Abstract
Intraoral somatosensory sensitivity in patients with atypical odontalgia (AO) has not been investigated systematically according to the most recent guidelines. The aims of this study were to: 1. Examine intraoral somatosensory disturbances in AO patients using healthy subjects as reference and 2. Evaluate the percent agreement between intraoral quantitative sensory testing (QST) and qualitative sensory testing (QualST). Forty-seven AO patients and 69 healthy controls were included at Universities of Washington, Malmö and Aarhus. In AO patients, intraoral somatosensory testing was performed on the painful site, the corresponding contralateral site and at thenar. In healthy subjects, intraoral somatosensory testing was performed bilaterally on the upper premolar gingiva and at thenar. Thirteen QST and 3 QualST parameters were evaluated at each site, z-scores were computed for AO patients based on the healthy reference material and LossGain scores were created. 87.3% of AO patients had QST abnormalities compared with controls. The most frequent somatosensory abnormalities in AO patients were somatosensory gain with regard to painful mechanical and cold stimuli and somatosensory loss with regard to cold detection and mechanical detection. The most frequent LossGain code was L0G2 (no somatosensory loss with gain of mechanical somatosensory function)(31.9% of AO patients). Percent agreement between corresponding QST and QualST measures of thermal and mechanical sensitivity ranged between 55.6 and 70.4% in AO patients and between 71.1 and 92.1% in controls. In conclusion, intraoral somatosensory abnormalities were commonly detected in AO patients and agreement between quantitative and qualitative sensory testing was good to excellent.
Keywords: Atypical odontalgia, quantitative sensory testing (QST), neuropathic pain, orofacial pain, somatosensory sensitivity
1. Introduction
Atypical odontalgia (AO) is an enigmatic chronic orofacial pain condition with no objective signs of pathology [2,4,13,14,18,25,26]. AO has also been termed phantom tooth pain [17], persistent dentoalveolar pain (PDAP) [19], peripheral painful traumatic trigeminal neuropathy [8] and persistent idiopathic orofacial pain (PIOP) [1]. It is generally agreed that AO is not a suitable term, since it reveals nothing about the pain mechanisms. The most prevailing hypothesis about AO pain mechanisms is that it is a neuropathic pain condition [2,13,18,27]. However, it is difficult to do confirmatory tests of nerve pathology or damage intraorally. According to recent guidelines, both demonstration of somatosensory abnormalities and other confirmatory tests, such as electrophysiological tests or special neuro-imaging techniques, are required for a definite diagnosis of neuropathic pain [8,24]. The level of certainty of the pain being neuropathic is only “possible” or “probable” without such confirmatory tests.
Somatosensory sensitivity can be measured with quantitative sensory testing (QST) [10–12,16,20–24]. Fortunately, the latest years have provided much progress with regards to standardization of QST, starting with the formation of the German Research Network on Neuropathic Pain (DFNS) and the publication of a standardized QST protocol for examination and data analysis [16,21]. The German Network introduced the somatosensory profiles and LossGain scores based on z-scores computed using the means and standard deviations of a healthy reference material [12,16]. Basically, the LossGain scores allows condensation of somatosensory findings for all 13 QST parameters into one single score [12,16]. Recently, adaptations to this protocol for intraoral use was published and evaluated with regard to reliability in healthy subjects [20] and guidelines for intraoral somatosensory examination was published by a task-force group formed by the Special Interest Group for Orofacial Pain under the International Association for the Study of Pain (IASP) [22]. In earlier studies not using the standardized German protocol, we and others have shown somatosensory disturbances in the majority of AO cases [5,14,27]. However, no common pattern of somatosensory disturbances could be detected, which is in accordance with what the German Network found in, for example, postherpetic neuralgia and other neuropathic pain conditions [16]. So far, no studies have assessed intraoral somatosensory sensitivity in AO patients using the full standardized 13 parameter QST protocol.
Recently, we have also published results on reliability of simple chair-side qualitative somatosensory testing (QualST) and comparison of these simple tests between AO patients and healthy controls [7]. QualST has been used for many years in clinical settings and may serve as an initial screening of patients with persistent orofacial pain. However, to the best of our knowledge, no studies have investigated the agreement between standardized QST and QualST for any test site.
The aims of this multicenter study were to: 1. Examine intraoral somatosensory disturbances in AO patients using a healthy age- and gender-matched control group as reference material according to the most recent standardized protocol for intraoral QST and 2. Evaluate the level of agreement between intraoral QST and QualST in AO patients as well as healthy controls.
2. Methods
This investigation was a multicenter study involving Universities of Washington (USA), Malmö (Sweden) and Aarhus (Denmark). The study was performed in accordance with the Helsinki Declaration and written informed consent was obtained from all participants. The study protocol was approved by the ethics committees of all participating centers. The hypotheses of this study were that: 1. AO patients show somatosensory abnormalities in comparison with a healthy age- and gender-matched reference material and 2. Percent agreement between corresponding QST and QualST measures of thermal and/ or mechanical sensitivity is fair.
2.1 Participants
Forty-seven patients with AO(40 women, 7 men, mean age 55.2 ± 2.0 years) were included at Malmö University (Sweden), University of Washington (USA) and Aarhus University (Denmark). Sixty-nine age- and sex-matched healthy adult (> 18 years) controls (53 women, 16 men, mean age 51.8 ± 1.3 years) were included as a reference group. The healthy subjects were recruited through advertisements at the Universities and in Aarhus also through the webpage www.forsoegsperson.dk. Inclusion criteria for AO patients were: > 18 years old, pain for more than 6 months in a tooth or persistent pain after tooth extraction with no signs of pathology in clinical or radiographic examinations [2,3,6,13,14,18,25]. The AO pain should be non-paroxysmal and present during most of the day [25]. Exclusion criteria for AO patients were: presence of other known orofacial pain conditions, such as odontogenic pain, trigeminal neuralgia, cluster headache etc. Patients with temporomandibular disorders (TMD) were not excluded as long as the patient could clearly distinguish between the two pain conditions [7] and also that the AO pain was not influenced by palpation of masticatory muscles or the temporomandibular joints (TMJs) or by movement of the jaws. The reason that AO patients with co-morbid TMD were not excluded was that a large proportion of AO patients do in fact fulfill the Research Diagnostic Criteria for TMD [4] and therefore we chose the approach described above in order to be able to analyze a representative sample of a sufficient size. The examiners including patients in the study were all experienced trained orofacial pain clinicians and researchers. All included patients had been through a thorough clinical intra- and extra-oral examination with intraoral radiographs. In case of unclear diagnosis from standard intraoral radiographs, other imaging techniques were used (Cone-beam Computed Tomography (CBCT) and/ or magnetic resonance imaging (MRI)). Exclusion criteria for the healthy subjects were: orofacial pain, serious dental, medical, psychiatric or personality disorders [7]. Slight to moderate levels of depression were allowed, since such psychological co-morbidity is very common among AO patients [4].
