdm2 interaction (Figure S4F). Importantly, the binding of Mdm2 to p53 was not affected by depletion of CCTα and CCTε, an effect that is most clearly seen following stabilization of the p53 protein using the proteasome inhibitor MG132 (Figure S4F). Furthermore, the CCT nonbinding N-terminal deletion of p53 (Δ4–13) retained the ability to bind Mdm2, an activity that was lost in the more extensive N-terminal truncation Δ39 (Figure S4G).
We also examined the impact of loss of CCT binding on the interaction between p53 and Hsp70 (Figure 4G). Again, deletion of the extreme N terminus of p53 (Δ4–13) that abolished the CCT interaction did not prevent binding to Hsp70, although a more extensive deletion (Δ39) reduced Hsp70 binding. Taken together, these results show that CCT binding is not required for p53 to bind Mdm2 or Hsp70.
CCT Depletion Results in a Conformational Change in p53
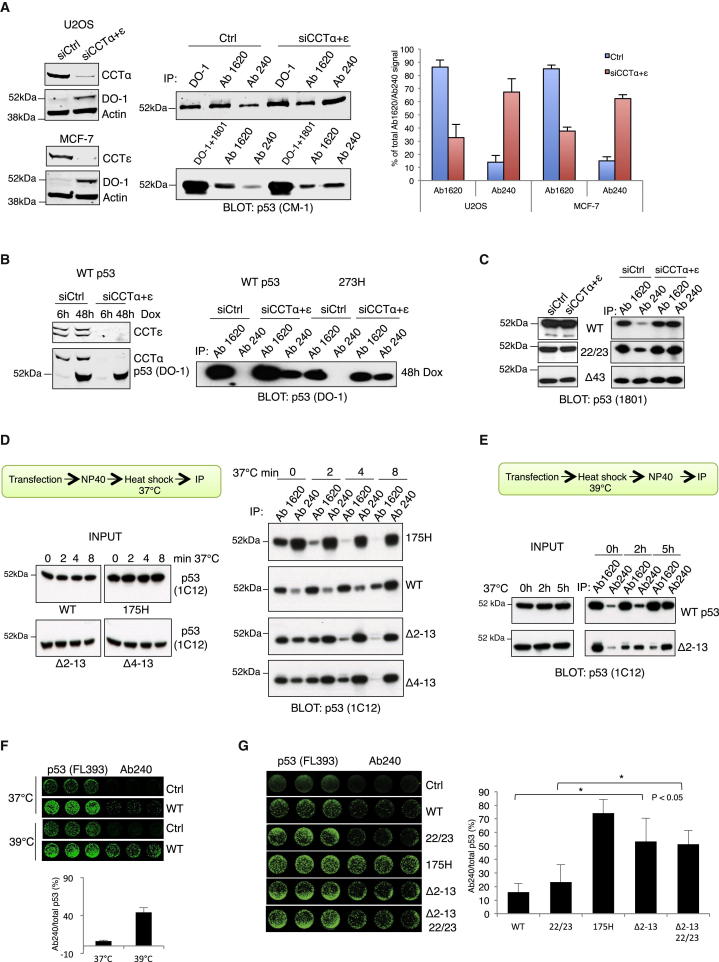
The interaction of p53 with the CCT complex suggests that this chaperonin may play a role in promoting the correct folding, and thus activity, of p53. The conformation of p53 can be assessed using two antibodies: Ab1620, which recognizes a domain only present in the correctly folded protein and therefore indicates functional wild-type p53 (Milner et al., 1987), and Ab240, which recognizes a cryptic epitope (amino acids 211–220) only exposed in the unfolded or denatured conformation (Gannon et al., 1990). The relative amounts of p53 recognized by Ab1620 and Ab240 provide an indication of the extent of p53 folding. To determine whether CCT contributes to p53 folding, we used small interfering RNA (siRNA) to deplete U2OS or MCF7 cells (that express endogenous wild-type p53) of CCTα and CCTε (Figure 5A). Immunoprecipitation of p53 with the conformation-specific antibodies showed a clear decrease in Ab1620-reactive p53 and an accumulation of Ab240-reactive p53 in cells depleted of CCT complex components (Figure 5A). A similar response was seen in A2780 cells (Figure S5A) and in cells where p53 protein was induced in response to doxycycline treatment (Figure 5B). In these cells, both wild-type p53 and the 273H mutant, which retains predominantly wild-type conformation, became misfolded (and Ab240 reactive) following CCT depletion (Figure 5B). During the course of these studies, we noted that depletion of both CCT subunits α and ε frequently led to an increase in p53 protein levels in cells expressing endogenous wild-type p53 cells (Figure 5A; Figure S5B), although this was not evident in cells expressing transfected mutant p53 (Figure 1E). The increase in p53 protein levels following CCT knockdown was accompanied by a slight increase in p53 half-life in both HCT116 and U2OS cells (Figure S5B), and depletion of CCT in otherwise unstressed cells resulted in a reduction in extent of ubiquitination of endogenous p53 (Figure S5C). Previous studies have shown that CCT depletion can result in cell-cycle arrest (Grantham et al., 2006; Liu et al., 2005), indicating that the incorrect folding of CCT client proteins is likely to promote a stress response that could indirectly signal to activate p53.
Figure 5.
