Abstract
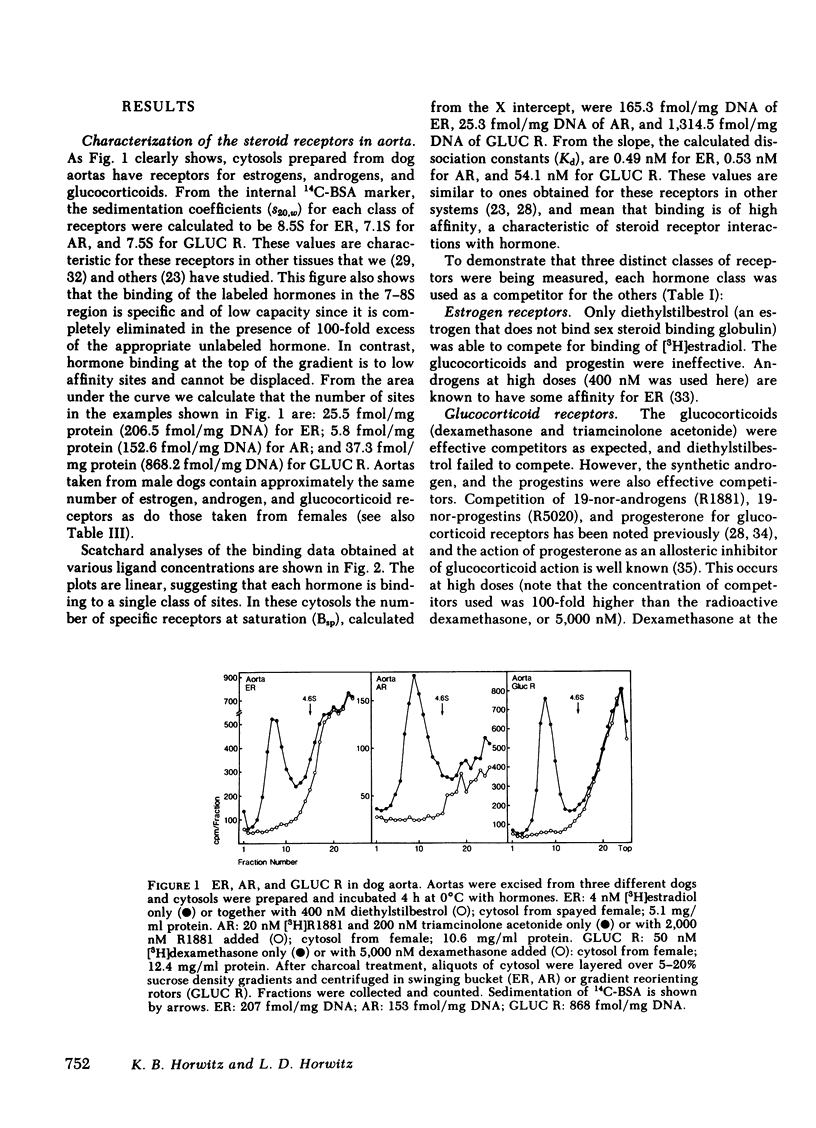
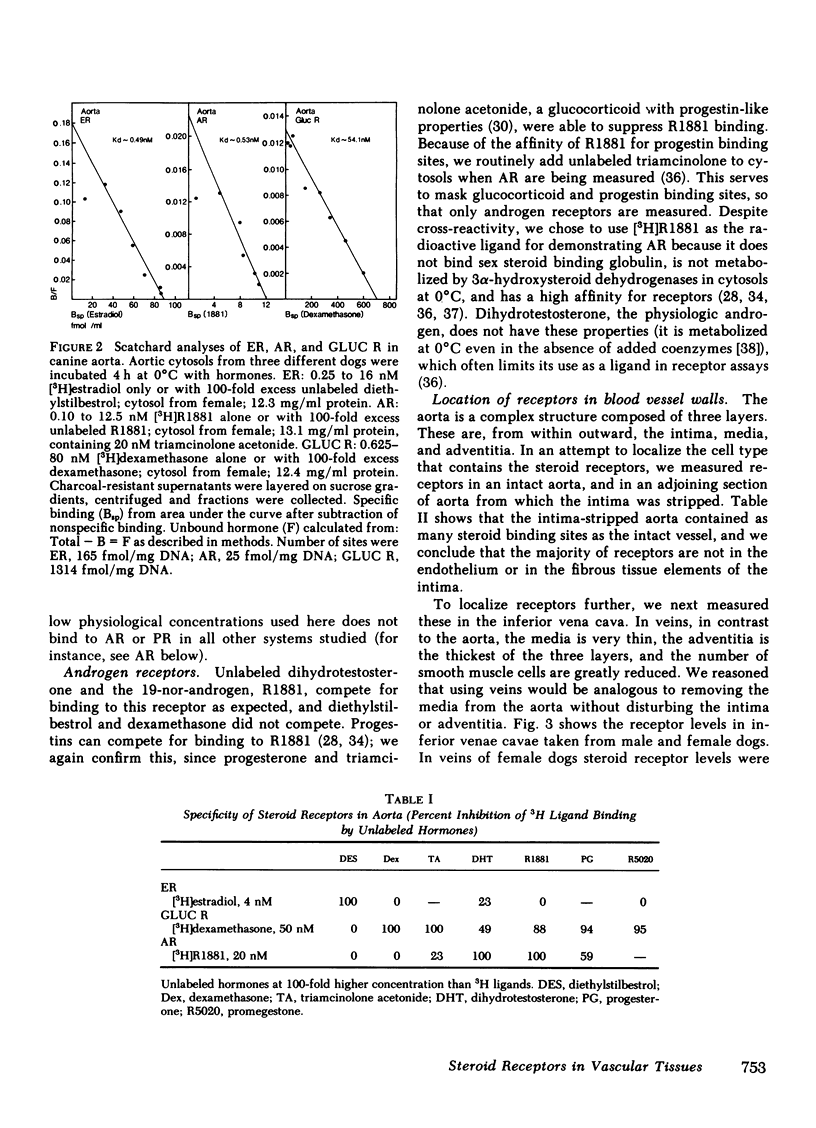
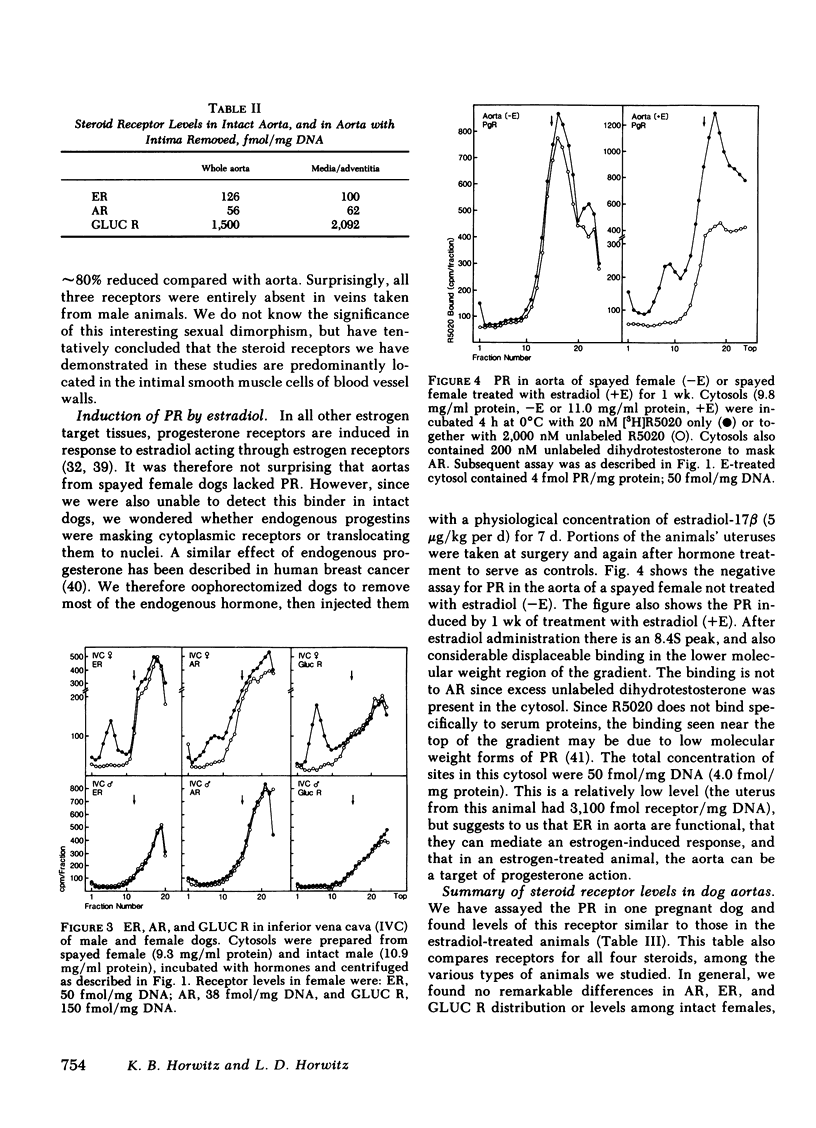
Sex differences and steroid hormones are known to influence the vascular system as shown by the different incidence of atherosclerosis in men and premenopausal women, or by the increased risk of cardiovascular diseases in women taking birth control pills or men taking estrogens. However, the mechanisms for these effects in vascular tissues are not known. Since steroid actions in target tissues are mediated by receptors, we have looked for cytoplasmic steroid receptor proteins in vascular tissues of dogs. We find specific saturable receptors, sedimenting at 8S on sucrose density gradients for estrogens (measured with [3H]estradiol +/- unlabeled diethylstilbestrol), androgens (measured with [3H]R1881 +/- unlabeled R1881 and triamcinolone acetonide), and glucocorticoids (measured with [3H]dexamethasone +/- unlabeled dexamethasone); they are absent for progesterone (measured with [3H]R5020 +/- unlabeled R5020 and dihydrotestosterone). Progesterone receptors can, however, be induced by 1-wk treatment of dogs with physiological estradiol concentrations (100 pg/ml serum estrogen), indicating a functional estrogen receptor. Receptor levels range from 20 to 2,000 fmol/mg DNA. They are specific for each hormone; unrelated steroids fail to complete for binding. Low dissociation constants, measured by Scatchard analyses, show that binding is of high affinity. Steroid binding sites are in the media and/or adventitia since they persist when the intima is removed. Compared with the arteries, receptor levels are reduced 80% in inferior venae cavae of females, and are absent in the venae cavae of males. We hypothesize that steroid hormones can have direct effects on vascular tissues medicated by specific receptors present in arterial blood vessel walls.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASSALI N. S., RAURAMO L., PELTONEN T. Measurement of uterine blood flow and uterine metabolism. VIII. Uterine and fetal blood flow and oxygen consumption in early human pregnancy. Am J Obstet Gynecol. 1960 Jan;79:86–98. doi: 10.1016/0002-9378(60)90367-7. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. J., Ramey E. R., Ramwell P. W. Androgen-mediated sex differences of cardiovascular responses in rats. Am J Physiol. 1978 Aug;235(2):H242–H246. doi: 10.1152/ajpheart.1978.235.2.H242. [DOI] [PubMed] [Google Scholar]
