Abstract
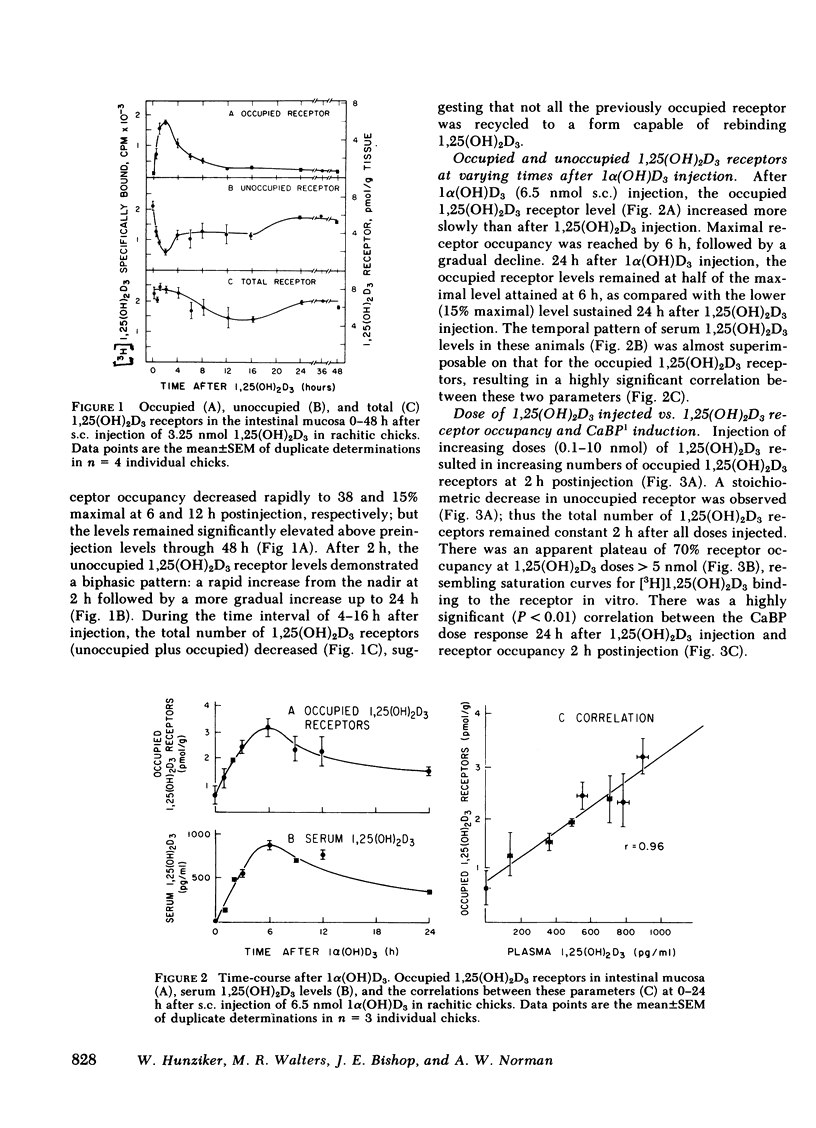
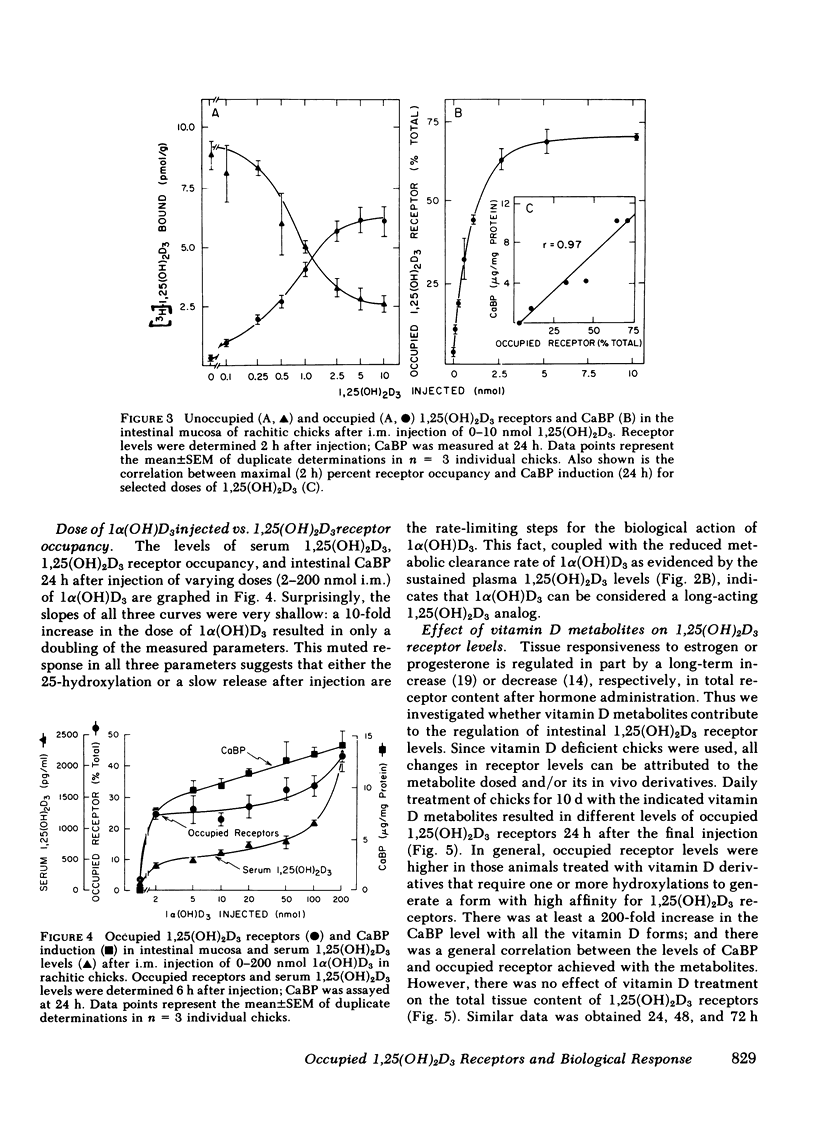
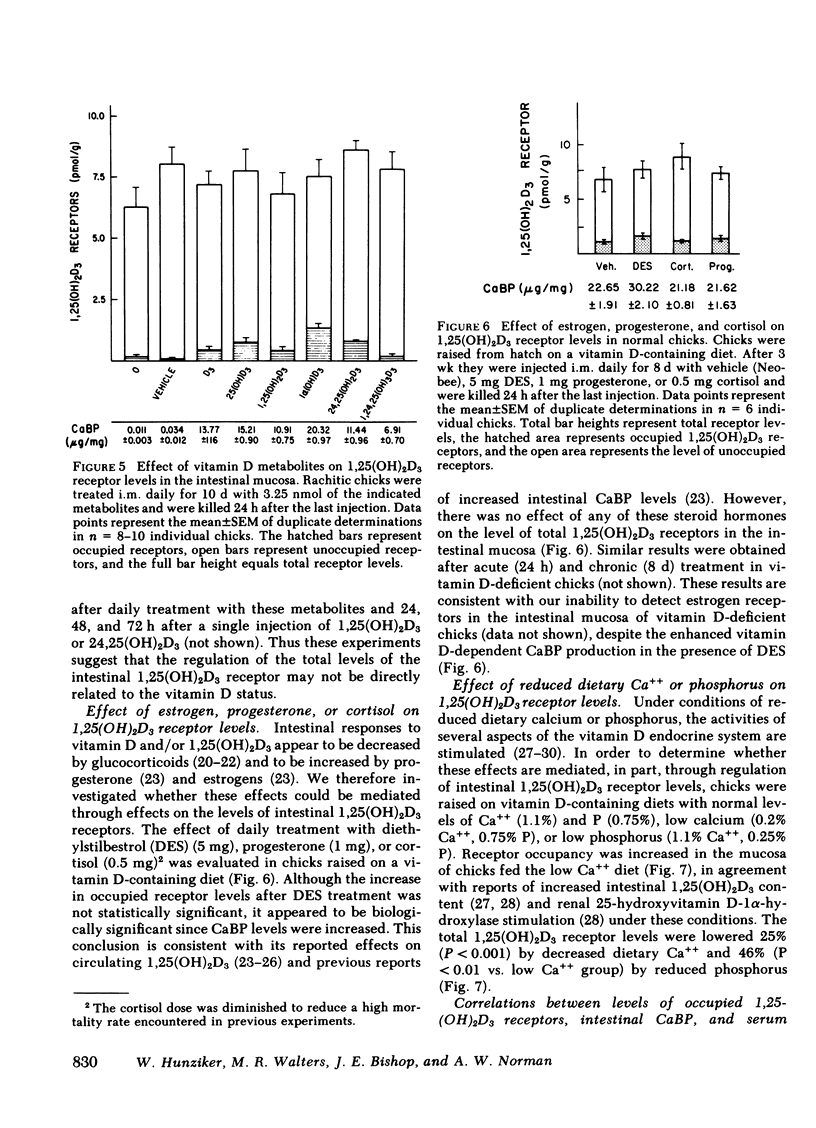
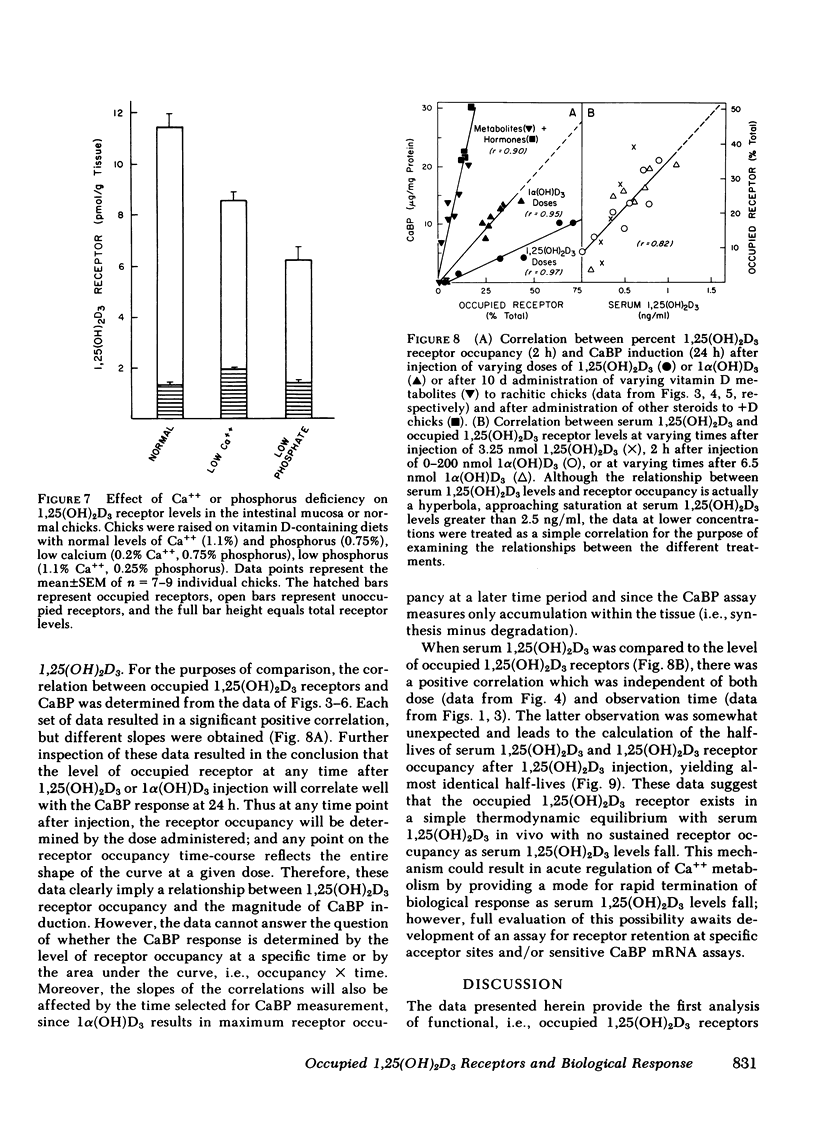
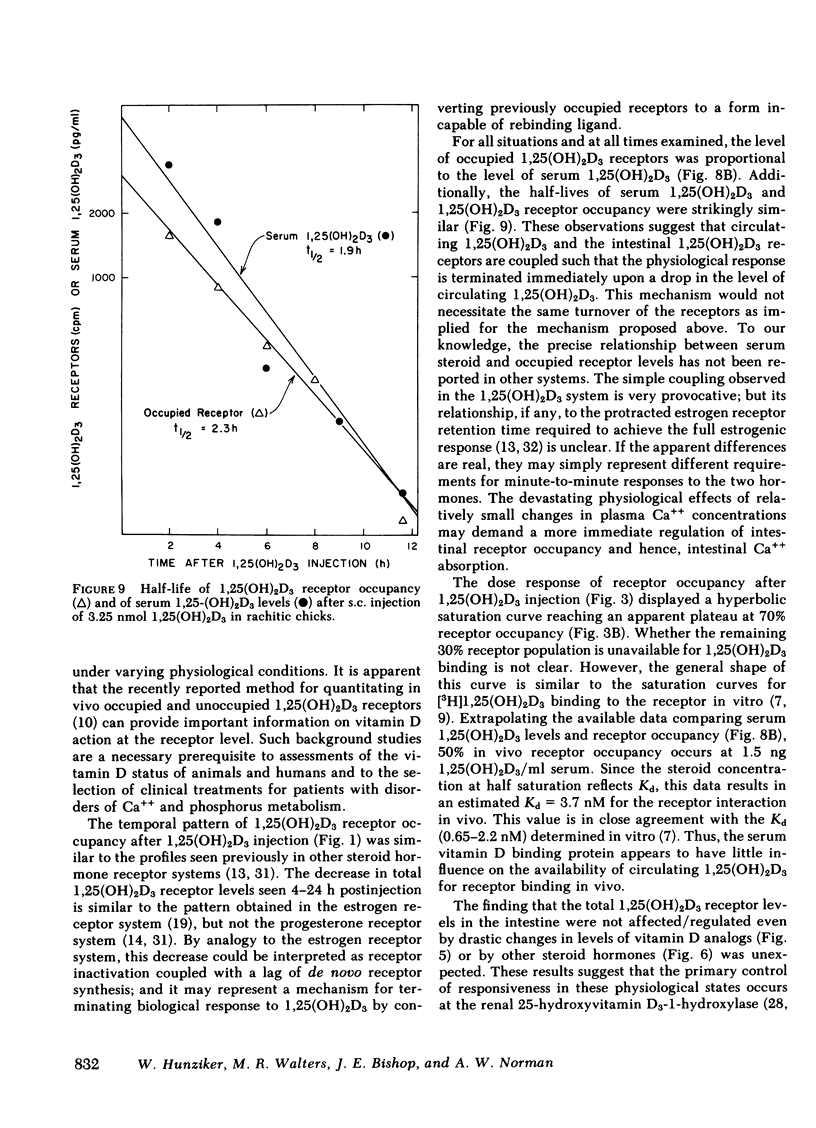
The dynamic equilibrium between in vivo occupied and unoccupied 1,25-dihydroxyvitamin D3[1,25(OH)2D3] receptors of the chick intestinal mucosa was investigated by the exchange assay previously reported [(1980). J. Biol. Chem. 255: 9534-9537]. These parameters and their correlation to biological response, i.e., the levels of intestinal vitamin D-dependent calcium binding protein (CaBP), were assessed under different physiological conditions. After a single 1,25(OH)2D3 injection (3.25 nmol), occupied receptor levels increased sharply to a maximum between 1 and 2 h, followed by a rapid decline. A single dose of 1α-hydroxy-vitamin D3 [1α(OH)D3], an analog that requires 25-hydroxylation for biological activity, resulted in a protracted, albeit lower, response with maximal receptor occupancy at 6 h and half maximal levels 24 h after injection. The intestinal receptor occupancy patterns mirrored the serum 1,25(OH)2D3 levels after either 1,25(OH)2D3 or 1α(OH)D3 treatment. Additionally, time-course (half-life) of blood disappearance of 1,25(OH)2D3 and occupied receptor levels were similar (1.9 and 2.3 h, respectively), suggesting that the amount of occupied 1,25(OH)2D3 receptor is determined by a simple equilibrium