Abstract
Renal transplant recipients who experience delayed graft function have increased risks of rejection and long-term graft failure. Ischemic damage is the most common cause of delayed graft function, and although it is known that tissue inflammation accompanies renal ischemia, it is unknown whether renal ischemia affects the production of antibodies by B lymphocytes, which may lead to chronic humoral rejection and allograft failure. Here, mice immunized with a foreign antigen 24–96 hours after renal ischemia-reperfusion injury developed increased levels of antigen-specific IgG1 compared with sham-treated controls. This amplified IgG1 response did not follow unilateral ischemia, and it did not occur in response to a T-independent antigen. To test whether innate immune activation in the kidney after ischemia affects the systemic immune response to antigen, we repeated the immunization experiment using mice deficient in factor B that lack a functional alternative pathway of complement. Renal ischemia-reperfusion injury did not cause amplification of the antigen-specific antibodies in these mice, suggesting that the increased immune response requires a functional alternative pathway of complement. Taken together, these data suggest that ischemic renal injury leads to a rise in antibody production, which may be harmful to renal allografts, possibly explaining a mechanism underlying the link between delayed graft function and long-term allograft failure.
Delayed graft function (DGF), defined as the need for dialysis in the first 7 days after transplantation surgery,1 is associated with an increased risk of acute rejection.2,3 The increased risk of rejection is seen with both cadaveric3 and live donor4 organs, and also, it is associated with increased recipient mortality.5,6 Furthermore, long-term survival is worse in DGF kidney grafts, and there is an increasing recognition that ongoing inflammation drives interstitial fibrosis/tubular atrophy (formerly known as chronic allograft nephropathy)7–9 and may limit average duration of transplant survival.
Although it is well known that a host of systemic inflammatory diseases cause renal injury, far less is known about the effect of AKI on the immune system. Animal and human data seem to be conflicting, indicating that AKI both is an intensely inflammatory event10–12 and may also have immunosuppressive effects.13 Ischemia-reperfusion injury (IRI) of native kidneys may also perturb systemic immune responses. AKI is associated with acute respiratory distress syndrome and synergistically increases patient mortality because of sepsis and multiorgan dysfunction syndrome.14
With the increasing incidence of AKI,15,16 it is important to understand the effects of AKI on immune function. Long-lasting immune lymphocyte activation has been shown after IRI.17 In the specific setting of renal transplantation, stubbornly high rates of late graft failure are now thought to involve persistent subclinical immune activity,18,19 and basic science work has shown a variety of immune factors that are active in the failing allograft, including T cells, selectins, integrins, and chemokine receptors such as chemokine receptor-2, chemokine receptor-7, and CXC chemokine receptor-4.8 There is an increasing awareness that ischemic kidney damage causes local inflammation in the kidney. In the case of renal allografts that suffer ischemic damage, it may be a pathologic process ultimately leading to increased antibody-mediated rejection.
These observations led us to hypothesize that IRI amplifies the humoral immune response to a newly presented antigen. To test this hypothesis, we subjected mice to bilateral renal IRI and then immunized them with nitrophenol keyhole limpet hemocyanin (NP-KLH), a large T cell-dependent antigen that is commonly used in humoral immunity experiments. We show the effect of IRI on antibody levels after immunization and explore mechanisms for the observed phenomena.
Results
Characterization of IRI
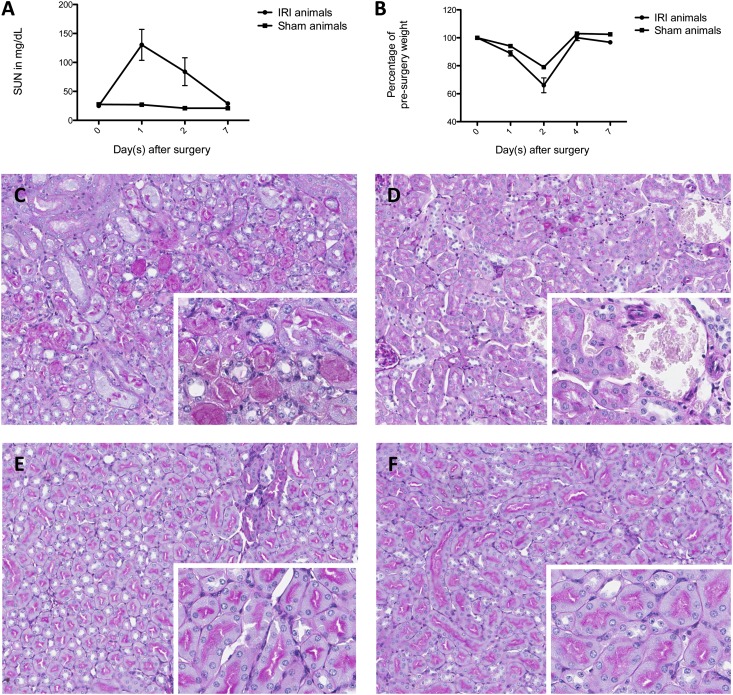
We characterized the kinetics of murine IRI over a 7-day period by subjecting mice to IRI or sham surgery and then collecting samples at prespecified time points. IRI surgery in our model is typified by elevated serum urea nitrogen (SUN) and loss of weight within 24 hours (Figure 1, A and B). The animals return to their presurgery body weight by day 4. The renal injury is primarily confined to the tubular compartment, where necrotic and sloughed tubular epithelial cells are observed (Figure 1, C–F).
Figure 1.
Murine IRI is characterized histologically. (A) SUN rises to a maximum at 24 hours and is back to presurgery value by 7 days. Shown are means ± SEM, with n=3 in the IRI-treated group. (B) Body weight changes after the surgery in both IRI- and sham-treated mice and return to presurgery values by 4 days; n=2 in the IRI-treated group. (C) Periodic acid-Schiff (PAS) staining of outer medullary tubules from an IRI-treated mouse at 48 hours reveals necrosis and sloughing of tubular epithelial cells. Original magnification: ×10; ×20 in inset. (D) PAS staining at 48 hours after sham surgery shows intact tubules. Original magnification: ×10; ×20 in inset. (E) PAS staining of IRI-treated mouse at 7 days with resolution of tubular necrosis. Original magnification: ×10; ×20 in inset. (F) PAS staining of sham-treated mouse at 7 days with normal tubules. Original magnification: ×10; ×20 in inset.
