Abstract
Clinicoanatomic correlation in the spinocerebellar ataxias (SCA) and Friedreich’s ataxia (FRDA) is difficult as these diseases differentially affect multiple sites in the central and peripheral nervous systems. A new way to study cerebellar ataxia is the systematic analysis of the “reciprocal cerebellar circuitry” that consists of tightly organized reciprocal connections between Purkinje cells, dentate nuclei (DN), and inferior olivary nuclei (ION). This circuitry is similar to but not identical to the “cerebellar module” in experimental animals. Neurohumoral transmitters operating in the circuitry are both inhibitory (γ-aminobutyric acid [GABA] in corticonuclear and dentato-olivary fibers) and excitatory (glutamate in olivocerebellar or climbing fibers). Glutamatergic climbing fibers also issue collaterals to the DN. The present study applied 5 immunohistochemical markers in six types of SCA (1, 2, 3, 6, 7, 17), genetically undefined SCA, FRDA, and FRDA carriers to identify interruptions within the circuitry: calbindin-D28k; neuron-specific enolase (NSE); glutamic acid decarboxylase (GAD); and vesicular glutamate transporters (VGluT) 1 and 2. Lesions of the cerebellar cortex, DN, and ION were scored according to a guide as 0 (normal); 1 (mild); 2 (moderate); and 3 (severe). Results of each of the 5 immunohistochemical stains were examined separately for each of the 3 regions. Combining scores of each anatomical region and each stain yielded a total score as an indicator of pathological severity. Total scores ranged from 16 to 38 in SCA-1 (9 cases); 22 to 39 in SCA-2 (6 cases); 9 to 15 in SCA-3 (4 cases); and 13 and 25 in SCA-6 (2 cases). In single cases of SCA-7 and SCA-17, scores were 16 and 31, respectively. In 2 genetically undefined SCA, scores were 36 and 37, respectively. In 9 cases of FRDA, total scores ranged from 11 to 19. The low scores in SCA-3 and FRDA reflect selective atrophy of the DN. The FRDA carriers did not differ from normal controls. These observations offer a semiquantitative assessment of the critical role of the DN in the ataxic phenotype of SCA and FRDA while other parts of the circuitry appear less important.
Keywords: Cerebellum, Dentate nucleus, Friedreich’s ataxia, Immunohistochemistry, Inferior olivary nucleus, Spinocerebellar ataxia
Introduction
Ataxia is defined as disturbed coordination of voluntary movements though neurologists use the term mostly for a disorder of gait and prefer the term “dysmetria” for limb ataxia. Traditionally, unilateral dysmetria is attributed to lesions of the ipsilateral cerebellar hemisphere, and ataxic gait to superior vermis and adjacent hemispheric cortex. This traditional clinico-anatomical correlation does not consider the corticonuclear organization of the cerebellum or the contribution of the inferior olivary nuclei (ION). The term “ataxia” will be used in this paper to include the disturbances of gait and limb movements. Recent advances in magnetic resonance imaging (MRI) allow a more precise localization of the ataxia-causing lesion and replace the time-honored though simplistic reliance on “lateralizing” signs. The dentate nucleus (DN) is the principal way station of cerebellar output. Its activity is largely controlled by inhibitory corticonuclear connections that utilize neurohumoral transmission by γ-aminobutyric acid (GABA), and excitatory glutamatergic collaterals arising from mossy and climbing fibers. The human DN also contains glycinergic neurons that contribute to normal cerebellar function [1]. The existence of a dentato-olivary connection and the clinical correlate of a lesion therein, namely, palatal myoclonus, has been known for nearly 100 years [2, 3]. Lapresle and Ben Hamida [4] extended our knowledge of the dentato-olivary connection by documenting the precise topistic relationship of human olivary hypertrophy and lesions of the DN. The discovery of GABA as the neurohumoral transmitter of the dentato-olivary tract is more recent [5, 6]. This work also provided the evidence that the DN supplies most GABA-ergic terminals to the ION (review in ref [7]). The “cerebellar module” (review in ref [8]) extends the topistic relationship between DN and ION to narrow parasagittal Purkinje cell zones. In experimental animals, the modular organization of the cerebellum is readily revealed by anatomical methods [8], but a comparable longitudinal zonation of the adult human cerebellum has been elusive. Voogd [9] considered it likely that it exists though the evidence is based on the study of developing human cerebellum rather than histochemistry or immununohistochemistry of the adult organ. In recognition of this uncertainty, this report will utilize the term “reciprocal cerebellar circuitry” instead of “cerebellar module”. Ataxia, dysmetria, dysarthria, and dysphagia are disabling manifestations in patients who experience diseases that interrupt the described connections between Purkinje cells, DN, and ION at one or more locations. The results reported here point to the DN as the critical dysfunctional component of the reciprocal cerebellar circuitry in the dominant ataxias (here termed spinocerebellar ataxias [SCA]) and the most common recessive ataxia, Friedreich’s ataxia (FRDA).
Material and methods
The authors received approval of their research by the Institutional Review Board of the Veterans Affairs Medical Center, Albany, N.Y., USA. Case material consisted of archival formaldehyde-fixed paraffin-embedded tissue samples that were collected over a period of 18 years through tissue donation programs of National Ataxia Foundation and Friedreich’s Ataxia Research Alliance. Specimens of one FRDA patient were made available through the courtesy of another investigator. The material also included two non-ataxic persons who were established FRDA carriers. To determine interruptions within the reciprocal cerebellar circuitry, each case required adequate sections of one cerebellar hemisphere, DN, and ION. The fixed cerebellum was sliced parasagittally rotating the knife in small steps around the estimated location of the hilum of the DN. Single tissue blocks representing DN and cerebellar cortex located just above the DN were embedded. In one case of SCA-6, a slice of cerebellar tonsil was also embedded. Among the cerebellar nuclei, only the DN proper was considered, ignoring emboliform, globose, and fastigial nuclei. Among the olivary nuclei, only the chief olivary nucleus was studied in detail. All specimens were first systematically examined after staining with hematoxylin and eosin. Cases with autolysis of the granular layer were excluded from the study. In 23 of 25 suitable cases of SCA, confirmation of pathogenic cytosine-adenine-guanine (CAG) trinucleotide repeat expansions was available as follows (number of cases in parentheses): SCA-1 (9); SCA-2 (6); SCA-3 (4); SCA-6 (2); SCA-7 (1); SCA-17 (1). In two patients with dominantly transmitted SCA, the mutation could not be established. Eight of 9 patients with FRDA had genetic confirmation of homozygous guanine-adenine-adenine (GAA) trinucleotide repeat expansions during life. The ninth patient was diagnosed clinically because of ataxia and cardiomyopathy but had no genetic confirmation. Her autopsy revealed typical FRDA lesions of a dorsal root ganglion; spinal cord; and DN. The heart contained iron-reactive granules. Frozen tissue for the extraction of deoxyribonucleic acid was not available.
