Summary
Immune tolerance requires regulatory T (Treg) cells to prevent autoimmune disease, with the transcription factor Foxp3 functioning as the critical regulator of Treg cell development and function. We report here that Foxp3 was lethal to developing Treg cells in the thymus because it induced a unique pro-apoptotic protein signature (Puma++p-Bim++p-JNK++DUSP6-) and repressed expression of pro-survival Bcl-2 molecules. However, Foxp3 lethality was prevented by common gamma chain (γc)-dependent cytokine signals that were present in the thymus in limiting amounts sufficient to support only ~1 million Treg cells. Consequently, most newly arising Treg cells in the thymus were deprived of this signal and underwent Foxp3-induced death, with Foxp3+CD25- Treg precursor cells being the most susceptible. Thus, we identify Foxp3 as a pro-apoptotic protein that requires developing Treg cells to compete with one another for limiting amounts of γc-dependent survival signals in the thymus.
Developing T cells express antigen receptors (TCRs) with diverse recognition specificities that are then screened in the thymus for usefulness and autoreactive potential. Thymocytes with useful TCR specificities are positively selected to differentiate into functionally mature CD4+ or CD8+ T cells, whereas thymocytes with autoreactive TCR specificities are negatively selected to undergo clonal deletion (Pobezinsky et al., 2012; Singer et al., 2008; Starr et al., 2003). Because thymic selection is imperfect, some potentially autoreactive T cells manage to escape into the periphery where their autoreactive potential must be suppressed. Regulatory T (Treg) cells are a specialized subpopulation of CD4+ T cells which prevent activation of autoreactive T cells (Josefowicz et al., 2012; Sakaguchi et al., 2008) so that animals lacking functional Treg cells develop severe autoimmunity and succumb to early death (Brunkow et al., 2001; Fontenot et al., 2003). The characteristic feature of Treg cells is their expression of the forkhead family transcription factor Foxp3 (Fontenot et al., 2003; Hori et al., 2003) whose function is incompletely understood, with little known about its effects on Treg cell viability.
For unclear reasons, there are striking similarities between the requirements for clonal deletion and Treg cell generation in the thymus. For example, Tregs resemble clonally deleted thymocytes in their expression of autoreactive TCRs (Apostolou et al., 2002; Hsieh et al., 2004; Jordan et al., 2001), although autoreactive TCRs on Tregs may be of somewhat lower affinity (Lee et al., 2012). Moreover, clonal deletion and Treg cell generation both require CD28 costimulation (Pobezinsky et al., 2012; Salomon et al., 2000; Tai et al., 2005). In developing Tregs CD28 costimulation induces Foxp3 expression probably through NFκB activation and binding of c-Rel to non-coding sequence elements in the Foxp3 gene (Isomura et al., 2009; Long et al., 2009; Zheng et al., 2010). Interestingly, Tregs are the only CD4+ T cells to survive CD28 costimulation in the thymus (Pobezinsky et al., 2012). Reciprocally, defects in the Bcl-2-regulated apoptotic pathway that impair clonal deletion disproportionately increase Treg cell numbers in the thymus (Zhan et al., 2011). These findings indicate an inverse relationship between clonal deletion and Treg generation and suggest that Foxp3 proteins might enhance cell viability.
Alternatively, Tregs are unique among newly arising CD4+ thymocytes in expressing CD25, a component of the high affinity IL-2 receptor, and in requiring cytokine signals (Bayer et al., 2008; Fontenot et al., 2005; Kramer et al., 1995; Malek et al., 2002; McCaughtry et al., 2012; Vang et al., 2008). Without signals induced by common γ chain (γc)-dependent cytokines, newly arising Tregs are markedly reduced in the thymus. Indeed the thymus of γc-deficient mice is virtually devoid of Tregs (Bayer et al., 2008; Burchill et al., 2007; Fontenot et al., 2005). While γc-mediated cytokines have been shown to signal developing Treg cells to express Foxp3 (Burchill et al., 2008; Lio and Hsieh, 2008), they also promote Treg cell survival. From this alternative perspective, it is possible that Foxp3 proteins impair cell viability and may be the reason that Treg cells require γc cytokine signals for survival.
The present study was undertaken to determine what effect, if any, Foxp3 protein expression actually has on cell viability. We report that Foxp3 is a potently pro-apoptotic transcription factor that is lethal to Treg cells unless its pro-apoptotic effects are counterbalanced by survival signals from γc-dependent cytokines. Thymocytes most prone to Foxp3 induced apoptosis are Foxp3+CD25− Treg precursor cells, which do not require γc-mediated signals to arise but do require γc cytokine signals to survive and complete their differentiation into mature Treg cells. As a result, newly arising Foxp3+ thymocytes must compete for limiting amounts of cytokine survival signals, with the result that most succumb to Foxp3 induced cell death. Thus, this study identifies a lethal function of Foxp3 that explains the strict requirement of Treg cells for γc-mediated survival signals and alters understanding of Treg generation in the thymus.
Results
Foxp3 induces cellular apoptosis
We first assessed the potential effects of Foxp3 on cell viability in heterozygous B6xScurfy female mice which contain two distinct Treg subpopulations. Because of random X chromosome inactivation, B6xScurfy female mice contain a Treg subpopulation that expresses functional Foxp3 proteins from the Xwt chromosome and another Treg cell subpopulation that expresses unstable mutant Foxp3 proteins from the Xsfy chromosome. Expression of the Foxp3GFP reporter transgene induced both Treg cell subpopulations to be GFP+ cells (Zhou et al., 2008). We then distinguished the two GFP+ Treg cell subpopulations from one another by intracellular staining with anti-Foxp3 which identified Xwt Treg cells as Foxp3+ and Xsfy Treg cells as Foxp3−/lo (Figure 1A, left and middle panels). Instead of containing equal numbers of Xwt and Xsfy Treg cells, 80% of GFP+CD4SP thymocytes were Foxp3+ (Xwt) while only 20% were Foxp3− (Xsfy) Treg cells (Figure 1A left). Such skewing in B6xScurfy female mice has been observed by others (Gavin et al., 2007; Lin et al., 2007) and was due, at least in part, to unstable and transient Foxp3GFP expression in Xsfy Tregs cells because they lack functional Foxp3 proteins to maintain Foxp3 gene expression (Zheng et al., 2010). Although lacking functional Foxp3 proteins, Xsfy CD4SP thymocytes were Treg lineage cells because, in addition to expressing Foxp3GFP, they expressed CTLA-4 and CD25 proteins, albeit in lower amounts than Xwt Tregs containing functional Foxp3 proteins (Figure 1A, middle) (Gavin et al., 2007; Lin et al., 2007). Most importantly, Xwt and Xsfy Treg cells differed in their susceptibility to spontaneous cell death, with significantly fewer Xwt Tregs surviving in overnight culture than Xsfy Tregs (Figure 1A, right). These findings indicate that functional Foxp3 proteins increase susceptibility to spontaneous cell death.
Figure 1. Foxp3 expression promotes cell death.
(A) Heterozygous B6xScurfy female mice with a Foxp3GFP reporter transgene (Zhou et al., 2008) contain two Treg subpopulations that express (Xwt) or lack (Xsfy) functional Foxp3 proteins. The frequency of each Treg subpopulation in the thymus, as well as their expression of intracellular Foxp3, intracellular CTLA-4, and surface CD25 is shown (left and middle panels). Right panel displays the relative percentage of each Treg subpopulation that survived in overnight culture. Mean ± SE of 3 mice aged 4-6 weeks.
(B) Foxp3 expression in thymocytes from Foxp3Tg mice (A10, C10, and T3) and B6 CD4SP thymocytes displayed as Mean Fluorescence Intensity (MFI). Data are representative of multiple experiments.
(C) Thymocyte numbers from age matched B6 and Foxp3Tg mice. Mean ± SE of 10 mice.
(D) Quantitation of Tunel+ cells in thymic sections from the indicated mice. Mean ± SE of triplicate samples in 2 experiments.
(E) Spontaneous thymocyte apoptosis in overnight medium culture. Frequencies of EtBr+ DP and CD4SP thymocytes from indicated mice were normalized to control B6 which was set equal to 0. Mean ± SE of triplicate cultures in 3 experiments.
(F) Thymocyte numbers from the indicated mice. Mean ± SE of 14 age matched mice in each group.
(G) Foxp3 protein expression in DP and CD4SP thymocytes (left) and frequency of apoptotic cells after overnight medium culture (right, mean ± SE of triplicate cultures). Data are representative of 3 experiments.
