Abstract
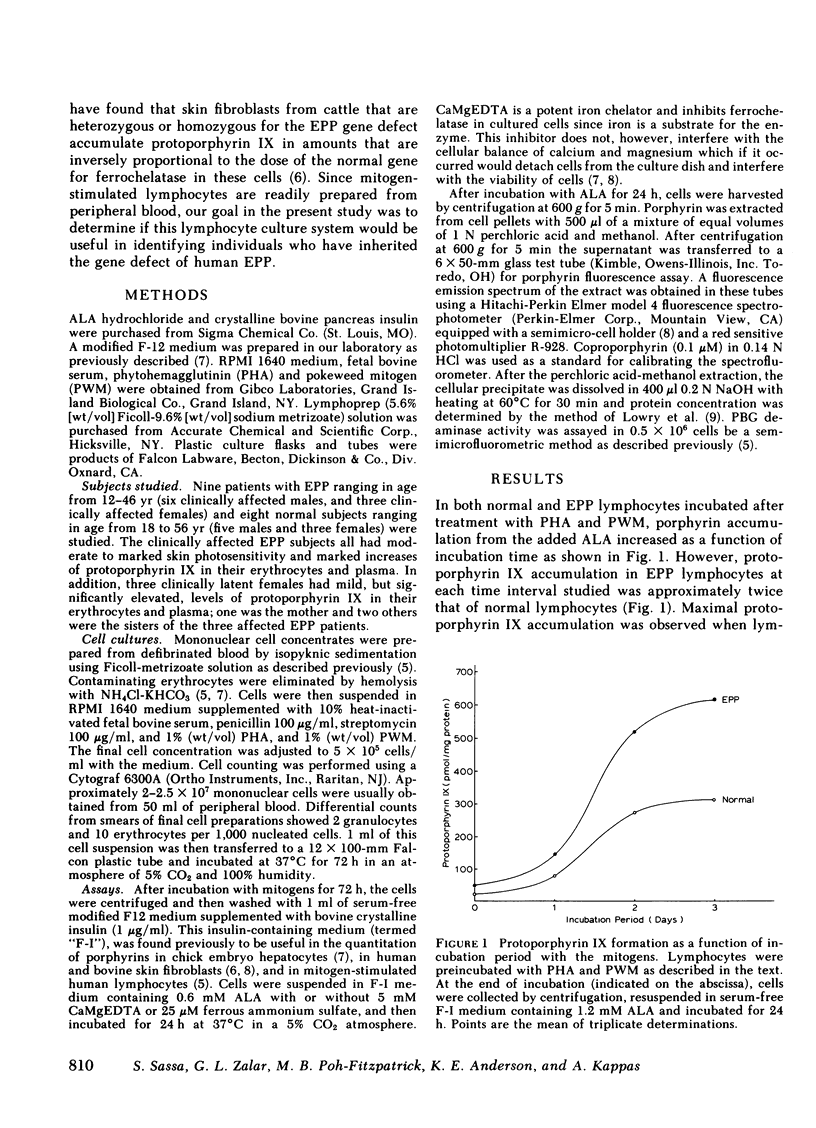
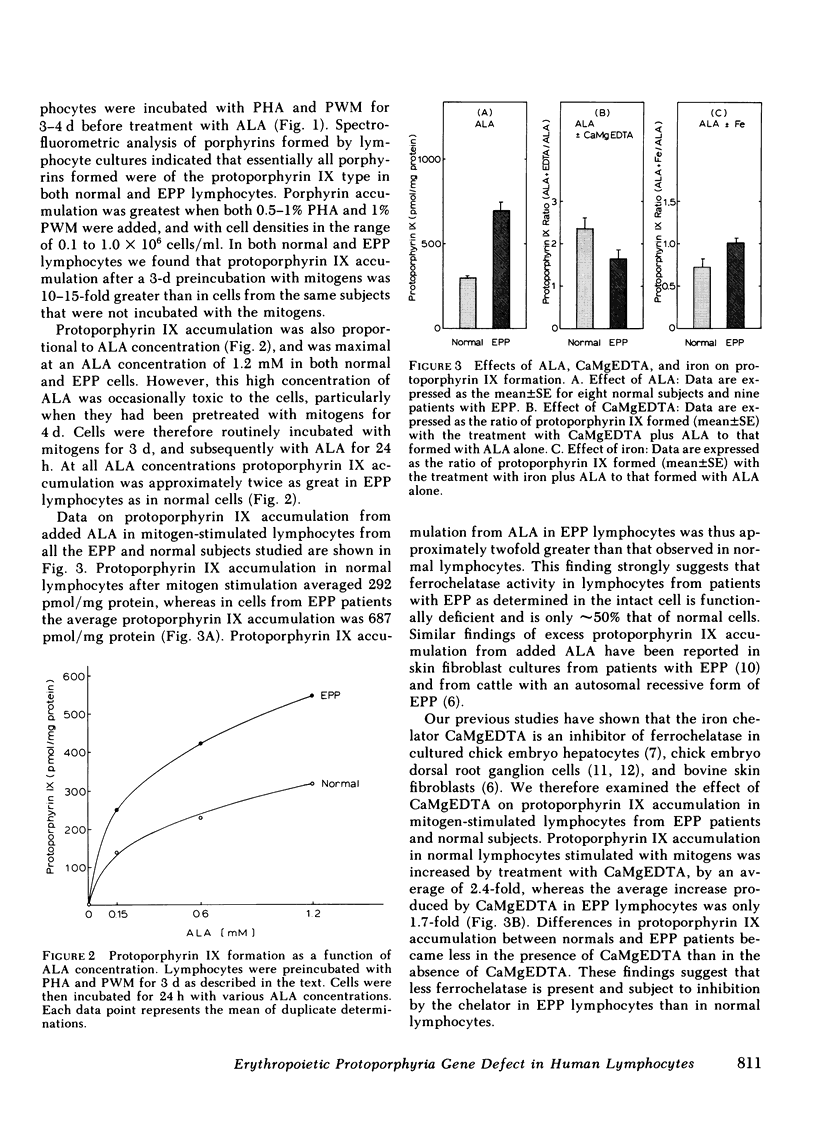
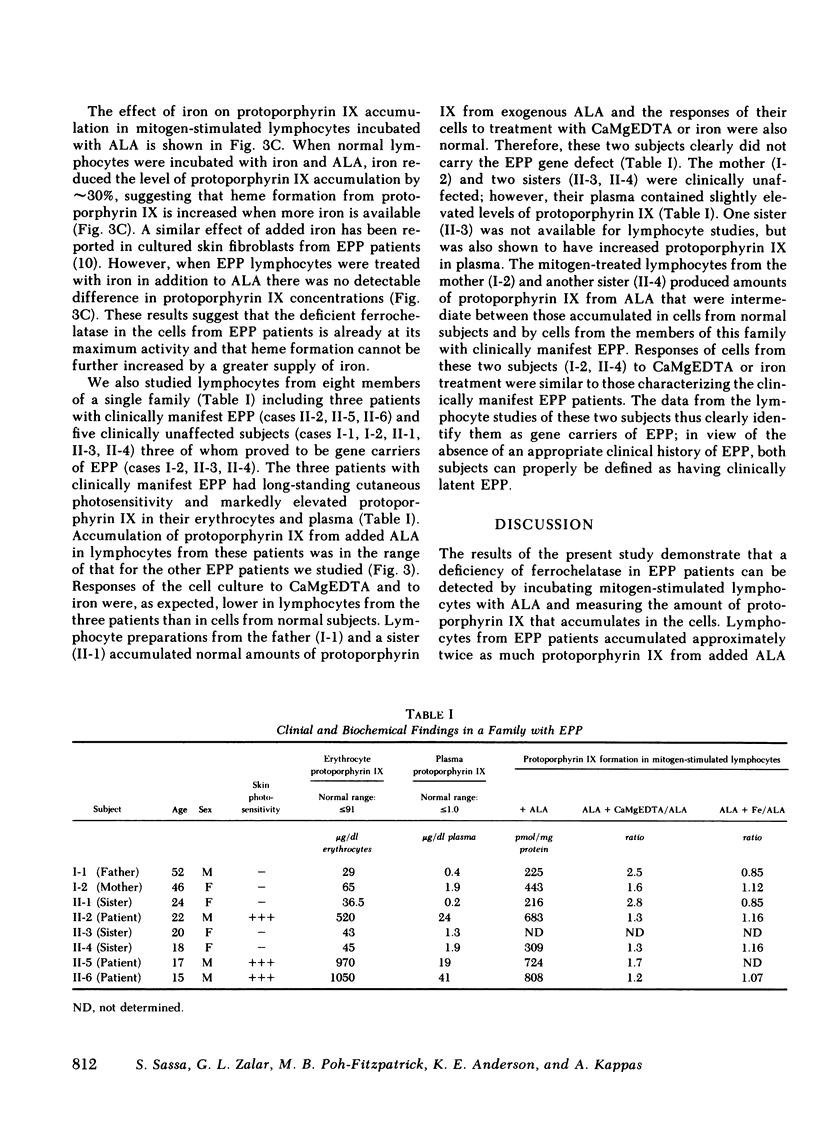
In this paper we show that the ferrochelatase defect in erythropoietic protoporphyria (EPP) can readily be identified in mitogen-stimulated lymphocytes since such cells from patients with EPP accumulate approximately twice as much protoporphyrin IX as cells from normal subjects when incubated with a porphyrin precursor, gamma-aminolevulinic acid (ALA). Treatment of cultures with ALA and with the iron chelator, CaMgEDTA significantly increased the level of protoporphyrin IX in mitogen-stimulated lymphocytes from normal subjects, while the same treatment failed to produce an increase in protoporphyrin IX in cell preparations from EPP patients. In contrast to the results with the chelator treatment, supplementation of the cultures with iron and ALA reduced the level of protoporphyrin IX in normal cells, but not in EPP cells. These findings are compatible with a partial deficiency of ferrochelatase in EPP lymphocytes. The gene defects of acute intermittent porphyria and hereditary coproporphyria have previously been identified using lymphocyte preparations from the gene carriers of these diseases. The present study demonstrates that EPP represents another form of human porphyria in which the gene defect of the disease can now be identified in lymphocyte preparations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. E., Bradlow H. L., Sassa S., Kappas A. Studies in porphyria. VIII. Relationship of the 5 alpha-reductive metabolism of steroid hormones to clinical expression of the genetic defect in acute intermittent porphyria. Am J Med. 1979 Apr;66(4):644–650. doi: 10.1016/0002-9343(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Bloomer J. R., Brenner D. A., Mahoney M. J. Study of factors causing excess protoporphyrin accumulation in cultured skin fibroblasts from patients with protoporphyria. J Clin Invest. 