The focused analysis of the reciprocal cerebellar circuitry utilized positive-contrast immunohistochemistry with antibodies to 5 well-established neuronal proteins: calbindin-D28k, neuron-specific enolase (NSE), glutamic acid decarboxylase (GAD), vesicular glutamate transporter 1 (VGluT1), and vesicular glutamate transporter 2 (VGluT2). Sources of the antibodies, antigen retrieval, and immunohistochemical steps were described previously [7]. Antibody concentrations, in μg protein/ml buffer, were as follows: polyclonal anti-calbindin-D28k, 0.35; monoclonal anti-NSE, 2; monoclonal anti-GAD, 2; monoclonal anti-VGluT1, 10; polyclonal anti-VGluT2, 1.4.
Abnormalities of the molecular layer, DN, and ION were graded by scoring them as “0” (normal), “1” (mild), “2” (moderate), or “3” (severe). Table 1 provides a guide for the grading of abnormalities in the reciprocal cerebellar circuitry, specifically Purkinje cells, DN, and ION. The approach excluded the granular layer though all stains yielded potentially important information on granule cells and Golgi neurons. Total scores were obtained by adding the individual scores of each of the 5 stains for each of the three anatomical sites. A normal total score was 0. Theoretically, the highest total score could be 45 (5 stains × 3 sites × a maximum score of 3). The maximum achievable score, however, was 42 since the human ION is VGluT1-negative.
Table 1.
Guide to the assessment of immunohistochemical findings
| Molecular layer | DN* | ION* | |
|---|---|---|---|
| Calbindin-D28k | Thickness (considering 250 μm or higher as normal); abundance of Purkinje cells and extent of arborization | Abundance of afferent axons and terminals; presence of grumose degeneration | Abundance of immunoreactive neurons and dendrites |
| NSE* | Thickness; number of negative images of Purkinje cells | Abundance of immunoreactive large and small neurons | Abundance of immunoreactive neurons and dendrites |
| GAD* | Thickness; abundance of Purkinje cells, immunoreactive terminal baskets and reactive GABA-ergic parallel fibers | Abundance of negative images of large GAD-negative neurons; abundance of GAD-positive small neurons; density of GAD-positive terminals; and presence of grumose degeneration | Abundance of GAD- positive nerve terminals |
| VGluT1* | Thickness; abundance of negative images of VGluT1-negative Purkinje cell bodies | Abundance of immunoreactive nerve terminals and of negative images of nerve cells | The human ION does not contain VGluT1- positive terminals [7] |
| VGluT2* | Thickness; abundance of immunoreactive climbing fibers | Abundance of immunoreactive nerve terminals and negative images of nerve cells | Abundance of immunoreactive terminals |
Abbreviations: DN, dentate nucleus; GAD, glutamic acid decarboxylase; ION, inferior olivary nucleus; NSE, neuron-specific enolase; VGluT1, vesicular glutamate transporter 1; VGluT2, vesicular glutamate transporter 2
Statistical analysis for ordinal scale data was by Spearman’s Rank Order test for correlations between scores in the molecular layer, DN, and ION; and by Kruskal-Wallis analysis of variance by ranks for differences among SCA types (Statistics Software, StatSoft, Tulsa, OK, USA). Significance was accepted at the 0.05 level.
Results
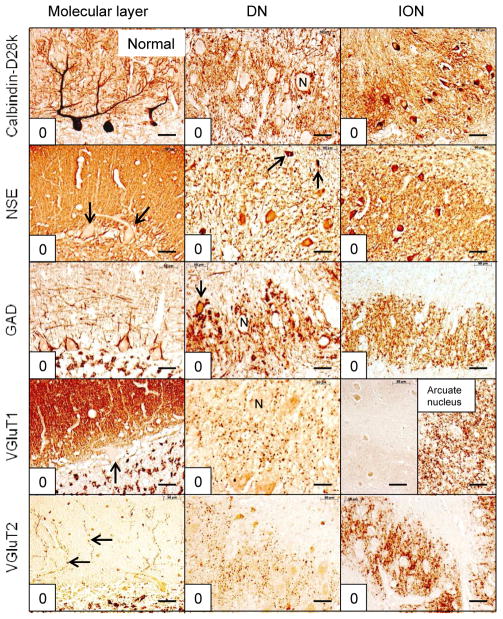
The localization of immunohistochemical reaction product provided information on the structural integrity and abundance of neuronal cell bodies, dendrites, axons, axon terminals, and axonal modifications such as grumose degeneration. Immunohistochemistry with anti-calbindin-D28k was most suitable to visualize perikarya and dendritic expanse of Purkinje cells, axons and terminals in the DN, and cell bodies and dendrites of the ION (fig. 1). Anti-NSE yielded information on axonal terminals in the molecular layer of the cerebellar cortex, with the added bonus of negative images of Purkinje cell bodies and their primary dendrites (fig. 1). NSE reaction product was also suitable to detect nerve cell bodies and dendrites in the DN and ION. Anti-GAD provided information on Purkinje cell bodies, the terminal baskets about these neurons, axons and terminals in the DN, perikarya of small neurons in the DN, and afferent GABA-ergic terminals in the ION (fig. 1). VGluT1 reaction product visualized the great abundance of glutamatergic terminals in the molecular layer and the normally sparse glutamatergic boutons in the DN (fig. 1). The inclusion of VGluT1-staining of the arcuate nucleus of the medulla oblongata served as an internal positive control to confirm the absence of VGluT1-immunoreactivity in the ION (fig. 1). The VGluT2-reaction provided selective visualization of climbing fibers in the molecular layer and glutamatergic afferents in DN and ION (fig. 1). Antibodies to calbindin-D28k and GAD were well suited to reveal the presence of grumose degeneration (figs. 4 and 6).