(H) Spleen CD4+ T cell numbers from B6 and Foxp3Tg mice. Mean ± SE of 6 mice per group.
*, p<0.01; ***, p<0.0001.
See also Figure S1.
To directly determine the effect of Foxp3 proteins on cell viability, we generated three lines of Foxp3 transgenic (Foxp3Tg) mice (A10, C10, and T3) that expressed different amounts of functional Foxp3 proteins, ranging from 40-110% of endogenous Foxp3 protein expression (Figure 1B). Unlike endogenous Foxp3 proteins which were expressed by ~3% of CD4SP thymocytes, the Foxp3 transgenes A10 and C10 were driven by human CD2 (hCD2) promoter-enhancer elements that induced Foxp3 expression in nearly all thymocytes (Figure S1A). In contrast, the Foxp3 transgene T3 was driven by E8III-CD8α promoter-enhancer elements (Sarafova et al., 2005) that actively induced Foxp3 expression only in pre-selection DP thymocytes (Figure S1A). We found that thymus cellularity was reduced in Foxp3Tg mice (Khattri et al., 2001) and that the reduction occurred in a Foxp3 dose-dependent manner (Figure 1C), indicating an effect of Foxp3 on cell viability.
To analyze the thymus of Foxp3Tg mice, we performed in situ tunel assays of thymic slices which revealed five-fold more apoptotic cells in Foxp3Tg than B6 thymi (Figure 1D; Figure S1B). Many of the Foxp3Tg thymocytes (both DP and CD4SP) that were viable in vivo nevertheless underwent spontaneous apoptosis after only a few hours in culture as determined by ethidium bromide (EtBr) staining (Figure 1E). Foxp3Tg thymocytes were protected from apoptosis by Bcl-2 overexpression as transgenic expression of human Bcl-2 (hBcl-2) reduced tunel+ apoptotic cells (Figure 1D), prevented spontaneous death of Foxp3Tg thymocytes (Figure 1E), and significantly increased in vivo thymus cellularity in Foxp3Tg mice (Figure 1F). The protective effect of Bcl-2 suggested that Foxp3 expression resulted in mitochondrial membrane damage, which was substantiated by low DiOC6 staining in Foxp3Tg thymocytes that was corrected by Bcl-2 over-expression (Figure S1C).
To document that spontaneous death of Foxp3Tg thymocytes was due to expression of functional Foxp3 proteins, we compared DP and CD4SP thymocytes from C10 and T3 Foxp3Tg mice for susceptibility to cell death (Figure 1G). Reflecting the different expression patterns of the C10 and T3 transgenes in vivo (Figure S1A), transgenic Foxp3 protein expression was increased in C10 mice but terminated in T3 mice during differentiation of DP into CD4SP thymocytes (Figure 1G, left). Importantly, termination of Foxp3 expression reversed susceptibility of T3 CD4SP thymocytes to spontaneous cell death (Figure 1G, right). In vivo cell numbers confirmed this point, as T3 mice contained 6-fold more peripheral splenic CD4+ T cells than C10 mice (Figure 1H) despite having fewer thymocytes (Figure 1C). Thus Foxp3 protein expression was associated with cell death.
Identification of a Foxp3 pro-apoptotic protein signature
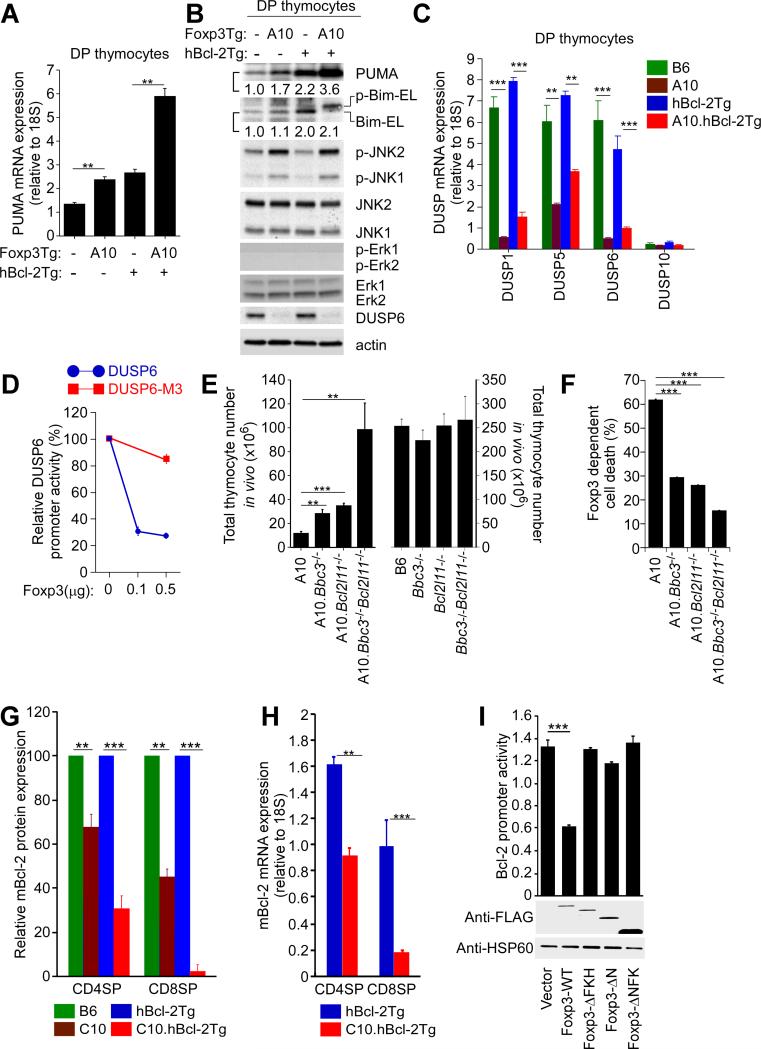
To understand the basis for the lethal effects of Foxp3, we examined the effect of Foxp3 on the pro-apoptotic molecules Puma and Bim because the first intron of the Bbc3 gene which encodes Puma and the promoter region of the Bcl2l1 gene which encodes Bim contain forkhead family consensus DNA binding sequences (Gilley et al., 2003; Sunters et al., 2003; You et al., 2006). We found that Foxp3Tg expression quantitatively increased Puma expression (both mRNA and protein; Figures 2A and 2B) but did not increase Bim expression (either mRNA or protein; Figure 2B; Figure S2A), even in hBcl-2Tg thymocytes with improved survival (Figure 2B). While Bim proteins were not quantitatively increased, we found that Bim proteins were phosphorylated in Foxp3Tg thymocytes, as seen by an upward mobility shift in SDS-PAGE that was reversed by λ–phosphatase treatment (Figure 2B; Figure S2B). Notably, expression of other pro-apoptotic proteins such as Bad were not altered by Foxp3 (Figure S2C). Thus, Foxp3 increased Puma expression but induced Bim protein phosphorylation.
Figure 2. Effect of Foxp3 on pro-apoptotic and pro-survival molecules.
(A) Puma mRNA expression in DP thymocytes from indicated mice. Mean ± SE of triplicate samples.
(B) DP thymocyte expression of pro-apoptotic proteins. Numbers in protein immunoblots refer to intensities of the indicated bands relative to B6 (i.e. Foxp3Tg−hBcl-2Tg−) which was set equal to 1.0. Data are representative of 3 experiments.
(C) DUSP mRNA expression in DP thymocytes. Mean ± SE of triplicate samples in 3 experiments.
(D) Co-transfection of Hela cells with a luciferase-encoding vector (pGL3) driven either by the DUSP6 or mutated DUSP6-M3 promoter, together with either a Foxp3-encoding or empty control vector (pcDNA3.1). DUSP6 promoter activity was determined 24h after transfection by relating luciferase activity to control renilla activity to normalize for transfection efficiency. Mean ± SE of triplicate samples in 5 experiments.
(E) Thymus cellularity in mice expressing the A10 Foxp3Tg (left) or not (right). Mean ± SE of 5 mice per group.
(F) Effect of pro-apoptotic gene deficiencies on Foxp3-dependent spontaneous apoptosis in overnight culture. Frequency of EtBr+ cells after overnight culture of DP thymocytes from A10 Foxp3Tg mice was normalized to that from the identical mice without the A10 Foxp3Tg. Mean ± SE of triplicate samples in 3 experiments.