The AO subjects included in the study were characterized according to present pain intensity on a 0–10 numerical rating scale (NRS), duration of the AO pain in months, depression and unspecific physical symptoms scores from the SCL-90 taken from the Axis II questionnaire of the RDC/TMD) [9].
2.2. Intraoral quantitative sensory testing
All investigators were carefully instructed and trained for at least one day with regard to performance of intraoral QST according to the latest guidelines[20–22] and practiced in healthy subjects[16]. In AO patients, intraoral QST was performed on the painful (or most painful) intraoral buccal gingival site, the corresponding contralateral “mirror-image” site and, as an extra-trigeminal control, at thenar on the right hand. Importantly, the painful site and thereby the test site of the AO patients could be situated both in the upper and lower jaw and at the level of incisors, premolars or molars. The QST data from contralateral “mirror-image” site was used to compute the side-to-side difference for evaluation of so-called “relative sensory abnormalities” (Please refer to section 2.4.2. for further information). In healthy subjects, intraoral QST was performed bilaterally on the attached gingiva buccal to the first premolar and in 33 healthy subjects also at thenar of the right hand. The full QST examination was performed three times, twice on the first day and once more on a separate occasion 1–2 weeks after the first session. Intra- and inter-examiner reliability was tested and will be reported in a separate manuscript. The mean value from each subject for each QST variable from these three examinations was used for the present analyses.
The standardized assessment of small and large fiber function involved 13 thermal and mechanical tests [16,20–22]: cold detection threshold (CDT), warmth detection threshold (WDT), thermal sensory limen (TSL), paradoxical heat sensation (PHS), cold pain threshold (CPT), heat pain threshold (HPT), mechanical detection threshold (MDT), mechanical pain threshold (MPT), mechanical pain sensitivity (MPS), dynamic mechanical allodynia (DMA), windup ratio (WUR), vibration detection threshold (VDT), and pressure pain threshold (PPT). For all parameters, loss of somatosensory function as well as gain of somatosensory function was assessed [16]. Due to the current lack of multicenter reference data for all intraoral regions, we included an age- and sex-matched reference group tested in the upper premolar region (please see above). Intraoral somatosensory sensitivity may vary slightly between different intraoral locations (upper jaw, lower jaw, incisor region, premolar region, molar region). However, as the German Research Network on Neuropathic Pain (DFNS) have used hand data as representative for upper body and foot data for the lower body with a few exceptions, the buccal gingival aspect of the upper first premolars was used in the present study as representative of all buccal gingival regions of the oral cavity [16].
2.2.1. Thermal thresholds and thermal sensory limen
Thermal testing was performed with Medoc Pathway, Medoc, Israel (University of Washington (UW)), Medoc TSA-II, Medoc, Israel (Aarhus University (AU)) and MSA Thermotest, SOMEDIC, Sweden (Malmö University (MU))[12,20–22]. At each center, two thermodes of different size were used for the assessments; a standard cutaneous thermode for extraoral measurements (SOMEDIC: 20 mm × 20 mm and Medoc: 30 mm × 30 mm, both square surface) and an intraoral thermode (SOMEDIC: 9 mm × 9 mm square surface; Medoc: 6 mm diameter round surface). CDT and WDT were measured first, followed by TSL with alternating cold and warm stimuli. For the TSL, the subject pressed a button when the ramped stimulus reached a temperature where the subject first perceived warmth. The temperature ramp then changed direction and the thermode cooled down and was again reversed when the subject perceived a temperature change and pressed the button. The number of paradoxical heat sensations (PHS) during this procedure was recorded. CPT and HPT were then determined. For all thermal testing, ramped stimuli of 1°C/s were used, and the procedure ended when the subject pressed a button. During testing, the subject could not observe the computer screen. Starting temperatures were 32°C on skin and 32°C (UW) or 37°C (MU, AU) on oral mucosa. Cutoffs temperatures were set at 0–50 °C (UW, AU) and 10–50 °C (MU; capacity limit for the SOMEDIC probe) for both intraoral and extraoral stimulation. Interstimulus interval was 4–6 seconds. For all thresholds, the mean of three threshold temperature measurements were calculated. If the thermode slipped during the examination and provoked a pain sensation, the registration was repeated.
2.2.2. Mechanical detection threshold
Mechanical detection thresholds(MDT) were measured using a standardized set of modified vonFrey filaments (OptiHair2, MARSTOCKnervtest, Marburg, Germany) [12,16,20,22]. The OptiHair2 set contains 12 monofilaments, exerting different forces upon bending; each monofilament increasing the force by a factor of 2, ranging from 0.25 mN to 512mN. To prevent filament slippage, intraoral examination sites were dried with gauze before testing. Care was also taken that the instrument did not accidentally touch other intraoral tissues (lip, tooth). The monofilament was applied perpendicularly to the examination site. Contact time was 1–2 s. Five threshold measurements were made, each through applying a series of ascending and descending stimulus intensities. By calculating the geometric mean of these five series, one threshold value was determined.
2.2.3. Mechanical pain threshold, mechanical pain sensitivity for pinprick stimuli, dynamic mechanical allodynia and wind-up ratio for repetitive pinprick stimuli
Weighted pinprick stimuli delivered with custom-made (Different sets manufactured at Johannes Gutenberg University of Mainz, Mainz, Germany: The Pin Prick; at Aarhus University, Aarhus, Denmark; at University of Washington, Seattle, USA) sets of seven stimulators were used to determine the mechanical pain threshold (MPT)[12,16,20–22]. Each stimulator had a flat contact surface of ø0.2 mm. The stimulators exerted forces ranging from8 to 512 mN. Contact time was ~2 s. All pinprick tests were made with the stimulator in a vertical position and perpendicular to the examination site. The “method of limits”, which was used to determine the MDT, was also used to determine the MPT.
