Abstract
Protein aggregation as a result of misfolding is a common theme underlying neurodegenerative diseases. Accordingly, most recent studies aim to prevent protein misfolding and/or aggregation as a strategy to treat these pathologies. For instance, state-of-the-art approaches, such as silencing protein overexpression by means of RNA interference, are being tested with positive outcomes in preclinical models of animals overexpressing the corresponding protein. Therapies designed to treat central nervous system diseases should provide accurate delivery of the therapeutic agent and long-term or chronic expression by means of a nontoxic delivery vehicle. After several years of technical advances and optimization, gene therapy emerges as a promising approach able to fulfill those requirements. In this review we will summarize the latest improvements achieved in gene therapy for central nervous system diseases associated with protein misfolding (e.g., amyotrophic lateral sclerosis, Alzheimer’s, Parkinson’s, Huntington’s, and prion diseases), as well as the most recent approaches in this field to treat these pathologies.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0191-8) contains supplementary material, which is available to authorized users.
Keywords: Gene therapy, Adeno-associated virus, Misfolding protein, CSF delivery, MRI-guided delivery
Introduction
Protein folding is vital for living organisms in order for proteins to be functionally active. With the assistance of proteins called chaperones, newly translated proteins fold into the three-dimensional shapes that are critical to their function. However, proteins do not always fold properly, despite the existence of a complex cellular quality control system. As a result, an elaborate pathway for sequestration [1] and degradation (for a review see [2]) of aberrant proteins exists in all cells because protein misfolding is frequently associated with the acquisition of new, deleterious properties not seen in the native analog. Of most relevance to the current topic, such aberrant structures sometimes generate fibrillar oligomers [3, 4]. Many neurodegenerative diseases have been associated with the accumulation of misfolded protein aggregates in the brain and other parts of the central nervous system (CNS). Dramatic examples of these pathologies include Alzheimer’s (AD), Parkinson’s (PD), Huntington’s (HD), amyotrophic lateral sclerosis (ALS), and prion diseases [5, 6]. In these neurodegenerative diseases, a minority of cases are caused by a genetic mutation and the remainder are sporadic. Interestingly enough, in some, mutations affect genes encoding proteins related to protein homeostasis (e.g., ubiquitin–proteasome system, lysosome–autophagy system) [7].
Unfortunately, to date, there is no treatment that alters the progression of any of these neurodegenerative diseases. Indeed, disease-modifying approaches specific to particular neurodegenerative diseases are currently being investigated clinically, none of which target protein misfolding directly. An attractive therapeutic strategy is to ameliorate the protein misfolding that is a shared characteristic of many neurodegenerative diseases by elevating the expression of chaperone proteins [8]. This hypothesis is now being explored as a potential therapeutic for neurodegenerative conformational diseases. Nevertheless, when designing a potential therapy for CNS diseases it should be kept in mind that any treatment would most probably need to be provided chronically, perhaps for life, with a correspondingly safe and comprehensive method of delivery of the therapeutic agent to the affected structures in order to obtain the best therapeutic results.
Gene therapy is a promising way to deliver therapeutic molecules to treat CNS diseases. It utilizes vectors to carry out gene transfer, resulting in targeted protein expression in specific regions of the brain (see Fig. 1) [9]. Recent advances have demonstrated that viral vectors, particularly adeno-associated vectors (AAV), can direct long-term expression levels in brain cells, both in the injection site and in distal interconnected structures after a single parenchymal injection [10–12]. AAV efficacy and safety has been amply demonstrated, and some AAV-based gene therapies have progressed to Phase I/II clinical trials [13, 14]. Even more recently, intravenous and cerebrospinal fluid (CSF) injections of AAV have demonstrated the possibility of employing less invasive gene delivery approaches [15–17].
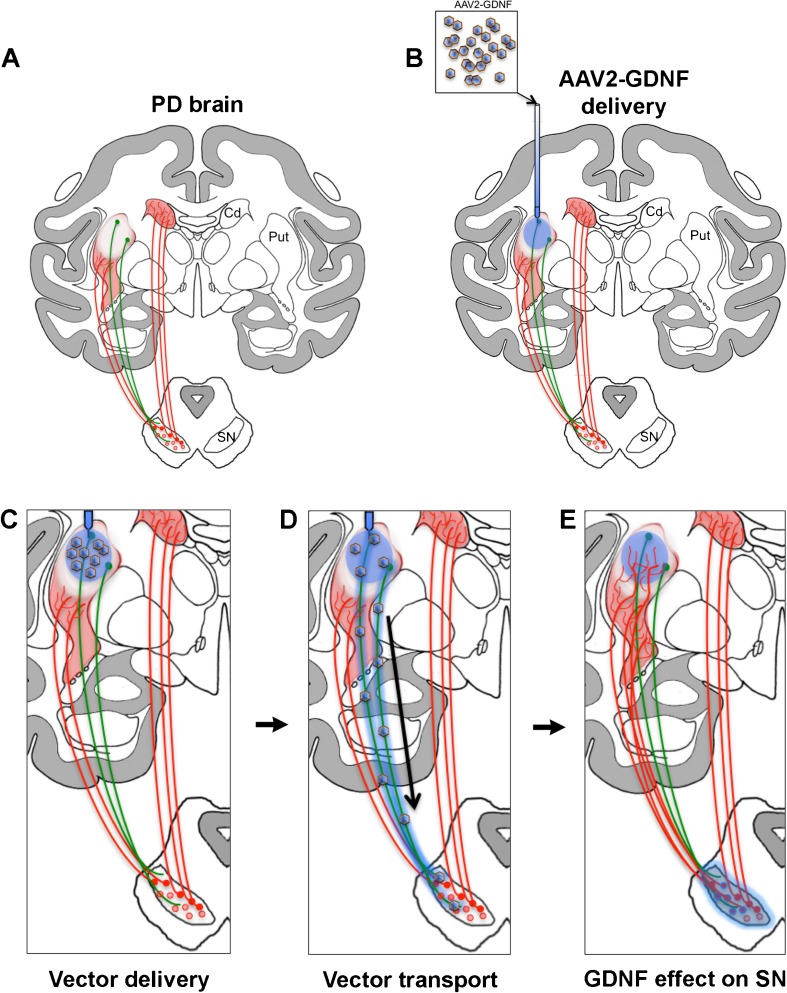
Fig. 1.
Adeno-associated virus 2 (AAV2)–glial cell line-derived neurotrophic factor (GDNF) gene therapy for Parkinson’s disease (PD). (a) Striatal dopamine in Parkinsonian brain (red shadow) is decreased owing to the loss of dopaminergic neurons and fibers from the substantia nigra (SN). Dopamine depletion is greater in the putamen (Put) than in the caudate nucleus (Cd). Gene therapy-based delivery of glial cell line-derived neurotrophic factor (GDNF), a well-known neuroprotective and neurorestorative factor for midbrain dopaminergic neurons (red dots and lines), rescues nigral neurons and, consequently, improves striatal dopaminergic tone in PD experimental models [104]. (b) AAV2 harboring GDNF transgene (blue shadow) is infused into putamen by convection-enhanced delivery to obtain an optimal distribution of the vector. (c) Viral particles transduce striatal medium spiny neurons (green dots and lines). (d) AAV2 is anterogradely transported through axons reaching the SN reticulata. Furthermore, some of the AAV2–GDNF particles are probably transported transynaptically and transduce nigral neurons (red and blue dots in SN). Although AAV2 is an anterograde vector, the dramatic loss of nigrostriatal fibers in the Parkinsonian brain prevents retrograde transport of GDNF protein through this pathway. (e) GDNF trophic effect on nigral neurons promotes neuronal survival and induces nigrostriatal dopaminergic sprouting seen as regrowth of axons in the striatum (note the larger number of dopaminergic fibers in Put) that results in the amelioration of Parkinsonian symptoms
In the following sections we will first review recent peer-reviewed research into optimization specifically of AAV-based gene therapy and then examine potential applications of this therapeutic modality to test the hypothesis that protein misfolding is central to major CNS diseases. Other vector systems, such as herpes simplex virus and lentivirus have also been explored to some extent, but AAV has been by far the most widely used vector system for neurologic gene therapy, and the delivery technology is correspondingly advanced.