(G) Effect of Foxp3 on endogenous Bcl-2 protein expression. mBcl-2 content (determined by intracellular staining) in thymocytes from mice with the C10 Foxp3Tg normalized to mice without it. Mean ± SE of 4 mice per group.
(H) Quantitative RT-PCR analysis of mBcl-2 mRNA in thymocytes from the indicated mice. Mean ± SE of triplicate samples in 2 experiments.
(I) mBcl-2 promoter activity was determined in Hela cells 24h after co-transfection with a luciferase-encoding vector (pGL3) driven by the mBcl-2 promoter, together with a vector (pcDNA3.1) encoding either Flag-tagged wild-type or truncated Foxp3 proteins. Anti-Flag immunoblots confirmed the presence of Flag-tagged Foxp3 proteins. Mean ± SE of triplicate samples in 3 experiments.
**, p<0.001; ***, p<0.0001.
See also Figure S2.
Bim proteins can be phosphorylated by either ERK or JNK MAP kinases with different consequences (Ley et al., 2005). ERK-mediated Bim phosphorylation targets Bim proteins for ubiquitylation and proteasomal degradation, thereby decreasing mitochondrial membrane damage and increasing cell survival. In contrast, JNK-mediated Bim phosphorylation disrupts Bim interaction with the dynein motor complex and induces Bim translocation to the mitochondria where it increases mitochondrial membrane damage and decreases cell survival (Ley et al., 2005). To determine if Bim phosphorylation was induced by ERK or JNK, thymocyte lysates from Foxp3Tg and wild-type thymocytes were immunoblotted for p-ERK and p-JNK, revealing that Foxp3Tg lysates contained elevated amounts of p-JNK1 and p-JNK2, but not p-ERK (Figure 2B; Figure S2D). Thus, Bim phosphorylation in Foxp3Tg thymocytes was mediated by p-JNK and so was pro-apoptotic.
Because Foxp3 is a transcription factor and not a protein kinase, JNK phosphorylation in Foxp3Tg thymocytes was likely due to a Foxp3 target gene that encoded a regulatory kinase or phosphatase. In this regard, we considered that JNK phosphorylation was reportedly increased by reduced expression of dual specific phosphatases (DUSPs) which de-phosphorylate both pthreonine and p-tyrosine residues (Chi et al., 2006; Liu et al., 2007). In fact DUSP1, DUSP5, and DUSP6 mRNA transcripts were highly expressed in wild-type and hBcl-2Tg thymocytes, but their expression was markedly diminished in Foxp3Tg thymocytes (Figure 2C). Expression of DUSP6 protein, the only DUSP protein we could unambiguously detect by immunoblotting, was undetectable in Foxp3Tg thymocytes (Figure 2B). We reasoned that if Foxp3 transgenic proteins directly downregulated DUSP6 expression, then DUSP6 proteins which were absent in all Foxp3Tg DP thymocytes would specifically reappear in T3 SP thymocytes that terminated Foxp3 expression. This is precisely what we observed (Figure S2E). Thus, Foxp3 proteins inhibited DUSP gene and protein expression and increased phosphorylation of JNK and Bim proteins.
To better understand the inhibition by Foxp3 of DUSP gene expression, we examined the promoter region of DUSP6 and found that it contained putative forkhead family consensus DNA binding sequences (Marson et al., 2007) (Figure S2F). To determine if Foxp3 inhibited DUSP6 promoter activity, we constructed a luciferase reporter that was driven either by DUSP6 or DUSP6-M3 promoter sequences with mutations of three putative forkhead family binding sequences (Figure 2D; Figure S2F). As assessed 24h after HeLa cell transfection, co-transfection with Foxp3 inhibited DUSP6, but not DUSP6-M3, promoter activity (Figure 2D), indicating that Foxp3 proteins directly downregulated DUSP6 gene expression, a result consistent with Foxp3 occupancy of the DUSP6 gene that was observed previously (Marson et al., 2007). We also found that co-transfection with Foxp3 DNA, compared to control pcDNA, induced HeLa cell death after 24h, as was reported in MCF-7 cells (Figure S2G; (Zuo et al., 2007).
These findings revealed that Foxp3 was pro-apoptotic and increased Puma expression and Bim activity. We then determined the involvement of Puma and Bim in Foxp3-induced cell death by making Foxp3Tg mice deficient in one or both molecules. Deficiency of either Puma or Bim increased in vivo numbers of Foxp3Tg thymocytes, but maximal in vivo numbers required double deficiency of both Puma and Bim (Figure 2E, left). Similarly, deficiency of Puma or Bim reduced spontaneous Foxp3-dependent cell death in vitro, but maximal reduction required deficiency of both Puma and Bim (Figure 2F). Note that Puma and/or Bim deficiency reduced spontaneous apoptosis of Foxp3Tg thymocytes to a substantially greater extent than non-Foxp3Tg thymocytes (Figure S2H). This analysis revealed that Foxp3-induced apoptosis involved Puma and Bim, and identified a Foxp3-induced pro-apoptotic protein signature abbreviated as Puma++p-Bim++p-JNK++DUSP6−.
Foxp3 inhibition of Bcl-2 expression
Having identified a Foxp3 pro-apoptotic protein signature, we examined the effect of Foxp3 on pro-survival proteins Bcl-xL and Bcl-2. Bcl-xL is expressed in DP and SP thymocytes, but Bcl-2 is expressed only in SP thymocytes and is their major pro-survival protein (Boise et al., 1993; Sentman et al., 1991). Expression of Bcl-xL proteins was not altered in Foxp3Tg mice (Figure S2I), but expression of endogenous murine Bcl-2 (mBcl-2) proteins was reduced (Figure 2G; Figure S2J). Endogenous mBcl-2 protein and mRNA levels were even more dramatically reduced in Foxp3Tg thymocytes with hBcl-2Tg, presumably because hBcl-2Tg maintained Foxp3Tg thymocytes with low mBcl-2 levels that otherwise failed to survive (Figures 2G and 2H; Figure S2J).
To determine if Foxp3 proteins influenced Bcl2 promoter activity, we performed transient transfections of HeLa cells with a Bcl2 promoter luciferase construct and observed that cotransfection with Flag-tagged Foxp3 significantly inhibited Bcl2 promoter activity (Figure 2I). We also performed co-transfection experiments with constructs encoding Flag-tagged Foxp3 deletion mutants lacking the C-terminal DNA binding domain (Foxp3-ΔFKH), the N-terminal repressor domain (Foxp3-ΔN), or both domains (Foxp3-ΔNFK) (Koh et al., 2009) (Figure S2K). Only intact Foxp3 molecules suppressed Bcl2 promoter activity (Figure 2I), documenting that inhibition of Bcl2 promoter activity required Foxp3 proteins with the forkhead DNA binding domain and the Foxp3 repressor domain, results consistent with occupancy by Foxp3 of the Bcl2 promoter region (Katoh et al., 2011).
Our results demonstrate that transgenic Foxp3 proteins are potent inducers of apoptosis that both upregulate pro-apoptotic factors and downregulate pro-survival factors.