- Ballard P. L., Baxter J. D., Higgins S. J., Rousseau G. G., Tomkins G. M. General presence of glucocorticoid receptors in mammalian tissues. Endocrinology. 1974 Apr;94(4):998–1002. doi: 10.1210/endo-94-4-998. [DOI] [PubMed] [Google Scholar]
- Beldekas J. C., Smith B., Gerstenfeld L. C., Sonenshein G. E., Franzblau C. Effects of 17 beta-estradiol on the biosynthesis of collagen in cultured bovine aortic smooth muscle cells. Biochemistry. 1981 Apr 14;20(8):2162–2167. doi: 10.1021/bi00511a014. [DOI] [PubMed] [Google Scholar]
- Bengtsson C. Ischaemic heart disease in women. A study based on a randomized population sample of women and women with myocardial infarction in Göteborg, Sweden. Acta Med Scand Suppl. 1973;549:1–128. [PubMed] [Google Scholar]
- Besse J. C., Bass A. D. Potentiation by hydrocortisone of responses to catecholamines in vascular smooth muscle. J Pharmacol Exp Ther. 1966 Nov;154(2):224–238. [PubMed] [Google Scholar]
- Bonne C., Raynaud J. P. Methyltrienolone, a specific ligand for cellular androgen receptors. Steroids. 1975 Aug;26(2):227–232. doi: 10.1016/s0039-128x(75)80023-7. [DOI] [PubMed] [Google Scholar]
- Cavallero C., Sarti P., Spagnoli L. G. Proliferation of arterial smooth muscle: glucocorticoid effect. Prog Biochem Pharmacol. 1977;13:88–93. [PubMed] [Google Scholar]
- Colburn P., Buonassisi V. Estrogen-binding sites in endothelial cell cultures. Science. 1978 Sep 1;201(4358):817–819. doi: 10.1126/science.684408. [DOI] [PubMed] [Google Scholar]
- DANFORTH D. N., MANALO-ESTRELLA P., BUCKINGHAM J. C. THE EFFECT OF PREGNANCY AND OF ENOVID ON THE RABBIT VASCULATURE. Am J Obstet Gynecol. 1964 Apr 1;88:952–962. doi: 10.1016/0002-9378(64)90741-0. [DOI] [PubMed] [Google Scholar]
- Dubé J. Y., Tremblay R. R., Chapdelaine P. Binding of methyltrienolone to various androgen-dependent and androgen-responsive tissues in four animal species. Horm Res. 1976;7(6):333–340. doi: 10.1159/000178747. [DOI] [PubMed] [Google Scholar]
- Elam M. B., Lipscomb G. E., Chesney C. M., Terragno D. A., Terragno N. A. Effect of synthetic estrogen on platelet aggregation and vascular release of PGI2-like material in the rabbit. Prostaglandins. 1980 Dec;20(6):1039–1051. doi: 10.1016/0090-6980(80)90058-1. [DOI] [PubMed] [Google Scholar]
- FABRICANT N. D. Sexual functions and the nose. Am J Med Sci. 1960 Apr;239:498–502. [PubMed] [Google Scholar]