Antigen-Specific IgG1 Is Augmented by IRI
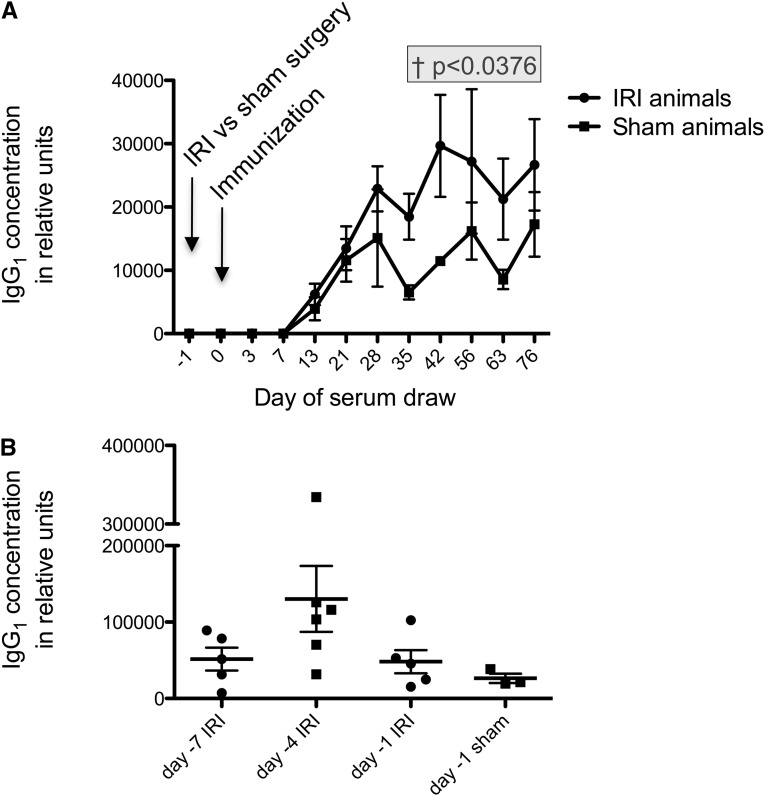
We next tested whether IRI affects the immune response to NP-KLH. IgG1 was studied, because it is the most abundant IgG subclass and raised by T-dependent antigens such as NP-KLH. In an early experiment, we performed intraperitoneal (ip) immunization in mice with NP-KLH 24 hours before IRI versus sham surgery, and we found that there was no difference between the two groups in their antigen-specific IgG1 titers (data not shown). Next, we performed ip immunization 24 hours after the surgery (at the height of renal failure) and found that there was a sustained increase in NP-KLH–specific IgG1 in IRI animals compared with sham animals (Figure 2A). This difference was statistically significant, with P=0.04 by mixed model analysis, for the time period beginning at 35 days until the end of the experiment.
Figure 2.
Antigen-specific IgG1 titers change over time. In all graphs, shown are means ± SEM. (A) Mice that underwent IRI versus sham surgery and then had ip immunization with 25 μg NP-keyhole limpet hemocyanin (KLH) 24 hours after surgery had sustained increases in NP-specific IgG1 titers (P=0.04 by mixed model analysis beginning at day 35). Data shown are from ELISA; n=5 for IRI-treated group and n=4 for sham-treated group. (B) The effect of reperfusion time on the immune response was tested. Although the mouse is in the recovery phase from IRI at 4 days, the augmentation effect was greatest at that time. Data shown are from day 35 after immunization. Data shown are from ELISA; n=5 for day −7 IRI, n=6 for day −4 IRI, n=5 for day −1 IRI, and n=3 for sham-treated. By ANOVA, P=0.10.
The amplified antibody titer was not seen in the case of IgM (Supplemental Figure 1A). To see the optimal time after surgery for the amplified antibody titer, we varied the time between IRI versus sham surgery and the immunization. We performed surgery at 1, 4, or 7 days before immunization and found that the highest antigen-specific IgG1 levels were present in mice that had IRI surgery 4 days before immunization (Figure 2B). This finding indicates that the amplification effect begins no later than 24 hours after IRI and lasts at least 96 hours. We waited 4 days after surgery before immunizing mice in subsequent experiments. Interestingly, this time frame includes both renal injury and recovery, suggesting that metabolic renal failure can be resolving at the time of immunization.
We sought to know if the secondary (memory) antibody response is also affected by IRI versus sham treatment. Mice were immunized with NP-KLH, had IRI versus sham surgery 20 days later, and were then reimmunized with NP-KLH after 24 hours of reperfusion. IRI had no effect on the amount of antigen-specific IgG1 (Supplemental Figure 1B). This result indicates that the primary, but not secondary, humoral immune response is amplified by IRI.
Control Experiments in Immunization
We sought to know if sham surgery has an effect on the humoral response to immunization. Therefore, an experiment was conducted in which a group of mice received no surgery, whereas another group underwent sham surgery on day 0. Both groups received ip immunization with NP-KLH on day 4. There was an increase in the antigen-specific IgG1 antibody titer in the sham-operated mice compared with the nonoperated controls (Supplemental Figure 1C) that narrowly missed significance, although the magnitude of the increase in antigen-specific IgG1 antibody titer was much smaller than the magnitude seen in mice with renal IRI. This finding suggests that inflammation from laparotomy augments the humoral immune response as assayed by antigen-specific antibody levels, although to a much lesser degree than seen with renal IRI.
To follow-up this result, we conducted an experiment, in which IRI-treated mice received ip versus subcutaneous (base of tail) immunization with identical quantities of NP-KLH. In this experiment, there was no difference in the antigen-specific IgG1 antibody titers between the ip- and subcutaneous-immunized animals (Supplemental Figure 1D). Therefore, the amplification of antigen-specific IgG1 is not restricted to antigens encountered in the inflamed peritoneum but seems to be a systemic effect.
Enhanced Immune Response after IRI Is Not Because of a Polyclonal Stimulation of B Cells
We sought to determine whether the amplified antigen-specific IgG1 against NP-KLH was caused by a general increase in the amount of IgG1 in the mouse. An ELISA was performed for total IgG1 rather than antigen-specific IgG1. There was no difference between the IRI and sham animals in the total amount of IgG1 in the serum (Supplemental Figure 2A).
We also sought to determine if there were differences in the total number of B lymphocytes or the expression of the B lymphocyte activation marker CD86. FACS was performed on fresh splenocytes taken from unimmunized animals euthanized 48 hours after IRI versus sham surgery. Compared with sham-treated mice, IRI-treated mice had equal proportions of B220hi lymphocytes, equal mean fluorescent intensity of CD86 staining in B lymphocytes, and equal proportions of CD86hi B lymphocytes (Supplement Figure 2, B–D). Viewing these findings along with the data on total Ig levels, it seems that there is not a polyclonal increase in B lymphocyte numbers, activation, or Ig products caused by IRI. The increased antibody response, therefore, seems to be specific for antigen first encountered in the period after IRI.