1977 Dec;60(6):1354–1361. doi: 10.1172/JCI108895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomer J. R. Characterization of deficient heme synthase activity in protoporphyria with cultured skin fibroblasts. J Clin Invest. 1980 Feb;65(2):321–328. doi: 10.1172/JCI109675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonkowsky H. L., Bloomer J. R., Ebert P. S., Mahoney M. J. Heme synthetase deficiency in human protoporphyria. Demonstration of the defect in liver and cultured skin fibroblasts. J Clin Invest. 1975 Nov;56(5):1139–1148. doi: 10.1172/JCI108189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottomley S. S., Tanaka M., Everett M. A. Diminished erythroid ferrochelatase activity in protoporphyria. J Lab Clin Med. 1975 Jul;86(1):126–131. [PubMed] [Google Scholar]
- DeLeo V. A., Poh-Fitzpatrick M., Mathews-Roth M., Harber L. C. Erythropoietic protoporphyria. 10 years experience. Am J Med. 1976 Jan;60(1):8–22. doi: 10.1016/0002-9343(76)90528-3. [DOI] [PubMed] [Google Scholar]
- Kappas A., Sassa S., Granick S., Bradlow H. L. Endocrine-gene interaction in the pathogenesis of acute intermittent porphyria. Res Publ Assoc Res Nerv Ment Dis. 1974;53:225–237. [PubMed] [Google Scholar]
- Kassner R. J., Walchak H. Heme formation from Fe(II) and porphyrin in the absence of ferrochelatase activity. Biochim Biophys Acta. 1973 Apr 28;304(2):294–303. doi: 10.1016/0304-4165(73)90247-x. [DOI] [PubMed] [Google Scholar]
