Abstract
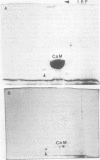
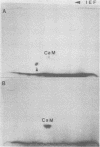
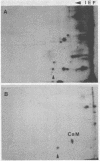
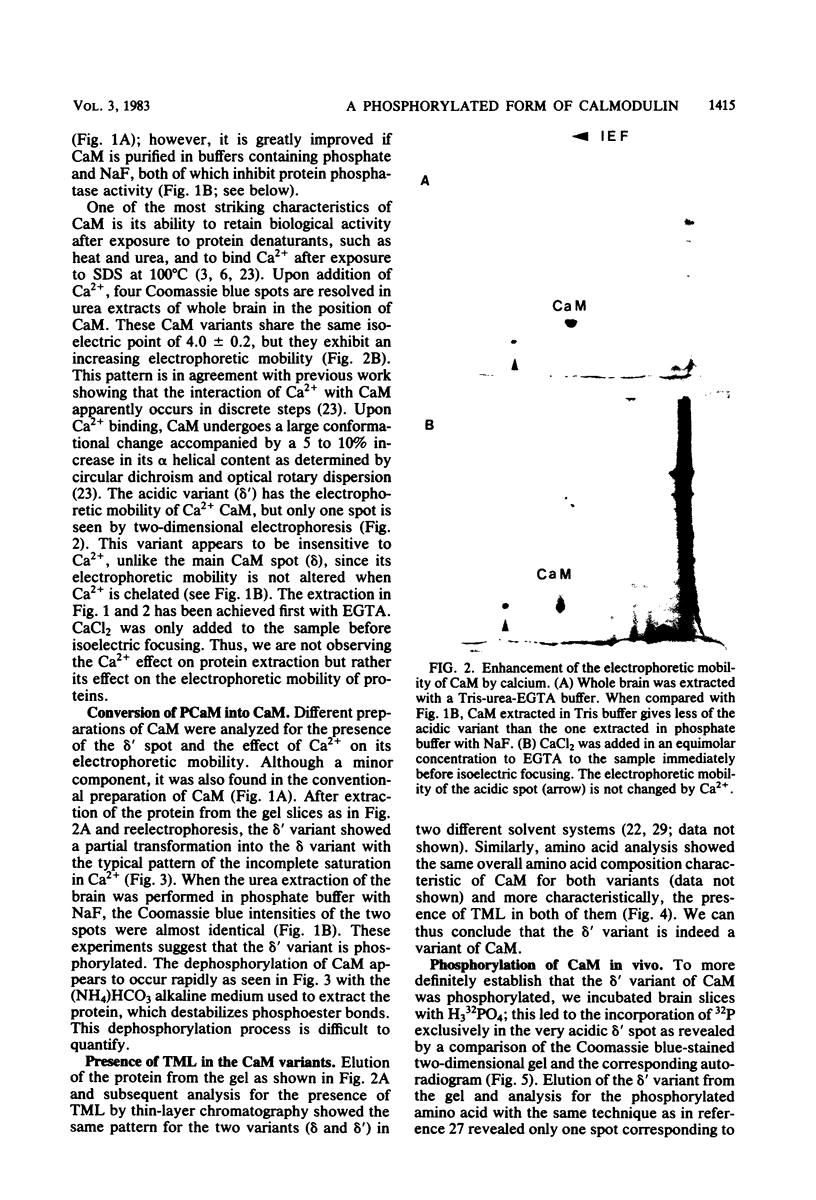
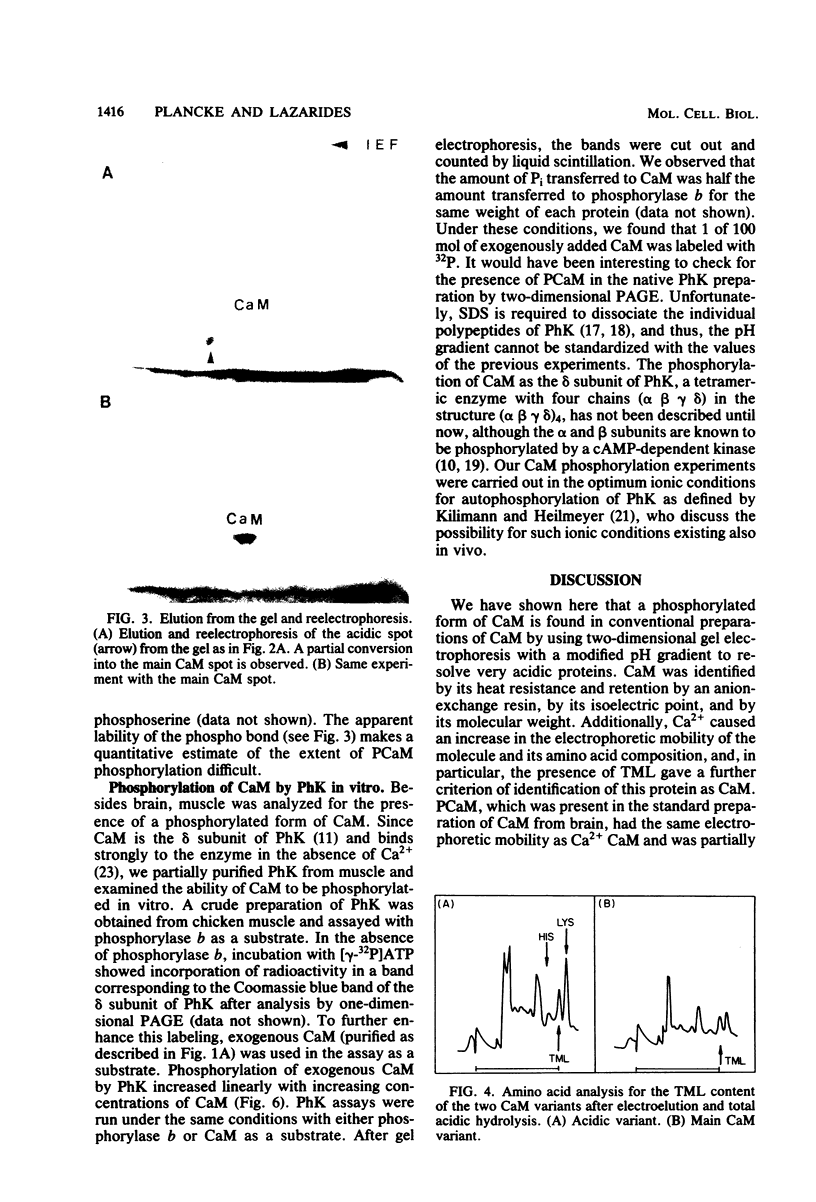
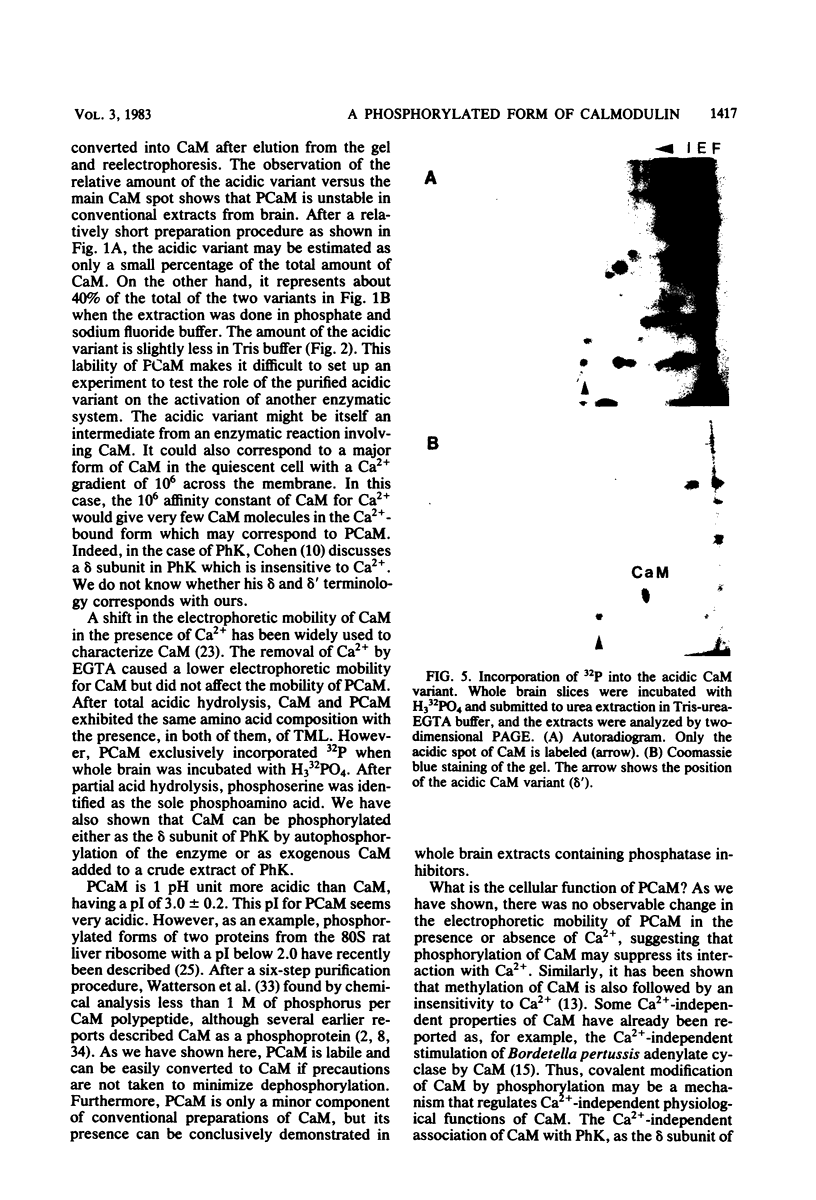
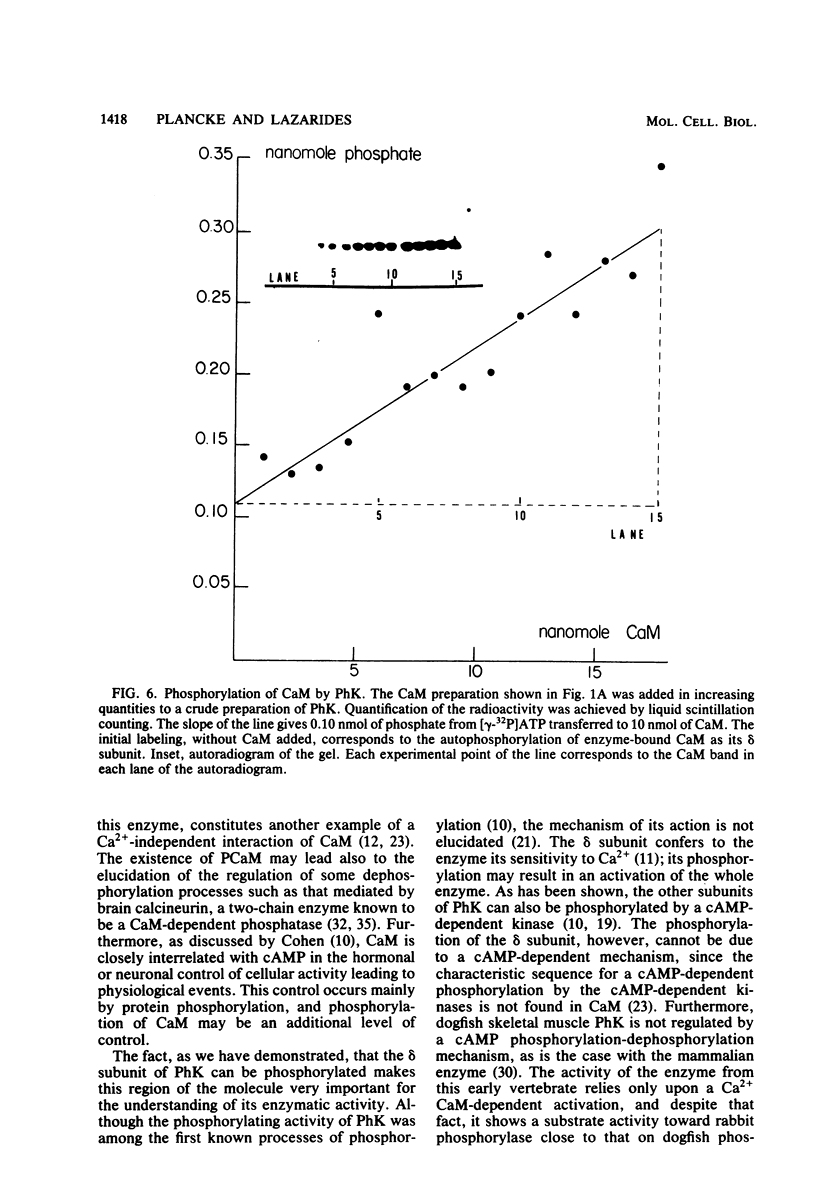
Phosphocalmodulin (PCaM) was identified after analysis of calmodulin (CaM) preparations by two-dimensional gel electrophoresis by using a modified ampholyte system to resolve very acidic proteins. The analysis of CaM prepared by the conventional procedure based upon its heat resistance and acidity as well as the analysis of whole urea extracts from brain showed that PCaM was a major component in this tissue. PCaM was 1 pH unit more acidic than CaM, and its electrophoretic mobility, unlike CaM, was not changed by either calcium or ethylene glycol-bis(beta-aminoethyl ether)-N,N-tetraacetic acid. In urea extracts of brain prepared in buffers containing phosphate and sodium fluoride, PCaM was as prominent as CaM; it was partially converted into CaM after elution from the gel and reelectrophoresis. Amino acid analysis of PCaM and CaM purified by two-dimensional gel electrophoresis showed the same composition for the two proteins, including their trimethyllysine content. Incorporation of 32P occurred exclusively into the acidic variant when brain slices were incubated with H332PO4; amino acid analysis showed that the phosphate was bound to serine residues. CaM was found also to be phosphorylated in vitro by a phosphorylase kinase preparation from skeletal muscle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper S. L., Palfrey H. C., DeRiemer S. A., Greengard P. Hormonal control of protein phosphorylation in turkey erythrocytes. Phosphorylation by cAMP-dependent and Ca2+-dependent protein kinases of distinct sites in goblin, a high molecular weight protein of the plasma membrane. J Biol Chem. 1980 Nov 25;255(22):11029–11039. [PubMed] [Google Scholar]