Total Number of Nitrophenol-Specific Lymphocytes Increases after IRI
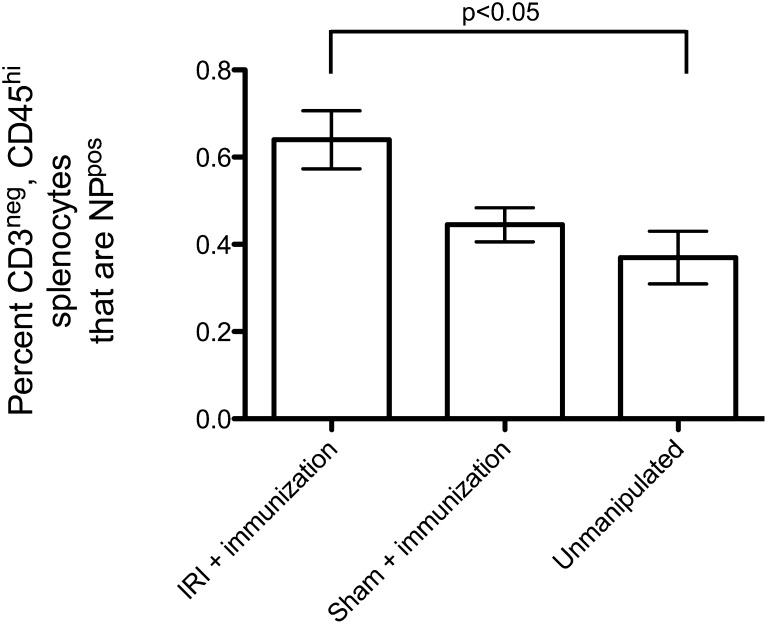
We next sought to determine whether renal IRI causes proliferation of NP-specific B lymphocytes. Mice were subjected to IRI versus sham surgery followed by immunization with NP-KLH 4 days later. FACS was performed 18 days after immunization using fluorophore-labeled NP to identify lymphocytes with NP-specific receptors. The percentage of overall splenocytes that bound NP was increased in mice that received IRI surgery (data not shown). Restricting our analysis to cells expressing B220 and negative for CD3—to isolate B lymphocytes and exclude T lymphocytes—we found that IRI and immunization significantly increased the number of NP-binding B lymphocytes compared with unmanipulated animals (Figure 3). Therefore, the amplified Ig titers are coming from a numerically expanded group of B lymphocytes with receptors for NP-KLH.
Figure 3.
IRI causes an increase in the number of NP-specific splenocytes. In this experiment, mice were treated with IRI (n=4) versus sham surgery (n=4) on day 0, immunized on day 4, and euthanized on day 22. Unmanipulated animals (n=3) underwent no surgery and were not immunized. Splenocytes were harvested and subjected to FACS. Back-gating was used to exclude CD3pos cells (which remove all T lymphocytes) and restrict analysis to B220hi cells (B lymphocytes). IRI and immunization combined raise the number of both antigen-binding B lymphocytes in the spleen after immunization (P<0.05).
Renal IRI Requires T Cell Activation and Signaling and T Cell Antigen Processing
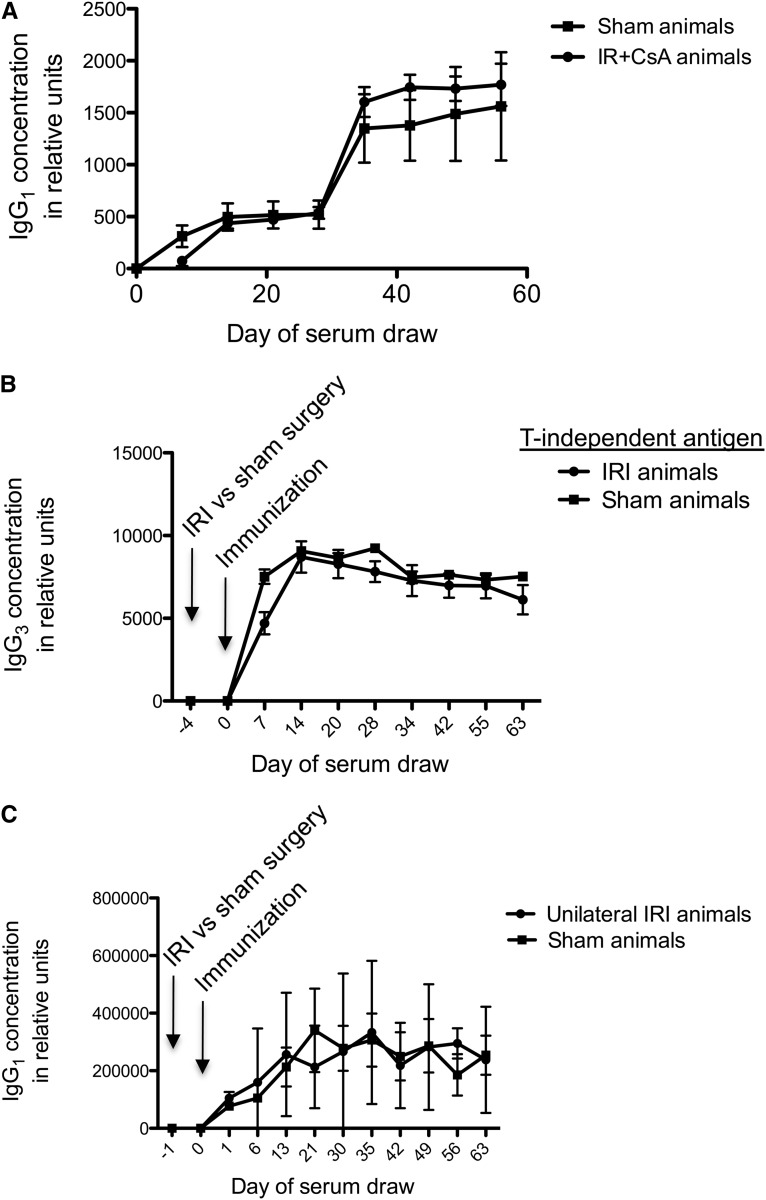
To determine whether a standard immunosuppressive agent would affect the amplification of the immune response in mice with renal IRI, we treated mice with cyclosporine, a calcineurin inhibitor that decreases T cell activation and signaling. We treated mice with subcutaneous cyclosporine from day 4 after immunization to week 6, and we found that the NP-specific IgG1 amplification was attenuated (Figure 4A). This finding indicates that T cell activation and signaling is involved in the observed phenomenon. It also indicates that calcineurin inhibitors, which are part of the standard immunosuppressive regimen after renal transplantation, reduce this effect.