Conformational Stability of p53 Is Dependent on CCT Binding
(A) Lysates from U2OS or MCF7 cells transfected with siRNA targeting CCTα and CCTε were immunoprecipitated with the p53 antibodies DO-1, 1801, Ab1620, or Ab240, followed by western blotting with CM-1. Knockdown was confirmed by western blotting total cell lysates (left). The extent of folded or misfolded p53 (right) is calculated as a percent of the sum of the band densities of p53 precipitated by Ab1620 and Ab240 for each condition. The graph shows the mean ±SEM from three independent experiments.
(B) Expression of wild-type p53 and 273H in H1299 cells transfected with CCTα and ε siRNA was induced by doxycycline treatment. Lysates were immunoprecipitated with the conformational antibodies and proteins detected by western blot.
(C) CCTα+ε subunits were depleted in H1299 cells as in (B), followed by transfection with wild-type (WT), 22/23, and Δ43 p53 constructs. After 48 hr, lysates were immunoprecipitated with the conformational antibodies and p53 detected by western blot.
(D) H1299 cells were transfected with the indicated p53 constructs (175H, WT, Δ2–13, and Δ4–13) and lysates incubated at 37°C for 0–8 min. p53 proteins were immunoprecipitated with Ab1620 or Ab240 followed by western blotting to detect p53.
(E) H1299 cells were transfected with the indicated p53 constructs, then incubated at 39°C for 0–5 hr before harvesting. p53 conformation was determined as in (B).
(F) H1299 cells were transfected with an empty plasmid (Ctrl) or wild-type p53 and incubated at 39°C for 24 hr. After fixation, cells were stained with FL-393 (to detect total p53) or Ab240.
(G) H1299 cells transfected with different p53 constructs were processed as in (F). The graphs represent the Ab240 reactive signal as a percentage of the total p53 signal and show the mean ±SEM of three replicates from three independent experiments. ∗p < 0.05. See also Figure S5.
Loss of CCT Binding Leads to Conformational Instability of p53
Following transient expression of p53, both wild-type and transactivation domain mutant 22/23 retained predominantly wild-type (Ab1620) conformation, while depletion of CCT resulted in an increase in misfolded p53 detected by Ab240 (Figure 5C). By contrast, p53Δ43, lacking the CCT binding and transactivation domain, adopted a more unfolded conformation that was not further affected by CCT depletion (Figure 5C). To more closely define the effect of CCT binding, we examined the smaller N-terminal deletions of p53. Using standard extraction procedure (lysing cells and immunoprecipitating at 4°C), we found variable results with the CCT nonbinding p53 mutants (Δ2–13 and Δ4–13), which showed a slightly higher ratio of Ab240 reactivity in some assays but mainly Ab1620 reactivity similar to wild-type p53 in others (for example, compare control lanes in Figures 5D and 5E). Previous studies have shown that p53 has a low thermodynamic stability in vitro, which is further reduced by mutations within the DNA binding domain (Bullock et al., 1997; Mayer et al., 2007). We therefore considered whether the p53 mutants that are unable to bind to CCT might show a greater sensitivity to temperature-induced denaturation. To test this, we examined the conformation of p53 in cell extracts following incubation at 37°C (Figure 5D). As previously described (Bullock et al., 2000), the 175H mutant was highly unstable compared to the wild-type p53 protein, which became predominantly Ab240 reactive only after 8 min at 37°C. Interestingly, both p53Δ2–13 and p53Δ4–13 were clearly less stable than the wild-type protein in this assay (Figure 5D). To extend these studies, we incubated p53-transfected cells at 39°C for 2 or 5 hr immediately before lysis and carried out conformation-specific immunoprecipitation (Figure 5E). These studies again showed that p53Δ2–13 was less stable than wild-type p53, shifting significantly to the unfolded, Ab240-reactive conformation after only 2 hr heat shock, at which time point the wild-type protein remained predominantly Ab1620 reactive.
To look directly at the conformation of p53 in cells, we used an immunofluorescence assay in which we compared the signal from total p53 (as detected using a p53 polyclonal antibody) with the signal using Ab240 (Figure 5F). To validate the assay, we showed that heat shock induced the expected increase in Ab240 reactivity in cells expressing wild-type p53, consistent with the results shown in Figure 5E. Turning to p53 mutants, both wild-type and transactivation domain mutant 22/23 showed low Ab240 reactivity in cells incubated at 37°C, while the 175H mutant reacted strongly with this antibody (Figure 5G), as expected. In this assay, deletion within the N-terminal 13 amino acids (Δ2–13 and Δ2–13, 22/23) resulted in a significant acquisition of Ab240 reactivity. Taken together, these results show that deletion of the CCT binding domain in the N terminus of p53 results in a protein that is structurally less stable than wild-type and more sensitive to unfolding under temperature stress.
We also examined the contribution of Hsp70 to the conformation of p53. Wild-type p53 showed a shift to the misfolded Ab240 reactive conformation following treatment of cells with an Hsp70 inhibitor alone or in combination with heat shock (Figure S5D). A similar shift in the conformation of wild-type p53 following treatment with the Hsp70 inhibitor was seen in cells using the immunofluorescence assay (Figure S5E). These results indicate that both CCT complex and Hsp70 can promote the folding of p53 and that impeding either chaperone impacts the conformation of p53.