Mechanical pain sensitivity (MPS) and dynamic mechanical allodynia (DMA) was evaluated using two sets of instruments in a stimulus–response assessment [12,16,20–22]. To determine MPS, seven weighted pinprick stimulators (as for MPT) were used. Three tactile stimulators were used to determine DMA: a cotton wisp, a cotton wool tip (Q-tip) attached to a flexible handle and a disposable toothbrush (Top Dent®, Meda AB, Solna, Sweden). The tactile stimulator was applied in a single stroke over about 1–2 cm in length of skin/mucosa. A series of 10 measurements was made five times, each with the 10 stimulators (seven pinprick and three tactile stimulators) applied in a different order, as specified in the DFNS protocol [20,21]. For each of the resulting 50 stimuli, the subject rated the pain on a 0 to100 numerical scale with the endpoints ‘0’ indicating “no pain” and ‘100’ indicating “most intense pain imaginable”. MPS was calculated as the geometric mean of all numerical ratings for pinprick stimuli. DMA was calculated as the geometric mean of all numerical ratings for tactile stimuli.
To measure the wind-up ratio (WUR) for repetitive pinprick stimuli, the perceived magnitude on a 0–100 numerical rating scale of a train of 10 pinprick stimuli repeated at a rate of 1Hz and kept constant using a metronome (MA-30 Digital metronome, KORG®, Tokyo, Japan) was divided by that of a single pinprick stimulus applied with the same force [12,16,20–22]. The custom-made pinprick stimulators used in the MPT determinations were used for WUR assessment. An instrument that delivered a force, which the subject perceived as “slightly painful”, was selected and the 128-mN stimulator was tried first. If the response was 0 (not painful), the test was repeated using a greater force. If the subject perceived the stimulus as intolerable, less force was used. If a subject did not perceive the 512-mN stimulator to be painful, the test was abandoned. The WUR test was repeated three times and the mean WUR was used for analysis.
2.2.4. Vibration detection threshold
The vibration detection threshold (VDT) was measured using a Rydel–Seiffer graded tuning fork (64 Hz, 8/8 scale) [12,16,20–22]. At the gingival sites, the fork was set in motion and placed over a bony prominence (maxilla or mandible). The subject indicated when the vibration could no longer be sensed. On the 9-point (0–8) scale measuring intensity of vibration, values were recorded to an accuracy of 0.5 units. The mean of three trials was calculated.
2.2.5. Pressure pain threshold
The pressure pain threshold (PPT) was measured with the use of an electronic pressure algometer (SOMEDIC Algometer®, SOMEDIC Sales AB, Sweden) with two probes with different surface area sizes : 1 cm2 was used at thenar and 0.18 cm2(ø 4.8 mm) at intraoral gingival sites [20,22]. During the test, pressure was increased at a rate of 50 kPa/s. At the first sensation of pain, the subjects pressed a button to interrupt stimulation, thereby indicating the pressure pain threshold. The PPT was determined as the mean of three recordings.
2.3. Agreement of intraoral qualitative sensory testing with QST
A subgroup of 65 participants (27 AO patients and 38 from reference group) were also examined with qualitative sensory tests (QualST) [7] with the purpose of evaluating the agreement between intraoral QST and QualST. The reliability and comparison between groups with regard to QualST has been published separately [7]. Sensitivity to touch, cold, and pinprick stimuli was evaluated on the same site as for the QST. The stimuli were always applied to the non-painful side first followed by the painful side and always in the same order: 1. touch, 2. cold, 3. pinprick pain. No qualitative tests of warmth detection, thermal pain, wind-up, pressure pain or vibration were applied. The touch stimulus was applied with a Q-tip in a single stroke over 1–2 cm of oral mucosa [7]. The cold stimulus was applied with a stainless steel dental spatula (kept cool in ice water, ~ 0 °C) for 1–2 s [7]. The pinprick stimulus was applied with a dental examination probe with moderate force on the gingiva for 1–2 s. Patients were asked to report hyper-, hypo-, or normosensitivity or -algesia to touch, cold and painful stimuli on the painful site compared with the non-painful contralateral site. In the reference group, the tests were performed bilaterally on the buccal gingiva adjacent to the canines and first maxillary premolars, right side before left side [7]. They were asked to compare sensitivity between sides. If sides were not perceived to be equally sensitive, they were asked to report any difference (hyper- or hyposensitivity) on the left side using the right side as the reference [7].
2.4. Data analysis and statistics
All absolute QST scores are presented as means +/− standard deviation (SD). Due to the different thermal devices and thermode sizes applied at the different centers, the possible differences in thermal thresholds in healthy subjects between centers were tested with unpaired t-tests. The thermal thresholds of the healthy controls were not statistically significantly different between centers (P > 0.369).
2.4.1. Z-transformation of QST data
Cold and heat pain thresholds as well as vibration detection thresholds were normally distributed. All other parameters were normally distributed in log-space and were log-transformed before analysis [16,21]. A z-transformation of each variable was performed based on the reference group data with adjustment of sign in such a way that positive z-scores indicated gain of somatosensory function (hyperesthesia, hyperalgesia, allodynia) and negative z-scores indicated loss of function (hypoesthesia, hypoalgesia) [16,21]. The individual pain site z-scores were calculated as (meanreference group – individual value)/SDreference group [15,21]. Values of z above 1.96 and below −1.96 indicate values outside of the 95% confidence interval (CI) of the reference group data. Such values were considered as absolute abnormalities [12,16]. Also, the side-to-side differences of each intraoral QST parameter were compared with the 95% CI of the side-to-side differences of the reference group [16]. If the side-to side differences were larger than the upper limit of the 95% CI of the reference group, the value was considered a relative abnormality [16]. In accordance with Maier et al. (2010), the assessment of frequencies of loss and gain of somatosensory function include a combination of absolute and relative (side-to-side) abnormalities (Please see below).
2.4.2. Assessment of somatosensory loss and gain of function
The LossGain coding system was applied [12,16]. As mentioned above, this system combines absolute and relative abnormalities into one single sensitivity measure per patient. The LossGain score combines a score of somatosensory loss of function (L0, L1, L2, or L3) with a score of somatosensory gain of function (G0, G1, G2, or G3) [11,14]. The number after the ‘L’ or ‘G’ indicates whether the somatosensory abnormality is related to the thermal modalities alone (1), mechanical modalities alone (2) or mixed (3) (thermal and mechanical). If measures of thermal and/ or mechanical detection (CDT, WDT, MDT or VDT) were abnormal on the affected side in comparison with the reference data (absolute abnormality) or if abnormally large side-to-side differences were detected (relative abnormality), it was recorded as one of the following: L1—isolated loss of small fiber function (if abnormal thermal detection thresholds (CDT or WDT) alone); L2—isolated loss of large fiber function (if abnormal mechanical detection thresholds (MDT or VDT) alone), or L3—mixed loss of function (if loss of both small and large fiber function) [12,16]. Likewise for somatosensory gain, thermal hyperalgesia (G1) was recorded, if gain of function in cold or heat pain thresholds (CPT or HPT) were found (absolute or relative abnormality). Mechanical hyperalgesia (G2) was recorded, if gain of function (absolute or relative abnormality) was detected for mechanical pain threshold (MPT), mechanical pain sensitivity (MPS), pressure pain threshold (PPT) or if the dynamic mechanical allodynia (DMA) score exceeded 0. Mixed gain (G3) was recorded in individuals with gain of both thermal and mechanical somatosensory function. L0 was scored if no loss of somatosensory function was present and G0 if no gain of somatosensory function was detected.
