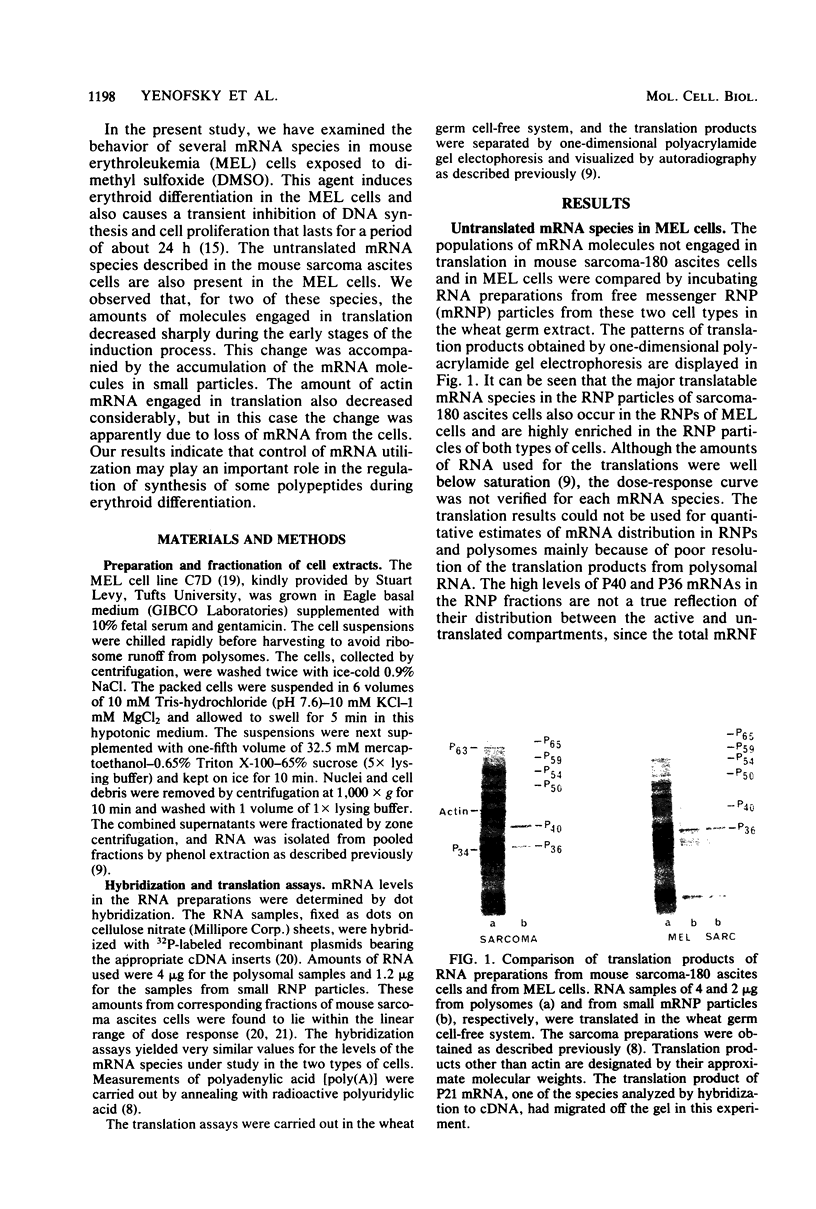
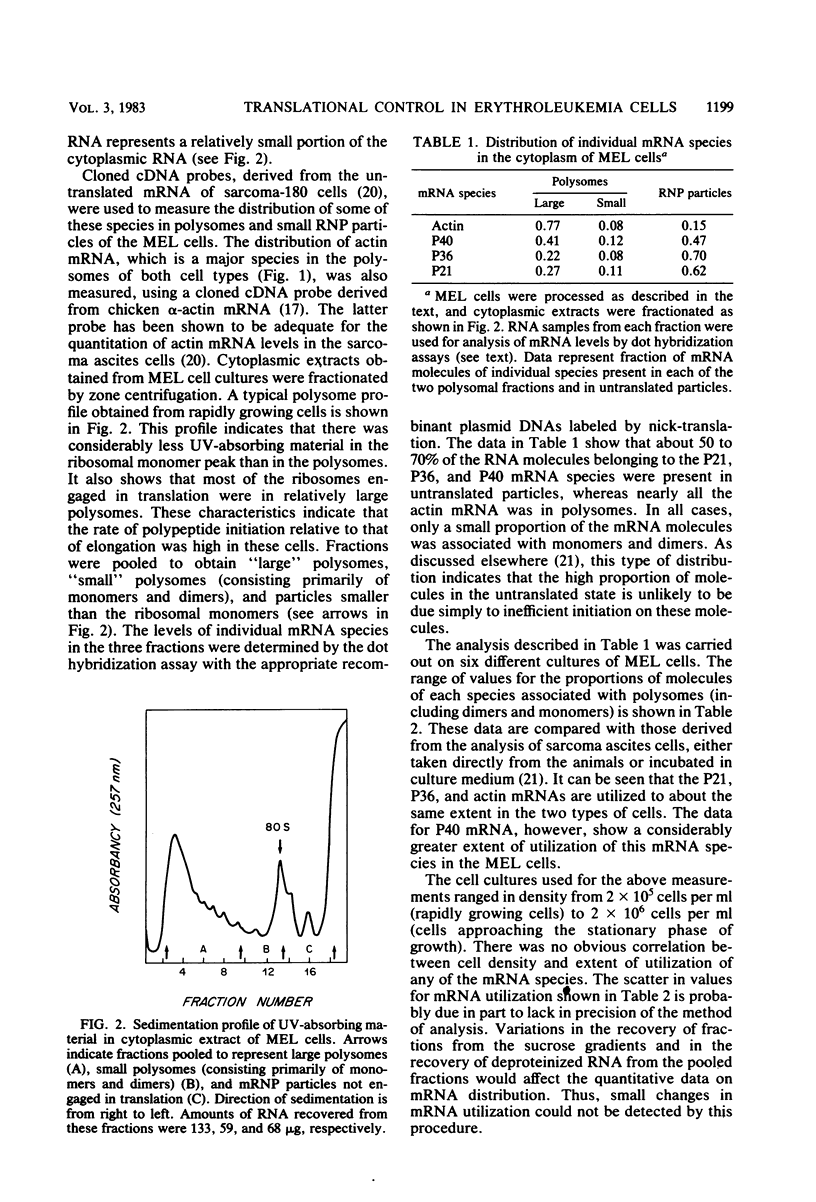
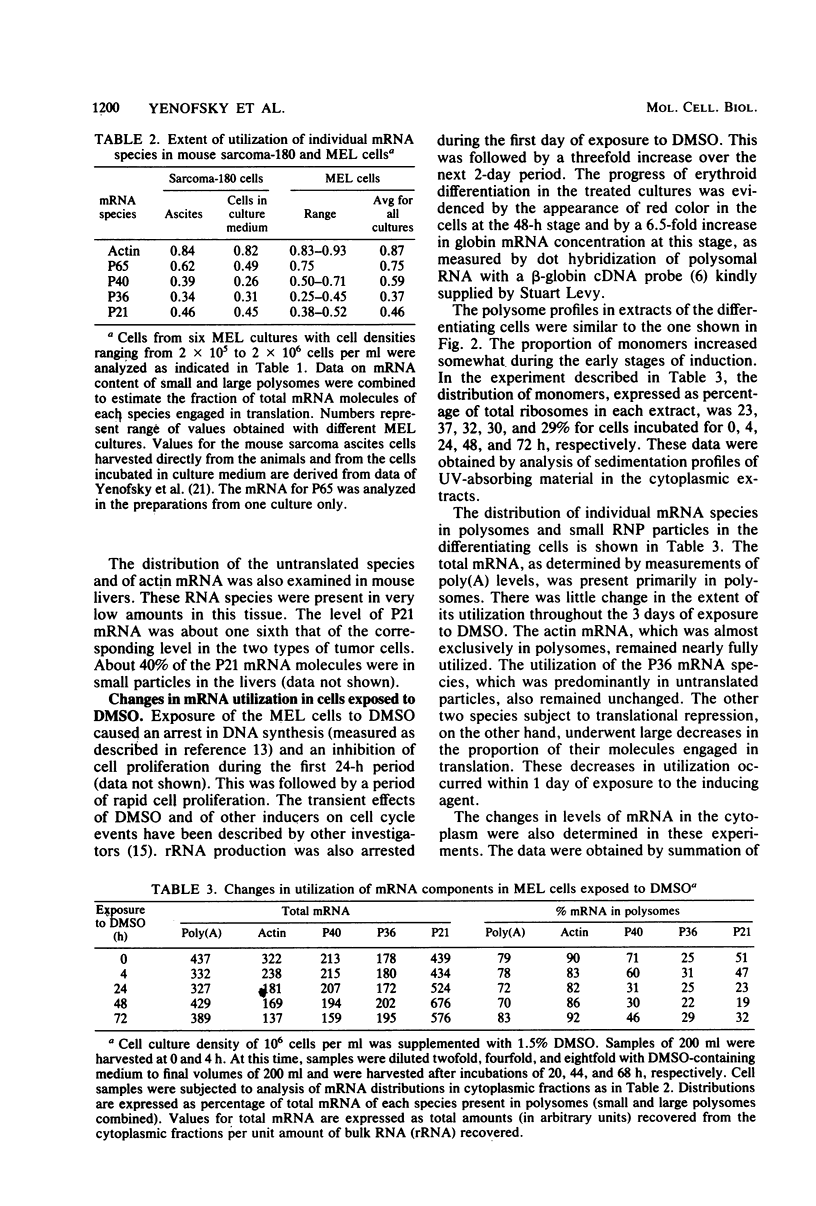
Abstract
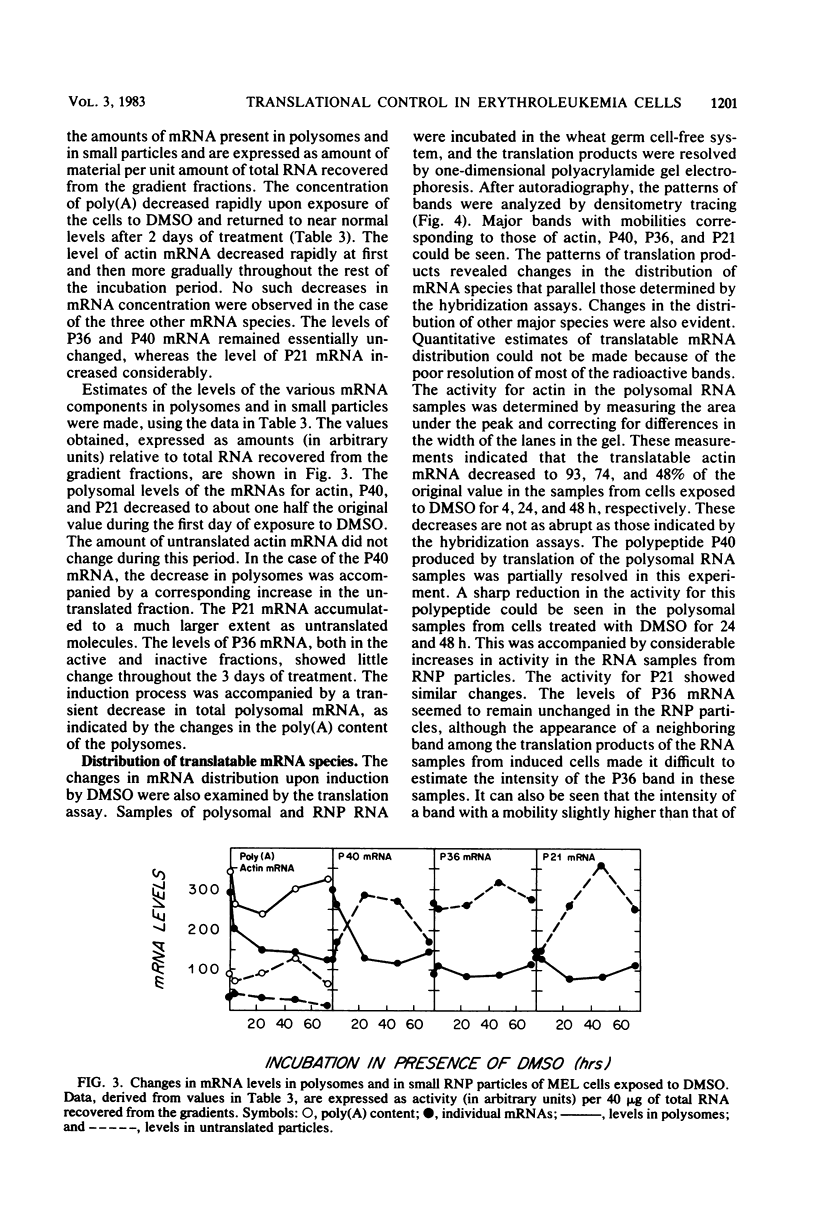
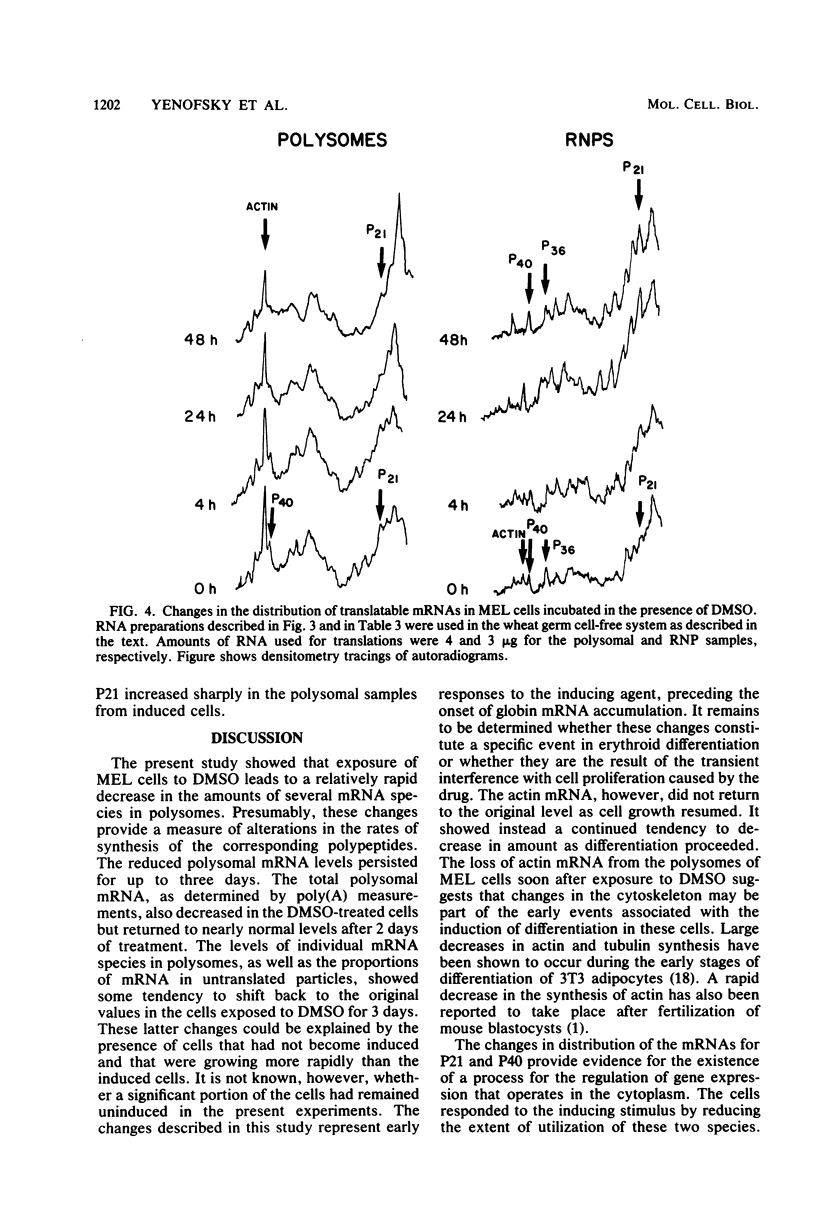
Mouse erythroleukemia cells contain several abundant mRNA species that occur to a considerable extent as untranslated molecules. For two of these species, which code for polypeptides P40 and P21, the proportion of molecules engaged in translation decreases rapidly after exposure of the cells to dimethyl sulfoxide. The extent of utilization of a third species, the P36 mRNA, is not altered. The rate of production of the P40 mRNA does not appear to be affected in the dimethyl sulfoxide-treated cells. The P21 mRNA appears to be produced in increasing amounts, leading to a large accumulation of untranslated molecules in the cytoplasm. The mRNA for actin remains nearly fully utilized during this process, but its intracellular concentration decreases, thus resulting in a reduction in the amounts present in polysomes. The results indicate that some mRNA species in mouse tumor cells are subject to a translational repression process that can serve to regulate selectively the extent of expression of the corresponding genes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abreu