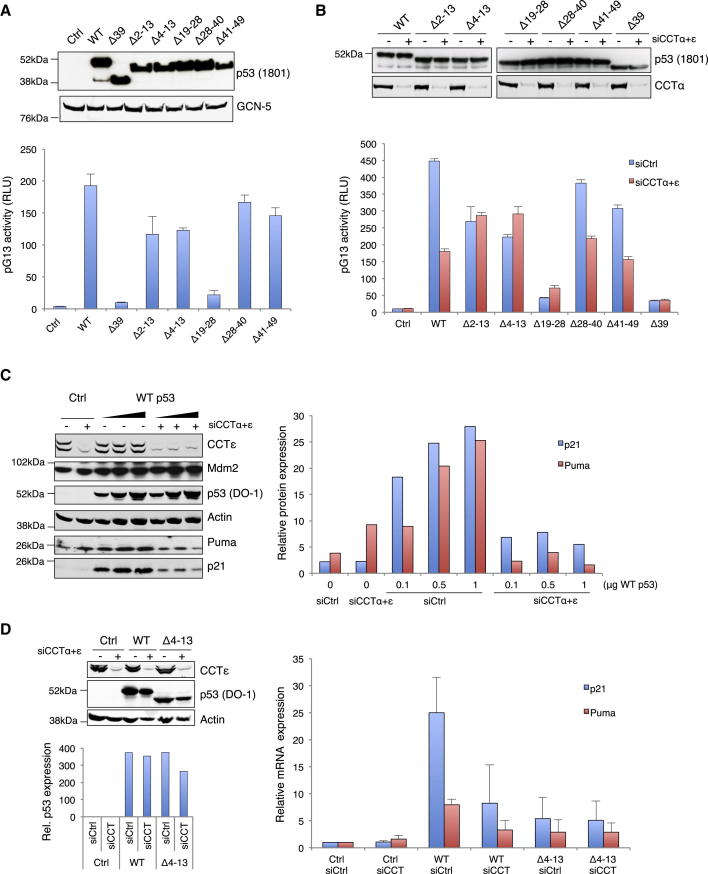
CCT Binding Regulates Transcriptional Activity of p53
p53 has two transactivation domains: AD1, which is located to residues 14–28, and AD2, which is located to residues 38–61 (Candau et al., 1997; Lee et al., 2009). The transcriptional activity of the N-terminal p53 mutants described here was therefore assessed using a p53-responsive promoter (PG13 luciferase; Figure 6A). As expected, deletion of residues within AD1 (p53Δ39 and Δ19–28) significantly impaired the transcriptional activity of p53, which was largely retained in a mutant deleted of residues outside this region (p53Δ28–40 and p53Δ41–49; Figure 6A). However, the CCT binding mutants p53Δ2–13 and p53Δ4–13 (which would not be predicted to impinge on AD1) showed reduced activity, consistent with the misfolding of these proteins. As seen in earlier assays, the levels of transfected p53 protein were not increased following CCT depletion (Figure 6B), indicating that ectopic overexpressed protein is less sensitive to the endogenous p53 degradation machinery. However, the activity of wild-type p53 and transcriptionally active mutants that retained the N-terminal CCT binding domain (p53Δ28–40, p53Δ41–49) was reduced following knockdown of CCTα and CCTε subunits (Figure 6B), consistent with a loss of wild-type conformation. By contrast, the lower level of transcriptional activity retained by p53Δ2–13 and p53Δ4–13 was not further compromised by inhibition of CCT complex (Figure 6B), supporting our observation that these mutants no longer interact with, and are therefore not subject to, regulation by CCT.
Figure 6.
Impairment of p53-CCT Binding Decreases p53 Transcriptional Activity
(A) H1299 transfected with pG13 firefly luciferase, TK Renilla luciferase, and p53 constructs (as indicated). Top: western blot of p53 protein expression with GCN5 loading control. The bottom graph shows luciferase activity. The graph shows means ± SEM of three replicates from three independent experiments.
(B) Cells treated as (A), following siRNA depletion of CCTα and CCTε. Top: p53 and CCT expression levels.
(C) H1299 cells were transfected with empty vector (Ctrl) and increasing amounts of wild-type p53 following depletion of CCTα and ε. Total protein expression levels were visualized with antibodies for p21, p53, Mdm2, Puma, and actin. The graph represents the quantification of p21 and Puma protein expression normalized to actin levels.
(D) After 24 hr of CCTα+ε depletion, H1299 cells were transfected with wild-type p53 and Δ4–13. p53, CCTε, and actin expression were assessed by western blot, and the graph represents quantification of p53 levels normalized to actin expression. mRNA expression for p21 and Puma is shown on the right. The graph shows the mean ±SEM from three independent experiments. See also Figure S6.
The modulation of p53’s transcriptional activity by CCT was also apparent when examining the activation of endogenous p53-dependent target genes. p53-induced expression of both Puma and p21 was clearly impeded by the depletion of CCT, both at the protein and messenger RNA (mRNA) level (Figures 6C and 6D; Figures S6A and S6B). Interestingly, however, Mdm2 expression was not affected by CCT depletion, suggesting that the ability of p53 to induce the expression of Mdm2 is less dependent on p53 conformation. Expression of p53Δ4–13 also showed a decreased ability to activate expression of p21 and Puma (Figure 6D). As seen with the p53 reporter assays, depletion of CCT reduced the transcriptional activity of wild-type p53 but did not impact the lower activity of the Δ4–13 mutant (Figure 6D).