Axonal Transport
AAV serotypes evince diverse tropisms in the CNS with attendant advantages and disadvantages. We have favored the use of neuron-specific serotypes primarily for safety reasons, as this restriction avoids targeting of antigen-presenting cells, dealt with in more detail later. AAV2, the most widely used serotype clinically, is neuron-specific in the brain and we recognized its propensity for anterograde axonal transport some years ago [18, 19]. Thus, AAV2 is transported from the soma to terminals projecting to distal structures, and this transport results in release of intact AAV-particles from axon terminals to transfect other nearby cells in the distal structure. For example, striatal infusion of AAV2 revealed a strong fiber transduction in the globus pallidus, substantia nigra (SN) pars reticulata, and subthalamic nucleus defining an anterograde striato-pallidal transport in nonhuman primate (NHP) brain [20]. Similarly, thalamic infusion of AAV2 resulted, via robust anterograde thalamo-cortical transport, in strong transgene expression in motor and sensory cortical regions in rats [18, 21] and NHP [19]. In contrast, AAV6 is transported in a retrograde direction. Recently, we showed that striatal infusion of AAV6 resulted in extensive transduction of cortical and SN pars compacta neurons, with both structures innervating the striatum. AAV6 not only was transported almost exclusively in a retrograde direction but also transduced rat neurons several-fold better than AAV2 [22].
This remarkable directional divergence in axonal transport properties, apart from being intriguing at the molecular level, has clear therapeutic implications. By means of either AAV2 or AAV6, it is possible to target different distal structures even when the same anatomic region is the primary infusion site. Thus, the ability of AAV2 to be transported anterogradely supports its use in the treatment of PD motor deficits [23, 24] where transport of the vector throughout affected basal ganglia results in transduction of many affected structures [18, 19]. The obverse application of AAV6 may be in HD where the pathology is mediated by damage to both striatal medium spiny neurons and cortico-striatal projections.
CSF Delivery of Viral Particles
Parenchymal delivery of AAV has unique strengths, but, in some cases, global distribution is desirable [25]. Intravenous and intrathecal delivery are both promising routes of delivery because, respectively, they exploit the intense vascularization of the brain and CSF flow through perivascular pathways. In this regard, AAV9 is especially interesting in view of its ability to cross the blood–brain barrier after being injected into the circulation [16, 26]. This approach is not without its limitations, of course. Other tissues, particularly the liver, are transduced by AAV9 when it is injected intravenously, although efforts have been made to limit this transduction by modifying AAV9 capsid [27]. Intravenous infusion of AAV9 primarily transduces perivascular astrocytes and it is thought that this is mediated through binding of the vector to astrocytic protrusions into the vasculature [26]. Some brain neurons are also transduced, but, apparently, little has been done to identify whether they conform to a particular phenotype. Further exploration of the interaction between AAV9 and astrocytes may help to improve transduction efficiency. A further and far more significant problem is that of circulating anti-AAV antibodies, which display a remarkable ability to block brain transduction by this route. This problematic role of AAV immunity is dealt with in more detail later.
Alternatively, administration of AAV9 encoding different transgenes into the CSF results in strong transduction levels by different serotypes mainly after intrathecal injection [28–30]. We have explored the injection of AAV9 into the cisterna magna (CM). CM injection may be performed with relative safety in an anesthetized patient. Cisternal punctures are performed by inserting a hypodermic needle in the midline, just between the skull and the arch of C1, through the atlanto-occipital membrane and into the expanded dural sac for the rostral cervical spine. Advancing the needle in a stepwise manner allows passage through the membrane and into the CSF. There is a risk of the infusion needle touching or entering the spinal cord, causing temporary or permanent injury, but fluoroscopy or X-ray guidance can minimize this risk. Recently, we showed that AAV9 delivered by this route yields stronger levels of transduction than by intravascular delivery. In that study, AAV9 transduced astrocytes more efficiently than it did neurons in NHP [17]. CM injection directed strong expression of transgene throughout the cortex and cerebellum, mainly in astrocytes. Interestingly, we also confirmed that animals with a significant pre-existing anti-AAV antibody titer abrogated brain transduction, demonstrating that delivery of AAV9 into the CSF, as with intravenous or arterial injection, does not shield against AAV antibodies.
Immunologic Response
AAV2 is the most common vector used in gene therapy for neurological disease, mainly, because AAV2 is a neuron-specific serotype with no described adverse effects in the CNS, although previous studies report development of immune response after AAV2 delivery into rat liver [31]. Little or no detectable innate immune response has been described after injecting AAV2 into rat brain [32, 33] with only a modest transient astrocytic activation at the injection site [34]. Injection of AAV2 into rat brain, however, generates a humoral anti-capsid immunity that depends on vector dose [35, 36]. Moreover, high titers of existing anti-AAV2 capsid antibody produced by prior peripheral immunization blocked transduction of rat striatum by AAV2–human amino acid decarboxylase (hAADC), but did not trigger any cell-mediated response and did not eliminate already transduced striatal neurons [36]. Other studies [33, 37] have reported significant evidence of a cell-mediated response in a similar type of experiment in which delayed striatal administration of AAV2 vectors resulted in significant inflammation. However, we have seen little effect of humoral immunity on transduction by AAV2 in NHP with parenchymal delivery and do not routinely screen candidate animals for antibody titers, even though significant anti-AAV titers are very common in primates, both human [38–40] and nonhuman [16, 17]. The lowest titers are usually found in infants and young children [40].
The strong immunologic safety profile of AAV2 can be ascribed very much to its neuronal specificity because it avoids presentation of antigenic peptides on the surface of cells to engage the adaptive immune system. A dramatically different picture emerged, however, when we used AAV9 to deliver nonself genes parenchymally to rat brain [41]. To do this, we used two transgenes, hAADC and green fluorescent protein (GFP), and manufactured AAV2 and AAV9 versions. Both proteins are, of course, foreign to the rat immune system. We found that striatal infusion of AAV9, but not AAV2, encoding these proteins activated a massive immune response characterized by glial major histocompatibility complex II upregulation, CD8+ lymphocytic infiltration, and neuronal death in the transduced cerebral regions. In these experiments, hAADC appeared to be a much more aggressive driver of cell-mediated and humoral immunity. These results have clear implications on the future clinical use of AAV9. The use of AAV9 is, of course, not precluded, but care has to be taken in the design of preclinical experiments, specifically with respect to maintaining syngeneic expression. It is particularly noteworthy that GFP is a widely used reporter gene because it is regarded as quite innocuous. That is clearly not the case when GFP is expressed by antigen-presenting cells. Interestingly, similar phenomena have also been observed in NHP brain with AAV1-GFP [42] and liver, where AAV7 elicited similar GFP-specific immune responses [43]. Clearly then, this issue of antigen presentation by AAV serotypes that are not neuron-specific presents a real challenge to gene therapists.
Real-Time MRI-Guided AAV Delivery
Limited distribution of the therapeutic agent seems to be a recurrent problem when trying to reproduce preclinical results in clinical trials, probably owing to the use of simple injection for parenchymal delivery [44–46]. To date, convection-enhanced delivery (CED) seems to be a more efficient system in order to achieve complete coverage of target structures. CED, first described by Oldfield and colleagues [47], is a parenchymal infusion technique that, by means of a pressure gradient from a cannula tip positioned within the target structure, generates bulk flow of macromolecules within the interstitial fluid space. This method allows higher quantities of therapeutic agents to be distributed through large volumes of brain tissue from a single cannula, not by simple diffusion kinetics but by a different mechanism that requires a pressure-driven engagement of the perivasculature to propel infusate over significant distances [48]. Our group has extensively optimized this method over the years in the NHP model [9, 49–52]. Our current technique allows us to monitor parenchymal infusions in a MR scanner by including free gadoteridol, a tracer visible with MR, in the therapeutic agent preparation. This method greatly enhances the accuracy and viability of, for example, AAV delivery in NHP as it provides real-time visualization of the infusion by using gadoteridol as a surrogate marker for parenchymal AAV2 distribution [51, 53]. Thus, performing infusions by interventional MRI represents a tremendous advantage in predicting the infusion outcome, as well as being able to achieve optimal cannula placement within target structures or monitor possible hemorrhages or infusate leakage outside the target structure [50]. In fact, MRI tracers for real-time CED have been already used in two patients treated at NIH and shown to be safe within the human brain parenchyma [54].