- Fischer G. M., Swain M. L. Effect of sex hormones on blood pressure and vascular connective tissue in castrated and noncastrated male rats. Am J Physiol. 1977 Jun;232(6):H617–H621. doi: 10.1152/ajpheart.1977.232.6.H617. [DOI] [PubMed] [Google Scholar]
- GOODRICH S. M., WOOD J. E. PERIPHERAL VENOUS DISTENSIBILITY AND VELOCITY OF VENOUS BLOOD FLOW DURING PREGNANCY OR DURING ORAL CONTRACEPTIVE THERAPY. Am J Obstet Gynecol. 1964 Nov 15;90:740–744. doi: 10.1016/0002-9378(64)90935-4. [DOI] [PubMed] [Google Scholar]
- Ginsburg J., Duncan S. L. Peripheral blood flow in normal pregnancy. Cardiovasc Res. 1967 Apr;1(2):132–137. doi: 10.1093/cvr/1.2.132. [DOI] [PubMed] [Google Scholar]
- HODGKINSON C. P. Physiology of the ovarian veins during pregnancy. Obstet Gynecol. 1953 Jan;1(1):26–37. [PubMed] [Google Scholar]
- Hagino N. The effect of synthetic corticosteroids on ovarian function in the baboon. J Clin Endocrinol Metab. 1972 Nov;35(5):716–721. doi: 10.1210/jcem-35-5-716. [DOI] [PubMed] [Google Scholar]
- Harder D. R., Coulson P. B. Estrogen receptors and effects of estrogen on membrane electrical properties of coronary vascular smooth muscle. J Cell Physiol. 1979 Aug;100(2):375–382. doi: 10.1002/jcp.1041000218. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Costlow M. E., McGuire W. L. MCF-7; a human breast cancer cell line with estrogen, androgen, progesterone, and glucocorticoid receptors. Steroids. 1975 Dec;26(6):785–795. doi: 10.1016/0039-128x(75)90110-5. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., Koseki Y., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer: role of estradiol and antiestrogen. Endocrinology. 1978 Nov;103(5):1742–1751. doi: 10.1210/endo-103-5-1742. [DOI] [PubMed] [Google Scholar]
- Horwitz K. B., McGuire W. L. Estrogen control of progesterone receptor in human breast cancer. Correlation with nuclear processing of estrogen receptor. J Biol Chem. 1978 Apr 10;253(7):2223–2228. [PubMed] [Google Scholar]
- Inman W. H., Vessey M. P., Westerholm B., Engelund A. Thromboembolic disease and the steroidal content of oral contraceptives. A report to the Committee on Safety of Drugs. Br Med J. 1970 Apr 25;2(5703):203–209. doi: 10.1136/bmj.2.5703.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jick H., Dinan B., Herman R., Rothman K. J. Myocardial infarction and other vascular diseases in young women. Role of estrogens and other factors. JAMA. 1978 Dec 1;240(23):2548–2552. [PubMed] [Google Scholar]