Interestingly, depletion of the CCT complex did not result in a profound decrease of endogenous p53 activity, either under basal conditions or following activation of p53 by treatment of the cells with Nutlin 3 or low levels of actinomycin D (Figures S6C and S6D). Although depletion of CCT resulted in an increase in endogenous p53 stability (Figure S5), no corresponding increase in transcriptional activity was detected either. Taken together, these results suggested that the response of endogenous p53 to CCT knockdown is complex and that the outcome is a balance between increased protein levels (due to reduced degradation of p53 as part of a general stress response) and decreased conformational integrity (due to defects in the protein folding process).
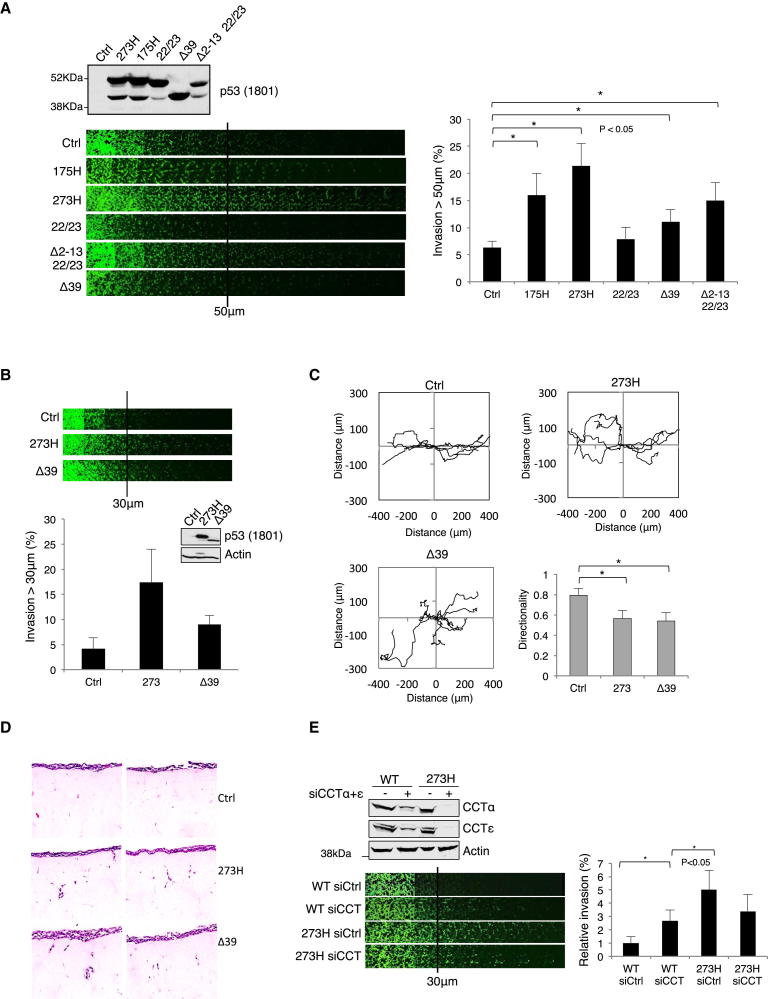
CCT Binding Regulates the Invasive Activity of p53
Tumor-derived p53 point mutations such as 175H and 273H acquire an ability to promote Matrigel invasion, invasion into organotypic assays, and random migration (Adorno et al., 2009; Muller et al., 2009; Muller et al., 2013). Expression of wild-type p53 in these cells inhibits proliferation and enhances cell death and cannot be used as a control for invasion. We therefore used the transcriptionally defective p53 22/23 mutant (which shows impaired growth inhibitory activity), which did not result in enhanced invasion above that seen with the empty vector control (Figure 7A). Interestingly transient expression of non-CCT binding mutants p53Δ2–13 22/23, p53Δ4–13 22/23, or p53Δ39 promoted invasion, albeit to a lesser extent than the tumor-derived 175H or 273H mutant (Figure 7A; Figure S7A). Notably, loss of the N terminus in 175H- or 273H-expressing cells did not impact invasive capacity (Muller et al., 2009). Further investigation of the ability of the non-CCT binding mutants of p53 to promote activities associated with tumor-derived mutants, such as random motility or invasion into organotypic assays, required cell lines that stably express these p53 proteins. Despite attempts to generate H1299 cell lines stably expressing p53 22/23, p53Δ2–13 22/23, p53Δ4–13 22/23, and p53Δ39, only p53Δ39-containing cells retained expression over time, although this was lower than 273H (Figure 7B; Figure S7B). These results are likely to reflect the retention of some residual growth inhibitory activity in the p53 22/23 mutant. Compared to empty vector transfected H1299 cells (Ctrl), p53Δ39 cells induced random motility and invasion into Matrigel and organotypic assays to a similar or slightly lesser extent than 273H (Figures 7B–7D). Furthermore, despite the loss of expression of transiently transfected p53 22/23 and p53Δ4–13 22/23 during the course of the experiment, both p53Δ4–13 22/23 and 175H 22/23 cells showed some invasion into organotypic plugs (Figure S7B).
Figure 7.
Impairment of p53-CCT Binding Enhances Invasion and Random Motility
(A) H1299 cells were transfected with mutant p53 constructs (as indicated) then placed onto Matrigel for 48 hr, and invasion was analyzed by confocal microscopy and quantified as described in Experimental Procedures. The graph shows the mean ±SEM from four independent experiments. ∗p < 0.05.
