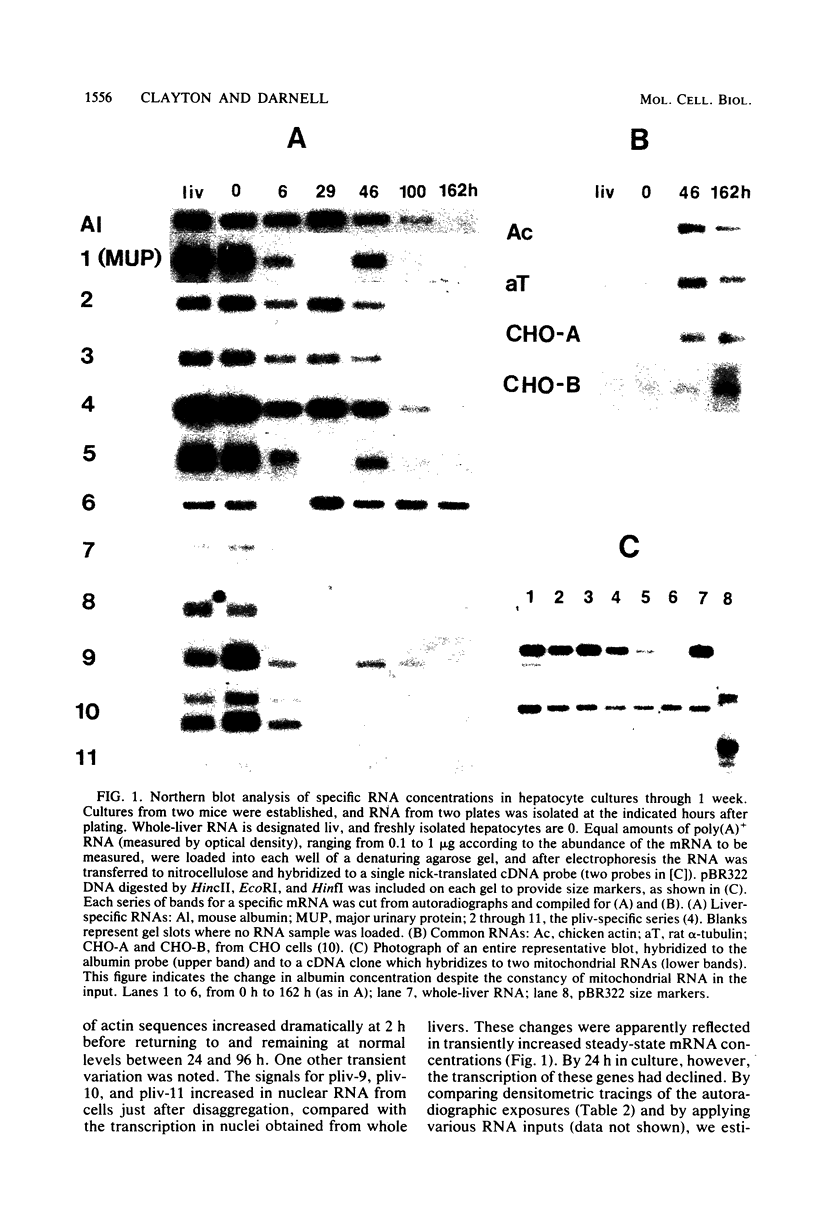
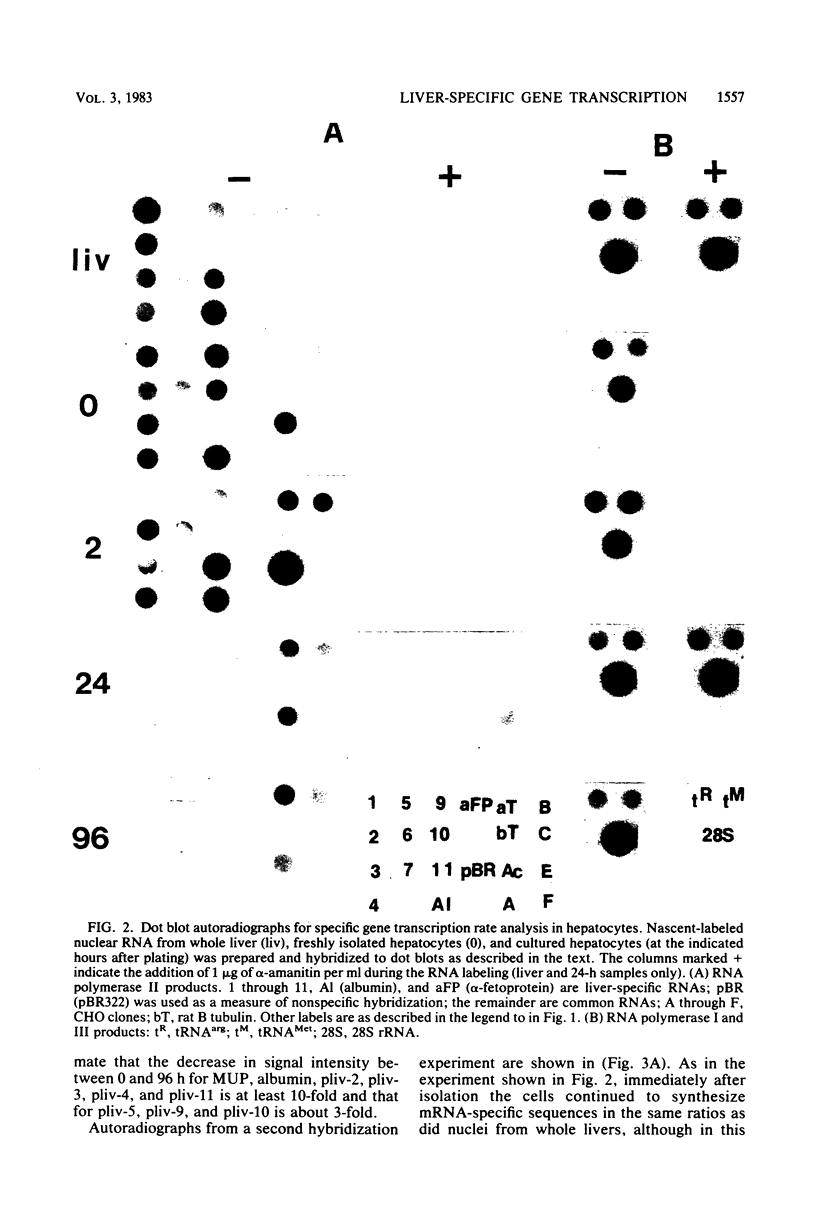
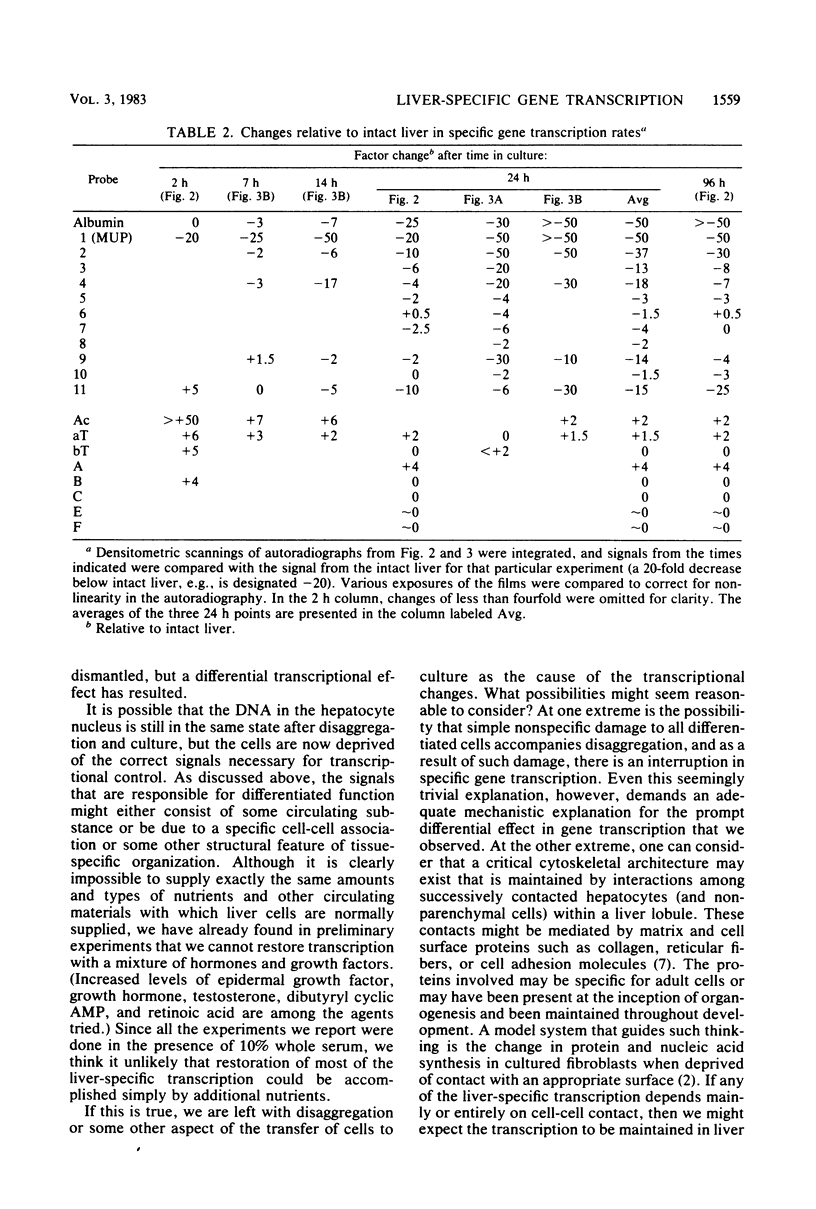
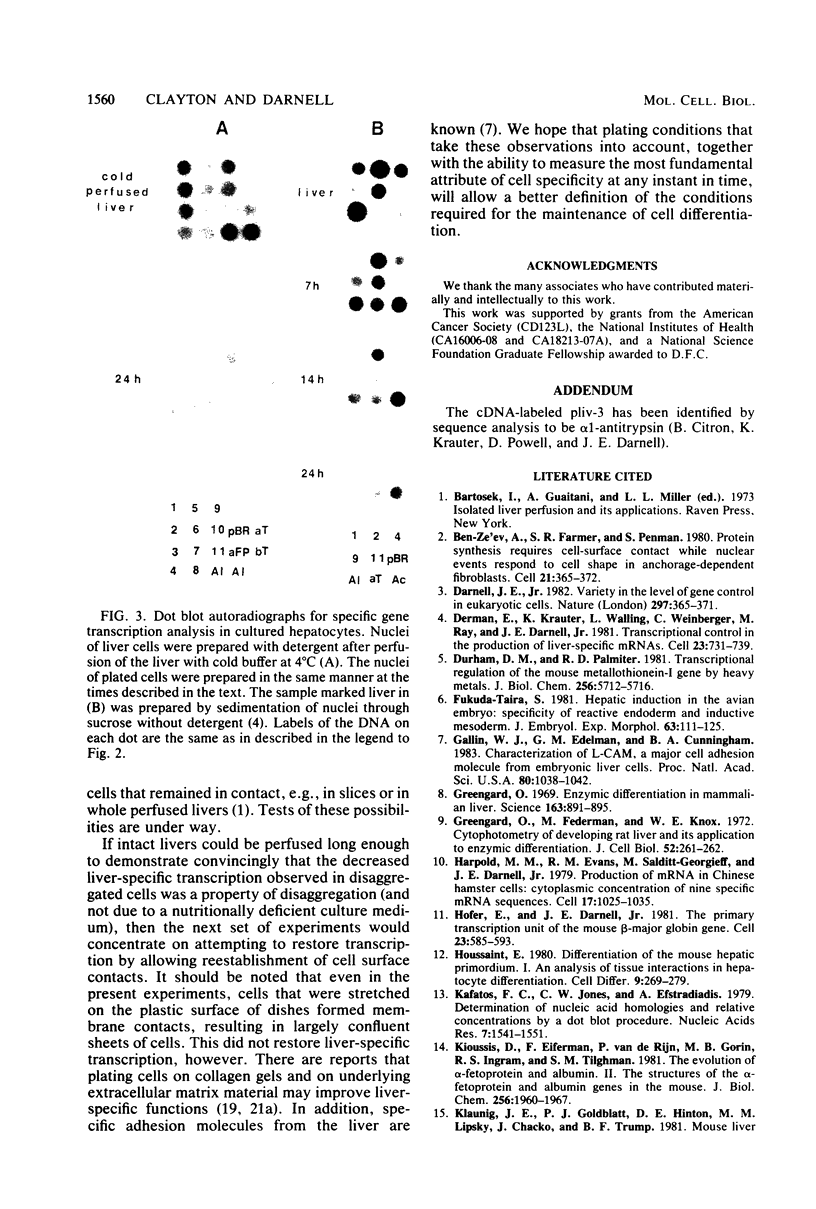
Abstract
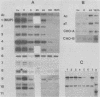
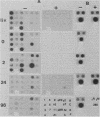
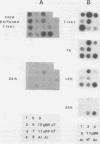
Liver-specific mRNA sequences were examined in primary cultures of mouse hepatocytes. After cell disaggregation by collagenase treatment and for at least 24 h in culture, little change in liver-specific mRNA concentrations was noted. Gradually over a period of 140 h, liver-specific mRNAs declined. In contrast, transcriptional assays in which liver cell nuclei were used to produce 32P-labeled nuclear RNA showed that liver-specific gene transcription was greatly diminished within 24 h, while polymerase II transcription of "common" genes and transcription of tRNA and rRNA did not decline. Thus, a prompt differential transcriptional effect seems to underlie the gradual loss of tissue specificity of the primary cultures.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Ze'ev A., Farmer S. R., Penman S. Protein synthesis requires cell-surface contact while nuclear events respond to cell shape in anchorage-dependent fibroblasts. Cell. 1980 Sep;21(2):365–372. doi: 10.1016/0092-8674(80)90473-0. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. Transcriptional regulation of the mouse metallothionein-I gene by heavy metals. J Biol Chem. 1981 Jun 10;256(11):5712–5716. [PubMed] [Google Scholar]