Figure 1.
Normal reciprocal cerebellar circuitry. The anatomical sites and stains are indicated. Individual scores are shown in the lower left corner of each microphotograph. The summed scores for cerebellar cortex = 0; DN = 0; and ION = 0, for a total score = 0. The ION is VGluT1-negative. The strongly reactive arcuate nucleus on the same section serves as an internal positive control. Purkinje cell cytoplasm is NSE-negative. Arrows in NSE/molecular layer and VGluT1/molecular layer indicate negative images of Purkinje cells. Arrows in NSE/DN and GAD/DN point to small neurons. The small neuron in GAD/DN (arrow) also reveals GAD-positive afferent terminals. Arrows in VGluT2/molecular layer indicate normal climbing fibers. “N” in calbindin-D28k/DN, GAD/DN, and VGluT1/DN highlight negative images of DN neurons. Abbreviations: DN, dentate nucleus; GAD, glutamic acid decarboxylase; ION, inferior olivary nucleus; NSE, neuron-specific enolase; VGluT1, vesicular glutamate transporter 1; VGluT2, vesicular glutamate transporter 2. Bars: 50 μm
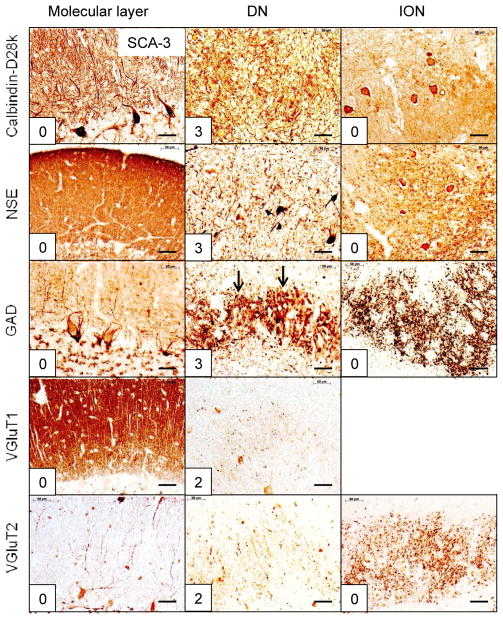
Figure 4.
The reciprocal cerebellar circuitry in SCA-3. The CAG trinucleotide repeats in this patient were 67 (expanded) and 23 (normal). Note the integrity of cerebellar molecular layer and ION. The lesion is restricted to the DN, accounting for all of the total score of 13. The arrows in GAD/DN indicate prominent grumose degeneration after visualization of GAD. Abbreviations as in fig. 1. Bars: 50 μm
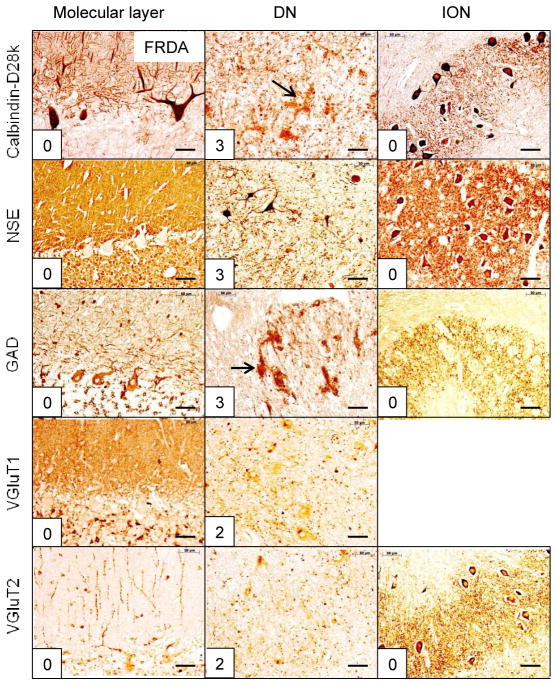
Figure 6.
The reciprocal cerebellar circuitry in FRDA. The homozygous GAA trinucleotide repeat expansions in this patient were 1070 and 1070 (normal under 50). Only the DN shows a measurable lesion: loss of large neurons (NSE) and grumose degeneration (calbindin-D28k and GAD, arrows). Small GAD-positive neurons remain intact. The DN score (13) accounts for the total score of 13. Abbreviations as in fig. 1. Bars: 50 μm
In figures 1–6 and supplemental figures 1–4, scores appear in the left lower corner of each microphotograph. Figures 2–6 show the most prominent abnormalities of the reciprocal cerebellar circuitry in SCA-1 (fig. 2); SCA-2 (fig. 3), SCA-3 (fig. 4); SCA-6 (fig. 5); and FRDA (fig. 6). Similar displays of SCA-7, SCA-17, SCA with unknown mutations; and an FRDA carrier appear as supplemental figures 1–4, respectively.
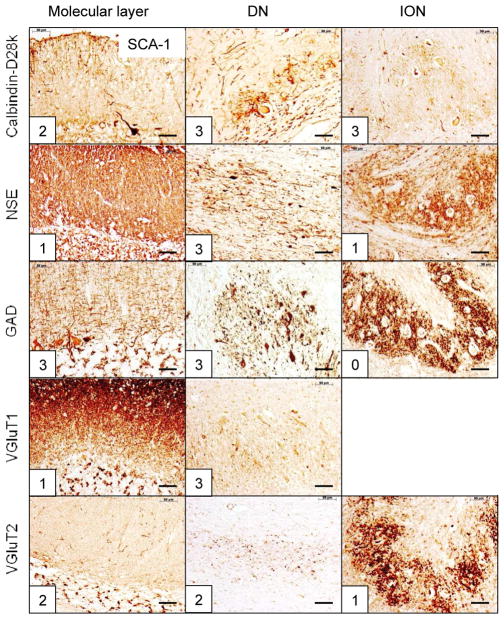
Figure 2.