- LABBE R. F., HUBBARD N. Preparation and properties of the iron-protoporphyrin chelating enzyme. Biochim Biophys Acta. 1960 Jul 1;41:185–191. doi: 10.1016/0006-3002(60)90001-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mailer K., Poulson R., Dolphin D., Hamilton A. D. Ferrochelatase: isolation and purification via affinity chromatography. Biochem Biophys Res Commun. 1980 Sep 30;96(2):777–784. doi: 10.1016/0006-291x(80)91422-9. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., JONES O. T. Studies on ferrochelatase. 1. Assay and properties of ferrochelatase from a pig-liver mitochondrial extract. Biochem J. 1963 Apr;87:181–185. doi: 10.1042/bj0870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUTH G. R., Schwartz S., Stephenson B. Bovine protoporphyria: the first nonhuman model of this hereditary photosensitizing disease. Science. 1977 Oct 14;198(4313):199–201. doi: 10.1126/science.905823. [DOI] [PubMed] [Google Scholar]
- Reed W. B., Wuepper K. D., Epstein J. H., Redeker A., Simonson R. J., McKusick V. A. Erythropoietic protoporphyria. A clinical and genetic study. JAMA. 1970 Nov 9;214(6):1060–1066. doi: 10.1001/jama.214.6.1060. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Kappas A. Induction of aminolevulinate synthase and porphyrins in cultured liver cells maintained in chemically defined medium. Permissive effects of hormones on induction process. J Biol Chem. 1977 Apr 10;252(7):2428–2436. [PubMed] [Google Scholar]
- Sassa S., Schwartz S., Ruth G. Accumulation of protoporphyrin IX from delta-aminolevulinic acid in bovine skin fibroblasts with hereditary erythropoietic protoporphyria. A gene-dosage effect. J Exp Med. 1981 May 1;153(5):1094–1101. doi: 10.1084/jem.153.5.