- Fukuda-Taira S. Hepatic induction in the avian embryo: specificity of reactive endoderm and inductive mesoderm. J Embryol Exp Morphol. 1981 Jun;63:111–125. [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard O. Enzymic differentiation in mammalian liver injection of fetal rats with hormones causes the premature formation of liver enzymes. Science. 1969 Feb 28;163(3870):891–895. doi: 10.1126/science.163.3870.891. [DOI] [PubMed] [Google Scholar]
- Greengard O., Federman M., Knox W. E. Cytomorphometry of developing rat liver and its application to enzymic differentiation. J Cell Biol. 1972 Feb;52(2):261–272. doi: 10.1083/jcb.52.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Evans R. M., Salditt-Georgieff M., Darnell J. E. Production of mRNA in Chinese hamster cells: relationship of the rate of synthesis to the cytoplasmic concentration of nine specific mRNA sequences. Cell. 1979 Aug;17(4):1025–1035. doi: 10.1016/0092-8674(79)90341-6. [DOI] [PubMed] [Google Scholar]
- Hofer E., Darnell J. E., Jr The primary transcription unit of the mouse beta-major globin gene. Cell. 1981 Feb;23(2):585–593. doi: 10.1016/0092-8674(81)90154-9. [DOI] [PubMed] [Google Scholar]