The reciprocal cerebellar circuitry in SCA-1. The CAG trinucleotide repeats in this patient were 47 (expanded) and 34 (normal). The lesion affects cerebellar cortex (summed score = 9); DN (summed score = 14); and ION (summed score = 5). The total score=28, and the DN lesion accounts for 50%. Abbreviations as in fig. 1. Bars: 50 μm
Figure 3.
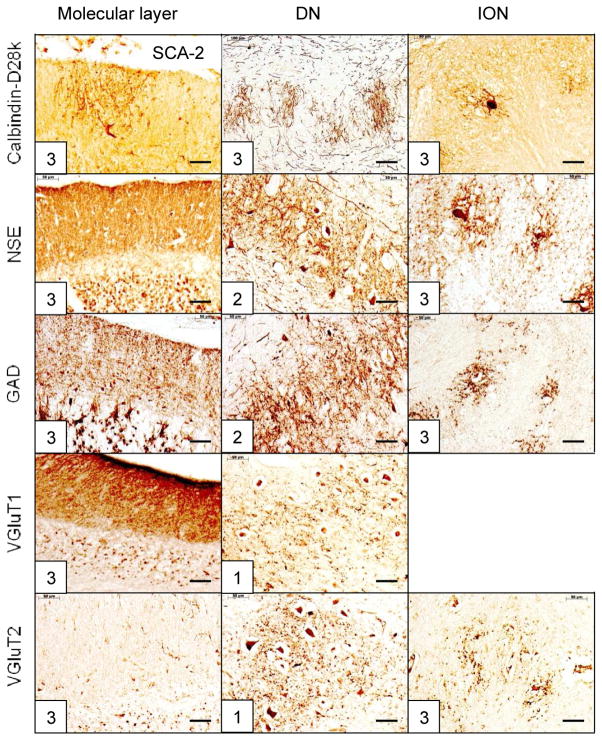
The reciprocal cerebellar circuitry in SCA-2. The CAG trinucleotide repeats in this patient were 50 (expanded) and 22 (normal). Similar to SCA-1, the lesion affects cerebellar cortex (summed score = 15); DN (summed score = 9); and ION (summed score = 12). Atrophy of ION is especially severe, displaying wide gaps between neurons on all stains. The total score = 36, and the DN lesion accounts for 25%. Abbreviations as in fig. 1. Bars: 50 μm
Figure 5.
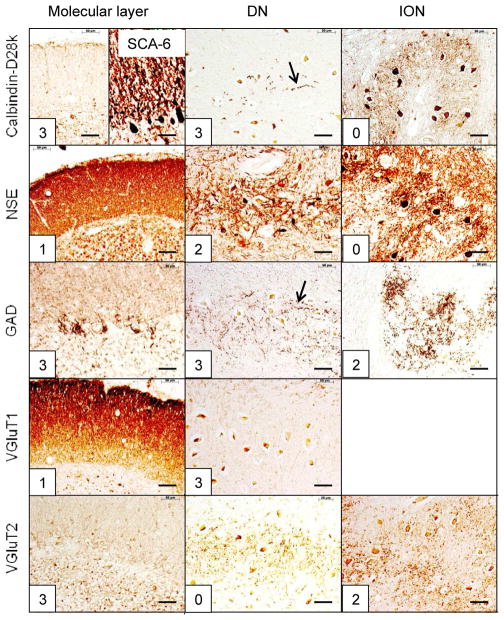
The reciprocal cerebellar circuitry in SCA-6. The CAG trinucleotide repeats in this patient were 26 (expanded) and 12 (normal). The total score in this case is 26, with the DN lesion accounting for 11 or 42.3%. The ION is normal after staining with anti-calbindin-D28k and NSE but shows moderate depletion of GAD and VGluT2 reaction products. An additional peculiarity of this case is the severe degeneration of calbindin-D28k- and GAD-reactive terminals (arrows) and subtotal loss of VGluT1-positive endings in the DN. The right half of the microphotograph identified as calbindin-D28k/molecular layer shows preservation of Purkinje cell bodies and dendrites in the tonsil. Abbreviations as in fig. 1. Bars, 50 μm.
SCA-1 (fig. 2), SCA-2 (fig. 3), SCA-6 (fig. 5), SCA-7 (supplemental fig. 1), SCA-17 (supplemental fig. 2), and SCA of unknown mutation (supplemental fig. 3) reveal lesions of cerebellar cortex, DN, and ION. ION atrophy is most severe in SCA-2 and SCA without known mutation, as evidenced by the wide separation of the remaining neuronal cell bodies displaying the characteristic arbor of ION neurons (figs. 3 and supplemental fig. 3, respectively). In contrast to SCA-1, SCA-2, SCA-6, SCA-7, SCA-17, and SCA with unknown mutation, all immunohistochemical stains in SCA-3 (fig. 4) and FRDA (fig. 6) show preservation of cerebellar cortex and ION. The DN of SCA-3 (fig. 4) and FRDA (fig. 6) show regions of grumose degeneration. While this peculiar modification of synaptic terminals is most prominent in SCA-3 and FRDA, similar smaller clusters also occur in SCA-7 (supplemental fig. 1). SCA-6 (fig. 5) presents several remarkable features. Calbindin-D28k immunohistochemistry shows total loss of reaction product in Purkinje cell bodies and dendrites over most of the cerebellar cortex. The cerebellar tonsil is an exception: Purkinje cells are normal. In the DN, calbindin-D28k- and GAD-positive axons and terminals are severely depleted. The large neurons of the DN are not identifiable (fig. 5). Antibodies to calbindin-D28k and NSE detect no convincing retrograde atrophy of the ION. In contrast, GAD and VGluT2 appear moderately reduced (fig. 5). Another notable discrepancy is the differential loss of VGluT1 and VGluT2 reaction product in the DN that is not unique to SCA-6. It also occurs in SCA-7 (supplemental fig. 1), SCA-17 (supplemental fig. 2), and SCA due to unknown mutation (supplemental fig. 3). The microphotographs of an FRDA carrier (supplemental fig. 4) disclose no abnormalities.