(B) Invasion of stable H1299 cells expressing vector control (Ctrl), mutant p53 (273H), and the N-terminal deletion Δ39 was assessed. Expression of 273H and p53Δ39 was verified by western blot.
(C) Motility of stable H1299 cells used in (B) was determined in wound scratch assays. The directionality was determined as described in Experimental Procedures, and error bars represent ±SEM of 108 track-plots from three independent experiments. ∗p < 0.05.
(D) Invasion of stable H1299 cells used in (B) in organotypic assays.
(E) RPE cells expressing endogenous wild-type p53 and transfected 273H were depleted of CCTα and CCTε and invasion measured as in (A). The graph shows the mean ±SEM from four independent experiments. ∗p < 0.05. Knockdown of CCT subunits and protein expression levels were verified by western blot with p53, actin, and CCT antibodies. See also Figure S7.
Finally, we examined whether depletion of the CCT complex would have an effect on cell invasion using RPE cells (expressing wild-type p53) and variants stably expressing 273H or 175H, mutants that promoted the invasive ability of these cells (Figure 7E; Figure S7C). Depletion of CCT complex by siRNA in either 175H- or 273H-expressing cells resulted in decreased invasion (Figure 7E; Figure S7C) that is likely to reflect the misfolding of other CCT client proteins like actin and tubulin, as previously described (Matus et al., 2010). In wild-type p53-expressing cells, we were unable to achieve a complete depletion of CCT complex proteins, indicating that only cells with partial knockdown continued proliferation, consistent with our observation that CCT complex depletion resulted in a stress response that activated p53 (Figure 6). Interestingly, cells that survived with a partial depletion of CCT showed a reproducible increase in invasion (Figure 7E), consistent with a role of CCT in maintaining wild-type conformation and function of p53. Depletion of wild-type p53 in these cells resulted in enhanced invasion (Figure S7D), so it was not possible to demonstrate p53 dependence of the increased invasion in CCT-depleted wild-type p53-expressing cells. Importantly, however, depletion of both p53 and CCT decreased invasion (Figure S7D), similar to the effect seen in mutant p53-expressing cells. These results show that despite a general inhibition of invasive behavior in response to CCT depletion, wild-type p53-expressing cells show enhanced invasion that correlates with the misfolding of the p53 protein.
Discussion
Correct protein folding is essential for function, and this process is assisted by molecular chaperones. The chaperonin CCT assists in specialized folding of selected proteins that are not fully folded by the nonspecialized chaperones alone. In this study, we demonstrate that p53 is a substrate for CCT and provide evidence that this chaperonin regulates p53 folding and activity. Binding of p53 to Mdm2 and Hsp70 was not dependent on CCT interaction.
Many previous studies have shown that tumor-derived p53 point mutants result in the failure of p53 to adopt the wild-type conformation, leading to the concept that p53 can either be in the wild-type or mutant (i.e., misfolded) conformation. Misfolding of p53 due to cancer-associated DNA binding domain mutations, which enhances the thermoinstability of p53 (Bullock et al., 1997), results in the loss of wild-type DNA binding activity and the acquisition of transforming functions, including the ability to promote invasion. Our studies suggest that CCT plays a specific role in maintaining a pool of wild-type, functional p53 in the cell and that failure of p53 to bind to CCT results in the accumulation of misfolded p53, which then acquires activities characteristic of tumor-derived mutant p53s. While the most straightforward model for the role of CCT is to assist in the correct folding of newly synthesized p53 (Mayer, 2010), it is also possible that CCT binding to p53 prevents its aggregation, as has been shown for other proteins. CCT would function to allow the correct folding of p53 that occurs either spontaneously or through a cooperation with other chaperones, for example with Hsp70. In either case, the consequence of loss or modulation of the CCT-p53 interaction would be the accumulation of misfolded p53 with invasive capacity.
Based on these results, we suggest that the regulation of wild-type p53 folding may be part of the normal biology of p53. Regulation of the interaction of p53 with CCT could determine the generation of folded or unfolded p53, without the requirement for mutations in the DNA binding domain. Our study shows that the effect of loss of CCT binding on p53 conformation is subtle, supporting in vitro studies in which wild-type p53 can spontaneously fold into an active conformation. Nevertheless, the role of CCT in protein folding becomes more apparent under stress, as shown in the heat shock experiments described here. It seems possible that the role of CCT will also be evident under other physiological stress conditions, such as hypoxia, reactive oxygen species, and loss of cell adhesion.
Several recent studies have shown that CCT activity can be controlled by phosphorylation by RSK and S6K (Abe et al., 2009) or deacetylation by Sirt1 (Liu et al., 2010). In addition, phosphorylation of serine 6 and 9 in p53 has also been identified (Meek and Anderson, 2009), so further studies will determine whether the p53/CCT interaction can be actively modulated through changes in posttranslational modifications of either protein. Active control of CCT binding in this way could allow for the expression of wild-type p53 in either folded or unfolded conformations resulting in distinct activities. According to this model, DNA binding domain point mutations that are selected in cancers would force p53 to constitutively adopt the unfolded conformation. The activity of mutant p53 would therefore be the inappropriate manifestation of a normal state of wild-type p53 rather than the acquisition of a completely new activity. The accumulation of unfolded p53 may lead to the formation of aggregates, which would not be reversible (Ano Bom et al., 2012). However, in vitro studies have shown that there is an initial reversible step in the denaturation of p53 (Wilcken et al., 2012), and in cells, temperature-sensitive p53 mutants can shift from mutant to wild-type forms (Friedlander et al., 1996; Michalovitz et al., 1990; Zhang et al., 1994). Understanding when wild-type p53 adopts the denatured or misfolded conformation, how this is regulated, and the functional consequences of this conformational switching in vivo will be an interesting future challenge.
