Abstract
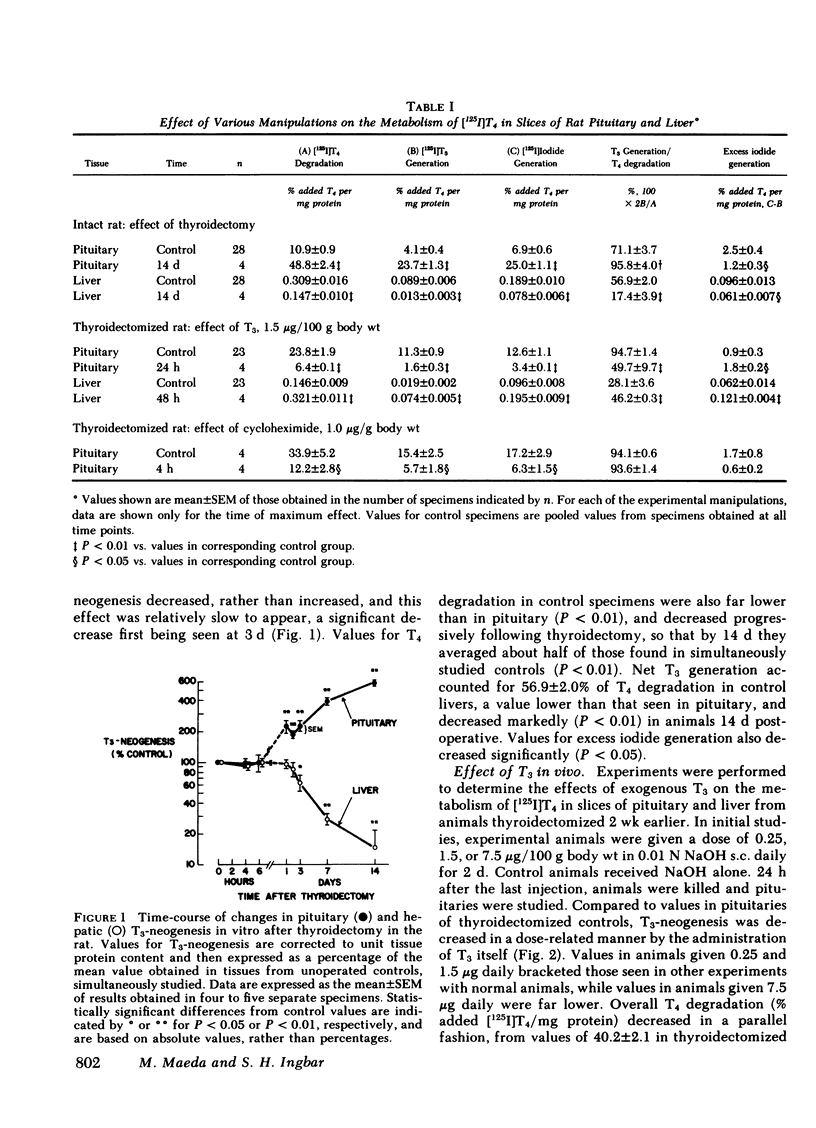
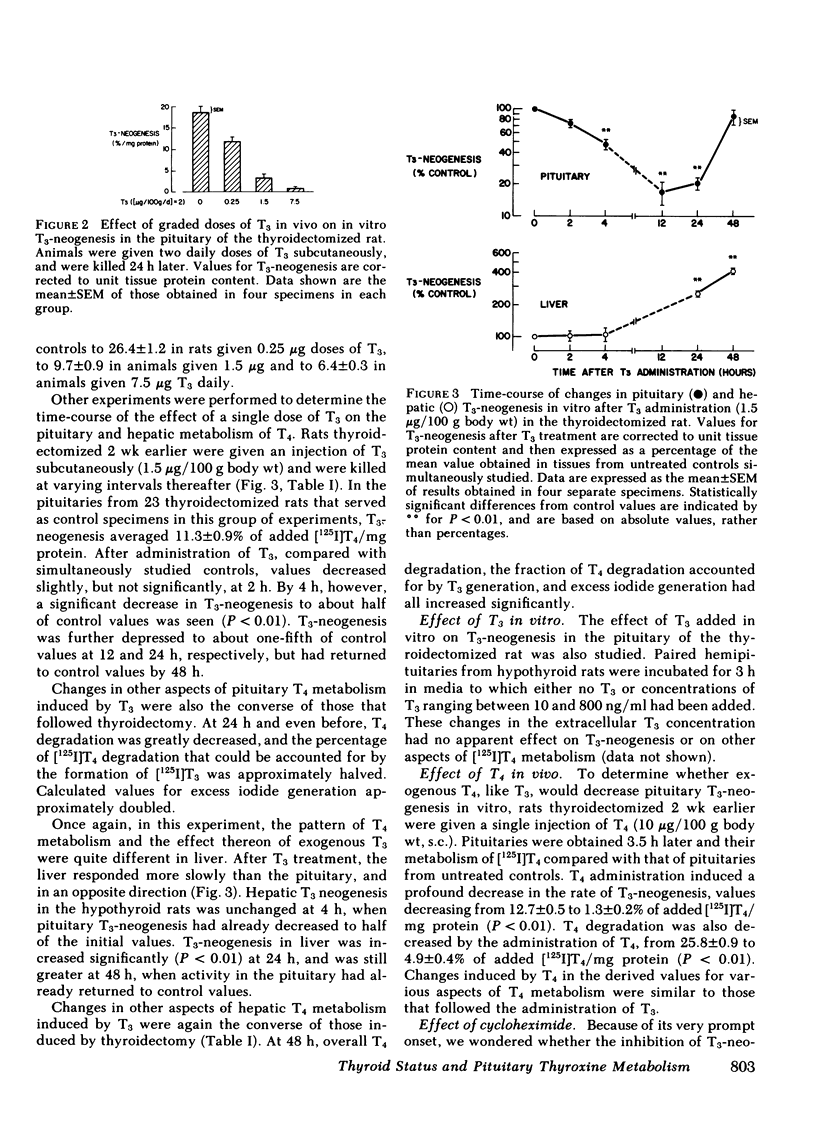
The metabolism of thyroxine (T4) was studied in slices of rat pituitary gland and liver from the same animal incubated in vitro with [125I]T4 and 10 mM dithiothreitol. In the pituitary gland, generation of 125I-labeled 3,5,3′-triiodothyronine (T3), as well as overall T4 degradation, increased significantly at 24 h after thyroidectomy and by 2 wk were approximately five times control values. Conversely, following a single injection of T3 (1.5 μg/100 g body wt), values for both functions were significantly decreased at 4 h, and reached a nadir of ∼20% of control values at 12 and 24 h. Net T3-neogenesis accounted for ∼70% of T4 degradation in control pituitaries from intact rats. This proportion was increased by thyroidectomy and decreased by T3 replacement. Indirect evidence indicated that thyroidectomy decreased, and T3 administration increased, non-T3 generating pathways of T4 metabolism, probably 5-monodeiodination leading to formation of 3,3′5′-triiodothyronine (rT3). As judged from studies by others, the prompt changes in T4 metabolism that followed thyroidectomy or T3 administration could not be explained by changes in pituitary cell type. Changes in T3-neogenesis in liver were the converse of those in pituitary, and were much slower to occur.