Integrated Delivery Platform for Parenchymal Delivery
Real-time CED described in the previous section led to the development of a clinical platform for MRI-monitored CED in the brain. In collaboration with MRI Interventions Inc. (Irvine, CA; formerly Surgivision Inc.) and BrainLab Inc. (Heimstetten, Germany), we have developed an MRI-compatible delivery platform that includes a skull-mounted aiming device (SmartFrame), a reflux-resistant CED cannula (SmartFlow) and a MRI-integrated software package (Clearpoint) that communicates with both the console and the operating neurosurgeon in the MRI suite [49]. This device results in cannula tip placement that is within 1 mm of the visually identified target site, greatly increasing our ability to safely and reliably deliver gene therapy vectors as we recently ascertained in NHP for infusion in different regions of the brain [49]. As CED is a pressurized infusion method, under certain conditions, such as a high flow rate, the pressure generated at the cannula tip can exceed the shear modulus of the tissue–cannula contact surface and result in a reflux of the infusate through the outside of the cannula [55]. In order to avoid this phenomenon, we designed a ceramic, fused silica reflux-resistant cannula with a 3-mm stepped tip that is now the Food and Drug Administration-approved SmartFlow® catheter [51, 55, 56]. We found out that, with this reflux-resistant cannula, flow rates up to 5 μl/min [55] and even 10 μl/min (KSB, unpublished data) could be safely achieved, significantly reducing the procedure time. For this custom-designed cannula, we also identified specific cannula placement zone within different target structures, or “green” zone, that would ensure anatomically contained infusion of the therapeutic agent [57, 58].
Gene Therapy Applications for Neurodegenerative Diseases
AD
AD is the most common cause of dementia, characterized by a progressive neurodegenerative disorder in which synaptic loss is followed by neuronal death. This process follows a typical anatomic progression with early olfactory degeneration followed by later hippocampal and cortical degeneration [59, 60]. As each region is attacked, the accumulation of somewhat fibrillary deposits chiefly composed of amyloid protein is a defining feature. Both familial and sporadic disease have been intensively studied for many years (for some excellent recent reviews, see [61–65]). A major focus of translational research has been the small protein β-amyloid (Aβ). Because early-onset familial disease is characterized by mutations in Aβ or in proteases that cleave the precursor into fragments, some of which evince various toxicities both in cell culture and in transgenic mice, attention has been directed very much at the development of therapies that seek to deplete the brain of such toxic species with the expectation that this alone would alter the course of the vastly more prevalent sporadic disease, itself not associated with over-production of toxic Aβ much as poor clearance [66]. Both active immunization with synthetic Aβ42 peptide and passive immunization with anti-Aβ antibodies were the most plausible immunotherapeutic approaches for AD. However, the presence of CNS inflammatory reaction in patients after active immunization [67], and few cognitive benefits after antibody injection [68], prompted a reassessment of whether toxic Aβ is really a driver of AD pathology or just one of a number of participants [69]. Interestingly, gene therapy-based delivery in experimental models suggested that this might be a useful approach for AD immunotherapy. Effective passive immunization was achieved by cerebroventricular delivery of recombinant anti-Aβ single-chain variable fragments (scFv) driven by AAV transduction, and this resulted in reduction of Aβ deposition in the mouse model of Aβ toxicity by 25–50 % [70]. Further, hippocampal infusion of AAV1-Aβ-scFv not only lowered levels of insoluble Aβ but animals also exhibited lower levels of hyperphosphorylated tau protein and a significant improvement in cognitive function [71]. Interestingly, peripheral expression of therapeutic agents driven by viral vectors has also been effective. Muscular injection (hind limb) of AAV-encoding neprilysin, a potent peptidase able to degrade Aβ induced a 60 % reduction of soluble Aβ peptides and 50 % reduction of amyloid deposits in the brain of amyloid over-expressing transgenic mice [72]. These approaches all suffer the same problem. The so-called “AD model” is really a model of Aβ toxicity in which mice have been engineered to massively overexpress mutated forms of Aβ. These mice, however, differ in important and perhaps crucial ways from humans with AD. First, the amyloid plaques: they differ significantly in appearance from human plaque in that they lack the fibrillary appearance of the human plaques [73]. Second, even though the mice show dramatic and progressive neurite retraction, they show little neuronal death—a hallmark of the human disease [74]. That strategies designed to ameliorate an engineered toxicity should do so, veers close to a tautology; thus, they should be interpreted and extrapolated to human disease with caution. The fact that anti-amyloid strategies have so far failed in the clinic is, perhaps, telling.
This fading faith in the amyloid hypothesis reminds us that AD is a complex disease in which multiple factors may be responsible for onset and progression. Neuroinflammation is another etiologic characteristic sign in AD. Astrocytosis and microgliosis are triggered by Aβ accumulation and direct upregulation of cytokines and chemokines that lead to chronic brain inflammation in AD patients [75]. Few studies have focused on neuronal anti-inflammatory cytokine signaling as an alternative to anti-amyloid strategies. Sustained expression of IL-4 by AAV-mediated hippocampal injection in transgenic “AD mice”, revealed a significant reduction of neuroinflammatory symptoms, as well as Aβ reduction, enhanced neurogenesis, and spatial learning restoration [76]. Recent experiments demonstrated that IL-10 expression mediated by AAV1 in hippocampal neurons resulted in amelioration of cognitive dysfunction, reduction of astro/microgliosis, neurogenesis, and Aβ clearance. Moreover, like IL-4, sustained overexpression of IL-10 also improved spatial learning [77]. Gene therapy for AD is an active area with many therapies in the pipeline. All of them will require mastery of delivery technology in the clinic. Inadequate targeting or distribution will doom candidate therapies, no matter how effective they may appear in more limited preclinical settings.
PD
PD is a neurodegenerative movement disorder characterized principally by the selective loss of dopaminergic neurons in the SN pars compacta, although other areas of the nervous system are frequently also affected [78, 79]. The main histopathologic hallmark of this disorder is the presence of intracellular inclusions called Lewy bodies inside the surviving dopaminergic neurons. Accordingly, association of mutations in specific genes (e.g., α-synuclein) and of genetic susceptibility variants in PD patients has implicated abnormalities in protein homeostasis, or the management and elimination of misfolded proteins, in the pathogenesis of this disorder that result in the presence of these inclusions [80]. As protein aggregates seem to play a major role in PD, strategies targeting the cellular systems that regulate proteostasis, such as the chaperone system (folding of proteins and refolding of misfolded proteins) and ubiquitin-proteasome system (elimination of misfolded proteins), are being explored (for a review see [7]). Gene therapy may provide strategies to upregulate the expression and/or activity of neuroprotective chaperones in neurons that are vulnerable to neurodegeneration in PD. Preclinical studies in animal models of PD have provided evidence for the potential of viral-mediated heat shock protein 70 (Hsp70) expression. One study in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mice demonstrated that Hsp70 gene transfer to striatal dopaminergic neurons by a recombinant AAV could protect against MPTP-induced dopaminergic cell death and associated decline in striatal dopamine levels [81]. Another study in a rat model of PD showed that AAV-mediated overexpression of Hsp70, but not Hsp40, protected against dopaminergic neurodegeneration [82]. However, the neuroprotective role of Hsp70 is still controversial as a recent study of overexpression of Hsp70 in transgenic mice did not reduce dopaminergic neuron loss or damage to striatal terminals after either acute or chronic treatment with MPTP [83]. Also, several studies have established that α-synuclein (the main component of Lewy bodies) overexpression, and subsequent aggregation, is deleterious for dopaminergic neurons in α-synuclein-overexpressing rodents [84–86] and primates [87, 88]. Thus, recent studies silenced human α-synuclein (hα-syn) expression both in vitro and in vivo by means of RNA interference (RNAi). Bohn and colleagues used a lentivirus to ectopically express hα-syn in the striatum of rats and co-infused them with a short hairpin (shRNA) against hα-syn harbored in a lentivirus [89]. Co-infusion of the lentiviral hα-syn and shRNA resulted in an almost complete elimination of hα-syn in the striatum. More recently, the same group used a similar approach to silence ectopic hα-syn in the SN of rats, although this time both hα-syn transgenes were harbored in AAV [90]. Animals were injected unilaterally and the experimental group that received AAV-hα-syn showed contralateral forelimb deficits, as well as nigral cell loss 9 weeks after AAV infusion. In contrast, animals that were co-infused with AAV-hα-syn and AAV-shRNA evinced normal movement of the contralateral forelimb at 9 weeks. Unfortunately, AAV-shRNA expression did not protect against nigral neuronal loss in these animals and, when injected alone, AAV-shRNA expression alone resulted in dopaminergic cell loss in the SN [90]. Interestingly, embedding the shRNA used in the latter study into a microRNA resulted in reduced toxicity in cell cultures, but still achieved a significant silencing of hα-syn (up to 60 % less hα-syn expression) [91]. Unfortunately, and like the comments made earlier about AD models, rodents engineered to overexpress hα-syn are not good models of PD and, probably, any pathology corresponds more to the toxic effect of hα-syn overexpression per se. This fact makes it rather difficult to translate preclinical data obtained in these models into therapies for PD patients.