between serum 1,25(OH)2D3 and unoccupied receptors. A dose-response study after intramuscular 1,25(OH)2D3 injection yielded a hyperbolic curve with an apparent plateau at 70% receptor occupancy, corresponding to 5 nmol 1,25(OH)2D3 injected. Half-maximal occupancy was reached after a dose of 1 nmol 1,25(OH)2D3, corresponding to 1.5 ng 1,25(OH)2D3/ml serum. From this value the apparent Kd in vivo is 3.7 nM, which is similar to that determined in vitro. A 10-fold increase in the 1α(OH)D3 dose resulted in less than a doubling of the levels of serum 1,25(OH)2D3, occupied 1,25(OH)2D3 receptors, or CaBP. Under all experimental conditions, there was a positive correlation between occupied receptor and CaBP levels; however, the slope of the lines depended on the times chosen for the assays due in part to the lag period for CaBP induction and its accumulation within the cell. Conversely, the correlation between serum 1,25-(OH)2D3 levels and occupied receptor levels yielded a single regression line independent of the observation time. Short and long-term treatment with different vitamin D metabolites, estrogen, progesterone, or cortisol did not affect the levels of total intestinal 1,25(OH)2D3 receptor. Under normal physiological conditions, only 10-15% of the total 1,25(OH)2D3 receptor population was occupied by ligand. These studies provide a basis for further investigations of physiological and biochemical parameters of the vitamin D endocrine system and their clinical applications.