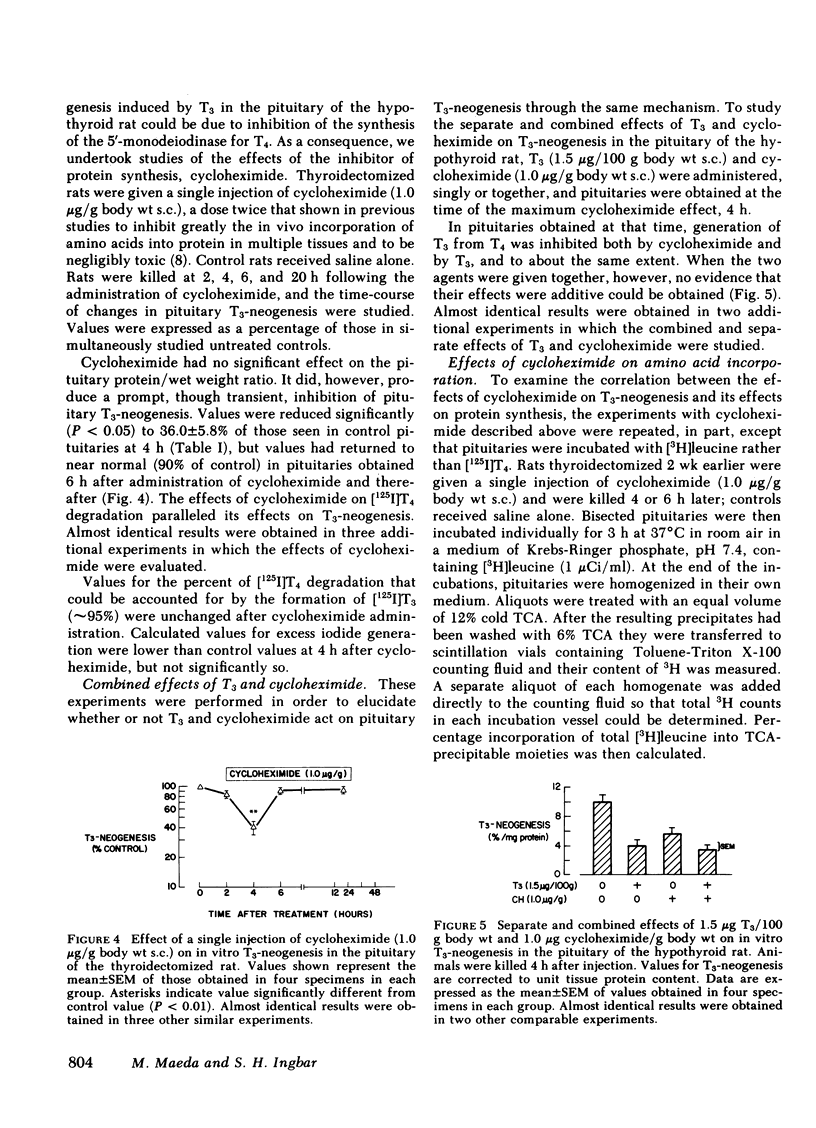
In the thyroidectomized rat, administration of cycloheximide resulted in an ∼60% inhibition of pituitary T3-neogenesis and T4-degradation at 4 h, a time-course of inhibition similar to that produced by T3. Unlike T3, cycloheximide did not alter the proportion of T4 degradation that could be accounted for by T3 neogenesis, and appeared, therefore, to inhibit both T3 generating and non-T3 generating pathways. The time-course of the inhibitory effect of cycloheximide on the incorporation of [3H]leucine into hemipituitaries in vitro was parallel to its effect on T3-neogenesis. The inhibition of T3-neogenesis that occurred when T3 and cycloheximide were given together did not exceed the effect of T3 alone, suggesting a common mechanism of action of the two agents.
From the foregoing information, the following tentative conclusions are drawn: (a) turnover of the 5′-monodeiodinase for T4 in rat pituitary is rapid, substantially more so than in liver; (b) thyroidectomy enhances, and T3 inhibits, the conversion of T4 to T3 in the pituitary; these manipulations have opposite effecs on the non-T3 generating pathways of T4 metabolism, probably the 5-monodeiodination of T4 that produces rT3; (c) these changes are probably the result of parallel effects on the synthesis of the corresponding enzymes; (d) the changes in T3-neogenesis described may permit an intrapituitary feedback mechanism that damps the changes in TSH secretion mediated by classical feedback regulatory control; (e) the effects of hypothyroidism and T3-replacement on T3-neogenesis and overall T4 degradation in liver were opposite to those produced in the pituitary. Hence, among differing tissues, the same stimuli may produce greatly different responses in pathways of peripheral T4 metabolism, thus making possible differing metabolic sequelae within each.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balsam A., Ingbar S. H. The influence of fasting, diabetes, and several pharmacological agents on the pathways of thyroxine metabolism in rat liver. J Clin Invest. 1978 Aug;62(2):415–424. doi: 10.1172/JCI109143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsam A., Sexton F., Ingbar S. H. The effect of thyroidectomy, hypophysectomy, and hormone replacement on the formation of triiodothyronine from thyroxine in rat liver and kidney. Endocrinology. 1978 Nov;103(5):1759–1767. doi: 10.1210/endo-103-5-1759. [DOI] [PubMed] [Google Scholar]
- Cheron R. G., Kaplan M. M., Larsen P. R. Physiological and pharmacological influences on thyroxine to 3,5,3'-triiodothyronine conversion and nuclear 3,5,3'-triiodothyronine binding in rat anterior pituitary. J Clin Invest. 1979 Nov;64(5):1402–1414. doi: 10.1172/JCI109598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFesi C. R., Astier H. S., Surks M. I. Kinetics of thyrotrophs and somatotrophs during development of hypothyroidism and L-triiodothyronine treatment of hypothyroid rats. Endocrinology. 1979 Apr;104(4):1172–1180. doi: 10.1210/endo-104-4-1172. [DOI] [PubMed] [Google Scholar]