Table 2 presents the range of total scores and disease duration for all SCA and FRDA. Means and standard deviations (S.D.) were calculated for disease duration when three or more representative cases were available. The range of total scores in SCA-3 and FRDA was lower than in SCA-1, SCA-2, SCA-6, SCA-7, SCA-17, and SCA without known mutation, but none of the ataxia scores showed a distinct correlation with disease duration (data not shown). The highest total score, 39, occurred in SCA-2, the lowest, 9, in SCA-3.
Table 2.
Range of total scores and disease duration
| Type of ataxia | Number of cases | Total scores (range) | Disease duration in years (mean±S.D.*) |
|---|---|---|---|
| Dominant ataxias | |||
| SCA-1 | 9 | 16–38 | 18±6 |
| SCA-2 | 6 | 22–39 | 23±8 |
| SCA-3 | 4 | 9–15 | 14±4 |
| SCA-6 | 2 | 13, 25 | 40, 54 |
| SCA-7 | 1 | 16 | 11 |
| SCA-17 | 1 | 31 | 12 |
| SCA, unknown mutation | 2 | 36, 37 | 29, 39 |
| FRDA* | |||
| FRDA | 9 | 11–19 | 27±14 |
Abbreviations: FRDA, Friedreich’s ataxia; SCA, spinocerebellar ataxia; S.D., standard deviation (for means of 3 or more subjects)
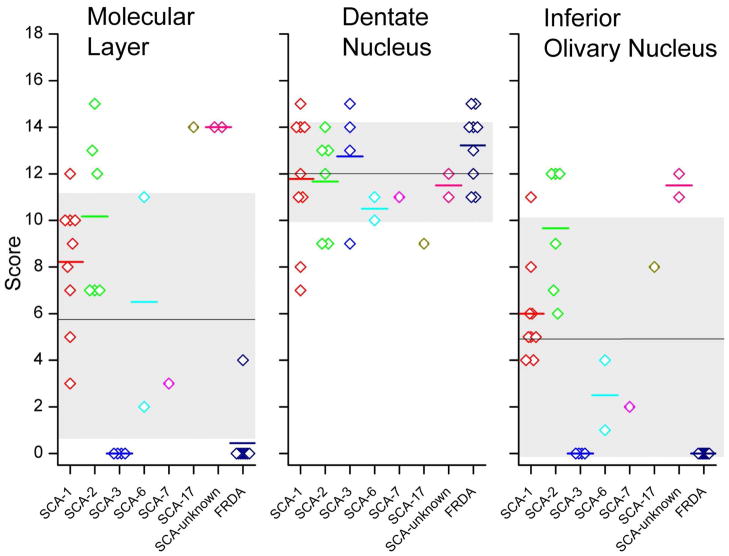
Figure 7 presents a display of scores in all patients and all three anatomical sites across all studied types of hereditary ataxia; and the means ± S.D. of all observations irrespective of ataxia type. Two observations stand out: first, the variability of scores in the molecular layer and ION is much greater than in the DN; and second, the lesion of the DN accounts for nearly all of the total scores in SCA-3 and FRDA. Only one patient with FRDA had a measurable lesion of the molecular layer (fig. 7).
Figure 7.
Individual scores for all patients and all three anatomical sites across all described types of SCA and FRDA. The short horizontal lines represent the means of the observations when 2 or more cases were available for study. The longer horizontal lines are the means of all scores in molecular layer, DN, and ION, irrespective of ataxia type. The gray zones represent the matching standard deviations. Scores are much less variable in the DN than in the molecular layer and ION. One patient with FRDA had Purkinje cell atrophy and received a score of 4 for the molecular layer. With this exception in FRDA, the DN accounts for the entire range of scores in SCA-3 and FRDA. Abbreviations: FRDA, Friedreich’s ataxia; SCA, spinocerebellar ataxia.
When comparing ataxia types, molecular layer scores and ION scores are significantly different among the ataxia types (p=0.001 and p<0.001; Kruskal-Wallis test) whereas the DN scores are not (p=0.369). Correlations were sought between DN, ION and ML scores in individual patients. There was a statistically significant correlation between ION and ML scores (Spearmans’s R=0.91; p<0.001), however, there was no correlation of DN scores to either ION or ML scores (Spearman’s R= −.21; p=.23 for DN and ION; Spearman’s R=−0.20; p=0.26 for DN and ML). Observations were too few and/or displayed too little variation in scores in the individual ataxias to determine whether or not ataxias differ with respect to relationships among the anatomical sites.
Discussion
Limitations and rationale for the use of immunohistochemical markers
Autolysis due to delay in the performance of the autopsy, formalin-fixation, and embedding in paraffin are recognized problems in immunohistochemistry. Not all available cases of SCA and FRDA were considered suitable for the described analysis. When successful, immunohistochemistry surpasses traditional cell and fiber stains [10] because it provides greater insight into the morphology of the lesions, such as atrophy of Purkinje cell dendrites (calbindin-D28k); a sharper distinction between large and small neurons of the DN (NSE, GAD); and information on the type of synaptic transmission (GAD, VGluT1, and VGluT2). The distinction between axosomatic and axodendritic synapses is made possible by GAD immunohistochemistry (fig. 1). GABA-ergic terminals abut large and small neurons of the DN (fig. 1). While large neurons are clearly surrounded by VGluT1- and VGluT2-positive terminals, these stains are not informative about glutamatergic afferents contacting small neurons (fig. 1). The technique also shows a degree of heterogeneity that would not be available with classic cell stains. The relatively recent introduction of VGluT2 immunohistochemistry to the human cerebellar cortex [7] allows an improved understanding of the state of climbing fibers in the hereditary ataxias. The differential involvement of VGluT1- and VGluT2-positive glutamatergic fibers in the DN (SCA-3 [fig. 4], SCA-6 [fig. 5], SCA-7 [supplemental fig. 1], SCA-17 [supplemental fig. 2], and SCA with unknown mutation [supplemental fig. 3]) adds to the complexity of the hereditary ataxias. Stains of VGluT1 and VGluT2 in the DN cannot distinguish climbing or mossy fiber collaterals, and the anatomical origin of the glutamatergic deficit remains uncertain. Immunohistochemical visualization of calbindin-D28k, NSE, GAD, VGluT1, and VGluT2 yields information on the state of “classic” retrograde atrophy of the ION [11] in SCA with cortical cerebellar atrophy (SCA-1, SCA-2, SCA-7, SCA-17, SCA with unknown mutation). Retrograde atrophy is not always commensurate with Purkinje cell atrophy, such as in SCA-6 (fig. 5) and SCA-17 (supplemental fig. 2).