- Houssaint E. Differentiation of the mouse hepatic primordium. I. An analysis of tissue interactions in hepatocyte differentiation. Cell Differ. 1980 Oct;9(5):269–279. doi: 10.1016/0045-6039(80)90026-3. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Eiferman F., van de Rijn P., Gorin M. B., Ingram R. S., Tilghman S. M. The evolution of alpha-fetoprotein and albumin. II. The structures of the alpha-fetoprotein and albumin genes in the mouse. J Biol Chem. 1981 Feb 25;256(4):1960–1967. [PubMed] [Google Scholar]
- Klaunig J. E., Goldblatt P. J., Hinton D. E., Lipsky M. M., Chacko J., Trump B. F. Mouse liver cell culture. I. Hepatocyte isolation. In Vitro. 1981 Oct;17(10):913–925. doi: 10.1007/BF02618288. [DOI] [PubMed] [Google Scholar]
- Klaunig J. E., Goldblatt P. J., Hinton D. E., Lipsky M. M., Trump B. F. Mouse liver cell culture. II. Primary culture. In Vitro. 1981 Oct;17(10):926–934. doi: 10.1007/BF02618289. [DOI] [PubMed] [Google Scholar]
- Leffert H. L., Moran T., Boorstein R., Koch K. S. Procarcinogen activation and hormonal control of cell proliferation in differentiated primary adult rat liver cell cultures. Nature. 1977 May 5;267(5606):58–61. doi: 10.1038/267058a0. [DOI] [PubMed] [Google Scholar]