2.4.3. Statistics
An unpaired t-test was used to compare age between groups. The gender distribution of the two groups was compared with a χ2-test. Distribution of frequencies of loss and gain of somatosensory function at the painful site according to the LossGain coding was evaluated with χ2-tests with Bonferroni adjustments for multiple comparisons. The occurrence of paradoxical heat sensations (PHS) during the TSL procedure, the measure of dynamic mechanical allodynia (DMA) and all 13 parameters measured at the thenar site were compared between groups with unpaired t-tests.
The agreement between QualST (3 modalities) and QST (LossGain codes) was tested for each group (AO and healthy controls) and modality separately. The agreement was calculated as the proportion of the group where hyposensitivity to touch in QualST was in agreement with a L2 or L3 score (both including tactile loss). Similar proportions were computed for the proportions of the groups showing agreement between hypersensitivity to touch (QualST) and G2 or G3 score; hyposensitivity to cold (QualST) and L1 or L3 score; hypersensitivity to cold (QualST) and G1 or G3 score; hypersensitivity to pinprick (QualST) and G2 or G3 score. No statistical tests were performed on these proportions.
Values of P less than 0.05 were considered statistically significant.
3. Results
3.1. Patients
The age- and sex-distribution did not differ significantly between groups (age: P = 0.144; gender: P = 0.288). The average present AO pain intensity on a 0–10 NRS was 2.9 ± 0.4. The range of AO pain duration was 18–240 months. The mean (± SEM) depression score from the SCL-90 in the AO patients was 0.81 ± 0.11 and the mean score of unspecific physical symptoms in AO patients was 0.88 ± 0.10.
3.2. Absolute abnormalities of QST z-scores and side-to-side differences
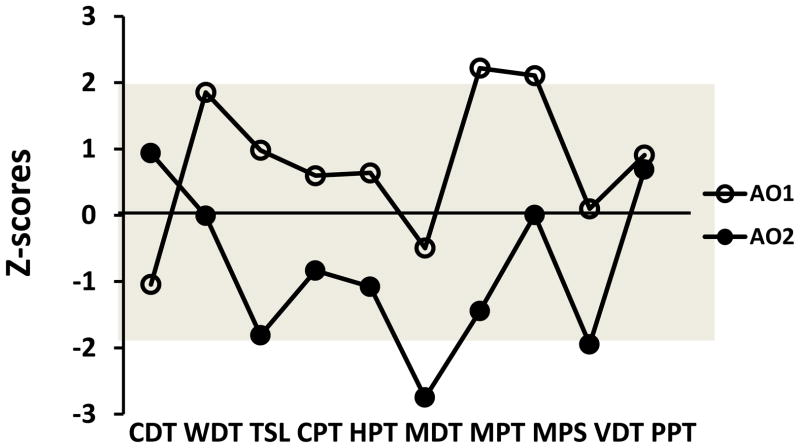
The frequencies of absolute abnormalities of QST z-scores (outside 95% CI of reference data) for both groups for each QST parameter are shown in Table 1a. The most frequent somatosensory absolute abnormalities found in the AO group (painful site) were (in order of frequency): somatosensory gain with regard to MPT, CPT, MPS and PPT; somatosensory loss with regard to CDT and MDT. Fig. 1 shows two examples of so-called somatosensory profiles based on the z-scores. As expected due to natural variation, a few abnormalities (values outside 95% CI) were found in the reference group (mean across parameters for somatosensory loss (1.0 ± 1.4%) and for somatosensory gain (2.5±2.1%)) (Table 1) [16]. In Table 1b, the absolute values of the side-to-side differences of the intraoral measurements in AO patient and the healthy reference group are displayed.
Table 1a.
Mean and standard deviation of the intraoral quantitative sensory testing (QST) parameters from the attached gingiva buccal to the first premolar before and after z-transformation in the age- and sex-matched reference group and from the painful intraoral site in patients with atypical odontalgia (AO). The table also shows the frequency of patients and reference group participants presenting with z-score values outside the reference 95% confidence interval (−1.96 < z-score < 1.96)
| Reference data | AO patient data | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Abs Mean (SD) | z-scores Mean (SD) | < −1.96a (%) | > 1.96a (%) | Abs Mean (SD) | z-scores Mean (SD) | < −1.96a (%) | > 1.96a (%) | |
| CDT | 16.4 (7.9) | 0.00 (1.00) | 2.9 | 0.0 | 12.7 (6.9) | −0.63 (1.30) | 8.5 | 0.0 |
| WDT | 12.0 (2.8) | 0.00 (1.00) | 0.0 | 0.0 | 11.8 (3.4) | 0.59 (0.74) | 0.0 | 4.3 |
| TSL | 25.2 (9.3) | 0.00 (1.00) | 1.4 | 1.4 | 26.6 (9.9) | −0.10 (1.16) | 2.1 | 6.4 |
| CPT | 10.2 (6.1) | 0.00 (1.00) | 0.0 | 2.9 | 13.1 (8.5) | 0.49 (1.40) | 0.0 | 14.9 |
| HPT | 47.6 (2.3) | 0.00 (1.00) | 0.0 | 2.9 | 47.5 (2.4) | 0.05 (1.04) | 0.0 | 4.3 |
| MDT | 25.2 (25.6) | 0.00 (1.00) | 0.0 | 5.8 | 47.3 (64.8) | −0.29 (1.19) | 6.4 | 0.0 |
| MPT | 197.1 (145.4) | 0.00 (1.00) | 0.0 | 5.8 | 156.6 (173.7) | 0.80 (1,54) | 0.0 | 27.7 |
| MPS | 1.3 (2.9) | 0.00 (1.00) | 1.4 | 2.9 | 3.1 (5.9) | 0.47 (1.10) | 2.1 | 8.5 |
| WUR | 6.5 (11.7) | 0.00 (1.01) | 0.0 | 2.9 | 5.4 (6.9) | −0.16 (0.69) | 0.0 | 0.0 |
| VDT | 6.9 (0.7) | 0.00 (1.00) | 4.3 | 0.0 | 6.9 (0.8) | 0.05 (1.13) | 2.1 | 0.0 |
| PPT | 152.0 (62.4) | 0.00 (1.00) | 1.4 | 2.9 | 133.6 (70.9) | 0.47 (1.36) | 2.1 | 8.5 |
|
| ||||||||
| all | Mean | Mean | Mean | Mean | ||||
| 1.0% | 2.5% | 2.1% | 6.8% | |||||
Abs: absolute values of QST findings in the healthy reference group. CDT: cold detection threshold (Δ°C), WDT: warmth detection threshold (Δ°C), TSL: thermal sensory limen (°C), CPT: cold pain threshold (°C), HPT: heat pain threshold (°C), MDT: mechanical detection threshold (mN), MPT: mechanical pain threshold (mN), MPS: mechanical pain sensitivity, WUR: wind-up ratio, VDT: vibration detection threshold (/8), PPT: pressure pain threshold (kPa).