The functional deficit of the reciprocal cerebellar circuitry in hereditary ataxia
The functional state of the reciprocal cerebellar circuitry was assessed by visualization of synaptic terminals. A previous systematic study of synapses in dominant and recessive ataxias revealed significant loss in the DN of patients with SCA-2, SCA-3, and FRDA [12]. The cited work used an antibody to synaptosome-associated protein 25 but provided no conclusions about inhibitory (GABA) or excitatory (glutamate) transmission. From the localization of VGluT1 reaction product, it is evident that excitatory terminals in the molecular layer remain intact in all cases of SCA with Purkinje cell atrophy. While the origin of VGluT1-positive glutamatergic endings from granule cells is certain, their functional role in the absence of target neurons (Purkinje cells) is unknown. Lack of retrograde atrophy may be explained by the persistence of stellate and basket neurons in SCA with otherwise severe involvement of the cerebellar cortex [10]. In contrast, VGluT2-positive climbing fibers disappear in step with dendritic atrophy of Purkinje cells (figs. 2–3, 5; supplemental figs. 2–3). In SCA-3 (fig. 4) and FRDA (fig. 6), the olivocerebellar connections persist, and it may be assumed that Purkinje cells continue to receive excitatory impulses from the ION. Purkinje cell axons provide the overwhelming majority of afferent terminals to the DN and are the likely source of grumose degeneration [7]. These peculiar structures contain synaptophysin [13] and GAD (figs. 3 and 6; supplemental fig. 1), implying a major disturbance of inhibitory transmission at the level of the DN. Grumose degeneration is non-specific but is most likely the consequence of primary atrophy of large DN neurons. It often does not affect the entire DN and is not present in all cases of SCA-3 or FRDA. Therefore, it may be an indication of failing corticonuclear GABA-ergic transmission only early in the course of the disease.
The reduction of glutamatergic terminals in the DN, as manifested by diminished reaction product with antibodies to VGluT1 and VGluT2 implies primary or secondary loss of excitatory input from at least two sources, namely, ION (VGluT2 only) and mossy fibers (VGluT1 and VGluT2) [review in ref 7].
The conclusion that the ION derives most of its GABA-ergic afferents from small neurons in the DN is based on animal experiments [5–6]. Interpreting lesions in the reciprocal cerebellar circuitry in hereditary ataxia may benefit from the pathology of acquired lesions, such as tumors, infarcts, or the plaques of multiple sclerosis that do not spare the small neurons of the DN or their efferent axons. If a destructive lesion interrupts the human dentato-olivary fibers in the hilum of the DN or the central tegmental tract of the pons, the ION reacts with neuronal hypertrophy, vacuolation, and bizarre large-cell gliosis [2–4]. This reaction is transsynaptic and differs greatly from retrograde olivary atrophy. Olivary hypertrophy was not present in any of the cases of SCA or FRDA reported here. This exemption highlights the importance of small neurons of the DN for the survival of ION neurons through trophic properties of GABA-ergic afferents [7]. Intact GABA-ergic dentato-olivary connections, however, do not assure the normal state of the ION if retrograde atrophy of olivocerebellar (climbing) fibers occurs at the same time. It is evident that the integrity of ION requires both intact climbing fiber targets (Purkinje cells) and unimpaired GABA-ergic dentato-olivary input. While VGluT2-positive terminals in the ION are not part of the reciprocal cerebellar circuitry, loss of reaction product is a compelling indicator of olivary atrophy. Evidence presented here suggests that nerve cells of the ION must remain intact to allow survival of afferent glutamatergic terminals. Experiments in cats indicated that the bulk of non-GABA-ergic, presumably excitatory glutamatergic, input to the ION derives from the upper brain stem [5, 14]. In humans, the parvocellular part of the red nucleus is the likely source of glutamatergic olivary afferents that travel in the ipsilateral central tegmental tract [9]. It is peculiar that this glutamatergic input to the ION is VGluT2-specific, similar to climbing fibers (fig. 1). Retrograde atrophy of ION neurons also causes loss of glutamatergic collaterals to the DN and, as expected, electrotonic coupling between atrophic olivary neurons [15]. The illustrations of SCA-6 (fig. 5) and SCA-17 (supplemental fig. 2), respectively, suggest that in certain cases, olivary neurons resist retrograde atrophy, at least to some degree. Holmes and Stewart [11] were aware of the fact that integrity of the cerebellar tonsil in a case of system atrophy was accompanied by integrity of matching ION neurons. Intact Purkinje cells in SCA-6 occur only in a restricted portion of the cerebellar cortex (fig. 5), which does not by itself explain the wider preservation of ION. An alternate explanation must be found. Partial survival of ION neurons after total loss of Purkinje cells is also known from the lurcher mouse that resembles SCA with cortical cerebellar atrophy [16]. If ION neurons are totally dependent on trophic support through climbing fibers, they may derive it from surviving collateral fibers to the DN (SCA-6, fig. 5; SCA-17, supplemental fig. 2).
Immunoreactivity with antibodies to glycine transporter 2 (GlyT2) has been validated as an indicator of glycinergic neurons, axons, and terminals [1, 17–18]. In the DN of FRDA, small GlyT2-positive nerve cells and varicose axons in the DN are few compared to normal, implying that not all small neurons survive (not illustrated). Resistance to FRDA may be an intrinsic property of GABA-ergic neurons but not of glycinergic cells.
The GAA trinucleotide repeat expansion in FRDA patients causes deficiency of frataxin, a small nuclear-genome encoded mitochondrial protein. The study of the reciprocal cerebellar circuit in FRDA carriers was of interest because these subjects have only 30–50% of normal tissue frataxin levels. The literature contains no systematic neuropathological study of FRDA carriers, but the normal microscopic anatomy illustrated in supplemental fig. 4 provides evidence that frataxin deficiency must reach a threshold before causing tissue damage. This threshold is unknown for the CNS, but immunofluorescence of the DN suggests that it is subtotal [13].