Induction of the pro-apoptotic protein signature by endogenous Foxp3
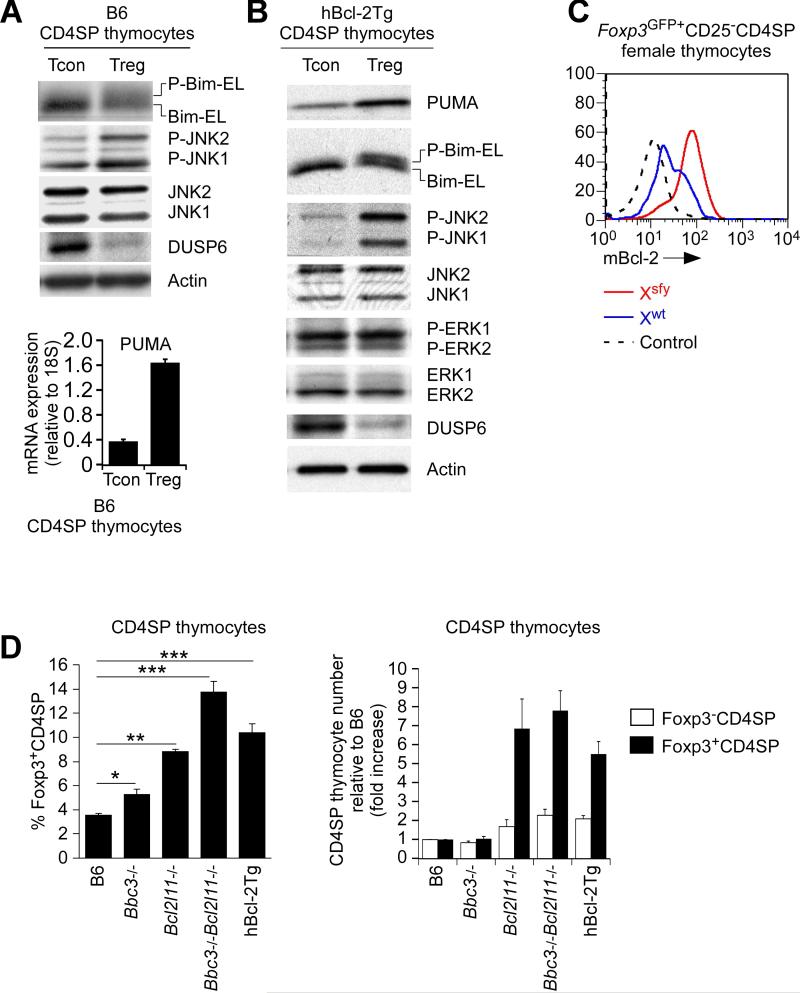
It was important to determine if endogenous Foxp3 proteins, like transgenic Foxp3 proteins, upregulated pro-apoptotic factors and repressed pro-survival factors. Compared to conventional (Foxp3−) CD4SP B6 thymocytes, Tregs contained increased Puma mRNA, increased p-Bim, increased p-JNK, and nearly absent DUSP6 protein (Figure 3A), all of which were concordant with the Foxp3 pro-apoptotic signature that we had identified in Foxp3Tg thymocytes. Expression of the Foxp3 protein signature (Puma++p-Bim++p-JNK++DUSP6−) was even better appreciated in Tregs from hBcl-2Tg mice, presumably because the hBcl-2Tg maintained cells that otherwise died from Foxp3-induced apoptosis (Figure 3B).
Figure 3. Normal Tregs display the Foxp3 pro-apoptotic protein signature.
(A and B) Protein immunoblots of conventional (Foxp3−CD25−) and regulatory (Foxp3+CD25+) CD4SP thymocytes from B6 (A) and hBcl-2Tg mice (B) containing the Foxp3-GFP reporter (Bettelli et al., 2006). Puma mRNA (A lower panel) was determined by quantitative RT-PCR relative to 18S rRNA (mean ± SE of triplicate samples). Data in A are representative of 2 experiments and data in B of 4 experiments.
(C) Intracellular mBcl-2 expression in Treg subpopulations (Xwt and Xsfy) in the thymus of heterozygous B6xScurfy female mice expressing the Foxp3GFP reporter. Dashed lines represent negative control staining. Data are representative of 2 experiments.
(D) Frequency (left) and relative number of Foxp3+CD4SP thymocytes compared to B6 (right). Mean ± SE of 5-10 mice per group.
*, p<0.01; **, p<0.001; ***, p<0.0001.
See also Figure S3.
Since Tregs were generated in the thymus by high affinity TCR engagements, it might be argued that the pro-apoptotic protein signature was not induced in Treg cells by Foxp3 but was induced by high affinity TCR engagements in the thymus. Consequently, we examined CD4SP thymocytes from AND TCR transgenic mice that were Rag2-/- on a B6xB10.A background and that had encountered their agonist ligand, pigeon cytochrome c (PCC) presented by I-Ek (Figure S3). However, the vast majority of PCC-I-Ek signaled AND thymocytes remained Foxp3− and did not display the Foxp3 pro-apoptotic protein signature (Figure S3). Instead they displayed a very different protein signature with increased Bim, mBcl-2, and DUSP6 proteins; unchanged p-JNK proteins; and unchanged or decreased Puma mRNA (Figure S3).
To determine the effect of endogenous Foxp3 proteins on pro-survival Bcl-2 proteins, we examined Bcl-2 expression in thymic Tregs from B6xScurfy female mice. Even though the two Treg subpopulations were from the same mice and had been generated by the same intrathymic signals, endogenous Bcl-2 expression was substantially lower in Foxp3+ (Xwt) than Foxp3− (Xsfy) Tregs (Figure 3C), demonstrating that functional Foxp3 proteins reduced endogenous Bcl-2 expression.
Induction of the Foxp3 pro-apoptotic protein signature together with reduced Bcl-2 expression raised the possibility that many newly arising Foxp3+ thymocytes normally died in the thymus. In fact, prevention of Foxp3-induced apoptosis disproportionately increased Foxp3+ relative to Foxp3− CD4SP thymocytes in both Puma-Bim double deficient and hBcl-2Tg mice (Figure 3D), as was reported recently but attributed to altered clonal deletion (Zhan et al., 2011). Notably, while Puma-Bim double deficiency and hBcl-2Tg expression increased conventional Foxp3−CD4SP thymocyte numbers 2-fold, they disproportionately increased Foxp3+CD4SP thymocyte numbers 8-fold (Figure 3D, right). As a result, their frequency of Foxp3+CD4SP thymocytes was increased to 14% compared to 4% in wildtype mice (Figure 3D, left).
Thus, endogenous Foxp3 proteins induce a distinct pro-apoptotic protein signature that makes Foxp3+ thymocytes prone to cell death.
γc-mediated cytokine signals rescue Tregs from the pro-apoptotic effects of Foxp3
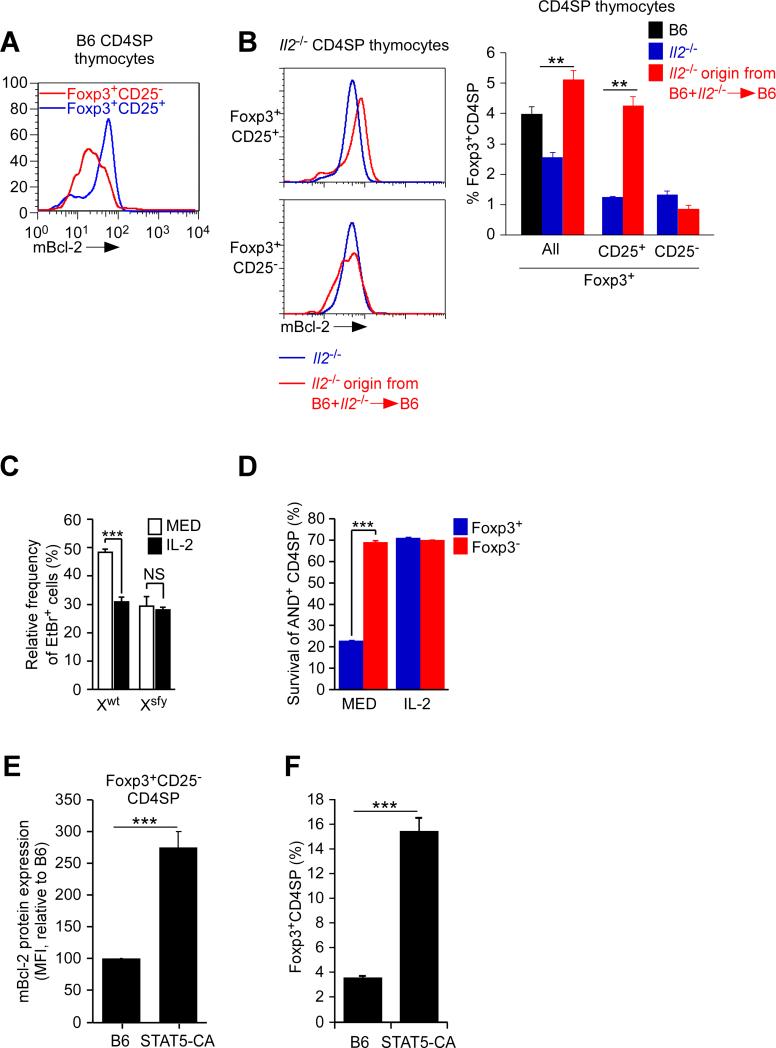
Tregs are known to depend on γc signals stimulated by such cytokines as IL-2, IL-7, and IL-15 (Bayer et al., 2008; Burchill et al., 2007; Fontenot et al., 2005; Kim et al., 2012; Vang et al., 2008). In fact, Foxp3+CD4SP thymocytes that were CD25+ and contained phosphorylated Stat5 proteins as a result of in vivo IL-2 signaling, also contained more endogenous Bcl-2 than Foxp3+CD4SP thymocytes that were CD25− (Figure 4A; Figure S4). To demonstrate that signaling by in vivo IL-2 upregulated Bcl-2 expression in Tregs, we compared Tregs that had never been exposed to in vivo IL-2 with Tregs that had. We did so by comparing Il2-/- Tregs from IL-2-deficient (Il2-/-) mice with genetically identical Il2-/- Tregs obtained from IL-2-sufficient mixed donor bone marrow chimeras (B6+ Il2-/- →B6) (Figure 4B). Comparing Il2-/-Foxp3+CD4SP thymocytes from these two sources, we found that in vivo IL-2 in the mixed chimeras had up-regulated Bcl-2 and increased Foxp3+CD25+ Tregs without affecting Foxp3+CD25− Tregs (Figure 4B). Thus, in vivo IL-2 signaling overcame Bcl-2 repression in Foxp3+CD25+ Tregs and increased their in vivo frequency.