z-transformed values outside the 95% confidence intervals of the normal range (a.m. [14]).
Fig. 1.
Example of somatosensory z-score profiles in two patients (AO1 and AO2) with atypical odontalgia (AO) indicating involvement of dysfunction of different primary afferent fibers. The grey area (−1.96 < z < 1.96) is the normal range based on the healthy reference material. CDT: cold detection threshold; WDT: warmth detection; TSL: thermal sensory limen; CPT: cold pain threshold; HPT: heat pain threshold; MDT: mechanical detection threshold; MPT: mechanical pain threshold; MPS: mechanical pain sensitivity; VDT: vibration detection threshold; PPT: pressure pain threshold.
Table 1b.
Mean values and standard deviations (SD) of absolute values of side-to-side differences at the intraoral sites in the patients with atypical odontalgia (AO) and the age- and sex-matched reference group. The upper limit of the 95% CI (95%CIup) of the side-to-side differences in the reference group is also given as it is used in the evaluation of relative abnormalities for the LossGain scores.
| Reference data | AO patient data | ||
|---|---|---|---|
|
| |||
| Mean (SD) | 95%CIup | Mean (SD) | |
| CDT | 5.0 (4.0) | 6.4 | 5.0 (4.3) |
| WDT | 2.0 (2.4) | 2.8 | 2.0 (2.1) |
| TSL | 4.8 (6.1) | 6.8 | 6.0 (4.0) |
| CPT | 3.3 (3.2) | 4.5 | 4.1 (4.2) |
| HPT | 2.4 (8.5) | 5.4 | 1.6 (1.5) |
| MDT | 15.6 (21.4) | 22.9 | 36.5 (46.7) |
| MPT | 68.2 (88.0) | 98.4 | 79.1 (80.4) |
| MPS | 0.6 (1.2) | 1.0 | 2.1 (5.3) |
| WUR | 2.7 (3.7) | 4.0 | 3.1 (7.0) |
| VDT | 0.4 (0.9) | 0.7 | 0.3 (0.3) |
| PPT | 25.6 (34.7) | 37.5 | 41.3 (33.2) |
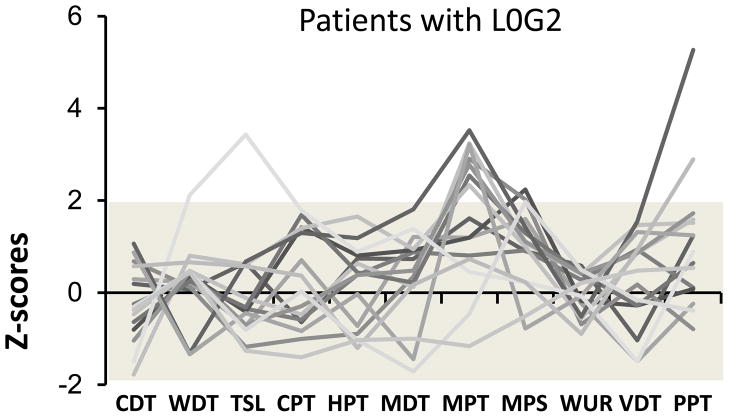
The distribution of the participants in each group according to the LossGain coding system including both absolute (abnormal z-scores) and relative (abnormal side-to-side difference) abnormalities is shown in Table 2. Only 12.7% of the AO patients had no somatosensory abnormalities at all in comparison with 63.8% of the reference group (P < 0.001) (Table 2). L0G2 (no somatosensory loss with gain of mechanical somatosensory function) was the most frequent coding in the AO group (31.9%), which was significantly different from the reference group (10.1%) (P = 0.048) (Table 2 and Fig. 2). The cumulative proportion of the groups exhibiting somatosensory loss without any gain (L1G0, L2G0, L3G0) was 10.7% in the AO group and 11.6% in the reference group (P > 0.05). In contrast, the cumulative proportion of subjects presenting with somatosensory gain without any loss was 57.5% in the AO group compared with 18.8% in the reference group (P =0.048) (Table 2, dark grey shading). The cumulative proportion of the groups showing mixed loss and gain (L1G1, L1G2, L1G3, L2G1, L2G2, L2G3, L3G1, L3G2or L3G3) was significantly higher in the AO group (31.9%) than in the reference group (5.8%) (P< 0.001) (Table 2, light grey shading).
Table 2.
LossGain distribution. Significant differences in LossGain score frequency between groups are shown in bold. Dark grey shading indicates no somatosensory loss (L0) in combination with somatosensory gain (G1-3) in AO patients. Light gray shading indicates mixed somatosensory loss (L1-3) and gain (G1-3) in AO patients.
| Loss | Gain | ||||
|---|---|---|---|---|---|
| G0 (No) | G1 (thermal) | G2 (mechanical) | G3 (both) | All | |
| AO patients | |||||
| L0 (No) | 6 (12.7%) | 3 (6.4%) | 15 (31.9%) | 3 (6.4%) | 27 (57.4%) |
| L1 (thermal) | 2 (4.3%) | 1 (2.1%) | 2 (4.3%) | 4 (8.5%) | 9 (19.1%) |
| L2 (mechanical) | 3 (6.4%) | 0 (0.0%) | 4 (8.5%) | 0 (0.0%) | 7 (14.9%) |
| L3 (both) | 0 (0.0%) | 2 (4.3%) | 2 (4.3%) | 0 (0.0%) | 4 (8.5%) |
| All | 11 (23.4%) | 6 (12.7%) | 23 (48.9%) | 7 (14.9%) | 47 (100%) |
| Reference | |||||
| L0 (No) | 44 (63.8%) | 3 (4.3%) | 7 (10.1%) | 3 (4.3%) | 57 (82.6%) |
| L1 (thermal) | 4 (5.8%) | 1 (1.4%) | 0 (0.0%) | 1 (1.4%) | 6 (8.7%) |
| L2 (mechanical) | 4 (5.8%) | 0 (0.0%) | 2 (2.9%) | 0 (0.0%) | 6 (8.7%) |
| L3 (both) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| All | 52 (75.4%) | 4 (5.8%) | 9 (13.0%) | 4 (5.8%) | 69 (100%) |
Fig. 2.