How does interruption of the reciprocal cerebellar circuitry generate ataxia?
Assignment of scores as indicators of pathological severity (Table 1) presents many difficulties, especially when the lesion does not occur along a gradient. Total scores (Table 2) are less informative than separating them according to anatomical site. Figure 7 highlights the importance of the DN in the ataxic phenotype. Low scores in the molecular layer and ION seem to be irrelevant for disease duration in SCA-3 and FRDA (Table 2). The central role of the DN in FRDA has only recently received the necessary attention [7].
The observations described in this report do not address the contribution of the basis pontis to the ataxic phenotype. Pontine atrophy is invariably present in SCA-2 and SCA-7, and nerve cell loss in the basis pontis also occurs in some cases of SCA-1 and SCA-3. While mossy fibers of pontine origin are technically not part of the reciprocal cerebellar circuitry, they occur in a spatial relationship to climbing fibers and share Purkinje cell targets through the granule cell-parallel fiber system in animals with zonal organization of the cerebellar cortex [19]. Some cases of hereditary ataxia are “granuloprival”, especially after long disease duration. Irrespective of type, long-standing SCA may show thinning of the granular layer and paucity of granule cells. The mechanism behind granular layer atrophy is unclear though transsynaptic atrophy may be the underlying cause in SCA with prominent loss of pontine neurons.
The described clinicoanatomic correlation does not consider that patients with hereditary ataxia often have proprioceptive and afferent spinocerebellar deficits due to lesions of dorsal root ganglia. FRDA and SCA-3 are examples. They share loss of neurons in Clarke’s column and fiber degeneration in the dorsal spinal columns (review in ref [10]).
The DN is the central component of the reciprocal cerebellar circuitry, and its function may be disabled by intrinsic disease of its neurons, atrophy of inhibitory corticonuclear fibers, or loss of excitatory connections from mossy and climbing fiber collaterals. The DN can no longer be regarded solely as the executor of Purkinje cell activity [17]. Glutamatergic and non-glutamatergic neurons in the DN fire spontaneously in vivo and in vitro despite large inhibitory input from the cerebellar cortex (review in [20]). Though the emerging neuronal complexity of the DN is based on animal experiments, a similar array of GABA-ergic, glutamatergic, and glycinergic neurons is also likely for the human cerebellum. The observations presented here identify loss of large DN neurons as a shared phenomenon in SCA and FRDA. The available special stains also identify an unexpected involvement of the DN in SCA-6 though this disease was previously thought to be “pure cortical cerebellar atrophy” or “cerebello-olivary atrophy” [10]. Only 2 cases of SCA-6 were available for the examination of the reciprocal cerebellar circuitry, and figure 5 may not fully represent this type of ataxia. It is of interest that the scores of the DN in SCA-1 and SCA-2 do not correlate with the scores of either the molecular layer or the ION, which contrasts with the correlation of molecular layer and ION.
The importance of the reciprocal cerebellar circuitry in the ataxic phenotype should bring about improved imaging of the cerebellum in the care of patients with hereditary ataxia. Due to its high iron content and consequent signal hypointensity, size and shape of the DN can be determined by T2-weighted MRI. Resolution has been further improved by susceptibility-weighted imaging [21]. While diffusion tensor imaging and diffusion spectrum imaging (DSI) are not routine MRI techniques, DSI can visualize all components of the reciprocal cerebellar circuitry [22]. Physicians undertaking clinical trials in hereditary ataxia should consider these complex connections and the central role of the DN, and students of animal models may wish to extend their work from Purkinje cells to cerebellar nuclei and ION.
Supplementary Material
Supplemental figure 1. Reciprocal cerebellar circuitry in SCA-7. The CAG trinucleotide repeats in this patient were 40 (expanded) and 10 (normal). The total score is 16, with the DN lesion accounting for 11 (68.8%). The DN shows loss of NSE, GAD, and VGluT1 reaction product. Grumose degeneration is present after staining with anti-GAD (arrow). Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 2. Reciprocal cerebellar circuitry in SCA-17. The CAG trinucleotide repeats in this patient were 55 (expanded) and (38 normal). The total score is 31, with the DN lesion accounting for 9 (29%). Thinning of the molecular layer (all stains) and lack of large neurons in the DN are prominent. Despite severe loss of Purkinje cells (calbindin-D28k/molecular layer), degeneration of the ION is moderate. The globular VGluT2 reaction product in the molecular layer is an artifact. Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 3. Reciprocal cerebellar circuitry in SCA due to an unknown mutation. The total score is 37, with the DN lesion accounting for 12 (32.4%). The lesion in the DN is manifest by loss of calbindin-D28k-, GAD-, and VGluT1-, and VGluT2-reactive terminals. Small neurons in the DN survive (NSE and GAD, arrows). The severe abnormalities in the molecular layer and ION are similar to those in SCA-2 (text fig. 3). Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 4. Reciprocal cerebellar circuitry in an FRDA carrier. The GAA trinucleotide repeats in this patient were 845 (expanded) and 30 (normal). All antibodies yield normal immunohistochemical reaction products. Abbreviations as in text fig. 1. Bars: 50 μm
Acknowledgments
The authors received financial support from the National Ataxia Foundation, Minneapolis, MN, USA; Friedreich’s Ataxia Research Alliance, Downingtown, PA, USA; Neurochemical Research, Inc., Glenmont, NY, USA; National Institutes of Health, Bethesda, MD, USA (Grant No. R01-NS069454); and University of Tübingen, Tübingen, Germany. This work was performed in the research laboratories of the Veterans Affairs Medical Center, Albany, NY, USA. Dr. Rodney D. McComb, Omaha, NE, USA, contributed one case of FRDA. Sarah Collins provided expert technical assistance in immunohistochemistry.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Uusisaari M, Knöpfel T. GlyT2+ neurons in the lateral cerebellar nucleus. Cerebellum. 2010;9:42–55. doi: 10.1007/s12311-009-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schaffer K. Gibt es eine cerebello-olivare Bahn? Zeitsch Ges Neurol Psychiat. 1915;30:70–83. [Google Scholar]