Experimental Procedures
Cell Culture and Constructs
H1299, HCT116, U2OS, MCF-7, and RPE cells were cultured as described in the Supplemental Experimental Procedures. Generation of stable cells lines expressing mutant p53 175H and 273H was performed as described elsewhere (Muller et al., 2009). All p53 mutant constructs have been described previously or created by site-directed mutagenesis as detailed in the Supplemental Information.
Immunoprecipitation and In Vitro Binding Assays
Details of immunoprecipitation and in vitro binding assays are described in the Supplemental Information.
Mass Spectrometry/Gel Filtration Chromatography
Mass spectrometry/gel filtration chromatography was performed as described in the Supplemental Information.
Electron Microscopy
Samples were applied onto carbon-coated copper grids previously glow-discharged and stained with 2% uranyl acetate. Micrographs were taken and individual particles and image classification were performed as described in the Supplemental Information.
Luciferase Assays
PG13 luciferase and Renilla luciferase constructs were transfected in combination with different p53 constructs and analyzed as described in the Supplemental Information.
mRNA Extraction and Quantitative PCR
Details of mRNA extraction and quantitative PCR are given in the Supplemental Information.
Invasion and Migration Assays
Matrigel assays were performed as described previously (Muller et al., 2009). Invasion toward a gradient of 10% fetal calf serum and a mixture of growth factors was measure by confocal microscopy in serial sections of 10 μm each, and quantification of invading cells was performed by ImageJ software. Organotypic invasion assays and migration assays (wound scratch) are detailed in the Supplemental Information.
Acknowledgments
We would like to thank David Lane for helpful discussion, Alan Fersht for providing the p53 purified protein used in electron microscopy analysis, Robert Ludwig for constructing the p53 mutants, and Bert Vogelstein and Ron Hay for reagents. Work at the Beatson Institute is supported by CR-UK. P.A.J.M. is supported by an AICR grant. Funding for J.M.V. was obtained through grants BFU2010-15703 and S2009MAT-1507.
Published: June 6, 2013
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.05.002.
Supplemental Information
References
- Abe Y., Yoon S.O., Kubota K., Mendoza M.C., Gygi S.P., Blenis J. p90 ribosomal S6 kinase and p70 ribosomal S6 kinase link phosphorylation of the eukaryotic chaperonin containing TCP-1 to growth factor, insulin, and nutrient signaling. J. Biol. Chem. 2009;284:14939–14948. doi: 10.1074/jbc.M900097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorno M., Cordenonsi M., Montagner M., Dupont S., Wong C., Hann B., Solari A., Bobisse S., Rondina M.B., Guzzardo V. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Andrews P., He Y.J., Xiong Y. Cytoplasmic localized ubiquitin ligase cullin 7 binds to p53 and promotes cell growth by antagonizing p53 function. Oncogene. 2006;25:4534–4548. doi: 10.1038/sj.onc.1209490. [DOI] [PubMed] [Google Scholar]
- Ano Bom A.P., Rangel L.P., Costa D.C., de Oliveira G.A., Sanches D., Braga C.A., Gava L.M., Ramos C.H., Cepeda A.O., Stumbo A.C. Mutant p53 aggregates into prion-like amyloid oligomers and fibrils: implications for cancer. J. Biol. Chem. 2012;287:28152–28162. doi: 10.1074/jbc.M112.340638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckerman R., Prives C. Transcriptional regulation by p53. Cold Spring Harb. Perspect. Biol. 2010;2:a000935. doi: 10.1101/cshperspect.a000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny M.V., Toretsky J., Bohen S., Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc. Natl. Acad. Sci. USA. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock A.N., Henckel J., DeDecker B.S., Johnson C.M., Nikolova P.V., Proctor M.R., Lane D.P., Fersht A.R. Thermodynamic stability of wild-type and mutant p53 core domain. Proc. Natl. Acad. Sci. USA. 1997;94:14338–14342. doi: 10.1073/pnas.94.26.14338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock A.N., Henckel J., Fersht A.R. Quantitative analysis of residual folding and DNA binding in mutant p53 core domain: definition of mutant states for rescue in cancer therapy. Oncogene. 2000;19:1245–1256. doi: 10.1038/sj.onc.1203434. [DOI] [PubMed] [Google Scholar]
- Candau R., Scolnick D.M., Darpino P., Ying C.Y., Halazonetis T.D., Berger S.L. Two tandem and independent sub-activation domains in the amino terminus of p53 require the adaptor complex for activity. Oncogene. 1997;15:807–816. doi: 10.1038/sj.onc.1201244. [DOI] [PubMed] [Google Scholar]