Figure 4.
Humoral immune amplification after IRI requires T lymphocyte processing and bilateral IRI. (A) Mice were immunized 4 days after IRI with NP-KLH and treated with daily injections of cyclosporine from day 4 to week 6 post-IRI. The augmentation effect of IRI on NP-specific IgG1 levels was reduced by cyclosporine treatment, indicating an important role for T cell processing and signaling; n=4 for both groups. (B) Mice were immunized 4 days after IRI with 5 µg NP-ficoll injected ip. No amplification of the antigen-specific IgG3 titer was seen in mice with IRI compared with sham-treated mice. IgG3 is the IgG subclass raised by T-independent antigens. Data shown are from ELISA; n=6 for IRI-treated group and n=5 for sham-treated group. (C) Mice were subjected to unilateral renal ischemia and injected with NP-KLH after 24 hours of reperfusion. Mice with unilateral IRI did not develop amplification of the immune response to NP-KLH compared with sham-treated controls. These data would suggest that more severe injury or metabolic renal failure with elevated SUN is required for the observed effect. Data shown are from ELISA; n=3 for unilateral IRI-treated group and n=5 for sham-treated group.
Next, we immunized mice with nitrophenol-ficoll, a repeating polysaccharide coupled to NP. This antigen raises humoral immunity through a T cell-independent mechanism.20 In this experiment, we detected IgG3 rather than IgG1, because IgG3 is the subclass raised by T-independent antigens.21 We found that IRI-treated mice had no increase in IgG3 specific for NP-ficoll (Figure 4B). Therefore, T cell processing of antigen seems to be necessary for the amplification of humoral immunity after renal IRI.
Unilateral IRI Is Not Sufficient to Cause the Amplified Immune Response to NP-KLH
An experiment was conducted to determine whether unilateral (rather than bilateral) kidney IRI is sufficient to cause the increase in antigen-specific IgG1. There was no difference between unilateral IRI- and sham-treated animals in antigen-specific IgG1 (Figure 4C). Furthermore, using a 45-minute period of unilateral IRI does not cause the IgG1 amplification (Supplemental Figure 3, top). Unilateral IRI does not cause elevated BUN, because the contralateral kidney can compensate metabolically (Supplemental Figure 3, bottom). These data indicate that less severe renal injury, lacking in metabolic derangement, does not cause the observed amplification of humoral immunity.
Complement Activation Is Critical for the Augmentation of the Antigen-Specific IgG1
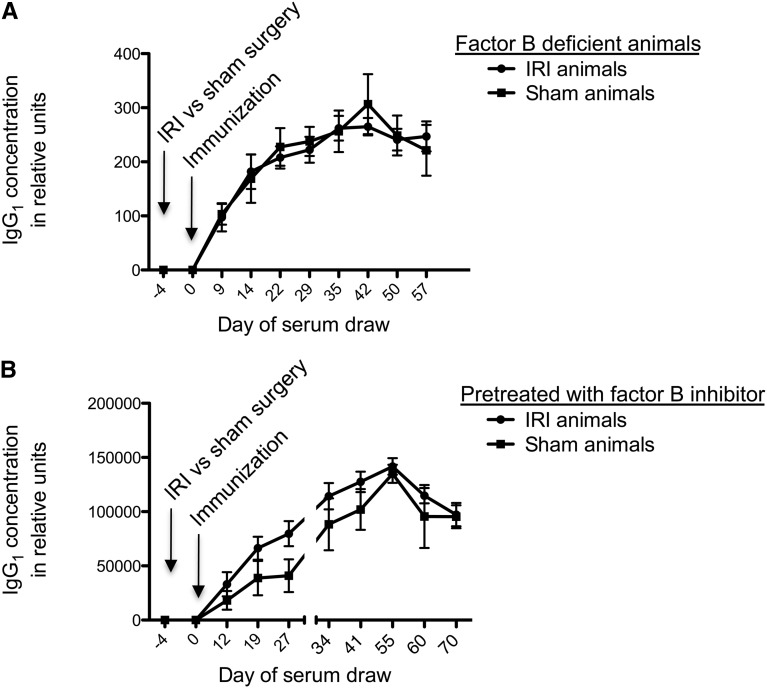
IRI causes inflammatory injury to the kidney, which our group has shown is typified by activation of the alternative complement pathway.22 Several complement activation products have been shown to promote an adaptive immune response.23–25 To determine whether activation of the complement cascade is critical for the amplified antibody titer specific for NP-KLH, we performed IRI versus sham surgery followed by immunization 4 days later in mice with targeted genetic deletions of the gene for factor B. Compared with sham-treated fB−/− mice, the fB−/− mice subjected to renal IRI did not show the amplified antibody titers seen in the earlier experiments (Figure 5A). This finding shows that the augmented antigen-specific Ig effect requires an intact alternative pathway of complement. To further explore this finding, we administered mAb 1379, a monoclonal antibody that inhibits the alternative pathway of complement for 48–72 hours,26 before subjecting mice to IRI versus sham surgery. The inhibitor-pretreated mice that received IRI did not have significantly amplified IgG1 levels compared with sham animals (Figure 5B). Treatment of mice with a control monoclonal antibody did not eliminate the IRI-induced immune amplification (Supplemental Figure 4). However, unmanipulated fB−/− also displayed anattenuated immune response to NP-KLH (Supplemental Figure 5), showing that the role of complement activation in the immune response to NP-KLH is not IRI specific.
Figure 5.
Mice deficient in the alternative pathway of complement do not exhibit amplified humoral immunity. (A) Mice that have a deletion of the gene for factor B (fB−/− mice), which causes inactivation of the alternative pathway of complement, do not have any difference in the antigen-specific IgG1 titers between IRI- and sham-treated groups. Data shown are from ELISA; n=5 for IRI-treated group and n=3 for sham-treated group. (B) Wild-type mice that were treated with a reversible inhibitor of factor B 2 hours before surgery had no significant amplification effect. Data shown are from ELISA; n=5 for IRI-treated group and n=4 for sham-treated group.