- 3.Guillain G, Mollaret P. Deux cas de myoclonies synchrones et rythmées vélo-pharyngo-laryngo-oculo-diaphragmatiques: Le problème anatomique et physiologique. Rev Neurol. 1931;2:545–566. [Google Scholar]
- 4.Lapresle J, Ben Hamida M. The dentato-olivary pathway. Somatotopic relationship between the dentate nucleus and the contralateral olive. Arch Neurol. 1970;22:135–143. doi: 10.1001/archneur.1970.00480200041004. [DOI] [PubMed] [Google Scholar]
- 5.De Zeeuw CI, Holstege JC, Ruigrok TJH, Voogd J. Ultrastructural study of the GABAergic, cerebellar, and mesodiencephalic innervation of the cat medial accessory olive: Anterograde tracing combined with immunocytochemistry. J Comp Neurol. 1989;284:12–35. doi: 10.1002/cne.902840103. [DOI] [PubMed] [Google Scholar]
- 6.Fredette BJ, Mugnaini E. The GABAergic cerebello-olivary projection in the rat. Anat Embryol. 1991;184:225–243. doi: 10.1007/BF01673258. [DOI] [PubMed] [Google Scholar]
- 7.Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich’s ataxia. Acta Neuropathol. 2011;122:323–330. doi: 10.1007/s00401-011-0844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruigrok TJH. Ins and outs of cerebellar modules. Cerebellum. 2011;10:464–474. doi: 10.1007/s12311-010-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Voogd J. Cerebellum and precerebellar nuclei. In: Paxinos G, Mai JK, editors. The human nervous system. 2. Elsevier Academic Press; Amsterdam: 2004. pp. 321–392. [Google Scholar]
- 10.Koeppen AH. Neuropathology of the inherited ataxias. In: Manto MU, Pandolfo M, editors. The cerebellum and its disorders. Cambridge University Press; Cambridge: 2002. pp. 387–405. [Google Scholar]
- 11.Holmes G, Stewart T. On the connection of the inferior olives with the cerebellum in man. Brain. 1908;31:125–137. [Google Scholar]
- 12.Koeppen AH, Dickson AC, Lamarche JB, Robitaille Y. Synapses in the hereditary ataxias. J Neuropathol Exp Neurol. 1999;58:748–764. doi: 10.1097/00005072-199907000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Koeppen AH. Friedreich’s ataxia: Pathology, pathogenesis, and molecular genetics. J Neurol Sci. 2011;303:1–12. doi: 10.1016/j.jns.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Zeeuw CI, Simpson JI, Hoogenraad C, Galjart N, Koekkoek SKE, Ruigrok TJH. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- 15.Llinás R, Baker R, Sotelo C. Electrotonic coupling between neurons in cat inferior olive. J Neurophysiol. 1974;37:560–571. doi: 10.1152/jn.1974.37.3.560. [DOI] [PubMed] [Google Scholar]
- 16.Heckroth JA, Eisenman LM. Olivary morphology and olivocerebellar topography in adult lurcher mutant mice. J Comp Neurol. 1991;312:641–651. doi: 10.1002/cne.903120413. [DOI] [PubMed] [Google Scholar]
- 17.Uusisaari M, Knöpfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2011;10:637–646. doi: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zafra F, Aragón C, Olivares L, Danbolt NC, Giménez C, Storm-Mathisen J. Glycine transporters are differentially expressed among CNS cells. J Neurosci. 1995;15:3952–3969. doi: 10.1523/JNEUROSCI.15-05-03952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pijpers A, Apps A, Pardoe J, Voogd J, Ruigrok TJH. Precise spatial relationship between mossy fibers and climbing fibers in rat cerebellar cortical zones. J Neurosci. 2006;26:12067–12080. doi: 10.1523/JNEUROSCI.2905-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uusisaari M, Obata K, Knöpfel T. Morphological and electrophysiological properties of GABAergic and non-GABAergic cells in the deep cerebellar nuclei. J Neurophysiol. 2007;97:901–911. doi: 10.1152/jn.00974.2006. [DOI] [PubMed] [Google Scholar]
- 21.Küper M, Thürling M, Maderwald S, Ladd ME, Timmann D. Structural and functional magnetic resonance imaging of the human cerebellar nuclei. Cerebellum. 2012;11:314–324. doi: 10.1007/s12311-010-0194-5. [DOI] [PubMed] [Google Scholar]
- 22.Granziera C, Schmahmann JD, Hadjikhani N, Meyer H, Meuli R, Wedeen V, Krueger G. Diffusion spectrum imaging shows the structural basis of functional cerebellar circuits in the human cerebellum in vivo. PLoS One. 2009;4:e5101, 1–6. doi: 10.1371/journal.pone.0005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Reciprocal cerebellar circuitry in SCA-7. The CAG trinucleotide repeats in this patient were 40 (expanded) and 10 (normal). The total score is 16, with the DN lesion accounting for 11 (68.8%). The DN shows loss of NSE, GAD, and VGluT1 reaction product. Grumose degeneration is present after staining with anti-GAD (arrow). Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 2. Reciprocal cerebellar circuitry in SCA-17. The CAG trinucleotide repeats in this patient were 55 (expanded) and (38 normal). The total score is 31, with the DN lesion accounting for 9 (29%). Thinning of the molecular layer (all stains) and lack of large neurons in the DN are prominent. Despite severe loss of Purkinje cells (calbindin-D28k/molecular layer), degeneration of the ION is moderate. The globular VGluT2 reaction product in the molecular layer is an artifact. Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 3. Reciprocal cerebellar circuitry in SCA due to an unknown mutation. The total score is 37, with the DN lesion accounting for 12 (32.4%). The lesion in the DN is manifest by loss of calbindin-D28k-, GAD-, and VGluT1-, and VGluT2-reactive terminals. Small neurons in the DN survive (NSE and GAD, arrows). The severe abnormalities in the molecular layer and ION are similar to those in SCA-2 (text fig. 3). Abbreviations as in text fig. 1. Bars: 50 μm
Supplemental figure 4. Reciprocal cerebellar circuitry in an FRDA carrier. The GAA trinucleotide repeats in this patient were 845 (expanded) and 30 (normal). All antibodies yield normal immunohistochemical reaction products. Abbreviations as in text fig. 1. Bars: 50 μm