- KNOWLTON A. I., LOEB E. N., STOERK H. C., WHITE J. P., HEFFERNAN J. F. Induction of arterial hypertension in normal and adrenalectomized rats given cortisone acetate. J Exp Med. 1952 Sep;96(3):187–205. doi: 10.1084/jem.96.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannel W. B., Feinleib M. Natural history of angina pectoris in the Framingham study. Prognosis and survival. Am J Cardiol. 1972 Feb;29(2):154–163. doi: 10.1016/0002-9149(72)90624-8. [DOI] [PubMed] [Google Scholar]
- Kannel W. B., Hjortland M. C., McNamara P. M., Gordon T. Menopause and risk of cardiovascular disease: the Framingham study. Ann Intern Med. 1976 Oct;85(4):447–452. doi: 10.7326/0003-4819-85-4-447. [DOI] [PubMed] [Google Scholar]
- Krakoff L. R., Selvadurai R., Sutter E. Effect of methylprednisolone upon arterial pressure and the renin angiotensin system in the rat. Am J Physiol. 1975 Feb;228(2):613–617. doi: 10.1152/ajplegacy.1975.228.2.613. [DOI] [PubMed] [Google Scholar]
- Krakoff L., Nicolis G., Amsel B. Pathogenesis of hypertension in Cushing's syndrome. Am J Med. 1975 Feb;58(2):216–220. doi: 10.1016/0002-9343(75)90572-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laragh J. H. Oral contraceptives and hypertensive disease: a cybernetic overview. Circulation. 1970 Dec;42(6):983–985. doi: 10.1161/01.cir.42.6.983. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Brånemark P. I., Birch J. [Microvascular changes in cheek-pouch wounds of oophorectomized hamsters following intramuscular injections of female sex hormones]. J Periodontal Res. 1968;3(3):180–186. doi: 10.1111/j.1600-0765.1968.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Lindhe J., Brånemark P. I. The effects of sex hormones on vascularization of granulation tissue. J Periodontal Res. 1968;3(1):6–11. [PubMed] [Google Scholar]
- Lund C. J., Donovan J. C. Blood volume during pregnancy. Significance of plasma and red cell volumes. Am J Obstet Gynecol. 1967 Jun 1;98(3):394–403. [PubMed] [Google Scholar]
- Mann J. I., Vessey M. P., Thorogood M., Doll S. R. Myocardial infarction in young women with special reference to oral contraceptive practice. Br Med J. 1975 May 3;2(5965):241–245. doi: 10.1136/bmj.2.5965.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire W. L., Horwitz K. B., Pearson O. H., Segaloff A. Current status of estrogen and progesterone receptors in breast cancer. Cancer. 1977 Jun;39(6 Suppl):2934–2947. doi: 10.1002/1097-0142(197706)39:6<2934::aid-cncr2820390680>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Mowszowicz I., Bardin C. W. In vitro androgen metabolism in mouse kidney: high 3-keto-reductase (3alpha-hydroxysteroid dehydrogenase) activity relative to 5alpha-reductase. Steroids. 1974 Jun;23(6):793–807. doi: 10.1016/0039-128x(74)90054-3. [DOI] [PubMed] [Google Scholar]