IL-10 Is Critical for the Observed Increase in Humoral Immunity after Renal IRI
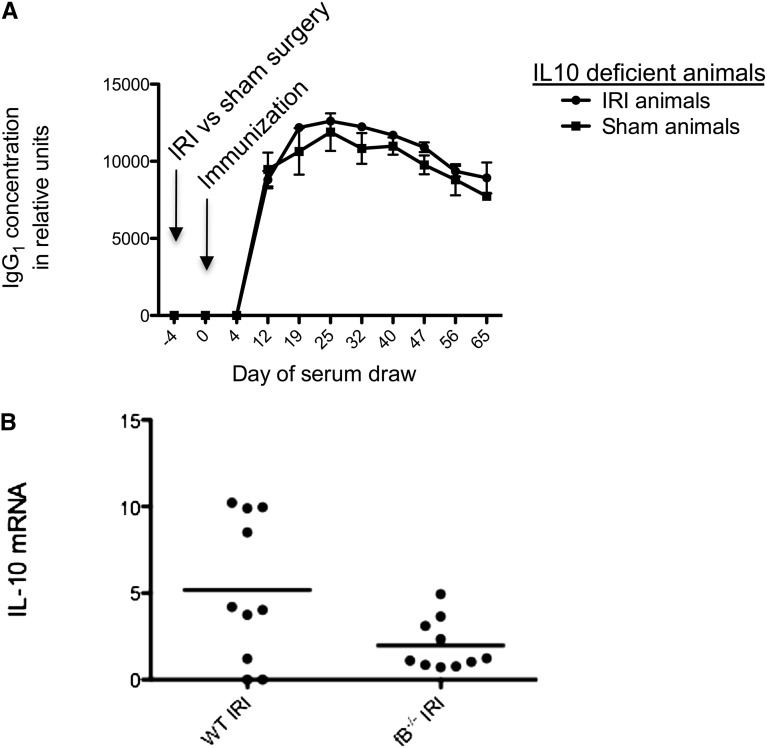
It was recently shown that complement activation induces the differentiation of a T cell subset that promotes the humoral immune response, an effect that requires IL-10 production.27 To test whether IL-10 is active in the complement-dependent increase in production of antibody to NP-KLH after renal IRI, we performed IRI versus sham surgery in IL-10–deficient mice and then immunized the mice with NP-KLH 4 days later. In this experiment, there was no observed amplification of the antigen-specific IgG1 levels (Figure 6A). Furthermore, quantitative PCR (qPCR) data from IRI- versus sham-treated kidneys revealed that fB−/− mice have less induction of IL-10 from the ischemic organ (Figure 6B). These data suggest that complement activation and IL-10 generation are intertwined and likely both necessary for the antigen-specific IgG1 amplification effect of IRI. However, deficiency of IL-10 did not detectably affect the antibody titer in unmanipulated mice. This result suggests that IL-10 does not play a critical role in the normal response to NP-KLH but that IL-10 induced during renal IR contributes to the amplified immune response to foreign antigens.
Figure 6.
Mice that cannot produce IL-10 do not display the observed effect after IRI. (A) Mice with a targeted genetic deletion of the gene for IL-10 were subjected to renal IRI or sham treatment, and they were immunized with NP-KLH after 24 hours of reperfusion. The IL-10−/− mice subjected to IRI did not show increased titers of anti-NP IgG1 compared with sham-treated animals. Data shown are from ELISA; n=3 for IRI-treated group and n=3 for sham-treated group. (B) qPCR data from IRI- versus sham-treated kidneys show that WT mice produce IL-10 within the kidney after renal IRI. The fB−/− mice produced significantly lower amounts of IL-10 after renal IRI than wild-type animals. Data are reported relative to WT sham-treated kidneys. Primer t test P=0.10.
Discussion
We have found that there is a sustained amplification of IgG1 antibodies directed against an antigen encountered in the days after renal IRI. This time period is characterized by tubular necrosis, inflammation, and resolving metabolic renal failure. The total amount of IgG1 (that is, not antigen-specific) and number of B lymphocytes are not changed by IRI, but the number of antigen-specific lymphocytes is increased. T lymphocyte processing of antigen and bilateral renal IRI are necessary for the observed amplification effect. Significantly, in alternative pathway-deficient mice, the effect is not observed, and it is not seen in IL-10–deficient mice. These data support the hypothesis that renal IRI amplifies the humoral immune response to heterologous antigens.
Our experiments indicate that renal IRI does not cause a change in general or polyclonal B lymphocyte activity but rather, that it specifically amplifies the humoral response to antigens encountered after reperfusion. This finding was consistent, whether by antibody levels, FACS counting of B lymphocytes, or measurement of CD86 expression (a marker of B cell activation). There seems to be a time window in which the amplification of an antigen-specific antibody titer occurs, which may be because of a transient effect caused by the release of immune factors from the reperfused kidney.
It is now well accepted that AKI causes distant organ effects.28 Additionally, in the setting of kidney transplantation, the increased rate of rejection and decreased graft survival persist for years after transplantation surgery. Remote effects in time and space reflect the pathophysiologic complexity of renal IRI. The effects of IRI on T lymphocytes, inflammatory cytokines, complement, and inflammatory cell infiltration have been well studied.29 However, to our knowledge, this study is the first study in which the direct effects of renal IRI on the humoral immune response have been examined. The enduring effect of renal IRI on antigen-specific antibody titers may be partially related to the long half-life (21 days) of IgG1, but more likely, they reflect a durable impact of renal IRI on humoral immunity, because IgG1 is not renally cleared. The ability of renal IRI to amplify the immune response may also be important in patients with autoimmune disease. Our results raise the possibility that IRI can trigger or exacerbate autoimmune disease activity in these patients.
Observational studies show that there is a relationship between DGF and renal allograft survival. The pathophysiologic basis of this relationship is not known. However, it is noteworthy that the long-term association persists for years after recovery from DGF. In up to one third of cellular rejection cases, there is evidence of antibody-mediated injury, which is typified by endothelial inflammation (likely caused by HLA I and II expression) and C4d deposition in the peritubular capillaries.30 It is increasingly thought that interstitial fibrosis/tubular atrophy (formerly chronic allograft nephropathy) is related to low-grade antibody-mediated rejection.18 There is evidence that renal tubular epithelial cells from the renal allograft produce C3, which can enhance the rejection process.31
DGF around the time of transplantation surgery increases the expression of graft antigens, such as MHCs, and based on our data, it also may augment the immune system’s response to those exposed antigens. DGF has been to shown to increase with cold ischemia time,32 and because acute tubular necrosis of the allograft is the dominant cause of DGF, IRI is an obvious experimental model to investigate these phenomena. We immunized mice with an exogenous protein antigen. Unlike proteins expressed on cells, the immunogen load of this antigen is unlikely to be affected by the transplant procedure. However, this model more closely resembles the indirect pathway of alloantigen recognition. Direct allorecognition of MHC on allografts may happen more rapidly, and the kinetics of the direct response may be different than the kinetics of the immune response to NP-KLH. Treatment of the mice with cyclosporine reduced the effect of renal IRI on the immune response to NP-KLH, indicating that standard immunosuppressive therapy may prevent this phenomenon in transplant patients. However, therapy with calcineurin inhibitors is frequently delayed in patients with DGF to facilitate renal recovery.