Full text
PDF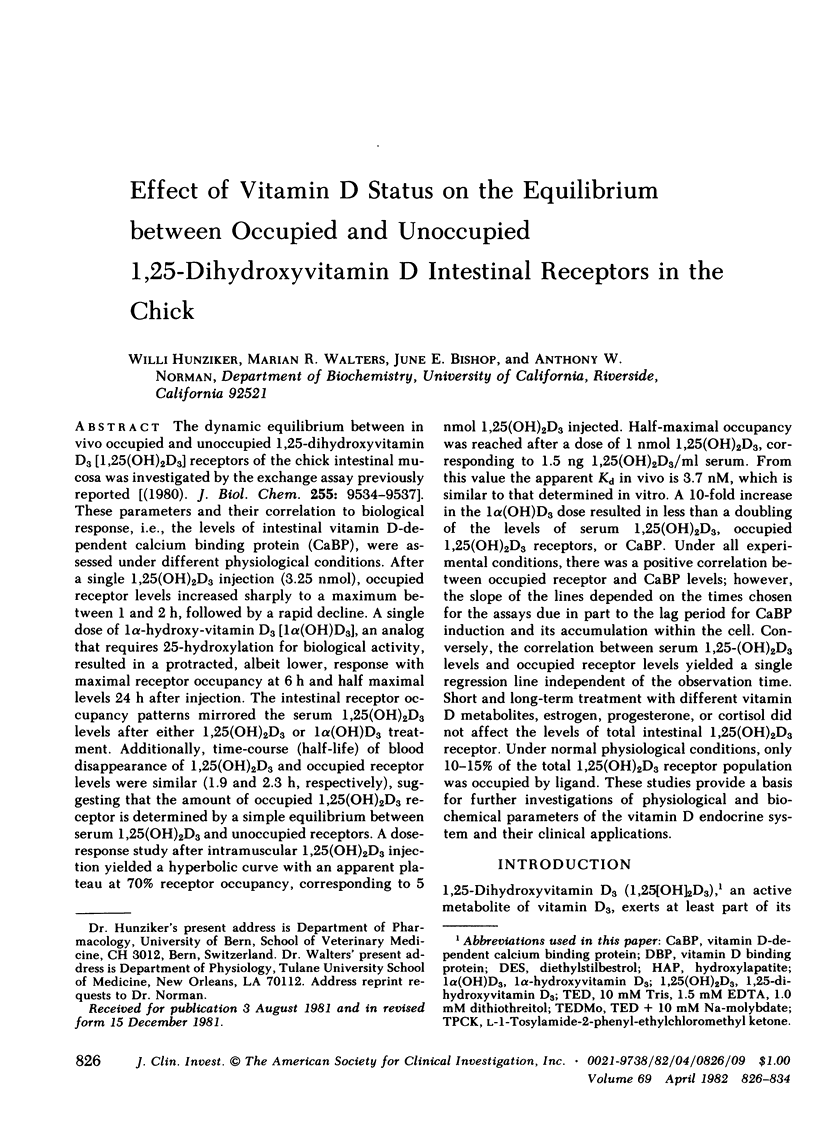



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. N., Peck E. J., Jr, Clark J. H. Estrogen-induced uterine responses and growth: relationship to receptor estrogen binding by uterine nuclei. Endocrinology. 1975 Jan;96(1):160–167. doi: 10.1210/endo-96-1-160. [DOI] [PubMed] [Google Scholar]
- Anderson J., Clark J. H., Peck E. J., Jr Oestrogen and nuclear binding sites. Determination of specific sites by ( 3 H)oestradiol exchange. Biochem J. 1972 Feb;126(3):561–567. doi: 10.1042/bj1260561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumbaugh P. F., Haussler M. R. Nuclear and cytoplasmic binding components for vitamin D metabolites. Life Sci. 1975 Feb 1;16(3):353–362. doi: 10.1016/0024-3205(75)90256-8. [DOI] [PubMed] [Google Scholar]
- Christakos S., Friedlander E. J., Frandsen B. R., Norman A. W. Studies on the mode of action of calciferol. XIII. Development of a radioimmunoassay for vitamin D-dependent chick intestinal calcium-binding protein and tissue distribution. Endocrinology. 1979 May;104(5):1495–1503. doi: 10.1210/endo-104-5-1495. [DOI] [PubMed] [Google Scholar]
- Clark J. H., Peck E. J. Nuclear retention of receptor-oestrogen complex and nuclear acceptor sites. Nature. 1976 Apr 15;260(5552):635–637. doi: 10.1038/260635a0. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F., Schnoes H. K. Metabolism and mechanism of action of vitamin D. Annu Rev Biochem. 