- Nakao J., Chang W. C., Murota S. I., Orimo H. Estradiol-binding sites in rat aortic smooth muscle cells in culture. Atherosclerosis. 1981 Jan-Feb;38(1-2):75–80. doi: 10.1016/0021-9150(81)90105-2. [DOI] [PubMed] [Google Scholar]
- Nuwayhid B., Brinkman C. R., 3rd, Woods J. R., Jr, Martinek H., Assali N. S. Effects of estrogens on systemic and regional circulations in normal and renal hypertensive sheep. Am J Obstet Gynecol. 1975 Nov 1;123(5):495–504. doi: 10.1016/0002-9378(75)90037-x. [DOI] [PubMed] [Google Scholar]
- Oka M., Horrobin D. F., Manku M. S. Progesterone interferes with the actions of prostaglandin (PG) E1 but not those of PGE2 or PGF2 alpha in a rat vascular preparation. Prostaglandins Med. 1979 Feb;2(2):161–164. doi: 10.1016/0161-4630(79)90051-x. [DOI] [PubMed] [Google Scholar]
- Rosenberg L., Hennekens C. H., Rosner B., Belanger C., Rothman K. J., Speizer F. E. Oral contraceptive use in relation to nonfatal myocardial infarction. Am J Epidemiol. 1980 Jan;111(1):59–66. doi: 10.1093/oxfordjournals.aje.a112874. [DOI] [PubMed] [Google Scholar]
- Rousseau G. G., Baxter J. D., Tomkins G. M. Glucocorticoid receptors: relations between steroid binding and biological effects. J Mol Biol. 1972 Jun 14;67(1):99–115. doi: 10.1016/0022-2836(72)90389-0. [DOI] [PubMed] [Google Scholar]
- SCHWARZ O. T., HAWKER W. D. Hyperplasia and hypertrophy of the uterine vessels during various stages of pregnancy. Am J Obstet Gynecol. 1950 Nov;60(5):967–976. doi: 10.1016/0002-9378(50)90502-3. [DOI] [PubMed] [Google Scholar]
- SIMS E. A., KRANTZ K. E. Serial studies of renal function during pregnancy and the puerperium in normal women. J Clin Invest. 1958 Dec;37(12):1764–1774. doi: 10.1172/JCI103769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPAZIANI E., SZEGO C. M. Further evidence for mediation by histamine of estrogenic stimulation of the rat uterus. Endocrinology. 1959 May;64(5):713–723. doi: 10.1210/endo-64-5-713. [DOI] [PubMed] [Google Scholar]
- Saez S., Martin P. M., Chouvet C. D. Estradiol and progesterone receptor levels in human breast adenocarcinoma in relation to plasma estrogen and progesterone levels. Cancer Res. 1978 Oct;38(10):3468–3473. [PubMed] [Google Scholar]