Mechanistically, it seems that an intact alternative pathway of complement is necessary for the amplified response to an antigen after IRI. Genetically deficient animals and reversible inhibitors (but not control monoclonal antibodies) abolish the observed effect. Animal data indicate that the complement receptors are necessary for a robust primary and secondary response to antigen.33 IL-10 has been found to be protective in renal IRI34,35 and even distant organ effects of renal IRI.12 Nevertheless, IL-10 seems to be a necessary component in our finding of amplified humoral immunity. A related question is the effect of B lymphocytes on renal IRI. The role of B lymphocytes in IRI has been studied by several groups, with differing findings as to whether B cells are ultimately protective or harmful to the ischemic kidney.36–38 B cells have repair, antigen-presenting, and natural antibody-producing functions in addition to their well known role in the adaptive immune system. These various functions suggest that B cells may influence renal injury and recovery after IRI. The results of this study show that IRI also influences B cell function. B cell therapies now are used in the treatment of renal diseases, making these important interactions to understand. Our results suggest that therapies that prevent complement activation or block IL-10 signaling may have a durable effect on the B cell response against the injured allograft.
There are a number of limitations to our study. First, it should be pointed out that alternative pathway-deficient mice (whether genetically deficient mice or mice pretreated with an inhibitor) develop less severe AKI than wild-type mice.22,26 Unmanipulated fB−/− mice also generate a weaker immune response against NP-KLH than unmanipulated wild-type mice. Thus, the finding that complement-deficient mice do not develop an amplified immune response after renal IRI may be an indirect effect of the milder renal injury in these animals or their reduced immune response independent of IRI. Second, it is not clear the stage of the immune response that is affected by alternative pathway activation. It is impossible to know from our experiments if the inhibitor influences renal injury, antigen processing and presentation, or antibody fixation of complement. Third, our study is a simplified model that is meant to directly examine the effects of renal IRI on the response to antigen, but this model does not fully reflect the complexity of transplant recipients who also have systemic illness, have fluctuating levels of renal function, and receive multiple immunosuppressive therapies.
In conclusion, we have found that reversible ischemic AKI causes a lasting increase in antibodies against antigens encountered during the period of renal failure. This effect requires injury severe enough to cause metabolic renal failure, and it also requires an intact alternative pathway and signaling through IL-10. Because factor B and IL-10 are required for this effect, both are potential therapeutic targets. Additional studies examining the effect of renal IRI on the immune system will focus on these initial mechanism candidates. A wealth of data indicates a relationship between ischemic kidney damage and immune system perturbation. Our findings show, for the first time, that renal ischemia likely influences the humoral response to an antigen. Therapies targeting this phenomenon may have salutary effects on the humoral immune response to renal allografts.
Concise Methods
Reagents
NP-KLH and NP-ficoll were obtained from Biosearch Technologies (Novato, CA). Of note, the hapten is the nitrophenol moiety. Ovalbumin was obtained from Sigma.
The mAbs 1379 (factor B inhibitor) and 171 (a murine IgG1 against the human CR2 receptor) were made by our laboratory as previously described39,40; 2 mg antibodies were injected ip 2 hours before surgery.
Cyclosporine (Novartis Sandimmune) was obtained from Sigma, filtered through a 0.22-μm poly(vinylidene difluoride) syringe filter, and stored in a glass vial protected from light. The cyclosporine was injected subcutaneously at a dose of 4 mg/kg per day. Injections were performed from day 4 after immunization to week 6 after immunization.
SUN Measurements
SUN was determined for each mouse using an Alfa-Wassermann ACE spectrophotometer.
Immunization
Animals were immunized through the ip route, except where otherwise noted, with 25 µg NP-KLH, or 5 µg NP-ficoll. Ovalbumin was injected ip at a dose of 100 µg per animal. Subcutaneous immunizations were performed near the base of the tail.
NP-Specific ELISAs
To measure NP-specific serum antibody responses, 96-well flat-bottom high-binding Costar 3690 plates (Corning Incorporated) were coated with 1 µg/ml NP-KLH diluted in 15 mM Na2CO3 and 35 mM NaHCO3 coating buffer overnight at 4°C. Plates were washed three times (PBS, 0.05% Tween-20; Fisher Scientific) and then blocked with 1% BSA (EMD Millipore) in PBS for 2 hours at 37°C. Plates were washed three times (PBS, 0.05% Tween-20; Fisher Scientific). For capture of antigen-specific antibodies, sera were diluted 1:100 and allowed to dwell for 1 hour at 37°C. Unbound antibody was washed three times (PBS, 0.05% Tween-20; Fisher Scientific). A 1:800 preparation of goat anti-mouse IgG1–horseradish peroxidase (Invitrogen), goat anti-mouse µ-chain–specific IgM–horseradish peroxidase (Jackson Laboratories), and goat anti-mouse IgG3–horseradish peroxidase (Southern Biotech), depending on the experiment, was added and allowed to dwell for 1 hour at 37°C. After three washes (PBS, 0.05% Tween-20; Fisher Scientific), the plates were developed with Ultra TMB (Fisher Scientific) diluted 1:1 in sterile H2O, and reactions were stopped with 0.1 N H2SO4. Absorbance was read at 450 nm.
The standard wells were coated with donkey anti-mouse IgG and IgM (Jackson Laboratories) 1:1200 in 15 mM Na2CO3 and 35 mM NaHCO3 coating buffer overnight at 4°C. Mouse IgG and IgM purified in our laboratory were used to form a standard curve, with the maximal value being 500–2000 ng/ml. Detection and development was using the techniques described above.
For mice immunized with NP-ficoll, Immulon 1B plates (Thermo Labsystems) were used. They were coated as above, because the nitrophenol hapten is the same in both NP-KLH and NP-ficoll.