1976;45:631–666. doi: 10.1146/annurev.bi.45.070176.003215. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Harell A., Bar A., Hurwitz S. The functional metabolism of vitamin D in chicks fed low-calcium and low-phosphorus diets. Biochim Biophys Acta. 1975 Apr 7;385(2):438–442. doi: 10.1016/0304-4165(75)90376-1. [DOI] [PubMed] [Google Scholar]
- Edelstein S., Noff D., Sinai L., Harell A., Puschett J. B., Golub E. E., Bronner F. Vitamin D metabolism and expression in rats fed on low-calcium and low-phosphorus diets. Biochem J. 1978 Feb 15;170(2):227–233. doi: 10.1042/bj1700227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher J. J., Wasserman R. H. Intestinal calcium-binding protein and calcium absorption in cortisol-treated chicks: effects of vitamin D3 and 1,25-dihydroxyvitamin D3. Endocrinology. 1979 Feb;104(2):547–551. doi: 10.1210/endo-104-2-547. [DOI] [PubMed] [Google Scholar]
- Friedlander E. J., Henry H. L., Norman A. W. Studies on the mode of action of calciferol. Effects of dietary calcium and phosphorus on the relationship between the 25-hydroxyvitamin D3-1alpha-hydroxylase and production of chick intestinal calcium binding protein. J Biol Chem. 1977 Dec 10;252(23):8677–8683. [PubMed] [Google Scholar]
- Haussler M. R., McCain T. A. Basic and clinical concepts related to vitamin D metabolism and action (first of two parts). N Engl J Med. 1977 Nov 3;297(18):974–983. doi: 10.1056/NEJM197711032971804. [DOI] [PubMed] [Google Scholar]