Other approaches to gene therapy of PD, not targeted to protein aggregation such as α-synuclein, actually represent the great majority of clinical neurologic gene therapy trials in terms of subjects [14, 92]. Two major classes of intervention have been advanced: 1) modulation of neurotransmitter synthesis (AAV-GAD [93, 94], AAV2-hAADC [23, 95]) and 2) use of growth factors [neurturin [24, 44, 46], glial cell line-derived neurotrophic factor (GDNF) [96–99], NCT01621581] to stimulate regeneration (see [100] for review). Approaches based on neurotransmitter function showed good results in Phase I trials as seen by a reduction in the UPDRS scores after each treatment [23, 93, 95]. Based on those encouraging results, an AAV-GAD Phase II trial was performed where treated patients showed a reduction of UPDRS scores compared with the placebo group [94]. However, that improvement was no better than that seen with deep brain stimulation, a surgical therapy already well established for the symptomatic treatment of PD. However, a Phase II clinical trial is already planned for AAV2–hAADC treatment of PD patients with higher vector doses and an optimized delivery platform based on promising preclinical results in NHP [101]. Similarly, therapeutic approaches using neurotrophic factors, such as neurturin (NTN) or GDNF, showed promising results in Phase I clinical trials [24, 96, 98, 102]. An AAV2–NTN vector was further tested for efficacy in a Phase II study; however, only secondary measures were significantly improved in patients receiving AAV2–NTN compared with a placebo group [46, 103]. A new Phase II study is ongoing with a higher vector dose. Phase I clinical trials testing GDNF protein delivery yielded positive behavioral results in PD patients [96, 98, 102]. Unfortunately, those results could not be replicated in a following Phase I/II study and some patients treated participating in the clinical trial generated anti-GDNF protein antibodies [97]. Preclinical studies in NHP recently demonstrated significantly recovery of MPTP-induced parkinsonism and that the adverse effects associated to delivering naked GDNF protein were not present when administering GDNF harbored by an AAV2 vector [104]. Consequently, a Phase I single-center, open-label, dose escalation, safety and tolerability study of AAV2–GDNF administered by CED to the putamen is now in progress for patients with advanced PD (NCT01621581, see Fig. 1). We do not know, however, whether any of these interventions, especially growth factors, modify protein aggregates in PD patients or, indeed, whether they might be effective in only certain subsets of PD.
HD
HD is an autosomal dominant neurodegenerative disease caused by a CAG expansion in exon 1 of the huntingtin (Htt) protein gene [105] with 36 or more CAG repeats conferring a gain of functional toxicity [106]. Patients with HD display chorea, cognitive deficits, and psychiatric symptoms. Currently, there is no effective therapy that either prevents the onset or modifies the progression of the disease. HD neuropathology is characterized by the atrophy of primarily the striatum, with massive degeneration of the striatal medium spiny neurons and cortex; however, other brain regions (e.g., thalamus, hippocampus, and white matter) are also affected. One neuropathologic hallmark is the presence of neuronal nuclear inclusions or cytoplasmic aggregates of misfolded mutant Htt (mHtt) protein [107]. Because it is an autosomal dominant inherited disease, several research groups have tried to reduce mHtt expression in a variety of HD experimental models [108]. Yamamoto and colleagues demonstrated with a tet-regulated conditional model of HD that the blockade of mHtt expression in these mice resulted in a behavioral improvement and a reduction of inclusion bodies [109]. More recent research is focused in silencing mHtt mRNA by means of RNAi or antisense oligonucleotide (ASOs) (see [110, 111] for reviews). Since that first proof-of-concept work, several studies in different rodent HD models have reported that suppressing ~70 % of mHtt expression over months by means RNAi is effective in reducing neuropathology, improving motor behavior, and prolonging survival [112–118]. Furthermore, these studies also showed that reduction in Htt could be tolerated for up to 9 months, even though the wild-type Htt protein has several physiological activities important for neuronal cells [119]. Indeed, a recent study in NHP demonstrated that a reduction of 45 % in striatal Htt expression in Rhesus macaques does not induce motor deficits, neuronal degeneration, astrogliosis, or an immune response, suggesting that partial suppression of wild-type Htt expression is well tolerated in the primate for up to 6 weeks [120]. Nevertheless, the long-term tolerability to wild-type Htt reduction remains unknown. Therefore, as HD patients will be treated chronically, a selective silencing of mHtt, such as targeting CAG expansions [121, 122] or single nucleotide polymorphisms associated only with the mutant allele [123–125], would be more desirable.
An alternative approach to RNAi is to infuse single-stranded ASO. These short complementary strands are chemically engineered to bind to mHtt mRNA and recruit RNase H that will efficiently degrade the mRNA paired to the ASO [126]. A few studies have investigated this potential antisense therapy for HD with encouraging results [121–125]. Particularly, Kordasiewicz and colleagues [127] have reported a study that provides strong preclinical evidence to support the use of ASO as an Htt-lowering therapeutic for HD . In this study, the authors demonstrated that temporary infusion of ASO against mHtt in the CSF of 3 HD mouse models resulted in a ~75 % reduction of mHtt levels and a sustained reversal of the disease even after the treatment was stopped. Furthermore, infusion of an ASO specific for monkey Htt in the CSF of Rhesus macaques yielded the same results as those seen in transgenic rodent models and, after 21 days of intrathecal infusion (4 mg/day), Htt suppression lasted for 8 weeks after treatment cessation. In addition, widespread expression of ASO in most regions of the brain and spinal cord, and subsequent suppression of wild-type Htt expression, was well tolerated. Despite these encouraging results, Htt silencing in NHP striatum, a region primarily affected in HD, was only about 25 % in the caudate and no Htt suppression data in putamen were reported. The authors argued that, based on the data obtained from the transgenic models, targeting striatum selectively would not result in a better outcome than CSF delivery. However, in our opinion, aiming primarily at affected structures as striatum, rather than engaging more widespread mHtt suppression, would likely result in a more effective therapy. In agreement with this, a recent study in NHP has shown that continuous CED of small interfering RNA yields widespread distribution in the putamen and a reduction in Htt mRNA of ~45 % [128], indicating that improving coverage of target structures would also result in better suppression of mHtt and, consequently, a potentially better clinical outcome.
ALS
ALS is a relentlessly degenerative disease of the CNS in which upper and lower motor neurons gradually die, causing paralysis and death. The first symptoms of the disease are muscle weakness in one or more limbs spreading progressively to all limbs and muscle groups, including diaphragm, that results in respiratory failure. About 50 % of patients die within 18 months of diagnosis. Despite the testing of a number of drugs, no treatment has ever extended life expectancy a few months beyond historical controls [129].
One of the most important discoveries in ALS research has been the identification of genetic mutations that cause familial ALS. Approximately 10 % of ALS is caused by mutations of known and unknown genes, including superoxide dismutase 1 (SOD1). A common feature of all ALS, both sporadic and familial, is the presence of large aggregates of proteins, including SOD1, in motor neuron cell bodies. This observation, together with genetic data, supports the idea that SOD1 plays a central role in familial and, possibly, sporadic ALS pathology. Indeed, generation of transgenic mice and rats that overexpress human SOD1 with point mutations (mSOD1) corresponding to those found in humans resulted in animals that expressed many of the hallmarks of the human disease.