Experimental Animals
Generation of the factor B-deficient mice has been previously described.41 These mice have been backcrossed nine generations on a C57/B6 background. The IL-10−/− mice were obtained from Jackson Laboratories and backcrossed onto a C57/B6 background. All experimental animals were male mice between 10 and 12 weeks old at the time of initial manipulation. They were fed ad libitum, and cages were kept on a 12-hour light–dark cycle. The mice were housed and maintained in the University of Colorado Denver Health Science Center’s Office of Laboratory Animal Resources in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Surgical Techniques and IRI Protocol
Mice weighing 20–25 g were anesthetized with 50 µl ketamine 50 mg/ml (Bioniche Pharma) and 12.5 µl xylazine 100 mg/ml (Lloyd Inc.) injected ip. The mice were placed on a heating pad to maintain their body temperature during surgery. Laparotomies were performed, and then, the renal pedicles were located and isolated by blunt dissection. One or both of the pedicles were clamped with atraumatic clamps (Schwartz vascular clip; Miltex Instrument), and occlusion of blood flow was confirmed by visual inspection of the kidneys. The clamps were left in place for 24 minutes and then released. The time of ischemia was chosen to obtain a reversible model of ischemic AKI with a minimum of vascular thrombosis and avoid animal mortality. In one experiment involving unilateral ischemia, the clamps were left in place for 45 minutes. The fascia and skin were sutured with 4–0 silk (US Surgical, Norwalk, CT). Sham surgery was performed in an identical fashion, except that the renal pedicles were not clamped. The mice were volume resuscitated with 0.5 ml PBS and kept 2 hours in an incubator at 29°C to maintain body temperature.
In the experiments of primary immune response, mice were immunized 1–7 days after IRI versus sham surgery. In an experiment investigating secondary (memory) response to immunization, mice were immunized at day −21, received surgery on day −1, and then, received a second immunization at day 0.
Renal Morphology
After the kidneys were removed from the mice, sagittal sections were fixed in 4% paraformaldehyde. After being embedded in paraffin, 4-µm sections were cut and stained with periodic acid-Schiff.
FACS
To quantify the number of B lymphocytes and their activation status shortly after ischemic renal failure, IRI versus sham surgery was performed, and then, unimmunized animals were exsanguinated 48 hours after surgery. Spleens were harvested and minced. Cells were passed through a 70-µM filter, and then, they were centrifuged and resuspended two times. Cells were suspended in a 36% solution of Percoll overlying a 72% solution of Percoll (MP Biomedicals, LLC) and spun for 30 minutes. Thus, a density interface was established, and from that interface, cells were extracted. Additional washes were performed. Cells were stained with rat anti-mouse B220 (B220) -FITC 0.5 mg/ml (BD Pharmingen), and isotype control staining was done with rat IgG2a-FITC 0.5 mg/ml (Southern Biotech). Cells were also stained with rat anti-mouse CD86-PE (BD Pharmingen), and isotype control staining was done with rat IgG2b-PE (BD Pharmingen). Cytometry was performed with a FACSCalibur (BD Biosciences), and analysis was performed with CellQuest Pro (BD Biosciences).
Later, we sought to know how many NP-PEpos cells are present 18 days after immunization in animals that had undergone IRI versus sham surgery. Animals were euthanized, and spleens were harvested and processed as described above. Staining was performed with NP-PE made by the laboratory of R.L., Armenian hamster anti-mouse CD3-PerCP 0.2 mg/ml (BioLegend) with isotype control staining done with Armenian hamster IgG-PerCP 0.2 mg/ml (BioLegend), and rat anti-mouse B220-FITC 0.5 mg/ml (BD Pharmingen) with isotype control staining done with rat IgG2a-FITC 0.5 mg/ml (Southern Biotech).
qPCR
Real-time qPCR was performed to analyze IL-10 mRNA expression in mouse kidneys after IRI. Total RNA (1 μg) isolated from tissues using Trizol (Invitrogen) was reverse transcribed using iSCript (Roche) according to the manufacturer's protocol. Aliquots (2.5 μl) of 1:10 dilutions of reverse transcription reactions were subjected to PCR in 25-μl reaction mixtures using 2× Power Sybr Green PCR Master Mix (Applied Biosystems, Foster City, CA) and a Roche 480 Thermal Cycler (Roche, CA). Primers were designed using a Beacon Designer Program (Premier Biosoft International). The primers for IL-10 were: 5′-GGT TGC CAA GCC TTA TCG-3′ (forward) and 5′-TCT TCA CCT GCT CCA CTG-3′ (reverse). Cyclophilin mRNA levels were used as a control housekeeping gene for normalization of the different mRNA expression levels, and the corrected mRNA levels are presented in arbitrary units. The primers for cyclophilin were 5′-TGG AGA GCA CCA AGA CAG ACA-3′ (forward) and 5′-TGC CGG AGT CGA CAA TGA T-3′ (reverse).
Statistical Analyses
Comparisons between two groups were performed using t tests—unpaired and two-tailed. ANOVA was used for comparisons between more than two groups. A value of P<0.05 was considered statistically significant. Results are reported as mean ± SEM. To determine if differing antibody titers were statistically significant over multiple time points, a longitudinal statistical test was performed using mixed model analysis.
Disclosures
J.M.T. serves as a consultant to Alexion Pharmaceuticals, a maker of complement inhibitor agents.
Supplementary Material
Acknowledgments
We were assisted by Dirk Homann at the University of Colorado at Denver; he offered advice and assistance with some of the assays. We were also assisted by Kim McFann and Pamela Mettler at the University of Colorado at Denver; they performed the mixed model analyses of antibody titers.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2012060560/-/DCSupplemental.