- Brooks J. C., Siegel F. L. Purification of a calcium-binding phosphoprotein from beef adrenal medulla. Identity with one of two calcium-binding proteins of brain. J Biol Chem. 1973 Jun 25;248(12):4189–4193. [PubMed] [Google Scholar]
- Burgess W. H., Jemiolo D. K., Kretsinger R. H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Jun 26;623(2):257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D. The loss of muscarinic acetylcholine receptors in synaptic membranes under phosphorylating conditions is dependent on calmodulin. FEBS Lett. 1981 May 5;127(1):144–148. doi: 10.1016/0014-5793(81)80361-4. [DOI] [PubMed] [Google Scholar]
- Chan K. F., Graves D. J. Isolation and physicochemical properties of active complexes of rabbit muscle phosphorylase kinase. J Biol Chem. 1982 May 25;257(10):5939–5947. [PubMed] [Google Scholar]
- Chan K. F., Graves D. J. Rabbit skeletal muscle phosphorylase kinase. Interactions between subunits and influence of calmodulin on different complexes. J Biol Chem. 1982 May 25;257(10):5956–5961. [PubMed] [Google Scholar]
- Chan K. F., Hurst M. O., Graves D. J. Phosphorylase kinase specificity. A comparative study with cAMP-dependent protein kinase on synthetic peptides and peptide analogs of glycogen synthase and phosphorylase. J Biol Chem. 1982 Apr 10;257(7):3655–3659. [PubMed] [Google Scholar]
- Cheung W. Y., Bradham L. S., Lynch T. J., Lin Y. M., Tallant E. A. Protein activator of cyclic 3':5'-nucleotide phosphodiesterase of bovine or rat brain also activates its adenylate cyclase. Biochem Biophys Res Commun. 1975 Oct 6;66(3):1055–1062. doi: 10.1016/0006-291x(75)90747-0. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin. Sci Am. 1982 Jun;246(6):62–70. doi: 10.1038/scientificamerican0682-62. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Evidence for and properties of a protein activator. J Biol Chem. 1971 May 10;246(9):2859–2869. [PubMed] [Google Scholar]
