Abstract
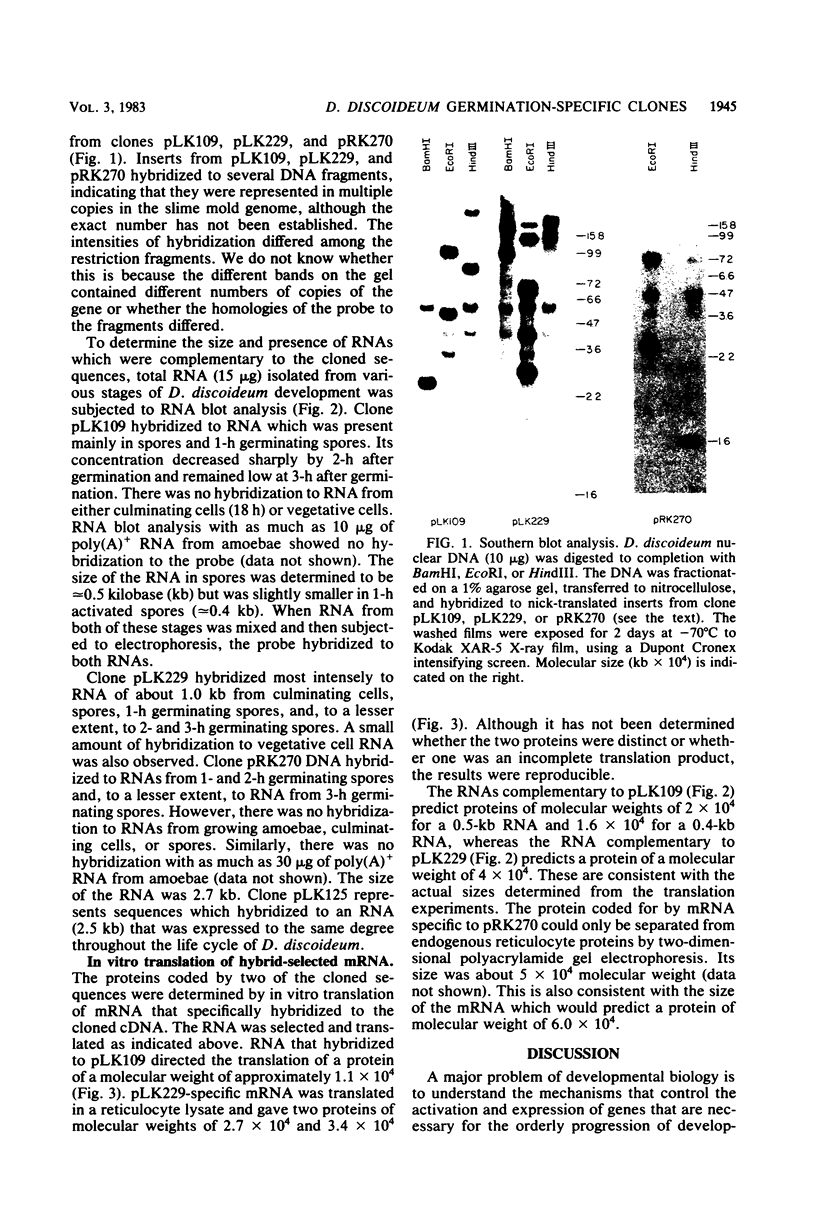
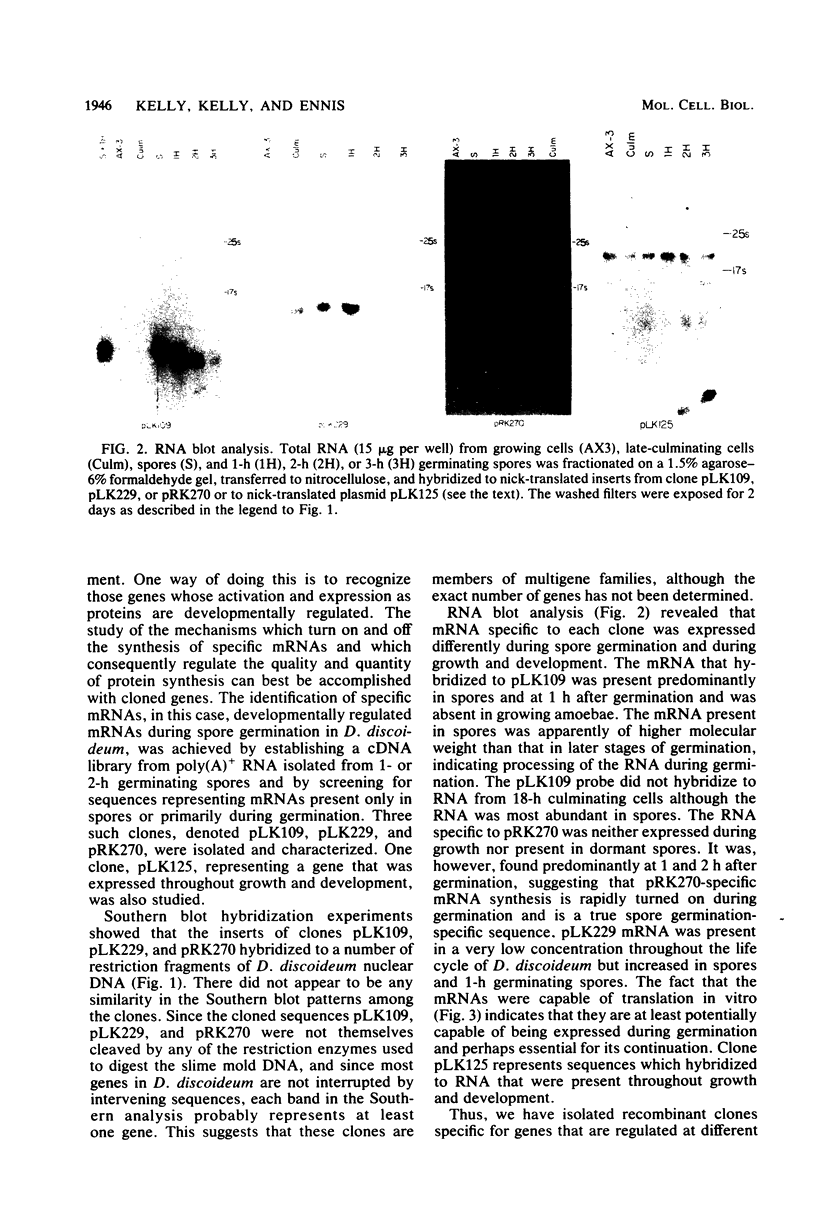
Spore germination in the slime mold Dictyostelium discoideum was used as a model to study the developmental regulation of protein and mRNA synthesis. Changes in the synthesis of these macromolecules occur during the transition from dormant spore to amoebae. The study of the mechanisms which regulate the quantity and quality of protein synthesis can best be accomplished with cloned genes. cDNA clones which hybridized primarily with mRNAs from only spores or germinating spores and not with growing amoebae were collected. Three such clones, denoted pLK109, pLK229, and pRK270, were isolated and had inserts of approximately 500, 1,200, and 690 base pairs, respectively. Southern blot hybridization experiments suggested that each of the genes is present in multiple copies in the D. discoideum genome. RNA blot hybridizations were performed to determine the sizes of the respective mRNAs and their developmental regulation. The mRNA that hybridized to pLK109 DNA was present predominantly in spores and at 1 h after germination but was absent in growing amoebae. Its concentration dramatically dropped at 3 h. The mRNA present in spores is apparently larger (approximately 0.5 kilobase) than in the later stages of germination (0.4 kilobase), indicating processing of the RNA during germination. The mRNA that hybridized to pLK229 DNA was approximately 1.0 kilobase and was present in very low amounts during growth. Its concentration rose until 1 h after spore germination and decreased thereafter. pRK270-specific RNA was approximately 2.7 kilobases and was found predominantly at 1 h after germination. It was present in lower concentrations at 2 and 3 h after germination and was absent in spores and amoebae. In vitro translation of mRNA selected from 1-h polyadenylated RNA which was hybridized to pLK109 or pLK229 DNA gave proteins of molecular weights consistent with the sizes of the mRNAs as determined by the RNA blot analysis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Miura-Santo L. Y., Hohl H. R. Ultrastructural changes during germination of Dictyostelium discoideum spores. J Bacteriol. 1969 Nov;100(2):1020–1026. doi: 10.1128/jb.100.2.1020-1026.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Properties of germinating spores of Dictyostelium discoideum. J Bacteriol. 