- Coffill C.R., Muller P.A., Oh H.K., Neo S.P., Hogue K.A., Cheok C.F., Vousden K.H., Lane D.P., Blackstock W.P., Gunaratne J. Mutant p53 interactome identifies nardilysin as a p53R273H-specific binding partner that promotes invasion. EMBO Rep. 2012;13:638–644. doi: 10.1038/embor.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collavin L., Lunardi A., Del Sal G. p53-family proteins and their regulators: hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- Douglas N.R., Reissmann S., Zhang J., Chen B., Jakana J., Kumar R., Chiu W., Frydman J. Dual action of ATP hydrolysis couples lid closure to substrate release into the group II chaperonin chamber. Cell. 2011;144:240–252. doi: 10.1016/j.cell.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D.E., Spiess C., Howard D.E., Frydman J. Tumorigenic mutations in VHL disrupt folding in vivo by interfering with chaperonin binding. Mol. Cell. 2003;12:1213–1224. doi: 10.1016/s1097-2765(03)00423-4. [DOI] [PubMed] [Google Scholar]
- Freed-Pastor W.A., Prives C. Mutant p53: one name, many proteins. Genes Dev. 2012;26:1268–1286. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander P., Legros Y., Soussi T., Prives C. Regulation of mutant p53 temperature-sensitive DNA binding. J. Biol. Chem. 1996;271:25468–25478. doi: 10.1074/jbc.271.41.25468. [DOI] [PubMed] [Google Scholar]
- Frydman J., Nimmesgern E., Ohtsuka K., Hartl F.U. Folding of nascent polypeptide chains in a high molecular mass assembly with molecular chaperones. Nature. 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- Gannon J.V., Greaves R., Iggo R., Lane D.P. Activating mutations in p53 produce a common conformational effect. A monoclonal antibody specific for the mutant form. EMBO J. 1990;9:1595–1602. doi: 10.1002/j.1460-2075.1990.tb08279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Puertas P., Martín-Benito J., Carrascosa J.L., Willison K.R., Valpuesta J.M. The substrate recognition mechanisms in chaperonins. J. Mol. Recognit. 2004;17:85–94. doi: 10.1002/jmr.654. [DOI] [PubMed] [Google Scholar]
- Grantham J., Brackley K.I., Willison K.R. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp. Cell Res. 2006;312:2309–2324. doi: 10.1016/j.yexcr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Hartl F.U., Bracher A., Hayer-Hartl M. Molecular chaperones in protein folding and proteostasis. Nature. 2011;475:324–332. doi: 10.1038/nature10317. [DOI] [PubMed] [Google Scholar]
- Hayer-Hartl M.K., Ewbank J.J., Creighton T.E., Hartl F.U. Conformational specificity of the chaperonin GroEL for the compact folding intermediates of alpha-lactalbumin. EMBO J. 1994;13:3192–3202. doi: 10.1002/j.1460-2075.1994.tb06618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Hainaut P. Massively regulated genes: the example of TP53. J. Pathol. 2010;220:164–173. doi: 10.1002/path.2637. [DOI] [PubMed] [Google Scholar]
- Joerger A.C., Fersht A.R. Structural biology of the tumor suppressor p53 and cancer-associated mutants. Adv. Cancer Res. 2007;97:1–23. doi: 10.1016/S0065-230X(06)97001-8. [DOI] [PubMed] [Google Scholar]
- Joly E.C., Tremblay E., Tanguay R.M., Wu Y., Bibor-Hardy V. TRiC-P5, a novel TCP1-related protein, is localized in the cytoplasm and in the nuclear matrix. J. Cell Sci. 1994;107:2851–2859. doi: 10.1242/jcs.107.10.2851. [DOI] [PubMed] [Google Scholar]
- Kitamura A., Kubota H., Pack C.G., Matsumoto G., Hirayama S., Takahashi Y., Kimura H., Kinjo M., Morimoto R.I., Nagata K. Cytosolic chaperonin prevents polyglutamine toxicity with altering the aggregation state. Nat. Cell Biol. 2006;8:1163–1170. doi: 10.1038/ncb1478. [DOI] [PubMed] [Google Scholar]
- Kussie P.H., Gorina S., Marechal V., Elenbaas B., Moreau J., Levine A.J., Pavletich N.P. Structure of the MDM2 oncoprotein bound to the p53 tumor suppressor transactivation domain. Science. 1996;274:948–953. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Arai M., Martinez-Yamout M.A., Dyson H.J., Wright P.E. Mapping the interactions of the p53 transactivation domain with the KIX domain of CBP. Biochemistry. 2009;48:2115–2124. doi: 10.1021/bi802055v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Lin C.Y., Lei M., Yan S., Zhou T., Erikson R.L. CCT chaperonin complex is required for the biogenesis of functional Plk1. Mol. Cell. Biol. 2005;25:4993–5010. doi: 10.1128/MCB.25.12.4993-5010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Larsson L., Caballero A., Hao X., Oling D., Grantham J., Nyström T. The polarisome is required for segregation and retrograde transport of protein aggregates. Cell. 2010;140:257–267. doi: 10.1016/j.cell.2009.12.031. [DOI] [PubMed] [Google Scholar]
- Matus D.Q., Li X.Y., Durbin S., Agarwal D., Chi Q., Weiss S.J., Sherwood D.R. In vivo identification of regulators of cell invasion across basement membranes. Sci. Signal. 2010;3:ra35. doi: 10.1126/scisignal.2000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer M.P. Gymnastics of molecular chaperones. Mol. Cell. 2010;39:321–331. doi: 10.1016/j.molcel.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Mayer S., Rüdiger S., Ang H.C., Joerger A.C., Fersht A.R. Correlation of levels of folded recombinant p53 in escherichia coli with thermodynamic stability in vitro. J. Mol. Biol. 2007;372:268–276. doi: 10.1016/j.jmb.2007.06.044. [DOI] [PubMed] [Google Scholar]