- Cohen P., Burchell A., Foulkes J. G., Cohen P. T., Vanaman T. C., Nairn C. Identification of the Ca2+-dependent modulator protein as the fourth subunit of rabbit skeletal muscle phosphorylase kinase. FEBS Lett. 1978 Aug 15;92(2):287–293. doi: 10.1016/0014-5793(78)80772-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. The role of protein phosphorylation in neural and hormonal control of cellular activity. Nature. 1982 Apr 15;296(5858):613–620. doi: 10.1038/296613a0. [DOI] [PubMed] [Google Scholar]
- Cohen P. The subunit structure of rabbit-skeletal-muscle phosphorylase kinase, and the molecular basis of its activation reactions. Eur J Biochem. 1973 Apr 2;34(1):1–14. doi: 10.1111/j.1432-1033.1973.tb02721.x. [DOI] [PubMed] [Google Scholar]
- DeMartino G. N., Blumenthal D. K. Identification and partial purification of a factor that stimulates calcium-dependent proteases. Biochemistry. 1982 Aug 31;21(18):4297–4303. doi: 10.1021/bi00261a019. [DOI] [PubMed] [Google Scholar]
- Gagnon C., Kelly S., Manganiello V., Vaughan M., Odya C., Strittmatter W., Hoffman A., Hirata F. Modification of calmodulin function by enzymatic carboxylic methylation. Nature. 1981 Jun 11;291(5815):515–516. doi: 10.1038/291515a0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Bretscher A., Weber K. Calcium control of the intestinal microvillus cytoskeleton: its implications for the regulation of microfilament organizations. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6458–6462. doi: 10.1073/pnas.77.11.6458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenlee D. V., Andreasen T. J., Storm D. R. Calcium-independent stimulation of Bordetella pertussis adenylate cyclase by calmodulin. Biochemistry. 1982 May 25;21(11):2759–2764. doi: 10.1021/bi00540a028. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R. Calcium dependent phosphodiesterase activity and its activating factor (PAF) from brain studies on cyclic 3',5'-nucleotide phosphodiesterase (3). Biochem Biophys Res Commun. 1970 Dec 9;41(5):1104–1110. doi: 10.1016/0006-291x(70)90199-3. [DOI] [PubMed] [Google Scholar]
- Kilimann M. W., Heilmeyer L. M., Jr Multiple activities on phosphorylase kinase. 2. Different specificities toward the protein substrates phosphorylase b, troponin, and phosphorylase kinase. Biochemistry. 1982 Apr 13;21(8):1735–1739. doi: 10.1021/bi00537a005. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Furano A. V. Methylated amino acids in the proteins of bacterial and mammalian cells. Arch Biochem Biophys. 1975 Aug;169(2):529–539. doi: 10.1016/0003-9861(75)90196-4. [DOI] [PubMed] [Google Scholar]
- Klee C. B., Crouch T. H., Richman P. G. Calmodulin. Annu Rev Biochem. 1980;49:489–515. doi: 10.1146/annurev.bi.49.070180.002421. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Nelson W. J. Expression of spectrin in nonerythroid cells. Cell. 1982 Dec;31(3 Pt 2):505–508. doi: 10.1016/0092-8674(82)90306-3. [DOI] [PubMed] [Google Scholar]
- MacConnell W. P., Kaplan N. O. The activity of the acidic phosphoproteins from the 80 S rat liver ribosome. J Biol Chem. 1982 May 25;257(10):5359–5366. [PubMed] [Google Scholar]