S. L., Brinster R. L. Actin and tubulin synthesis in murine blastocyst outgrowths. Exp Cell Res. 1978 Aug;115(1):89–94. doi: 10.1016/0014-4827(78)90405-6. [DOI] [PubMed] [Google Scholar]
- Braude P., Pelham H., Flach G., Lobatto R. Post-transcriptional control in the early mouse embryo. Nature. 1979 Nov 1;282(5734):102–105. doi: 10.1038/282102a0. [DOI] [PubMed] [Google Scholar]
- Cascio S. M., Wassarman P. M. Program of early development in the mammal: post-transcriptional control of a class of proteins synthesized by mouse oocytes and early embryos. Dev Biol. 1982 Feb;89(2):397–408. doi: 10.1016/0012-1606(82)90328-1. [DOI] [PubMed] [Google Scholar]
- Cervera M., Dreyfuss G., Penman S. Messenger RNA is translated when associated with the cytoskeletal framework in normal and VSV-infected HeLa cells. Cell. 1981 Jan;23(1):113–120. doi: 10.1016/0092-8674(81)90276-2. [DOI] [PubMed] [Google Scholar]
- Curtis P. J., Mantei N., Weissmann C. Characterization and kinetics of synthesis of 15S beta-globin RNA, a putative precursor of beta-globin mRNA. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):971–984. doi: 10.1101/sqb.1978.042.01.098. [DOI] [PubMed] [Google Scholar]