Figure 4. Effect of IL-2 on Bcl-2 expression and survival of thymic Tregs.
(A) Intracellular mBcl-2 expression in CD4SP thymocytes from B6 mice. Data are representative of 6 experiments.
(B) Intracellular mBcl-2 expression in Foxp3+CD4SP thymocytes of Il2-/- origin from intact Il2-/- mice and B6+Il2-/-→B6 mixed donor chimeras (left). Frequencies of Il2-/- origin Foxp3+CD4SP thymocyte subsets are also shown (right). Mean ± SE of 6 mice per group.
(C) Spontaneous thymocyte apoptosis in overnight culture of Xwt and Xsfy Tregs from B6 and Scurfy male mice expressing the Foxp3GFP reporter. Frequencies of Foxp3GFP+CD4SP thymocytes that were EtBr+ were normalized to Foxp3GFP-CD4SP thymocytes which were set equal to 0. Mean ± SE of triplicate cultures in 3 experiments.
(D) Survival of CD4SP thymocytes from AND.Rag2-/-.PCC (on B6xB10.A background) in overnight cultures. Mean ± SE of triplicate cultures in 3 experiments.
(E) mBcl-2 protein expression in Foxp3+CD25− CD4SP thymocytes from mice expressing constitutively active Stat5b transgenic molecules (STAT5-CA) normalized to B6 mice which was set equal to 100. Mean ± SE of 5 mice per group.
(F) Frequency of Foxp3+ CD4SP thymocytes. Mean ± SE of 5 mice.
**, p<0.001; ***, p<0.0001.
See also Figure S4.
To directly assess IL-2's ability to prevent Foxp3-induced apoptosis, we compared the impact of IL-2 on spontaneous apoptosis of Xwt and Xsfy Tregs from B6 and scurfy male mice (Figure 4C). Addition of IL-2 to overnight culture significantly reduced spontaneous apoptosis of Foxp3+ (Xwt) Tregs to levels identical with that of Foxp3− (Xsfy) Tregs (Figure 4C), indicating that IL-2 prevented cell death from Foxp3-induced apoptosis. To assess this same issue in a different way, we took advantage of the fact that, among CD4SP thymocytes in AND-PCC double transgenic mice that were on a Rag2-deficient B6xB10.A background, 8% were Foxp3+ and 92% were Foxp3− even though all AND CD4SP thymocytes had been signaled by identical PCC-I-Ek complexes in vivo. Only 20% of the Foxp3+ AND thymocytes survived in overnight culture compared to 70% of the Foxp3− AND thymocytes (Figure 4D). Most importantly, IL-2 improved the survival of Foxp3+ AND thymocytes to the identical level as Foxp3− AND thymocytes (Figure 4D), demonstrating that IL-2 cytokine signaling prevented Foxp3-induced apoptosis.
Having learned that IL-2 signaling increased Bcl-2 expression and prevented Foxp3-induced apoptosis, it would be expected that in vivo Treg frequencies would be increased by increased IL-2 signaling. In fact transgenic expression of constitutively active transgenic STAT5b molecules, a downstream effector of γc-mediated cytokine signaling, increased endogenous Bcl-2 expression in Foxp3+CD25− CD4SP thymocytes and increased in vivo Treg frequencies (Figures 4E and 4F), the latter reported previously (Burchill et al., 2007). Notably, constitutively active STAT5b transgenic mice displayed the same disproportionately high thymic Treg frequencies (~16%) (Figure 4F) as found in hBcl-2Tg and in Puma- and Bim-deficient mice (Figure 3D).
Effect of γc-deficiency on generation of Foxp3+ CD4SP thymocytes
Because IL-2 signaling prevented Foxp3-induced apoptosis and increased γc signaling increased thymic Treg cells, it might be inferred that γc-dependent cytokines were not present in sufficient amounts to signal all Foxp3+ thymocytes to survive. However, such a conclusion would require that thymocytes could express Foxp3 without having been cytokine signaled, which conflicts with the prevailing view that IL-2 signals are required to induce Foxp3 expression (Burchill et al., 2008; Lio and Hsieh, 2008). Consequently, we experimentally examined if γc-dependent cytokine signals were necessary for Foxp3 expression in developing thymocytes.
To do so, we introduced the hBcl-2Tg or Bim-deficiency into γc-deficient Il2rg-/- mice that otherwise contained very few Tregs or other thymocytes (Bayer et al., 2008; Burchill et al., 2007; Fontenot et al., 2005; Figure 5A). However, Il2rg-/- mice with either the hBcl-2Tg or Bim-deficiency contained significantly more Foxp3+CD4SP thymocytes (Figure 5A), documenting that γC signals were not required for Foxp3 expression if thymocytes were protected from Foxp3-induced apoptosis. Interestingly, the Foxp3+CD4SP thymocytes that were present in Il2rg-/- mice were phenotypically CD25- (Figure 5A). To confirm these findings in mice with physiologic numbers of thymocytes, we conditionally deleted γC in pre-selection DP thymocytes in mice containing floxed Il2rg alleles and the E8IIICre transgene (McCaughtry et al., 2012). We confirmed that DP thymocytes and their CD4SP progeny in E8IIICre-Il2rgfl/fl mice lacked γC expression (Figure 5B, left). γC-deficiency abrogated the appearance of Foxp3+CD4SP thymocytes but did not effect Foxp3−CD4SP thymocyte numbers (Figure 5B, middle). As in germline Il2rg-/- mice, the hBcl-2Tg restored Foxp3+CD4SP thymocyte numbers (Figure 5B, middle) and the rescued Foxp3+CD4SP thymocytes were CD25− (Figure 5B, right). Thus, Foxp3+CD4SP thymocytes can arise without γc-dependent cytokine signals but, in the absence of γc-dependent cytokine signals, the Foxp3+ cells remain CD25- and susceptible to Foxp3-induced cell death.
Figure 5. γc-independent expression of Foxp3.
(A) Foxp3+CD4SP thymocytes in germline Il2rg-/- (γc-deficient) mice. Numbers in histograms indicate frequency of cells within box. Mean ± SE of 5-8 mice per group.
(B) γc expression and its effect on DP and CD4SP thymocytes from the indicated mice. Numbers in histograms indicate frequency of cells within box. Mean ± SE of 6 mice per group.