- Henry H. L., Midgett R. J., Norman A. W. Regulation of 25-hydroxyvitamin D3-1-hydroxylase in vivo. J Biol Chem. 1974 Dec 10;249(23):7584–7592. [PubMed] [Google Scholar]
- Henry H. L., Norman A. W. Studies on calciferol metabolism. IX. Renal 25-hydroxy-vitamin D3-1 hydroxylase. Involvement of cytochrome P-450 and other properties. J Biol Chem. 1974 Dec 10;249(23):7529–7535. [PubMed] [Google Scholar]
- Hunziker W., Walters M. R., Norman A. W. 1,25-dihydroxyvitamin D3 receptors. Differential quantitation of endogenously occupied and unoccupied sites. J Biol Chem. 1980 Oct 25;255(20):9534–9537. [PubMed] [Google Scholar]
- Kenny A. D. Vitamin D metabolism: physiological regulation in egg-laying Japanese quail. Am J Physiol. 1976 Jun;230(6):1609–1615. doi: 10.1152/ajplegacy.1976.230.6.1609. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Baerg R. D., Gershon E., Graudusius R. T. Effect of cortisone treatment on the active transport of calcium by the small intestine. J Clin Invest. 1971 Jun;50(6):1309–1321. doi: 10.1172/JCI106610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navickis R. J., Dial O. K., Katzenellenbogen B. S., Nalbandov A. V. Effects of gonadal hormones on calcium-binding protein in chick duodenum. Am J Physiol. 1979 Nov;237(5):E408–E417. doi: 10.1152/ajpendo.1979.237.5.E409. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Bishop J. E. Enzymic preparation of [3H]1,25-dihydroxyvitamin D3. Methods Enzymol. 1980;67:424–426. doi: 10.1016/s0076-6879(80)67050-5. [DOI] [PubMed] [Google Scholar]