Therapeutic interventions with gene therapy have, as for PD, followed a dual path. The earliest approaches used growth factors designed to protect motor neurons from ALS-mediated toxicity. Kaspar and colleagues [130] injected AAV2–insulin-like growth factor 1 into quadriceps muscle of mSOD1 mice, relying on a weak retrograde transport of vector up the sciatic nerve, and showed a significant extension of lifespan.
Antisense-mediated SOD1 silencing by viral vectors may be an effective therapeutic tool in ALS [131]. In this regard, spinal injection of RNAi–lentiviral vectors against SOD1 demonstrated significant improvement in motor impairment and motor neuron survival in a transgenic ALS model [132]. Consistent with this, muscular injection revealed that silencing SOD1 expression by lentivirus-mediated shRNA significantly reduced the progression of the pathology [133]. In 2006, Isis Pharmaceuticals (Carlsbad, CA, USA) initiated a Phase I study to evaluate the safety of a single intrathecal infusion of an ASO, called ISIS-SOD1Rx, designed to interfere with the production of mSOD1 (NCT01041222). Currently, early data report that the drug is safe and well tolerated by patients receiving lower doses of the synthetic molecules. Although no signs of clinical benefit have been reported yet, the authors have reported a strong correlation between mRNA target knockdown in the brain and its reduction of target protein in CSF [134].
Recently, a study revealed the possibility of allele-specific suppression of SOD1 by RNAi-mediated SOD1 silencing [135]. Unfortunately, the variability between different studies and the unresolved challenges in delivery and off-target effects place the clinical development of these candidate therapies at a very early stage of development [136].
Prion Disease
Prion diseases or transmissible spongiform encephalopathies (TSE) are a family of rare progressive and invariably fatal neurodegenerative disorders that affect both humans and animals. Prions cause neurodegenerative disease by aggregating extracellularly within the CNS to form amyloid plaques [137]. The highly-conserved host-encoded cellular prion protein (PrPC) undergoes a conformational change in which it is converted into a partially protease-resistant amyloidogenic isoform, PrPSc (disease-associated isoform of PrP), resulting in misfolding and aggregation [138, 139]. This latter isoform is also associated with infectivity (the ‘protein-only’ hypothesis) [140]. At present, there is no effective therapy for clinically affected TSE patients available, such that TSE are usually fatal. However, very interesting gene therapy-based approaches have been reported recently.
One of the more studied approaches to treat TSE is immunotherapy. This strategy is based on the finding that PrP-specific antibodies antagonize prion propagation both in vitro [141–143] and in vivo [144]. As an alternative to immunization and to by-pass the problems inherent in the use of full length antibodies, Wuertzer and colleagues [145] developed an AAV2 vector that expressed linearized fragments of antibodies (scFv) against PrP that retain binding properties similar to the monoclonal or Fab counterpart. In addition, scFv delivery by means of a viral vector, such as AAV2, ensured the long-term expression of the transgene. One month after AAV2-scFv infusion into the brain (striatum and thalamus), mice were intraperitoneally challenged with PrPSc [146]. AAV2-scFv-treated animals showed an improvement of clinical signs accompanied by an extended incubation period and lower PrPSc levels in the brain. However, the protection against TSE was partial, probably owing to the fact that the scFv could only act on brain propagation, and prion amplification was still going on peripherally [146].
Alternatively, Mallucci and colleagues [147] developed a strategy that targeted PrPC rather than the disease-associated PrPSc isoform. As PrPC is the substrate for conversion in prion disease, its removal would alter the progression of the disease. In order to prove this hypothesis, Mallucci and colleagues [147] generated an adult-onset PrP-knockout mice (NFH-Cre/tg37), with neuron-specific deletion of PrPC at 9 weeks of age, and proved that depletion of PrPC in neurons cured clinical disease and reversed pathology, even though there was still accumulation of extra-neuronal PrPSc [148]. More recently, this group developed a more therapeutic approach aimed at reducing endogenous PrPC expression by means of RNAi-driven gene silencing. Tg37 mice received a focal infusion of lenti-shRNA against PrPC or an empty lentivirus in both hippocampi [149]. The resulting down-regulation of PrPC was able to slow down the progression of TSE. Lenti-RNAi delivery to mice with established prion disease significantly prolonged survival time (19 % and 24 % longer than empty-lentivirus-treated or untreated mice, respectively), reduced spongiform degeneration, and protected against neuronal loss. Unfortunately, the treatment did not cure any of the treated animals and all of them eventually died. The authors of this study speculated that optimization of vector delivery to achieve a more extensive expression of the RNAi might result in more extensive neuroprotection [150], a speculation with which we wholeheartedly concur.
Overall, there are two key and challenging aspects that need to be addressed in order to promote a more effective translation of experimental therapies results. Interestingly, the authors of most of the proposed approaches to treat misfolding protein diseases of the CNS reviewed earlier agree that achieving accurate targeting and optimal distribution of the therapeutic agent is a very challenging, but critical, goal in order to achieve full and effective translation of preclinical results into the clinic. Although this represents a serious challenge in the diseased CNS scenario, in our opinion the more recent advances and optimizations accomplished in particular for the delivery of gene-based therapies, such as real-time imaging of delivery and CED, will help to achieve an adequate coverage of the target structure and, consequently, avoid unwanted off-target effect of therapeutic agents. In some cases, researchers also agree on the importance of the experimental models available to test new therapeutic approaches considering it a critical aspect of the translation of positive preclinical results into actual therapies for patients. Although it is extremely important to our understanding of the disease mechanisms and candidate therapies, experimental models need to provide the closest scenario possible to that of the human disease. Moreover, animal models need to be incorporated into an overall narrative of each neurodegenerative disease, particularly with respect to the natural origins of the disease process. This will ensure a faster, more accurate, and effective generation of therapies.
Conclusion
CNS diseases associated with protein misfolding are prevalent among neurodegenerative pathologies. Unfortunately, at present, there is no disease-modifying or curative therapy to treat them despite the fact that many positive results have been reported in preclinical work. Studies on potential strategies with encouraging results argue that better results could be achieved by improving therapeutic agent delivery and long-term expression. In the last decade, gene therapy for CNS has been greatly refined for optimal accuracy, coverage, and safety. Gene therapy is now considered an increasingly feasible approach owing to an improved accuracy of targeting, optimal delivery of the therapeutic agent by ensuring almost complete coverage of the target structure, and a safe and persistent expression of the transgene by viral vectors. Also, capsid engineering to avoid off-target cell transduction and characterization of AAV serotypes tropism and transport are important in order to select the more suitable vector for each pathology. Nevertheless, many safety and efficacy challenges still need to be addressed, such as regulated transgene expression (for reviews see [151] and [152]). Many regulatable systems are now under development and some, i.e., the tetracycline-dependent transcriptional system, have been used widely for in vivo preclinical applications. However, inducible gene-expression systems still need to pass a few milestones. Regulation of transgene expression should be possible with drug-like molecules that, like the genetic response elements themselves, must be nontoxic and nonimmunogenic. A clinically viable gene regulation system must have acceptable pharmacokinetics such that gene expression can be turned “on” or “off” quickly and effectively.
It is also important, particularly in the protein misfolding field, to develop experimental models that reproduce CNS diseases as faithfully as possible. Unfortunately, for many CNS pathologies, especially nonfamilial types, the agent causing the disease remains unknown. Models based on overexpressing the aggregating protein associated with the disease are at risk of assuming this phenomenon to be the ultimate causing factor and, consequently, result in the development of therapies optimized for the treatment of a protein overexpression-induced toxicity. Therefore, when brought to patients, these therapeutic strategies will not cure or change the progression of the disease because the human disease is not the result of only a misfolded protein. Nevertheless, these experimental models are very valuable and allow researchers to elucidate the mechanisms underlying protein misfolding, protein aggregation, as well as to identify key players in the pathology of CNS diseases associated with protein misfolding.
Gene therapy is coming of age, as indicated by the plethora of gene therapy products being tested in clinical trials and by the recent approval in Europe of a commercial gene therapy to treat lipoprotein lipase deficiency, the first to be approved in the Western world. Further experiments to develop more accurate experimental models for neurodegenerative diseases along with those aimed to optimize gene therapy will provide better translation of positive preclinical results into actual therapies for patients.