The individual z-score profiles of the 15 patients (31.9%)with atypical odontalgia (AO) with a LossGain score of L0G2 (no somatosensory loss with gain of mechanical somatosensory function). The grey area (− 1.96 < z < 1.96) is the normal range based on the healthy reference material. CDT: cold detection threshold; WDT: warmth detection; TSL: thermal sensory limen; CPT: cold pain threshold; HPT: heat pain threshold; MDT: mechanical detection threshold; MPT: mechanical pain threshold; MPS: mechanical pain sensitivity; WUR: windup ratio; VDT: vibration detection threshold; PPT: pressure pain threshold.
In AO patients, the occurrence of PHS and DMA at the painful site was significantly more frequent than in the reference group (mean PHS: 1.10 ± 0.14 out of 3 for AO compared to 0.62 ± 0.09 for reference, P = 0.003; mean DMA: 0.23 ± 0.11 for AO compared to 0.03 ± 0.02 for reference, P = 0.037). In the reference group, 43.5% reported no PHS occurrence in comparison with 21.3% of the AO group (P = 0.014). 82.6% in the reference group and 59.6% in the AO group reported 1 or less occurrence of PHS (out of 3 possible; P = 0.006).
Overall, the absolute values on the thenar site were not significantly different between groups for any of the 13 QST parameters (P> 0.103). The individual thenar z-scores for each patient for each stimulus modality based on the means and SDs of the healthy reference material showed that 13.6% of the AO patients had gain of function with regard to MPT (6.1% of healthy), 11.4% had gain of function regarding CPT (3.0% of healthy), 9.1% had gain of function regarding HPT (3.0% of healthy) and 9.1% had loss of function with regard to MDT (3.0% of healthy) (For further results, please refer to Table 3).
Table 3.
Frequency (%) of patients with atypical odontalgia and healthy reference participants presenting with thenar site z-score values outside the reference 95% confidence interval of the reference material (−1.96 < z < 1.96).
| Reference data | Patient data | |||
|---|---|---|---|---|
|
| ||||
| < −1.96a (%) | > 1.96a (%) | < −1.96a (%) | > 1.96a (%) | |
| CDT | 0.0 | 0.0 | 0.0 | 6.8 |
| WDT | 3.0 | 0.0 | 0.0 | 0.0 |
| TSL | 0.0 | 0.0 | 2.3 | 0.0 |
| CPT | 0.0 | 3.0 | 0.0 | 11.4 |
| HPT | 0.0 | 3.0 | 0.0 | 9.1 |
| MDT | 3.0 | 0.0 | 9.1 | 0.0 |
| MPT | 3.0 | 6.1 | 0.0 | 13.6 |
| MPS | 3.0 | 3.0 | 6.8 | 0.0 |
| WUR | 0.0 | 3.0 | 2.3 | 4.5 |
| VDT | 3.0 | 0.0 | 6.8 | 0.0 |
| PPT | 0.0 | 6.1 | 2.3 | 2.3 |
|
| ||||
| Mean all | 1.4 | 2.2 | 2.7 | 4.3 |
z-transformed values outside the 95% confidence intervals of the normal range (a.m. [14]). CDT: cold detection threshold, WDT: warmth detection threshold, TSL: thermal sensory limen, CPT: cold pain threshold, HPT: heat pain threshold, MDT: mechanical detection threshold, MPT: mechanical pain threshold, MPS: mechanical pain sensitivity, WUR: wind-up ratio, VDT: vibration detection threshold, PPT: pressure pain threshold.
3.3. Agreement between QualST and QST
The percent agreement between corresponding measures from QST and the three modalities of QualST (for example the percent agreement between Touch hyposensitivity from QualST and mechanical loss (L2 or L3) from QST and between Cold hyposensitivity from QualST and thermal loss (L1 or L3) from QST) ranged from 55.6–70.4% in the AO group and from 71.1–92.1% in the reference group (for further results, please refer to Table 4).
Table 4.
Agreement between the corresponding measures from quantitative and qualitative sensory tests. Number (and % proportion of group) of patients with atypical odontalgia (AO) or reference group participants (Ref).
| N = 65 | Touch hyposensitivity in agreement with mechanical loss (L2 or L3) | Touch hypersensitivity in agreement with mechanical gain (G2 or G3) | Cold hyposensitivity in agreement with thermal loss (L1 or L3) | Cold hypersensitivity in agreement with thermal gain (G1 or G3) | Pinprick hyperalgesia in agreement with mechanical gain (G2 or G3) |
|---|---|---|---|---|---|
| AO (n = 27) | 19 (70.4%) | 18 (66.7%) | 15 (55.6%) | 16 (59.3%) | 19 (71.4%) |
| Ref (n = 38) | 35 (92.1%) | 30 (78.9%) | 33 (86.8%) | 31 (81.6%) | 27 (71.1%) |
4. Discussion
This is the first study to apply the full battery of 13 standardized intraoral QST parameters in a chronic intraoral pain condition [12,16,20–22]. The main finding of this study was that 87.3% of the AO patients presented with somatosensory abnormalities in terms of loss or gain of somatosensory function in comparison with an age- and sex-matched reference group. Earlier studies have also demonstrated sensory disturbances in AO patients [5,14,27] but in these studies, overall means were compared between AO patients and a control group, which did not take into account that using the mean values of patients with gain of function (positive values) and patients with patients with loss of function (negative values) results in an underestimation of the sensory abnormalities. The most frequent LossGain score encountered in this material was L0G2 (no somatosensory loss combined with gain of mechanical somatosensory function). Interestingly, this corresponds with the score that was most frequently found in patients with the trigeminal neuropathic pain condition trigeminal neuralgia (TN) but was much less common in other non-trigeminal neuropathic pain conditions in the study by Maier et al.(2010) [16]. Looking at the individual QST parameters, somatosensory gain with regards to MPT (mechanical pain threshold), and CPT (cold pain threshold), i.e. mechanical and thermal allodynia, were the most frequently encountered abnormalities (27.7 and 14.9% of AO patients, respectively). This corresponds well with earlier studies in AO [14,27].