- Norman A. W., Wong R. G. Biological activity of the vitamin D metabolite 1,25-dihydroxycholecalciferol in chickens and rats. J Nutr. 1972 Dec;102(12):1709–1718. doi: 10.1093/jn/102.12.1709. [DOI] [PubMed] [Google Scholar]
- Pike J. W., Spanos E., Colston K. W., MacIntyre I., Haussler M. R. Influence of estrogen on renal vitamin D hydroxylases and serum 1alpha,25-(OH)2D3 in chicks. Am J Physiol. 1978 Sep;235(3):E338–E343. doi: 10.1152/ajpendo.1978.235.3.E338. [DOI] [PubMed] [Google Scholar]
- Sarff M., Gorski J. Control of estrogen binding protein concentration under basal conditions and after estrogen administration. Biochemistry. 1971 Jun 22;10(13):2557–2563. doi: 10.1021/bi00789a022. [DOI] [PubMed] [Google Scholar]
- Spanos E., Colston K. W., Macintyre I. Effect of glucocorticoids on vitamin D metabolism. FEBS Lett. 1977 Mar 15;75(1):73–76. doi: 10.1016/0014-5793(77)80056-2. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Castillo L., DeLuca H. F. Control of renal vitamin D hydroxylases in birds by sex hormones. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2701–2705. doi: 10.1073/pnas.73.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Baelen H., Bouillon R., De Moor P. Binding of 25-hydroxycholecalciferol in tissues. J Biol Chem. 1977 Apr 25;252(8):2515–2518. [PubMed] [Google Scholar]