Electronic supplementary material
(PDF 550 kb)
Acknowledgments
This work was supported, in part, by NIH grant R01NS073940 to KSB.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Kaganovich D, Kopito R, Frydman J. Misfolded proteins partition between two distinct quality control compartments. Nature. 2008;454:1088–1095. doi: 10.1038/nature07195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roth J, Yam GH, Fan J, et al. Protein quality control: the who’s who, the where’s and therapeutic escapes. Histochem Cell Biol. 2008;129:163–177. doi: 10.1007/s00418-007-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luheshi LM, Dobson CM. Bridging the gap: from protein misfolding to protein misfolding diseases. FEBS Lett. 2009;583:2581–2586. doi: 10.1016/j.febslet.2009.06.030. [DOI] [PubMed] [Google Scholar]
- 4.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 5.Bellotti V, Mangione P, Stoppini M. Biological activity and pathological implications of misfolded proteins. Cell Mol Life Sci. 1999;55:977–991. doi: 10.1007/s000180050348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobson CM. Protein misfolding, evolution and disease. Trend Biochem Sci. 1999;24:329–332. doi: 10.1016/S0968-0004(99)01445-0. [DOI] [PubMed] [Google Scholar]
- 7.Kalia SK, Kalia LV, McLean PJ. Molecular chaperones as rational drug targets for Parkinson’s disease therapeutics. CNS Neurol Disord Drug Targets. 2010;9:741–753. doi: 10.2174/187152710793237386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011;10:930–944. doi: 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson RM, Varenika V, Forsayeth JR, et al. Future applications: gene therapy. Neurosurg Clin North Am. 2009;20:205–210. doi: 10.1016/j.nec.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mandel RJ, Burger C, Snyder RO. Viral vectors for in vivo gene transfer in Parkinson’s disease: properties and clinical grade production. Exp Neurol. 2008;209:58–71. doi: 10.1016/j.expneurol.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kells AP, Forsayeth J, Bankiewicz KS. Glial-derived neurotrophic factor gene transfer for Parkinson’s disease: anterograde distribution of AAV2 vectors in the primate brain. Neurobiol Dis. 2012;48:228–235. doi: 10.1016/j.nbd.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao PJ, Lentz TB, Samulski RJ. Recombinant adeno-associated virus: clinical application and development as a gene-therapy vector. Ther Deliv. 2012;3:835–856. doi: 10.4155/tde.12.63. [DOI] [PubMed] [Google Scholar]
- 13.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2011;11:181–188. doi: 10.2174/156652311795684759. [DOI] [PubMed] [Google Scholar]
- 14.Forsayeth J, Bankiewicz KS, Aminoff MJ. Gene therapy for Parkinson’s disease: where are we now and where are we going? Exp Rev Neurother. 2010;10:1839–1845. doi: 10.1586/ern.10.161. [DOI] [PubMed] [Google Scholar]
- 15.Federici T, Taub JS, Baum GR, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2012;19:852–859. doi: 10.1038/gt.2011.130. [DOI] [PubMed] [Google Scholar]
- 16.Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23:382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciesielska A, Mittermeyer G, Hadaczek P, et al. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol Ther. 2011;19:922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kells AP, Hadaczek P, Yin D, et al. Efficient gene therapy-based method for the delivery of therapeutics to primate cortex. Proc Natl Acad Sci U S A. 2009;106:2407–2411. doi: 10.1073/pnas.0810682106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankiewicz KS, Eberling JL, Kohutnicka M, et al. Convection-enhanced delivery of AAV vector in parkinsonian monkeys; in vivo detection of gene expression and restoration of dopaminergic function using pro-drug approach. Exp Neurol. 2000;164:2–14. doi: 10.1006/exnr.2000.7408. [DOI] [PubMed] [Google Scholar]
- 21.Hadaczek P, Mirek H, Bringas J, et al. Basic fibroblast growth factor enhances transduction, distribution, and axonal transport of adeno-associated virus type 2 vector in rat brain. Hum Gene Ther. 2004;15:469–479. doi: 10.1089/10430340460745793. [DOI] [PubMed] [Google Scholar]
- 22.Salegio EA, Samaranch L, Kells AP, et al. Axonal transport of adeno-associated viral vectors is serotype-dependent. Gene Ther. 2013;20:348–352. doi: 10.1038/gt.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Christine CW, Starr PA, Larson PS, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73:1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 25.Forsayeth JR, Bankiewicz KS. AAV9: over the fence and into the woods. Mol Ther. 2011;19:1006–1007. doi: 10.1038/mt.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pulicherla N, Shen S, Yadav S, et al. Engineering liver-detargeted AAV9 vectors for cardiac and musculoskeletal gene transfer. Mol Ther. 2011;19:1070–1078. doi: 10.1038/mt.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Towne C, Pertin M, Beggah AT, et al. Recombinant adeno-associated virus serotype 6 (rAAV2/6)-mediated gene transfer to nociceptive neurons through different routes of delivery. Mol Pain. 2009;5:52. doi: 10.1186/1744-8069-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques SJ, Ahmed Z, Forbes A, et al. AAV8(gfp) preferentially targets large diameter dorsal root ganglion neurones after both intra-dorsal root ganglion and intrathecal injection. Mol Cell Neurosci. 2012;49:464–474. doi: 10.1016/j.mcn.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 30.Xu Q, Chou B, Fitzsimmons B, et al. In vivo gene knockdown in rat dorsal root ganglia mediated by self-complementary adeno-associated virus serotype 5 following intrathecal delivery. PloS one. 2012;7:e32581. doi: 10.1371/journal.pone.0032581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vandenberghe LH, Wang L, Somanathan S, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12:967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 32.Mandel RJ, Burger C. Clinical trials in neurological disorders using AAV vectors: promises and challenges. Curr Opin Mol Ther. 2004;6:482–490. [PubMed] [Google Scholar]
- 33.Peden CS, Burger C, Muzyczka N, et al. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reimsnider S, Manfredsson FP, Muzyczka N, et al. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- 35.Forsayeth JR. Influence of the Immune system on central nervous system gene transfer. In: Kaplitt MG, During MJ, editors. Gene therapy of the central nervous system - from bench to bedside. Academic Press: Amsterdam; 2006. pp. 45–52. [Google Scholar]
- 36.Sanftner LM, Suzuki BM, Doroudchi MM, et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Mastakov MY, Baer K, Symes CW, et al. Immunological aspects of recombinant adeno-associated virus delivery to the mammalian brain. J Virol. 2002;76:8446–8454. doi: 10.1128/JVI.76.16.8446-8454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21:704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 39.Madsen D, Cantwell ER, O’Brien T, et al. Adeno-associated virus serotype 2 induces cell-mediated immune responses directed against multiple epitopes of the capsid protein VP1. J Gen Virol. 2009;90:2622–2633. doi: 10.1099/vir.0.014175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calcedo R, Morizono H, Wang L, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18:1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ciesielska A, Hadaczek P, Mittermeyer G, et al. Cerebral infusion of AAV9 vector-encoding non-self proteins can elicit cell-mediated immune responses. Mol Ther. 2013;21:158–166. doi: 10.1038/mt.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadaczek P, Forsayeth J, Mirek H, et al. Transduction of nonhuman primate brain with adeno-associated virus serotype 1: vector trafficking and immune response. Hum Gene Ther. 2009;20:225–237. doi: 10.1089/hum.2008.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao G, Wang Q, Calcedo R, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther. 2009;20:930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartus RT, Herzog CD, Chu Y, et al. Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson’s disease and nonhuman primate brains. Mov Disord. 2011;26:27–36. doi: 10.1002/mds.23442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70:1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 46.Marks WJ, Jr, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9:1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 47.Bobo R, Laske D, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. PNAS. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hadaczek P, Yamashita Y, Mirek H, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther. 2006;14:69–78. doi: 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richardson RM, Kells AP, Martin AJ, et al. Novel platform for MRI-guided convection-enhanced delivery of therapeutics: preclinical validation in nonhuman primate brain. Stereotact Funct Neurosurg. 2011;89:141–151. doi: 10.1159/000323544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fiandaca MS, Varenika V, Eberling J, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47(Suppl. 2):T27–35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fiandaca M, Forsayeth J, Dickinson P, et al. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richardson RM, Kells AP, Rosenbluth KH, et al. Interventional MRI-guided putaminal delivery of AAV2-GDNF for a planned clinical trial in Parkinson’s disease. Mol Ther. 2011;19:1048–1057. doi: 10.1038/mt.2011.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su X, Kells AP, Aguilar Salegio E, et al. Real time MRI imaging with gadoteridol predicts distribution of transgenes after convection-enhanced delivery of AAV2 vectors. Mol Ther. 2010;18:1490–1495. doi: 10.1038/mt.2010.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lonser RR, Warren KE, Butman JA, et al. Real-time image-guided direct convective perfusion of intrinsic brainstem lesions. Technical note. J Neurosurg. 2007;107:190–197. doi: 10.3171/JNS-07/07/0190. [DOI] [PubMed] [Google Scholar]
- 55.Krauze MT, Saito R, Noble C, et al. Reflux-free cannula for convection-enhanced high-speed delivery of therapeutic agents. J Neurosurg. 2005;103:923–929. doi: 10.3171/jns.2005.103.5.0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito R, Krauze MT, Bringas JR, et al. Gadolinium-loaded liposomes allow for real-time magnetic resonance imaging of convection-enhanced delivery in the primate brain. Exp Neurol. 2005;196:381–389. doi: 10.1016/j.expneurol.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 57.Yin D, Richardson RM, Fiandaca MS, et al. Cannula placement for effective convection-enhanced delivery in the nonhuman primate thalamus and brainstem: implications for clinical delivery of therapeutics. J Neurosurg. 2010;113:240–248. doi: 10.3171/2010.2.JNS091744. [DOI] [PubMed] [Google Scholar]
- 58.Yin D, Valles FE, Fiandaca MS, et al. Optimal region of the putamen for image-guided convection-enhanced delivery of therapeutics in human and non-human primates. Neuroimage. 2011;54(Suppl. 1):S196–203. doi: 10.1016/j.neuroimage.2009.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 60.Kovacs T, Cairns NJ, Lantos PL. Olfactory centres in Alzheimer’s disease: olfactory bulb is involved in early Braak’s stages. Neuroreport. 2001;12:285–288. doi: 10.1097/00001756-200102120-00021. [DOI] [PubMed] [Google Scholar]
- 61.Hardy JA, Higgins GA. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 62.Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 63.Hardy J. The amyloid hypothesis for Alzheimer’s disease: a critical reappraisal. J Neurochem. 2009;110:1129–1134. doi: 10.1111/j.1471-4159.2009.06181.x. [DOI] [PubMed] [Google Scholar]
- 64.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 65.Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 66.Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420:879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- 68.Morgan D. Immunotherapy for Alzheimer’s disease. J Intern Med. 2011;269:54–63. doi: 10.1111/j.1365-2796.2010.02315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Birmingham K, Frantz S. Set back to Alzheimer vaccine studies. Nat Med. 2002;8:199–200. doi: 10.1038/nm0302-199b. [DOI] [PubMed] [Google Scholar]
- 70.Levites Y, Jansen K, Smithson LA, et al. Intracranial adeno-associated virus-mediated delivery of anti-pan amyloid beta, amyloid beta40, and amyloid beta42 single-chain variable fragments attenuates plaque pathology in amyloid precursor protein mice. J Neurosci. 2006;26:11923–11928. doi: 10.1523/JNEUROSCI.2795-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ryan DA, Mastrangelo MA, Narrow WC, et al. Abeta-directed single-chain antibody delivery via a serotype-1 AAV vector improves learning behavior and pathology in Alzheimer’s disease mice. Mol Ther. 2010;18:1471–1481. doi: 10.1038/mt.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Studzinski C, Beckett T, et al. Expression of neprilysin in skeletal muscle reduces amyloid burden in a transgenic mouse model of Alzheimer disease. Mol Ther. 2009;17:1381–1386. doi: 10.1038/mt.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kuo YM, Kokjohn TA, Beach TG, et al. Comparative analysis of amyloid-beta chemical structure and amyloid plaque morphology of transgenic mouse and Alzheimer’s disease brains. J Biol Chem. 2001;276:12991–12998. doi: 10.1074/jbc.M007859200. [DOI] [PubMed] [Google Scholar]
- 74.Richardson JA, Burns DK. Mouse models of Alzheimer’s disease: a quest for plaques and tangles. ILAR J. 2002;43:89–99. doi: 10.1093/ilar.43.2.89. [DOI] [PubMed] [Google Scholar]
- 75.Farfara D, Lifshitz V, Frenkel D. Neuroprotective and neurotoxic properties of glial cells in the pathogenesis of Alzheimer’s disease. J Cell Mol Med. 2008;12:762–780. doi: 10.1111/j.1582-4934.2008.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kiyota T, Okuyama S, Swan RJ, et al. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB J. 2010;24:3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiyota T, Ingraham KL, Swan RJ, et al. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Ther. 2012;19:724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/S0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 79.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 80.Tan JM, Wong ES, Lim KL. Protein misfolding and aggregation in Parkinson’s disease. Antioxid Redox Signal. 2009;11:2119–2134. doi: 10.1089/ars.2009.2490. [DOI] [PubMed] [Google Scholar]
- 81.Dong Z, Wolfer DP, Lipp HP, et al. Hsp70 gene transfer by adeno-associated virus inhibits MPTP-induced nigrostriatal degeneration in the mouse model of Parkinson disease. Mol Ther. 2005;11:80–88. doi: 10.1016/j.ymthe.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 82.Jung AE, Fitzsimons HL, Bland RJ, et al. HSP70 and constitutively active HSF1 mediate protection against CDCrel-1-mediated toxicity. Mol Ther. 2008;16:1048–1055. doi: 10.1038/mt.2008.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao L, Diaz-Martin J, Dillmann WH, et al. Heat shock protein 70 kDa over-expression and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced nigrostriatal degeneration in mice. Neuroscience. 2011;193:323–329. doi: 10.1016/j.neuroscience.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 84.Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson’s disease? Exp Neurol. 2008;209:22–27. doi: 10.1016/j.expneurol.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kirik D, Rosenblad C, Burger C, et al. Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci. 2002;22:2780–2791. doi: 10.1523/JNEUROSCI.22-07-02780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lo Bianco C, Ridet JL, Schneider BL, et al. alpha-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kirik D, Annett LE, Burger C, et al. Nigrostriatal alpha-synucleinopathy induced by viral vector-mediated overexpression of human alpha-synuclein: a new primate model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:2884–2889. doi: 10.1073/pnas.0536383100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–149. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 89.Sapru MK, Yates JW, Hogan S, et al. Silencing of human alpha-synuclein in vitro and in rat brain using lentiviral-mediated RNAi. Exp Neurol. 2006;198:382–390. doi: 10.1016/j.expneurol.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 90.Khodr CE, Sapru MK, Pedapati J, et al. An alpha-synuclein AAV gene silencing vector ameliorates a behavioral deficit in a rat model of Parkinson’s disease, but displays toxicity in dopamine neurons. Brain Res. 2011;1395:94–107. doi: 10.1016/j.brainres.2011.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Han Y, Khodr CE, Sapru MK, et al. A microRNA embedded AAV alpha-synuclein gene silencing vector for dopaminergic neurons. Brain Res. 2011;1386:15–24. doi: 10.1016/j.brainres.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fiandaca MS, Bankiewicz KS. Gene therapy for Parkinson’s disease: from non-human primates to humans. Curr Opin Mol Ther. 2010;12:519–529. [PubMed] [Google Scholar]