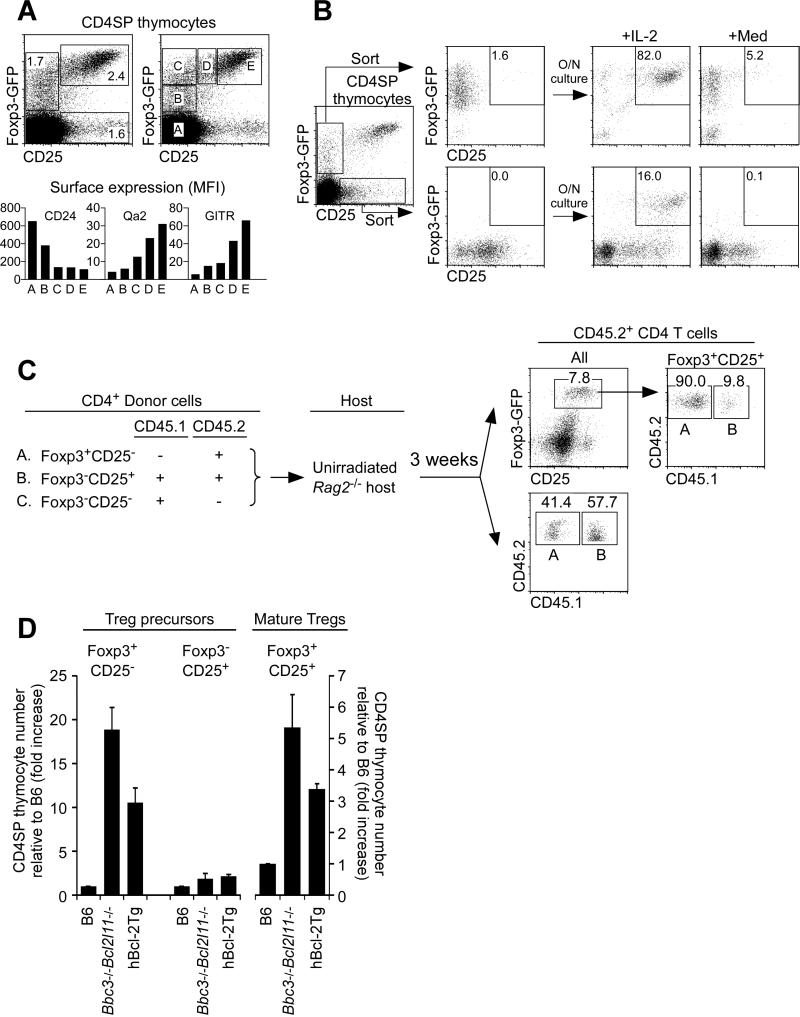
We then assessed if Foxp3+CD25− cells participate in normal Treg development by differentiating into mature Foxp3+CD25+ cells in the thymus. In principle, mature Foxp3+CD25+ cells could arise from CD4SP (Foxp3−CD25−) thymocytes along two developmental pathways: (a) by up-regulation of Foxp3 to become Foxp+CD25− precursors that rely on IL-2 signals to survive and differentiate into mature Tregs (i.e. Foxp3−CD25− →Foxp3+CD25− → Foxp3+CD25+), and (b) by up-regulation of CD25 to become Foxp3−CD25+ precursors that must then be signaled by IL-2 to express Foxp3 and differentiate into mature Treg cells (i.e. Foxp3−CD25− → Foxp3−CD25+ → Foxp3+CD25+). In fact both Foxp3+CD25− and Foxp3−CD25+ putative precursors were in normal B6 mice (Figure 6A, top), with the current view being that mature Tregs arise only from Foxp3−CD25+ precursors in response to IL-2 signaling (Burchill et al., 2008; Lio and Hsieh, 2008). However, analysis of intermediates along the Foxp3+CD25− precursor pathway revealed that surface CD24 expression progressively declined, while surface Qa-2 and GITR expression progressively increased, to become Foxp3+CD25+ mature Treg cells (Figure 6A, bottom), suggesting that mature Treg cells also developed from Foxp3+CD25− precursors.
Figure 6. Foxp3+CD25- precursors of Foxp3+CD25+ mature Tregs.
(A) CD4SP thymocytes from Foxp3-GFP reporter mice (Bettelli et al., 2006) (top) and flow cytometric analysis of gated subsets (A-E) (bottom). Data are representative of 4 experiments.
(B) In vitro differentiation of sorted Foxp3+CD25− and Foxp3−CD25+ CD4SP thymocytes. Data are representative of 6 experiments.
(C) In vivo development of Foxp3+CD25+ Tregs from two Treg precursor subsets. CD4 cells were electronically sorted into subsets of Foxp3-GFP+CD25− cells (A), Foxp3-GFP−CD25+ cells (B), and Foxp3-GFP−CD25− cells (C) as indicated (left) and transferred together into unirradiated Rag2-/- host mice. Each host mouse received 3×105 donor cells of A, 1×105 donor cells of B, and 1×106 donor cells of C. Three weeks later, host spleen and LN T cells were analyzed (right). Data are representative of 4 host mice analyzed in 2 independent experiments. (D) Effects of Puma-Bim double deficiency and hBcl-2Tg expression on precursor and mature thymic Tregs. Each CD4SP thymocyte subset was compared relative to B6 mice which was set equal to 1. Mean ± SE of 4-9 mice per group.
See also Figure S5.
To determine if Foxp3+CD25− precursors could actually differentiate into mature Treg cells, we placed them in overnight culture with IL-2 and found that they differentiated into Foxp3+CD25+ thymocytes even more extensively than did Foxp3−CD25+ precursors (Figure 6B). To see if Foxp3+CD25− precursors differentiated into mature Tregs in vivo, we adoptively transferred Foxp3+CD25− and Foxp3−CD25+ CD4 cells that were CD45.2 (along with Foxp3−CD25− CD4 cells that were CD45.1 as an in vivo source of IL-2) into unirradiated Rag2-/- host mice (Figure 6C). Three weeks later Foxp3+CD25− cells had differentiated into Foxp3+CD25+ mature Tregs, with 90% of the mature Tregs derived from Foxp3+CD25− precursors and 10% from Foxp3−CD25+ precursors, even though total CD45.2 CD4 T cells in host mice were derived almost equally from both (Figure 6C; Figure S5A). Notably, mature Tregs derived from Foxp3+CD25− precursor cells were functionally suppressive in vitro (Figure S5A, bottom).
We conclude that Foxp3+CD25− precursors do normally arise, but they require γc-mediated cytokine signals to survive and differentiate into mature Foxp3+CD25+ Tregs.
Competition for γc-dependent cytokines
Having determined that Foxp3+CD25− precursors can differentiate into Foxp3+CD25+ mature Tregs, we examined how they were affected by hBcl-2Tg expression and Puma-Bim deficiency. Both experimental maneuvers substantially increased Foxp3+CD25− precursors by10-20 fold and modestly increased Foxp3+CD25+ mature Tregs by 3-6 fold, but had little effect on Foxp3−CD25+ precursors (Figure 6D; Figure S5B). That Foxp3+CD25− precursors were markedly increased by Bcl-2Tg expression and Puma-Bim deficiency, and were increased to a much greater degree than Foxp3+CD25+ mature Tregs, indicated that: (a) most Foxp3+CD25− precursors normally fail to survive, and (b) there is insufficient γc-mediated signaling to support the differentiation of all Foxp3+CD25− precursors into Foxp3+CD25+ mature Tregs. This reasoning predicts a life-and-death competition among newly arising Foxp3+CD25- thymocytes for limiting amounts of γc cytokine signaling in the thymus.
To detect competition among Foxp3+ thymocytes for γc cytokine signaling, we utilized the Foxp3Cre transgene (Zhou et al., 2008) to delete γC expression only in Foxp3+ thymocytes in Il2rgfl/fl mice (Figure 7A). In Foxp3Cre-Il2rgfl/fl mice, Foxp3+CD4SP thymocytes were greatly reduced but were increased by the hBcl-2Tg which rescued mainly Foxp3+CD25− precursor cells (Figure 7A; Figure S6A). As a result of their deficiency in Foxp3+ cells, Foxp3Cre-Il2rgfl/fl mice suffered from aggressive autoimmune disease with stunted growth, multi-organ lymphocytic infiltrations, and death at 3 weeks of age, while hBcl-2Tg mice remained disease-free longer and survived until 6-7 weeks of age (Figure S6B-D).
Figure 7. Newly generated Foxp3+ thymocytes compete for γC-mediated survival signals.
(A) Effect of Foxp3-specific γc-deletion on γc expression (left) and Treg cell number (right) in thymi of 3-4 wk old mice. Mean ± SE of 4-5 mice per group.
(B) Frequency (left) and number (right) of Foxp3+ cells among CD4SP thymocytes in competitive mixed donor chimeras. CD4SP thymocytes of B6 donor origin are γc+ (closed symbols) and displayed in top panels; CD4SP thymocytes of Il2rgfl/fl donor origin are γcfl (open symbols) and displayed in bottom panels. Each circle and square represents an individual mouse. The star indicates intact B6 mice.
***, p<0.0001.
See also Figure S6.