1968 Nov;96(5):1680–1689. doi: 10.1128/jb.96.5.1680-1689.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter D. A., Raper K. B. Spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1966 Sep;56(3):880–887. doi: 10.1073/pnas.56.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dowbenko D. J., Ennis H. L. Regulation of protein synthesis during spore germination in Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1791–1795. doi: 10.1073/pnas.77.4.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maxam A. M., Maniatis T. Enzymatic in vitro synthesis of globin genes. Cell. 1976 Feb;7(2):279–288. doi: 10.1016/0092-8674(76)90027-1. [DOI] [PubMed] [Google Scholar]
- Ennis H. L., Sussman M. Mutants of Dictyostelium discoideum defective in spore germination. J Bacteriol. 1975 Oct;124(1):62–64. doi: 10.1128/jb.124.1.62-64.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Developmental changes in RNA and protein synthesis during germination of Dictyostelium discoideum spores. Dev Biol. 1978 Nov;67(1):189–201. doi: 10.1016/0012-1606(78)90308-1. [DOI] [PubMed] [Google Scholar]
- Giri J. G., Ennis H. L. Protein and RNA synthesis during spore germination in the cellular slime mold Dictyostelium discoideum. Biochem Biophys Res Commun. 1977 Jul 11;77(1):282–289. doi: 10.1016/s0006-291x(77)80194-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson A., Firtel R. A., Lodish H. F. Synthesis of messenger and ribosomal RNA precursors in isolated nuclei of the cellular slime mold Dictyostelium discoideum. J Mol Biol. 1974 Jan 15;82(2):213–230. doi: 10.1016/0022-2836(74)90342-8. [DOI] [PubMed] [Google Scholar]
- Mangiarotti G., Chung S., Zuker C., Lodish H. F. Selection and analysis of cloned developmentally-regulated Dictyostelium discoideum genes by hybridization-competition. Nucleic Acids Res. 1981 Feb 25;9(4):947–963. doi: 10.1093/nar/9.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgard M. V., Tocci M. J., Monahan J. J. On the cloning of eukaryotic total poly(A)-RNA populations in Escherichia coli. J Biol Chem. 1980 Aug 25;255(16):7665–7672. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sussman R., Sussman M. Cultivation of Dictyostelium discoideum in axenic medium. Biochem Biophys Res Commun. 1967 Oct 11;29(1):53–55. doi: 10.1016/0006-291x(67)90539-6. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]