- O'Connor C. M., Gard D. L., Lazarides E. Phosphorylation of intermediate filament proteins by cAMP-dependent protein kinases. Cell. 1981 Jan;23(1):135–143. doi: 10.1016/0092-8674(81)90278-6. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Oakley B. R., Kirsch D. R., Morris N. R. A simplified ultrasensitive silver stain for detecting proteins in polyacrylamide gels. Anal Biochem. 1980 Jul 1;105(2):361–363. doi: 10.1016/0003-2697(80)90470-4. [DOI] [PubMed] [Google Scholar]
- Pocinwong S., Blum H., Malencik D., Fischer E. H. Phosphorylase kinase from dogfish skeletal muscle. Purification and properties. Biochemistry. 1981 Dec 8;20(25):7219–7226. doi: 10.1021/bi00528a026. [DOI] [PubMed] [Google Scholar]
- Sobue K., Muramoto Y., Fujita M., Kakiuchi S. Calmodulin-binding protein of erythrocyte cytoskeleton. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1063–1070. doi: 10.1016/0006-291x(81)91931-8. [DOI] [PubMed] [Google Scholar]
- Stewart A. A., Ingebritsen T. S., Manalan A., Klee C. B., Cohen P. Discovery of a Ca2+- and calmodulin-dependent protein phosphatase: probable identity with calcineurin (CaM-BP80). FEBS Lett. 1982 Jan 11;137(1):80–84. doi: 10.1016/0014-5793(82)80319-0. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Harrelson W. G., Jr, Keller P. M., Sharief F., Vanaman T. C. Structural similarities between the Ca2+-dependent regulatory proteins of 3':5'-cyclic nucleotide phosphodiesterase and actomyosin ATPase. J Biol Chem. 1976 Aug 10;251(15):4501–4513. [PubMed] [Google Scholar]
- Wolff D. J., Brostrom C. O. Calcium-binding phosphoprotein from pig brain: identification as a calcium-dependent regulator of brain cyclic nucleotide phosphodiesterase. Arch Biochem Biophys. 1974 Jul;163(1):349–358. doi: 10.1016/0003-9861(74)90486-x. [DOI] [PubMed] [Google Scholar]
- Yang S. D., Tallant E. A., Cheung W. Y. Calcineurin is a calmodulin-dependent protein phosphatase. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1419–1425. doi: 10.1016/0006-291x(82)91272-4. [DOI] [PubMed] [Google Scholar]