The present study contributes with some reference data for standardized intraoral QST. A limitation to the study may be that for practical reasons, the upper premolar region served as the standard reference site for all patients, regardless of which was their painful region. However, the German Research Network on Neuropathic pain has similarly used hand data as representative for the upper body and foot data for the lower body and we suggest the present reference material to be equally appropriate [16]. However, more region-specific reference data are needed. Another limitation could be that some intraoral regions are more difficult to target than others, for example the most distal molars. In order to test such regions, the cheek needs to be gently retracted, which inevitably provides a sensory input to the patients. However, all buccal test regions involve cheek or lip retraction to some degree and the competing sensory input is therefore present in all patients and healthy subjects in this study.
Like in the study by Maier et al. (2010), some somatosensory deviations were also found in the healthy reference group, with a total of 36.2% showing one or more values outside the 95% CI. This frequency may seem high but is actually lower than would be expected based on simple calculation of the chance probability of being healthy and having at least one of 11 values being outside the 95% CI ((1 - 0.9511) = 43.1%) [16]. This high probability for healthy subjects of showing at least one abnormal value may be seen as a limitation to the very comprehensive QST protocol and emphasizes the importance of both within and between group comparisons. Also, it stresses that QST cannot stand alone in the evaluation of patients with possible neuropathic pain and should always be combined with a thorough clinical examination and other supplementary tests (for example imaging or neurophysiological tests) whenever possible in accordance with current guidelines [8,24].
The LossGain score includes evaluation of absolute (mean score outside 95% CI of reference material) and relative (side-to-side difference outside 95% CI of reference material) abnormalities [12,16]. We speculate that using absolute abnormalities alone may be too conservative (low diagnostic sensitivity, high specificity), mainly due to the large inter-individual variation in somatosensory sensitivity. Including the side-to-side difference in the evaluation could increase diagnostic sensitivity but would also inevitably result in lower specificity. Also, including the side-to-side differences in the evaluation of cases with bilateral somatosensory abnormalities presents further difficulties. The diagnostic accuracy of the different approaches needs to be tested against a gold standard, which at present is not available or agreed upon amongst experts in the field. In the diagnosis of possible neuropathic pain conditions, it is now generally agreed that if pain is present in a neuroanatomically relevant area and if somatosensory abnormalities within this area has been demonstrated in combination with other possible confirmatory tests (for example neurophysiological or imaging techniques), the pain can be considered a ‘definite’ neuropathic pain condition [8,24]. Development of gold standard diagnostic criteria for AO condition and other orofacial pain conditions is a relevant future research objective.
The occurrence of paradoxical heat sensations (PHS) seems to be higher intraorally in healthy subjects (up to 71 % [20]) than what has been reported at extraoral sites (2.4% [16]). This might suggest that in the clinical setting, the PHS measure is less useful to reveal neuropathic involvement in intraoral pain than what is considered for extraoral pain conditions. However, there was a significantly higher occurrence of intraoral PHS in the AO patients in the present study, suggesting that despite the relatively frequent encounter of this phenomenon in healthy subjects, paradoxical thermal sensations may have relevance when pain mechanisms are studied.
From the QST performed at the extra-trigeminal control site (the thenar of the right hand) no significant differences between groups were detected for the absolute values. However, z-scores outside the 95% CI were more frequently encountered for AO patients than for controls, especially with regards to gain of thermal and mechanical somatosensory function. This may suggest involvement of central mechanisms in accordance with findings from other studies [5,13,14,27].
Another main finding of this study was that there was a good to excellent agreement between three modalities of simple chair-side intraoral qualitative somatosensory testing (QualST) and the comprehensive and more time-consuming quantitative testing (QST). To our knowledge, this has not been systematically examined before in any intra- or extraoral pain condition. The agreement between tests was higher in the reference group than in AO patients, which probably reflects the much smaller proportion of abnormal scores in the reference group. It is evident that the agreement between the simple chair-side tests and full QST is not perfect but we suggest, based on the knowledge from the present study and our recent study showing good test-retest reliability of the QualST, that for the clinician, QualST is an appropriate screening tool for initial assessment of somatosensory function in trigeminal pain conditions where neuropathic pain is suspected. Further studies are needed to examine the influence of other orofacial pain conditions on intraoral somatosensory function.
5. Conclusions
In conclusion, in this first multi-center study using the full battery of QST tests recommended by the German Network on Neuropathic Pain, intraoral somatosensory abnormalities were detected in 87.3% of patients with atypical odontalgia, most frequently in the form of somatosensory gain to painful mechanical and thermal stimuli. We also conclude that agreement between quantitative and qualitative sensory testing was in the good to excellent range, suggesting that intraoral qualitative sensory tests may serve as an appropriate screening of patients with suspected neuropathic pain conditions before referral for further, more comprehensive investigation including quantitative sensory testing and neurophysiological testing.
Summary.
Intraoral somatosensory abnormalities were commonly detected in AO patients and agreement between quantitative and qualitative sensory testing was good to excellent.
Acknowledgments
This study was financially supported by the Faculty of Odontology, Malmö University and NIH grants K12 DE14069 and R21-DE018768.
Footnotes
All authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Lene Baad-Hansen, Section of Clinical Oral Physiology, Department of Dentistry, Aarhus University, Aarhus, Denmark.
Maria Pigg, Department of Endodontics, Faculty of Odontology, Malmö University, Malmö, Sweden.
Susanne El’Masry Ivanovic, Department of Stomatognathic Physiology, Faculty of Odontology, Malmö University, Malmö, Sweden.
Hanan Faris, Section of Clinical Oral Physiology, Department of Dentistry, Aarhus University, Aarhus, Denmark.
Thomas List, Department of Stomatognathic Physiology, Faculty of Odontology, Malmö University, Malmö, Sweden.
Mark Drangsholt, Department of Oral Medicine, School of Dentistry, University of Washington, Seattle, USA.
Peter Svensson, Section of Clinical Oral Physiology, Department of Dentistry, Aarhus University and MindLab, Center of Functionally Integrative Neuroscience (CFIN), Aarhus University Hospital, Aarhus, Denmark.