- Saruta T., Saade G. A., Kaplan N. M. A possible mechanism for hypertension induced by oral contraceptives. Diminished feedback suppression of renin release. Arch Intern Med. 1970 Oct;126(4):621–626. [PubMed] [Google Scholar]
- Sherman M. R., Tuazon F. B., Diaz S. D., Miller L. K. Multiple forms of oviduct progesterone receptors analyzed by ion exchange filtration and gel electrophoresis. Biochemistry. 1976 Mar 9;15(5):980–989. doi: 10.1021/bi00650a006. [DOI] [PubMed] [Google Scholar]
- Silva de Sá M. F., Meirelles R. S. Vasodilating effect of estrogen on the human umbilical artery. Gynecol Invest. 1977;8(5-6):307–313. doi: 10.1159/000301109. [DOI] [PubMed] [Google Scholar]
- Stokes T., Wynn V. Serum-lipids in women on oral contraceptives. Lancet. 1971 Sep 25;2(7726):677–680. doi: 10.1016/s0140-6736(71)92247-1. [DOI] [PubMed] [Google Scholar]
- Ueland K., Parer J. T. Effects of estrogens on the cardiovascular system of the ewe. Am J Obstet Gynecol. 1966 Oct 1;96(3):400–406. doi: 10.1016/0002-9378(66)90243-2. [DOI] [PubMed] [Google Scholar]
- Walters W. A., Lim Y. L. Haemodynamic changes in women taking oral contraceptives. J Obstet Gynaecol Br Commonw. 1970 Nov;77(11):1007–1012. doi: 10.1111/j.1471-0528.1970.tb03447.x. [DOI] [PubMed] [Google Scholar]
- Walters W. A., MacGregor W. G., Hills M. Cardiac output at rest during pregnancy and the puerperium. Clin Sci. 1966 Feb;30(1):1–11. [PubMed] [Google Scholar]
- Wolinsky H. Effects of androgen treatment on the male rat aorta. J Clin Invest. 1972 Oct;51(10):2552–2555. doi: 10.1172/JCI107071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolinsky H. Effects of estrogen and progestogen treatment on the response of the aorta of male rats to hypertension. Morphological and chemical studies. Circ Res. 1972 Mar;30(3):341–349. doi: 10.1161/01.res.30.3.341. [DOI] [PubMed] [Google Scholar]
- Zava D. T., Landrum B., Horwitz K. B., McGuire W. L. Androgen receptor assay with [3H]methyltrienolone (R1881) in the presence of progesterone receptors. Endocrinology. 1979 Apr;104(4):1007–1012. doi: 10.1210/endo-104-4-1007. [DOI] [PubMed] [Google Scholar]
- Zava D. T., McGuire W. L. Androgen action through estrogen receptor in a human breast cancer cell line. Endocrinology. 1978 Aug;103(2):624–631. doi: 10.1210/endo-103-2-624. [DOI] [PubMed] [Google Scholar]