- Meek D.W., Anderson C.W. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb. Perspect. Biol. 2009;1:a000950. doi: 10.1101/cshperspect.a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melville M.W., McClellan A.J., Meyer A.S., Darveau A., Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol. Cell. Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A.S., Gillespie J.R., Walther D., Millet I.S., Doniach S., Frydman J. Closing the folding chamber of the eukaryotic chaperonin requires the transition state of ATP hydrolysis. Cell. 2003;113:369–381. doi: 10.1016/s0092-8674(03)00307-6. [DOI] [PubMed] [Google Scholar]
- Michalovitz D., Halevy O., Oren M. Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell. 1990;62:671–680. doi: 10.1016/0092-8674(90)90113-s. [DOI] [PubMed] [Google Scholar]
- Milner J., Cook A., Sheldon M. A new anti-p53 monoclonal antibody, previously reported to be directed against the large T antigen of simian virus 40. Oncogene. 1987;1:453–455. [PubMed] [Google Scholar]
- Muller P.A., Caswell P.T., Doyle B., Iwanicki M.P., Tan E.H., Karim S., Lukashchuk N., Gillespie D.A., Ludwig R.L., Gosselin P. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Muller P.A., Vousden K.H., Norman J.C. p53 and its mutants in tumor cell migration and invasion. J. Cell Biol. 2011;192:209–218. doi: 10.1083/jcb.201009059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P.A., Trinidad A.G., Timpson P., Morton J.P., Zanivan S., van den Berghe P.V., Nixon C., Karim S.A., Caswell P.T., Noll J.E. Mutant p53 enhances MET trafficking and signalling to drive cell scattering and invasion. Oncogene. 2013;32:1252–1265. doi: 10.1038/onc.2012.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaev A.Y., Li M., Puskas N., Qin J., Gu W. Parc: a cytoplasmic anchor for p53. Cell. 2003;112:29–40. doi: 10.1016/s0092-8674(02)01255-2. [DOI] [PubMed] [Google Scholar]
- Siegers K., Waldmann T., Leroux M.R., Grein K., Shevchenko A., Schiebel E., Hartl F.U. Compartmentation of protein folding in vivo: sequestration of non-native polypeptide by the chaperonin-GimC system. EMBO J. 1999;18:75–84. doi: 10.1093/emboj/18.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam S., Geller R., Spiess C., Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat. Cell Biol. 2006;8:1155–1162. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thulasiraman V., Yang C.F., Frydman J. In vivo newly translated polypeptides are sequestered in a protected folding environment. EMBO J. 1999;18:85–95. doi: 10.1093/emboj/18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valpuesta J.M., Martín-Benito J., Gómez-Puertas P., Carrascosa J.L., Willison K.R. Structure and function of a protein folding machine: the eukaryotic cytosolic chaperonin CCT. FEBS Lett. 2002;529:11–16. doi: 10.1016/s0014-5793(02)03180-0. [DOI] [PubMed] [Google Scholar]
- Vousden K.H., Prives C. Blinded by the light: the growing complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Walerych D., Olszewski M.B., Gutkowska M., Helwak A., Zylicz M., Zylicz A. Hsp70 molecular chaperones are required to support p53 tumor suppressor activity under stress conditions. Oncogene. 2009;28:4284–4294. doi: 10.1038/onc.2009.281. [DOI] [PubMed] [Google Scholar]
- Wilcken R., Wang G., Boeckler F.M., Fersht A.R. Kinetic mechanism of p53 oncogenic mutant aggregation and its inhibition. Proc. Natl. Acad. Sci. USA. 2012;109:13584–13589. doi: 10.1073/pnas.1211550109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willison K., Lewis V., Zuckerman K.S., Cordell J., Dean C., Miller K., Lyon M.F., Marsh M. The t complex polypeptide 1 (TCP-1) is associated with the cytoplasmic aspect of Golgi membranes. Cell. 1989;57:621–632. doi: 10.1016/0092-8674(89)90131-1. [DOI] [PubMed] [Google Scholar]
- Xu J., Reumers J., Couceiro J.R., De Smet F., Gallardo R., Rudyak S., Cornelis A., Rozenski J., Zwolinska A., Marine J.C. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- Yam A.Y., Xia Y., Lin H.T., Burlingame A., Gerstein M., Frydman J. Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nat. Struct. Mol. Biol. 2008;15:1255–1262. doi: 10.1038/nsmb.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yébenes H., Mesa P., Muñoz I.G., Montoya G., Valpuesta J.M. Chaperonins: two rings for folding. Trends Biochem. Sci. 2011;36:424–432. doi: 10.1016/j.tibs.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Zhang W., Guo X.Y., Hu G.Y., Liu W.B., Shay J.W., Deisseroth A.B. A temperature-sensitive mutant of human p53. EMBO J. 1994;13:2535–2544. doi: 10.1002/j.1460-2075.1994.tb06543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.