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Whetsell W. O., Jr, Kappas A. Studies on porphyrin-heme biosynthesis in organotypic cultures of chick dorsal root ganglia. II. The effect of lead. Environ Res. 1979 Aug;19(2):415–426. doi: 10.1016/0013-9351(79)90066-5. [DOI] [PubMed] [Google Scholar]
- Sassa S., Zalar G. L., Kappas A. Studies in porphyria. VII. Induction of uroporphyrinogen-I synthase and expression of the gene defect of acute intermittent porphyria in mitogen-stimulated human lymphocytes. J Clin Invest. 1978 Feb;61(2):499–508. doi: 10.1172/JCI108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tephly T. R., Gibbs A. H., De Matteis F. Studies on the mechanism of experimental porphyria produced by 3,5-diethoxycarbonyl-1,4-dihydrocollidine. Role of a porphyrin-like inhibitor of protohaem ferro-lyase. Biochem J. 1979 Apr 15;180(1):241–244. doi: 10.1042/bj1800241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga R., Sano S. Comparative studies on nonenzymic and enzymic protoheme formation. Biochim Biophys Acta. 1972 Apr 21;264(2):263–271. doi: 10.1016/0304-4165(72)90290-5. [DOI] [PubMed] [Google Scholar]
- Whetsell W. O., Jr, Sassa S., Bickers D., Kappas A. Studies on porphyrin-heme biosynthesis in organotypic cultures of chick dorsal root ganglion. I. Observations on neuronal and non-neuronal elements. J Neuropathol Exp Neurol. 1978 Sep;37(5):497–507. doi: 10.1097/00005072-197809000-00005. [DOI] [PubMed] [Google Scholar]
- de Goeij A. F., Christianse K., van Steveninck J. Decreased haem synthetase activity in blood cells of patients with erythropoietic protoporphyria. Eur J Clin Invest. 1975 Sep 12;5(5):397–400. doi: 10.1111/j.1365-2362.1975.tb00470.x. [DOI] [PubMed] [Google Scholar]