References
- 1.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, Parikh CR: Marked variation in the definition and diagnosis of delayed graft function: A systematic review. Nephrol Dial Transplant 23: 2995–3003, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quiroga I, McShane P, Koo DD, Gray D, Friend PJ, Fuggle S, Darby C: Major effects of delayed graft function and cold ischaemia time on renal allograft survival. Nephrol Dial Transplant 21: 1689–1696, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL: Delayed graft function: Risk factors and implications for renal allograft survival. Transplantation 63: 968–974, 1997 [DOI] [PubMed] [Google Scholar]
- 4.Nogueira JM, Haririan A, Jacobs SC, Weir MR, Hurley HA, Al-Qudah HS, Phelan M, Drachenberg CB, Bartlett ST, Cooper M: The detrimental effect of poor early graft function after laparoscopic live donor nephrectomy on graft outcomes. Am J Transplant 9: 337–347, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Narayanan R, Cardella CJ, Cattran DC, Cole EH, Tinckam KJ, Schiff J, Kim SJ: Delayed graft function and the risk of death with graft function in living donor kidney transplant recipients. Am J Kidney Dis 56: 961–970, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, Kim SJ: Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol 21: 153–161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park WD, Griffin MD, Cornell LD, Cosio FG, Stegall MD: Fibrosis with inflammation at one year predicts transplant functional decline. J Am Soc Nephrol 21: 1987–1997, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scherer A, Gwinner W, Mengel M, Kirsch T, Raulf F, Szustakowski JD, Hartmann N, Staedtler F, Engel G, Klupp J, Korn A, Kehren J, Haller H: Transcriptome changes in renal allograft protocol biopsies at 3 months precede the onset of interstitial fibrosis/tubular atrophy (IF/TA) at 6 months. Nephrol Dial Transplant 24: 2567–2575, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Nakorchevsky A, Hewel JA, Kurian SM, Mondala TS, Campbell D, Head SR, Marsh CL, Yates JR, 3rd, Salomon DR: Molecular mechanisms of chronic kidney transplant rejection via large-scale proteogenomic analysis of tissue biopsies. J Am Soc Nephrol 21: 362–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murugan R, Karajala-Subramanyam V, Lee M, Yende S, Kong L, Carter M, Angus DC, Kellum JA, Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators : Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 77: 527–535, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, De Broe ME: Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant 15: 1562–1574, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hoke TS, Douglas IS, Klein CL, He Z, Fang W, Thurman JM, Tao Y, Dursun B, Voelkel NF, Edelstein CL, Faubel S: Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. J Am Soc Nephrol 18: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Singbartl K, Bishop JV, Wen X, Murugan R, Chandra S, Filippi MD, Kellum JA: Differential effects of kidney-lung cross-talk during acute kidney injury and bacterial pneumonia. Kidney Int 80: 633–644, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu KD, Matthay MA: Advances in critical care for the nephrologist: Acute lung injury/ARDS. Clin J Am Soc Nephrol 3: 578–586, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Star RA: Treatment of acute renal failure. Kidney Int 54: 1817–1831, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Ascon M, Ascon DB, Liu M, Cheadle C, Sarkar C, Racusen L, Hassoun HT, Rabb H: Renal ischemia-reperfusion leads to long term infiltration of activated and effector-memory T lymphocytes. Kidney Int 75: 526–535, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaunat O: Humoral immunity in chronic allograft rejection: Puzzle pieces come together. Transpl Immunol 26: 101–106, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Mannon RB: Immune monitoring and biomarkers to predict chronic allograft dysfunction. Kidney Int Suppl 119: S59–S65, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Haniuda K, Nojima T, Ohyama K, Kitamura D: Tolerance induction of IgG+ memory B cells by T cell-independent type II antigens. J Immunol 186: 5620–5628, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Honda S, Kurita N, Miyamoto A, Cho Y, Usui K, Takeshita K, Takahashi S, Yasui T, Kikutani H, Kinoshita T, Fujita T, Tahara-Hanaoka S, Shibuya K, Shibuya A: Enhanced humoral immune responses against T-independent antigens in Fc alpha/muR-deficient mice. Proc Natl Acad Sci U S A 106: 11230–11235, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thurman JM, Ljubanovic D, Edelstein CL, Gilkeson GS, Holers VM: Lack of a functional alternative complement pathway ameliorates ischemic acute renal failure in mice. J Immunol 170: 1517–1523, 2003 [DOI] [PubMed] [Google Scholar]
- 23.Ricklin D, Hajishengallis G, Yang K, Lambris JD: Complement: A key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dunkelberger JR, Song WC: Complement and its role in innate and adaptive immune responses. Cell Res 20: 34–50, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Sarma JV, Ward PA: The complement system. Cell Tissue Res 343: 227–235, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thurman JM, Royer PA, Ljubanovic D, Dursun B, Lenderink AM, Edelstein CL, Holers VM: Treatment with an inhibitory monoclonal antibody to mouse factor B protects mice from induction of apoptosis and renal ischemia/reperfusion injury. J Am Soc Nephrol 17: 707–715, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP: Activation of human CD4+ cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature 421: 388–392, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Grams ME, Rabb H: The distant organ effects of acute kidney injury. Kidney Int 81: 942–948, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Ioannou A, Dalle Lucca J, Tsokos GC: Immunopathogenesis of ischemia/reperfusion-associated tissue damage. Clin Immunol 141: 3–14, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Rowshani AT, Bemelman FJ, Lardy NM, Ten Berge IJ: Humoral immunity in renal transplantation: Clinical significance and therapeutic approach. Clin Transplant 22: 689–699, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Pratt JR, Basheer SA, Sacks SH: Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Opelz G, Döhler B: Multicenter analysis of kidney preservation. Transplantation 83: 247–253, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Fang Y, Xu C, Fu YX, Holers VM, Molina H: Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol 160: 5273–5279, 1998 [PubMed] [Google Scholar]
- 34.Jung M, Sola A, Hughes J, Kluth DC, Vinuesa E, Vinas JL, Perez-Ladaga A, Hotter G: Infusion of IL-10-expressing cells protects against renal ischemia through induction of lipocalin-2. Kidney Int 81: 969–982, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Deng J, Kohda Y, Chiao H, Wang Y, Hu X, Hewitt SM, Miyaji T, McLeroy P, Nibhanupudy B, Li S, Star RA: Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int 60: 2118–2128, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Renner B, Strassheim D, Amura CR, Kulik L, Ljubanovic D, Glogowska MJ, Takahashi K, Carroll MC, Holers VM, Thurman JM: B cell subsets contribute to renal injury and renal protection after ischemia/reperfusion. J Immunol 185: 4393–4400, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burne-Taney MJ, Ascon DB, Daniels F, Racusen L, Baldwin W, Rabb H: B cell deficiency confers protection from renal ischemia reperfusion injury. J Immunol 171: 3210–3215, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Jang HR, Gandolfo MT, Ko GJ, Satpute SR, Racusen L, Rabb H: B cells limit repair after ischemic acute kidney injury. J Am Soc Nephrol 21: 654–665, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thurman JM, Kraus DM, Girardi G, Hourcade D, Kang HJ, Royer PA, Mitchell LM, Giclas PC, Salmon J, Gilkeson G, Holers VM: A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 42: 87–97, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Lenderink AM, Liegel K, Ljubanović D, Coleman KE, Gilkeson GS, Holers VM, Thurman JM: The alternative pathway of complement is activated in the glomeruli and tubulointerstitium of mice with adriamycin nephropathy. Am J Physiol Renal Physiol 293: F555–F564, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Matsumoto M, Fukuda W, Circolo A, Goellner J, Strauss-Schoenberger J, Wang X, Fujita S, Hidvegi T, Chaplin DD, Colten HR: Abrogation of the alternative complement pathway by targeted deletion of murine factor B. Proc Natl Acad Sci U S A 94: 8720–8725, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.