To detect competition among Foxp3+ thymocytes for γc-mediated survival signals, we constructed mixed donor chimeras whose Foxp3+CD4SP thymocytes would be a mixture of γc+ and γc-deficient (γcfl) cells. The chimeras were designated B6+Foxp3Cre-Il2rgfl/fl →B6 and B6+Foxp3Cre-Il2rgfl/fl.hBcl-2Tg→B6 and remained healthy because of B6 origin Tregs. Importantly, B6 origin Tregs were the only Treg cells that expressed γc and would compete for γc-mediated survival signals in these chimeras. Indeed, in these chimeras, Foxp3+ thymocytes of B6 origin were overwhelmingly Foxp3+CD25+ indicating that they had received γc cytokine signals, whereas γcflFoxp3+ thymocytes of Il2rgfl/fl origin were mainly Foxp3+CD25− (Figure S6E). Analyzed 8-10 wks after construction, individual chimeras contained different numbers of B6 and Il2rg-/- origin CD4SP thymocytes (Figure 7B). Importantly, as the number of B6 donor origin CD4SP thymocytes increased, the frequency of B6 origin CD4SP thymocytes that were Foxp3+ declined (Figure 7B, top left) and their absolute number plateaued at ~0.8 million Foxp3+ cells per thymus (Figure 7B, top right). In contrast, γcfl cells in the same thymi behaved differently. As the number of Il2rgfl/fl donor origin CD4SP thymocytes increased, the frequency of Il2rgfl/fl origin CD4SP thymocytes that were Foxp3+ was constant (Figure 7B, bottom left) and their absolute number increased accordingly, which was best appreciated in hBcl-2Tg thymocytes because of their greater survival (Figure 7B, bottom).
Together, these results revealed that: (a) γc+ Treg cells in the thymus competed for γc-mediated survival signals, and (b) the thymus contained only enough γc cytokines to support fewer than 1 million Foxp3+ thymocytes.
Discussion
The present study provides a new perspective on the impact and sequence of Foxp3 expression during Treg cell development in the thymus. Foxp3 induced a characteristic proapoptotic protein signature that was lethal to developing Treg cells unless counterbalanced by γc-mediated survival signals. However, γc-mediated survival signals were present in the thymus in limiting amounts that could support fewer than 1 million Foxp3+ cells. As a result, most newly arising Foxp3+CD4SP thymocytes underwent Foxp3-induced cell death. The cells most prone to dying of Foxp3-induced apoptosis were Foxp3+CD25− thymocytes, which strictly required γc-mediated signals for their survival and further differentiation into mature Foxp3+CD25+ Treg cells.
Foxp3-induced apoptosis resulted from increased activity of the pro-apoptotic proteins Bim and Puma, and decreased expression of the pro-survival protein Bcl-2. Because the Foxp3 protein functions as a transcription factor, it was readily understandable how Foxp3 could increase Puma expression but it was unclear how Foxp3 could induce Bim protein phosphorylation. We determined that it did so by suppressing expression of at least three members of the DUSP gene family (DUSP1,5,6) that encode dual specific phosphatases that function as negative regulators of JNK kinases to prevent JNK induced Bim phosphorylation. Detailed analysis of the promoter region of one DUSP gene (DUSP6) revealed conserved forkhead family consensus binding motifs that were responsible for direct suppression by Foxp3 of DUSP promoter activity. As a result, expression of Foxp3 proteins resulted in a unique proapoptotic protein signature that we characterized as Puma++p-Bim++p-JNK++DUSP6−. That this pro-apoptotic protein signature promotes cell death was shown by significantly improved survival of Foxp3+ cells in vivo in mice deficient in Puma and Bim. Foxp3 was also shown to reduce Bcl-2 expression, and it did so by reducing Bcl2 promoter activity. Reciprocally, transgenic over-expression of Bcl-2 proteins prevented Foxp3-induced apoptosis.
Genetic disruption of intracellular death pathways by either transgenic over-expression of pro-survival proteins or deficient expression of pro-apoptotic proteins rescued newly arising Tregs from Foxp3-induced cell death and revealed that the actual frequency of newly arising Tregs among CD4SP thymocytes was substantially higher than the 3-5% normally observed. Notably, the basis for increased Tregs in the thymus of mice with impaired intracellular death pathways has been understood quite differently by others (Zhan et al., 2011). In their study, disruption of the Bcl-2 regulated apoptotic pathway was thought to increase Treg frequency in the thymus as a consequence of impaired TCR-signaled clonal deletion (Zhan et al., 2011). In contrast, the present study documents that Foxp3 proteins are themselves pro-apoptotic but require intact intracellular death pathways to induce apoptosis. Indeed, Foxp3 was shown to induce a characteristic pro-apoptotic protein signature (Puma++p-Bim++p-JNK++DUSP6−) and to promote apoptosis of all cell types in which Foxp3 was expressed, even cells that never expressed or were never signaled by their TCR. Foxp3-induced apoptosis was prevented by the γc-dependent cytokine IL-2 or, experimentally, by Puma-Bim deficiency or hBcl-2 transgene expression which explains why Bim deficiency was previously found to restore appearance of Foxp3+ cells in the absence of IL-2 signaling (Barron et al., 2010).
Even though γc-dependent cytokine signals are necessary for Treg survival, we were surprised that the absolute number of γc-sufficient Foxp3+ cells that could survive in an individual thymus was fewer than 1 million. These findings revealed that γc-dependent survival signals are not present in sufficient amounts to support all newly arising Tregs in the thymus, so that most succumb to Foxp3-induced apoptosis. Consequently, the frequency of γc+CD4SP thymocytes that expressed Foxp3 in competitive chimeras increased substantially when the absolute number was fewer than ~1 million. Indeed, the frequency of newly arising γc+Tregs in competitive chimeras with fewer than ~1 million competing γc+Tregs was as high as that observed in Bcl-2Tg and PUMA-Bim double deficient mice. Consequently, in addition to competing for limiting numbers of antigenic complexes (Bautista et al., 2009), newly arising Tregs must compete for limiting amounts of γc-survival signals.
It has been thought that CD4 thymocytes developing into Tregs first up-regulate CD25, a component of the high affinity IL-2 receptor complex, to become Foxp3−CD25+ precursors that are then signaled by IL-2 to initiate Foxp3 expression and become Foxp3+CD25+ mature Tregs (Burchill et al., 2008; Lio and Hsieh, 2008). While we think that Foxp3−CD25+ precursors constitute one pathway by which mature Tregs arise in the thymus, the present study revealed another pathway in which CD4 thymocytes first up-regulate Foxp3 to become Foxp3+CD25− precursors that are then subsequently signaled to become Foxp3+CD25+ mature Tregs. In fact, because Puma-Bim deficiency and hBcl-2Tg expression mainly rescued Foxp3+CD25− precursors, we think the Foxp3+CD25− precursor pathway is a major pathway by which mature Treg cells develop in the thymus.
While Foxp3+CD25- precursors can be maintained by IL-7 signals (Bayer et al., 2008), we think their expression of Foxp3 is the result of TCR-CD28 costimulation (Tai et al., 2005). Consistent with this view, enhancement of NF-κB activity, which is a downstream result of TCR-CD28 costimulation, markedly increased Foxp3+CD25− precursors in the thymus (Long et al., 2009). Because TCR-CD28 costimulation can also signal CD25 up-regulation, it was conceivable that TCR-CD28 costimulation also signaled Foxp3+CD25− precursors to express CD25 and to become mature Treg cells. However, Foxp3+CD25− precursors failed to become CD25+ in γc-deficient mice, even though γc-deficiency neither impairs TCR-CD28 costimulation nor CD25 expression. Consequently, we think that Foxp3+CD25− precursors are induced to express CD25 by γc-mediated signals transduced by intermediate affinity IL-2 βγ receptor complexes.
In conclusion, the present study has revealed that Foxp3 is pro-apoptotic and responsible for imposing cytokine dependence on newly arising Tregs. This study significantly alters understanding of Treg cell generation in the thymus and provides new fundamental insights into their development.
EXPERIMENTAL PROCEDURES
Animals
Two Foxp3 reporters were utilized: Foxp3GFP referred to FoxP3-GFP-hCre transgenic mice (Zhou et al., 2008); Foxp3-GFP referred to Foxp3-GFP knockin mice (Bettelli et al., 2006). All mice were cared for in accordance with National Institutes of Health guidelines.
Foxp3 transgenic mice
cDNA encoding mouse Foxp3 was introduced into a hCD2-based vector to generate Foxp3Tg lines A10 and C10, and was introduced into the E8III-CD8α enhancer-promoter construct TG-31 (Sarafova et al., 2005) to generate the Foxp3Tg line T3.
Targetting γc-deficiency to Foxp3+ cells
Male conditional knockout mice with targeted deletion of γc in Foxp3+ cells were generated by mating Il2rgfl/fl mice with Foxp3-GFP-hCre mice.