- Walters M. R., Clark J. H. Cytosol and nuclear compartmentalization of progesterone receptors of the rat uterus. Endocrinology. 1978 Aug;103(2):601–609. doi: 10.1210/endo-103-2-601. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Clark J. H. Relationship between the quantity of progesterone receptor and the antagonism of estrogen-induced uterotropic response. Endocrinology. 1979 Aug;105(2):382–386. doi: 10.1210/endo-105-2-382. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Clark J. H. Hydroxylapatite prevents nuclear receptor loss during the exchange assay of progesterone receptors. J Steroid Biochem. 1980 Oct;13(10):1129–1132. doi: 10.1016/0022-4731(80)90068-0. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Apparent nuclear localization of unoccupied receptors for 1,25-dihydroxyvitamin D3. Biochem Biophys Res Commun. 1981 Feb 27;98(4):990–996. doi: 10.1016/0006-291x(81)91208-0. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Cytosol preparations are inadequate for quantitating unoccupied receptors for 1,25-dihydroxyvitamin D3. J Recept Res. 1980;1(2):313–327. doi: 10.3109/10799898009044103. [DOI] [PubMed] [Google Scholar]
- Walters M. R., Hunziker W., Norman A. W. Unoccupied 1,25-dihydroxyvitamin D3 receptors. Nuclear/cytosol ratio depends on ionic strength. J Biol Chem. 1980 Jul 25;255(14):6799–6805. [PubMed] [Google Scholar]