- Harris A. R., Fang S. L., Vagenakis A. G., Braverman L. E. Effect of starvation, nutriment replacement, and hypothyroidism on in vitro hepatic T4 to T3 conversion in the rat. Metabolism. 1978 Nov;27(11):1680–1690. doi: 10.1016/0026-0495(78)90290-1. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M. Thyroxine 5'-monodeiodination in rat anterior pituitary homogenates. Endocrinology. 1980 Feb;106(2):567–576. doi: 10.1210/endo-106-2-567. [DOI] [PubMed] [Google Scholar]
- Kaplan M. M., Utiger R. D. Iodothyronine metabolism in liver and kidney homogenates from hyperthyroid and hypothyroid rats. Endocrinology. 1978 Jul;103(1):156–161. doi: 10.1210/endo-103-1-156. [DOI] [PubMed] [Google Scholar]
- LARSON F. C., TOMITA K., ALBRIGHT E. C. The deiodination of thyroxine to triiodothyronine by kidney slices of rats with varying thyroid function. Endocrinology. 1955 Sep;57(3):338–344. doi: 10.1210/endo-57-3-338. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Larsen P. R., Dick T. E., Markovitz B. P., Kaplan M. M., Gard T. G. Inhibition of intrapituitary thyroxine to 3.5.3'-triiodothyronine conversion prevents the acute suppression of thyrotropin release by thyroxine in hypothyroid rats. J Clin Invest. 1979 Jul;64(1):117–128. doi: 10.1172/JCI109430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Kaplan M. M., Cheron R. G., Dick T. E., Larsen P. R. Thyroxine to 3,5,3'-triiodothyronine conversion by rat anterior pituitary and liver. Metabolism. 1978 Nov;27(11):1601–1607. doi: 10.1016/0026-0495(78)90282-2. [DOI] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Contributions of plasma triiodothyronine and local thyroxine monodeiodination to triiodothyronine to nuclear triiodothyronine receptor saturation in pituitary, liver, and kidney of hypothyroid rats. Further evidence relating saturation of pituitary nuclear triiodothyronine receptors and the acute inhibition of thyroid-stimulating hormone release. J Clin Invest. 1978 May;61(5):1247–1259. doi: 10.1172/JCI109041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J. E., Larsen P. R. Pituitary nuclear 3,5,3'-triiodothyronine and thyrotropin secretion: an explanation for the effect of thyroxine. Science. 1977 Nov 11;198(4317):617–620. doi: 10.1126/science.199941. [DOI] [PubMed] [Google Scholar]
- Spira O., Birkenfeld A., Avni A., Gross J., Gordon A. TSH synthesis and release in the thyroidectomized rat: b) effect of T3. Acta Endocrinol (Copenh) 1979 Nov;92(3):502–511. doi: 10.1530/acta.0.0920502. [DOI] [PubMed] [Google Scholar]
- Spira O., Birkenfeld A., Gross J., Gordon A. TSH synthesis and release in the thyroidectomized rat: a) Effect of short- and long-term hypothyroidism. Acta Endocrinol (Copenh) 1979 Nov;92(3):489–451. doi: 10.1530/acta.0.0920489. [DOI] [PubMed] [Google Scholar]
- Vagenakis A. G., Ingbar S. H., Braverman L. E. The relationship between thyroglobulin synthesis and intrathyroid iodine metabolism as indicated by the effects of cycloheximide in the rat. Endocrinology. 1974 Jun;94(6):1669–1680. doi: 10.1210/endo-94-6-1669. [DOI] [PubMed] [Google Scholar]
- Visser T. J., Does-Tobé I., Docter R., Hennemann G. Subcellular localization of a rat liver enzyme converting thyroxine into tri-iodothyronine and possible involvement of essential thiol groups. Biochem J. 1976 Aug 1;157(2):479–482. doi: 10.1042/bj1570479. [DOI] [PMC free article] [PubMed] [Google Scholar]