- Leffert H., Moran T., Sell S., Skelly H., Ibsen K., Mueller M., Arias I. Growth state-dependent phenotypes of adult hepatocytes in primary monolayer culture. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1834–1838. doi: 10.1073/pnas.75.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalopoulos G., Pitot H. C. Primary culture of parenchymal liver cells on collagen membranes. Morphological and biochemical observations. Exp Cell Res. 1975 Aug;94(1):70–78. doi: 10.1016/0014-4827(75)90532-7. [DOI] [PubMed] [Google Scholar]
- Newman S., Guzelian P. S. Stimulation of de novo synthesis of cytochrome P-450 by phenobarbital in primary nonproliferating cultures of adult rat hepatocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2922–2926. doi: 10.1073/pnas.79.9.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. Methodology and utility of primary cultures of hepatocytes from experimental animals. Methods Cell Biol. 1980;21B:441–456. doi: 10.1016/s0091-679x(08)60697-4. [DOI] [PubMed] [Google Scholar]
- Salditt-Georgieff M., Harpold M., Sawicki S., Nevins J., Darnell J. E., Jr Addition of poly(A) to nuclear RNA occurs soon after RNA synthesis. J Cell Biol. 1980 Sep;86(3):844–848. doi: 10.1083/jcb.86.3.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiskocil R., Bensky P., Dower W., Goldberger R. F., Gordon J. I., Deeley R. G. Coordinate regulation of two estrogen-dependent genes in avian liver. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4474–4478. doi: 10.1073/pnas.77.8.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]