- Geoghegan T. E., Sonenshein G. E., Brawerman G. Characteristics and polyadenylate content of the actin messenger RNA of mouse sarcoma-180 ascites cells. Biochemistry. 1978 Oct 3;17(20):4200–4207. doi: 10.1021/bi00613a014. [DOI] [PubMed] [Google Scholar]
- Geoghegan T., Cereghini S., Brawerman G. Inactive mRNA-protein complexes from mouse sarcoma-180 ascites cells. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5587–5591. doi: 10.1073/pnas.76.11.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havaranis A. S., Heywood S. M. Cytoplasmic utilization of liposome-encapsulated myosin heavy chain messenger ribonucleoprotein particles. During muscle cell differentiation. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6898–6902. doi: 10.1073/pnas.78.11.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante A. A., Heilmann L. J. Distribution of messenger ribonucleic acid in polysomes and nonpolysomal particles of sea urchin embryos: translational control of actin synthesis. Biochemistry. 1981 Jan 6;20(1):1–8. doi: 10.1021/bi00504a001. [DOI] [PubMed] [Google Scholar]
- Krüger C., Benecke B. J. In vitro translation of Drosophila heat-shock and non--heat-shock mRNAs in heterologous and homologous cell-free systems. Cell. 1981 Feb;23(2):595–603. doi: 10.1016/0092-8674(81)90155-0. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Krsmanovic V., Brawerman G. Attachment of ribosomes to membranes during polysome formation in mouse sarcoma 180 cells. J Cell Biol. 1971 Jun;49(3):683–691. doi: 10.1083/jcb.49.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMullen M. D., Shaw P. H., Martin T. E. Characterization of poly(A+)RNA in free messenger ribonucleoprotein and polysomes of mouse Taper ascites cells. J Mol Biol. 1979 Aug 25;132(4):679–694. doi: 10.1016/0022-2836(79)90382-6. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Rifkind R. A., Marks P. A. Chemically induced murine erythroleukemic differentiation. Biochim Biophys Acta. 1980 Sep 22;605(3):325–346. doi: 10.1016/0304-419x(80)90015-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal E. T., Hunt T., Ruderman J. V. Selective translation of mRNA controls the pattern of protein synthesis during early development of the surf clam, Spisula solidissima. Cell. 1980 Jun;20(2):487–494. doi: 10.1016/0092-8674(80)90635-2. [DOI] [PubMed] [Google Scholar]
- Schwartz R. J., Haron J. A., Rothblum K. N., Dugaiczyk A. Regulation of muscle differentiation: cloning of sequences from alpha-actin messenger ribonucleic acid. Biochemistry. 1980 Dec 9;19(25):5883–5890. doi: 10.1021/bi00566a034. [DOI] [PubMed] [Google Scholar]
- Spiegelman B. M., Farmer S. R. Decreases in tubulin and actin gene expression prior to morphological differentiation of 3T3 adipocytes. Cell. 1982 May;29(1):53–60. doi: 10.1016/0092-8674(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Vinton E. C., Woytowicz J. M., Levy S. B. Biological characteristics of type C viruses isolated from different Friend erythroleukaemic cells. J Gen Virol. 1982 Mar;59(Pt 1):73–81. doi: 10.1099/0022-1317-59-1-73. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Cloned complementary deoxyribonucleic acid probes for untranslated messenger ribonucleic acid components of mouse sarcoma ascites cells. Biochemistry. 1982 Aug 17;21(17):3909–3913. doi: 10.1021/bi00260a001. [DOI] [PubMed] [Google Scholar]
- Yenofsky R., Bergmann I., Brawerman G. Messenger RNA species partially in a repressed state in mouse sarcoma ascites cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5876–5880. doi: 10.1073/pnas.79.19.5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zähringer J., Baliga B. S., Munro H. N. Novel mechanism for translational control in regulation of ferritin synthesis by iron. Proc Natl Acad Sci U S A. 1976 Mar;73(3):857–861. doi: 10.1073/pnas.73.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]