References
- 1.Abrahamsen R, Baad-Hansen L, Svensson P. Hypnosis in the management of persistent idiopathic orofacial pain--clinical and psychosocial findings. Pain. 2008;136:44–52. 21. doi: 10.1016/j.pain.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Baad-Hansen L. Atypical odontalgia - pathophysiology and clinical management. J Oral Rehabil. 2008;35:1–11. doi: 10.1111/j.1365-2842.2007.01813.x. [DOI] [PubMed] [Google Scholar]
- 3.Baad-Hansen L, Juhl GI, Jensen TS, Brandsborg B, Svensson P. Differential effect of intravenous S-ketamine and fentanyl on atypical odontalgia and capsaicin-evoked pain. Pain. 2007;129:46–54. doi: 10.1016/j.pain.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 4.Baad-Hansen L, Leijon G, Svensson P, List T. Comparison of clinical findings and psychosocial factors in patients with atypical odontalgia and temporomandibular disorders. J Orofac Pain. 2008;22:7–14. [PubMed] [Google Scholar]
- 5.Baad-Hansen L, List T, Jensen TS, Svensson P. Increased pain sensitivity to intraoral capsaicin in patients with atypical odontalgia. J Orofac Pain. 2006;20:107–114. [PubMed] [Google Scholar]
- 6.Baad-Hansen L, List T, Kaube H, Jensen TS, Svensson P. Blink reflexes in patients with atypical odontalgia and matched healthy controls. Exp Brain Res. 2006;172:498–506. doi: 10.1007/s00221-006-0358-1. [DOI] [PubMed] [Google Scholar]
- 7.Baad-Hansen L, Pigg M, Ivanovic SE, Faris H, List T, Drangsholt MT, Svensson P. Chair-side Intraoral Qualitative Somatosensory Testing (QualST) - Reliability and Comparison between Patients with Atypical Odontalgia and Healthy Controls. J Orofac Pain. 2013 doi: 10.11607/jop.1062. (In press) [DOI] [PubMed] [Google Scholar]
- 8.Benoliel R, Zadik Y, Eliav E, Sharav Y. Peripheral painful traumatic trigeminal neuropathy: clinical features in 91 cases and proposal of novel diagnostic criteria. J Orofac Pain. 2012;26:49–58. [PubMed] [Google Scholar]
- 9.Dworkin SF, LeResche L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J CraniomandibDisord. 1992;6:301–355. [PubMed] [Google Scholar]
- 10.Jääskeläinen SK. The utility of clinical neurophysiological and quantitative sensory testing for trigeminal neuropathy. J Orofac Pain. 2004;18:355–359. [PubMed] [Google Scholar]
- 11.Jääskeläinen SK, Teerijoki-Oksa T, Forssell H. Neurophysiologic and quantitative sensory testing in the diagnosis of trigeminal neuropathy and neuropathic pain. Pain. 2005;117:349–357. doi: 10.1016/j.pain.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 12.Krumova EK, Westermann A, Maier C. Quantitative sensory testing: a diagnostic tool for painful neuropathy. Future Neurol. 2010;5:721–733. [Google Scholar]
- 13.List T, Leijon G, Helkimo M, Öster A, Svensson P. Effect of local anesthesia on atypical odontalgia--a randomized controlled trial. Pain. 2006;122:306–314. doi: 10.1016/j.pain.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 14.List T, Leijon G, Svensson P. Somatosensory abnormalities in atypical odontalgia: A case-control study. Pain. 2008;139:333–341. doi: 10.1016/j.pain.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Lu S, Baad-Hansen L, List T, Zhang Z, Svensson P. Somatosensory profiling in healthy subjects modified by intraoral capsaicin and menthol. Eur J Oral Sci. doi: 10.1111/eos.12014. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maier C, Baron R, Tolle TR, Binder A, Birbaumer N, Birklein F, Gierthmuhlen J, Flor H, Geber C, Huge V, Krumova EK, Landwehrmeyer GB, Magerl W, Maihofner C, Richter H, Rolke R, Scherens A, Schwarz A, Sommer C, Tronnier V, Uceyler N, Valet M, Wasner G, Treede RD. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain. 2010;150:439–450. doi: 10.1016/j.pain.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Marbach JJ, Raphael KG. Phantom tooth pain: a new look at an old dilemma. Pain Med. 2000;1:68–77. doi: 10.1046/j.1526-4637.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- 18.Melis M, Secci S. Diagnosis and treatment of atypical odontalgia: a review of the literature and two case reports. J Contemp Dent Pract. 2007;8:81–89. [PubMed] [Google Scholar]
- 19.Nixdorf DR, Drangsholt MT, Ettlin DA, Gaul C, De Leeuw R, Svensson P, Zakrzewska JM, DE Laat A, Ceusters W. Classifying orofacial pains: a new proposal of taxonomy based on ontology. J Oral Rehabil. 2012;39:161–169. doi: 10.1111/j.1365-2842.2011.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pigg M, Baad-Hansen L, Svensson P, Drangsholt M, List T. Reliability of intraoral quantitative sensory testing (QST) Pain. 2010;148:220–226. doi: 10.1016/j.pain.2009.10.024. [DOI] [PubMed] [Google Scholar]
- 21.Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, Treede RD. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88. doi: 10.1016/j.ejpain.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 22.Svensson P, Baad-Hansen L, Pigg M, List T, Eliav E, Ettlin D, Michelotti A, Tsukiyama Y, Matsuka Y, Jääskeläinen SK, Essick G, Greenspan JD, Drangsholt M. Guidelines and recommendations for assessment of somatosensory function in oro-facial pain conditions - a taskforce report. J Oral Rehabil. 2011;38:366–394. doi: 10.1111/j.1365-2842.2010.02196.x. [DOI] [PubMed] [Google Scholar]
- 23.Svensson P, Baad-Hansen L, Thygesen T, Juhl GI, Jensen TS. Overview on tools and methods to assess neuropathic trigeminal pain. J Orofac Pain. 2004;18:332–338. [PubMed] [Google Scholar]
- 24.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain. Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 25.Woda A, Pionchon P. A unified concept of idiopathic orofacial pain: clinical features. J Orofac Pain. 1999;13:172–184. [PubMed] [Google Scholar]
- 26.Woda A, Tubert-Jeannin S, Bouhassira D, Attal N, Fleiter B, Goulet JP, Gremeau-Richard C, Navez ML, Picard P, Pionchon P, Albuisson E. Towards a new taxonomy of idiopathic orofacial pain. Pain. 2005;116:396–406. doi: 10.1016/j.pain.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Zagury JG, Eliav E, Heir GM, Nasri-Heir C, Ananthan S, Pertes R, Sharav Y, Benoliel R. Prolonged gingival cold allodynia: a novel finding in patients with atypical odontalgia. Oral Surg Oral Med Oral Pathol Oral RadiolEndod. 2011;111:312–319. doi: 10.1016/j.tripleo.2010.10.008. [DOI] [PubMed] [Google Scholar]