Bone marrow chimeras
Radiation bone marrow chimeras were constructed by reconstituting lethally irradiated (950R) host mice with a total of 10-15×106 T cell-depleted bone marrow cells 6h after irradiation. Chimeric mice were analyzed 8 weeks after reconstitution.
In vitro cultures
2×106 thymocytes were cultured overnight in medium or rIL-2 (200U/ml) and then stained with EtBr. Relative frequency of EtBr+ cells was calculated as follows: (%EtBr+ (experimental mice) - %EtBr+ (B6 mice))/(100 - %EtBr+ B6 mice). For DiOC6 staining, DiOC6 was added to culture 30 min before harvesting cells. Relative frequency of DiOC6lo cells was calculated using the same formula as EtBr+.
Adoptive transfer and in vitro functional assays
Where indicated, Rag2-/- mice were injected with 3 different CD45-distinct CD4 subpopulations: (a) Foxp3-GFP+CD25− (3×105); (b) Foxp3-GFP−CD25+ (1×105); and Foxp3-GFP−CD25− (1×106). 3 weeks later, donor origin LN and spleen cells were analyzed and assessed functionally in suppression assays utilizing CFSE-labeled CD4+CD25− responder T cells stimulated for 72h with anti-CD3 (1μg/ml) in the presence of added APCs.
Statistical analysis
Two-tailed Student's t-test was used for statistical analyses. P values of 0.01 or less were considered significant.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to Pierre Henkart for editorial assistance; J. Bluestone, V. Kuchroo, A. Strasser, and M.A. Farrar for mice; and R. Hodes, J.-H. Park, and N. Taylor for reading the manuscript. This research was supported by the Intramural Research Program of the NIH, NCI, Center for Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Apostolou I, Sarukhan A, Klein L, von Boehmer H. Origin of regulatory T cells with known specificity for antigen. Nat Immunol. 2002;3:756–763. doi: 10.1038/ni816. [DOI] [PubMed] [Google Scholar]
- Barron L, Dooms H, Hoyer KK, Kuswanto W, Hofmann J, O'Gorman WE, Abbas AK. Cutting edge: mechanisms of IL-2-dependent maintenance of functional regulatory T cells. J Immunol. 2010;185:6426–6430. doi: 10.4049/jimmunol.0903940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista JL, Lio CW, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, Hsieh CS. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat Immunol. 2009;10:610–617. doi: 10.1038/ni.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Boise LH, Gonzalez-Garcia M, Postema CE, Ding L, Lindsten T, Turka LA, Mao X, Nunez G, Thompson CB. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CW, Vegoe AL, Hsieh CS, Jenkins MK, Farrar MA. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity. 2008;28:112–121. doi: 10.1016/j.immuni.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- Chi H, Barry SP, Roth RJ, Wu JJ, Jones EA, Bennett AM, Flavell RA. Dynamic regulation of pro- and anti-inflammatory cytokines by MAPK phosphatase 1 (MKP-1) in innate immune responses. Proc Natl Acad Sci U S A. 2006;103:2274–2279. doi: 10.1073/pnas.0510965103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- Gavin MA, Rasmussen JP, Fontenot JD, Vasta V, Manganiello VC, Beavo JA, Rudensky AY. Foxp3-dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- Hsieh CS, Liang Y, Tyznik AJ, Self SG, Liggitt D, Rudensky AY. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J Exp Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, Caton AJ. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat Immunol. 2001;2:301–306. doi: 10.1038/86302. [DOI] [PubMed] [Google Scholar]
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30:531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Qin ZHS, Liu RH, Wang LZ, Li WQ, Li XZ, Wu LP, Du ZW, Lyons R, Liu CG, et al. FOXP3 Orchestrates H4K16 Acetylation and H3K4 Trimethylation for Activation of Multiple Genes by Recruiting MOF and Causing Displacement of PLU-1. Mol Cell. 2011;44:770–784. doi: 10.1016/j.molcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri R, Kasprowicz D, Cox T, Mortrud M, Appleby MW, Brunkow ME, Ziegler SF, Ramsdell F. The amount of scurfin protein determines peripheral T cell number and responsiveness. J Immunol. 2001;167:6312–6320. doi: 10.4049/jimmunol.167.11.6312. [DOI] [PubMed] [Google Scholar]
- Kim GY, Ligons DL, Hong C, Luckey MA, Keller HR, Tai X, Lucas PJ, Gress RE, Park JH. An in vivo IL-7 requirement for peripheral Foxp3+ regulatory T cell homeostasis. J Immunol. 2012;188:5859–5866. doi: 10.4049/jimmunol.1102328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh KP, Sundrud MS, Rao A. Domain requirements and sequence specificity of DNA binding for the forkhead transcription factor FOXP3. PLoS One. 2009;4:e8109. doi: 10.1371/journal.pone.0008109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer S, Schimpl A, Hunig T. Immunopathology of interleukin (IL) 2-deficient mice: thymus dependence and suppression by thymus-dependent cells with an intact IL-2 gene. J Exp Med. 1995;182:1769–1776. doi: 10.1084/jem.182.6.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity. 2012;37:1–12. doi: 10.1016/j.immuni.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley R, Ewings KE, Hadfield K, Cook SJ. Regulatory phosphorylation of Bim: sorting out the ERK from the JNK. Cell Death Differ. 2005;12:1008–1014. doi: 10.1038/sj.cdd.4401688. [DOI] [PubMed] [Google Scholar]
- Lin W, Haribhai D, Relland LM, Truong N, Carlson MR, Williams CB, Chatila TA. Regulatory T cell development in the absence of functional Foxp3. Nature Immunology. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- Lio CW, Hsieh CS. A two-step process for thymic regulatory T cell development. Immunity. 2008;28:100–111. doi: 10.1016/j.immuni.2007.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shepherd EG, Nelin LD. MAPK phosphatases--regulating the immune response. Nat Rev Immunol. 2007;7:202–212. doi: 10.1038/nri2035. [DOI] [PubMed] [Google Scholar]
- Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Malek TR, Yu A, Vincek V, Scibelli P, Kong L. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rbeta-deficient mice. Implications for the nonredundant function of IL-2. Immunity. 2002;17:167–178. doi: 10.1016/s1074-7613(02)00367-9. [DOI] [PubMed] [Google Scholar]
- Marson A, Kretschmer K, Frampton GM, Jacobsen ES, Polansky JK, MacIsaac KD, Levine SS, Fraenkel E, von Boehmer H, Young RA. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, Grinberg A, Love P, Feigenbaum L, Erman B, Singer A. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med. 2012;209:2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pobezinsky LA, Angelov GS, Tai X, Jeurling S, Van Laethem F, Feigenbaum L, Park JH, Singer A. Clonal deletion and the fate of autoreactive thymocytes that survive negative selection. Nat Immunol. 2012;13:569–578. doi: 10.1038/ni.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–440. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- Sarafova SD, Erman B, Yu Q, Van Laethem F, Guinter T, Sharrow SO, Feigenbaum L, Wildt KF, Ellmeier W, Singer A. Modulation of coreceptor transcription during positive selection dictates lineage fate independently of TCR/coreceptor specificity. Immunity. 2005;23:75–87. doi: 10.1016/j.immuni.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Sentman CL, Shutter JR, Hockenbery D, Kanagawa O, Korsmeyer SJ. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991;67:879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- Sunters A, Fernandez de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- Tai X, Cowan M, Feigenbaum L, Singer A. CD28 costimulation of developing thymocytes induces Foxp3 expression and regulatory T cell differentiation independently of interleukin 2. Nat Immunol. 2005;6:152–162. doi: 10.1038/ni1160. [DOI] [PubMed] [Google Scholar]
- Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoietin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Zhang Y, Gray D, Carrington EM, Bouillet P, Ko HJ, O'Reilly L, Wicks IP, Strasser A, Lew AM. Defects in the Bcl-2-regulated apoptotic pathway lead to preferential increase of CD25 low Foxp3+ anergic CD4+ T cells. J Immunol. 2011;187:1566–1577. doi: 10.4049/jimmunol.1100027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jeker LT, Fife BT, Zhu S, Anderson MS, McManus MT, Bluestone JA. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo T, Wang LZ, Morrison C, Chang X, Zhang HM, Li WQ, Liu Y, Wang Y, Liu XL, Chan MWY, et al. FOXP3 is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.