- 93.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 94.LeWitt PA, Rezai AR, Leehey MA, et al. AAV2-GAD gene therapy for advanced Parkinson’s disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10:309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 95.Valles F, Fiandaca MS, Eberling JL, et al. Qualitative imaging of adeno-associated virus serotype 2-human aromatic L-amino acid decarboxylase gene therapy in a phase I study for the treatment of Parkinson disease. Neurosurgery. 2010;67:1377–1385. doi: 10.1227/NEU.0b013e3181f53a5c. [DOI] [PubMed] [Google Scholar]
- 96.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat Med. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 97.Lang AE, Gill S, Patel NK, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann Neurol. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- 98.Patel NK, Gill SS. GDNF delivery for Parkinson’s disease. Acta Neurochir Suppl. 2007;97:135–154. doi: 10.1007/978-3-211-33081-4_16. [DOI] [PubMed] [Google Scholar]
- 99.Slevin JT, Gash DM, Smith CD, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J Neurosurg. 2007;106:614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- 100.Denyer R, Douglas MR. Gene therapy for Parkinson’s disease. Parkinsons Dis. 2012;2012:757305. doi: 10.1155/2012/757305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.San Sebastian W, Richardson RM, Kells AP, et al. Safety and tolerability of magnetic resonance imaging-guided convection-enhanced delivery of AAV2-hAADC with a novel delivery platform in nonhuman primate striatum. Hum Gene Ther. 2012;23:210–217. doi: 10.1089/hum.2011.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Patel NK, Bunnage M, Plaha P, et al. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Ann Neurol. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- 103.Huddleston DE, Factor SA. Of monkeys and men: analysis of the phase 2 double-blind, sham-surgery controlled, randomized trial of AAV2-neurturin gene therapy for Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:345–348. doi: 10.1007/s11910-011-0206-y. [DOI] [PubMed] [Google Scholar]
- 104.Kells AP, Eberling J, Su X, et al. Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF. J Neurosci. 2010;30:9567–9577. doi: 10.1523/JNEUROSCI.0942-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell 1993;72:971–983. [DOI] [PubMed]
- 106.Walker FO. Huntington’s disease. Lancet. 2007;369:218–228. doi: 10.1016/S0140-6736(07)60111-1. [DOI] [PubMed] [Google Scholar]
- 107.DiFiglia M, Sapp E, Chase KO, et al. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- 108.Ross CA, Tabrizi SJ. Huntington’s disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 109.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington’s disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 110.Vagner T, Young D, Mouravlev A. Nucleic acid-based therapy approaches for huntington’s disease. Neurol Res Int. 2012;2012:358370. doi: 10.1155/2012/358370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sah DW, Aronin N. Oligonucleotide therapeutic approaches for Huntington disease. J Clin Invest. 2011;121:500–507. doi: 10.1172/JCI45130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boudreau RL, McBride JL, Martins I, et al. Nonallele-specific silencing of mutant and wild-type huntingtin demonstrates therapeutic efficacy in Huntington’s disease mice. Mol The. 2009;17:1053–1063. doi: 10.1038/mt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DiFiglia M, Sena-Esteves M, Chase K, et al. Therapeutic silencing of mutant huntingtin with siRNA attenuates striatal and cortical neuropathology and behavioral deficits. Proc Natl Acad Sci U S A. 2007;104:17204–17209. doi: 10.1073/pnas.0708285104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Drouet V, Perrin V, Hassig R, et al. Sustained effects of nonallele-specific Huntingtin silencing. Ann Neurol. 2009;65:276–285. doi: 10.1002/ana.21569. [DOI] [PubMed] [Google Scholar]
- 115.Harper SQ, Staber PD, He X, et al. RNA interference improves motor and neuropathological abnormalities in a Huntington’s disease mouse model. Proc Natl Acad Sci U S A. 2005;102:5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Machida Y, Okada T, Kurosawa M, et al. rAAV-mediated shRNA ameliorated neuropathology in Huntington disease model mouse. Biochem Biophys Res Commun. 2006;343:190–197. doi: 10.1016/j.bbrc.2006.02.141. [DOI] [PubMed] [Google Scholar]
- 117.McBride JL, Boudreau RL, Harper SQ, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci U S A. 2008;105:5868–5873. doi: 10.1073/pnas.0801775105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rodriguez-Lebron E, Denovan-Wright EM, Nash K, et al. Intrastriatal rAAV-mediated delivery of anti-huntingtin shRNAs induces partial reversal of disease progression in R6/1 Huntington’s disease transgenic mice. Mol Ther. 2005;12:618–633. doi: 10.1016/j.ymthe.2005.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dragatsis I, Levine MS, Zeitlin S. Inactivation of Hdh in the brain and testis results in progressive neurodegeneration and sterility in mice. Nat Genet. 2000;26:300–306. doi: 10.1038/81593. [DOI] [PubMed] [Google Scholar]
- 120.McBride JL, Pitzer MR, Boudreau RL, et al. Preclinical safety of RNAi-mediated HTT suppression in the rhesus macaque as a potential therapy for Huntington’s disease. Mol Ther. 2011;19:2152–2162. doi: 10.1038/mt.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gagnon KT, Pendergraff HM, Deleavey GF, et al. Allele-selective inhibition of mutant huntingtin expression with antisense oligonucleotides targeting the expanded CAG repeat. Biochemistry. 2010;49:10166–10178. doi: 10.1021/bi101208k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hu J, Matsui M, Gagnon KT, et al. Allele-specific silencing of mutant huntingtin and ataxin-3 genes by targeting expanded CAG repeats in mRNAs. Nat Biotechnol. 2009;27:478–484. doi: 10.1038/nbt.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carroll JB, Warby SC, Southwell AL, et al. Potent and selective antisense oligonucleotides targeting single-nucleotide polymorphisms in the Huntington disease gene / allele-specific silencing of mutant huntingtin. Mol Ther. 2011;19:2178–2185. doi: 10.1038/mt.2011.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu W, Kennington LA, Rosas HD, et al. Linking SNPs to CAG repeat length in Huntington’s disease patients. Nat Methods. 2008;5:951–953. doi: 10.1038/nmeth.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pfister EL, Kennington L, Straubhaar J, et al. Five siRNAs targeting three SNPs may provide therapy for three-quarters of Huntington’s disease patients. Curr Biol. 2009;19:774–778. doi: 10.1016/j.cub.2009.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bennett CF, Swayze EE. RNA targeting therapeutics: molecular mechanisms of antisense oligonucleotides as a therapeutic platform. Annu Rev Pharmacol Toxicol. 2010;50:259–293. doi: 10.1146/annurev.pharmtox.010909.105654. [DOI] [PubMed] [Google Scholar]
- 127.Kordasiewicz HB, Stanek LM, Wancewicz EV, et al. Sustained therapeutic reversal of Huntington’s disease by transient repression of huntingtin synthesis. Neuron. 2012;74:1031–1044. doi: 10.1016/j.neuron.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stiles DK, Zhang Z, Ge P, et al. Widespread suppression of huntingtin with convection-enhanced delivery of siRNA. Exp Neurol. 2012;233:463–471. doi: 10.1016/j.expneurol.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 129.Miller RG, Gelinas D, O’Connor P. Amyotrophic lateral sclerosis. New York: American Academy of Neurology Press; 2005. [Google Scholar]
- 130.Kaspar BK, Frost LM, Christian L, et al. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 131.Mello CC, Conte D., Jr Revealing the world of RNA interference. Nature. 2004;431:338–342. doi: 10.1038/nature02872. [DOI] [PubMed] [Google Scholar]
- 132.Raoul C, Abbas-Terki T, Bensadoun JC, et al. Lentiviral-mediated silencing of SOD1 through RNA interference retards disease onset and progression in a mouse model of ALS. Nat Med. 2005;11:423–428. doi: 10.1038/nm1207. [DOI] [PubMed] [Google Scholar]
- 133.Ralph GS, Radcliffe PA, Day DM, et al. Silencing mutant SOD1 using RNAi protects against neurodegeneration and extends survival in an ALS model. Nat Med. 2005;11:429–433. doi: 10.1038/nm1205. [DOI] [PubMed] [Google Scholar]
- 134.Winer L, Srinivasan D, Chun S, et al. SOD1 in cerebral spinal fluid as a pharmacodynamic marker for antisense oligonucleotide therapy. JAMA Neurol. 2013;70:201–207. doi: 10.1001/jamaneurol.2013.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kubodera T, Yamada H, Anzai M, et al. In vivo application of an RNAi strategy for the selective suppression of a mutant allele. Hum Gene Ther. 2011;22:27–34. doi: 10.1089/hum.2010.054. [DOI] [PubMed] [Google Scholar]
- 136.Berry JD, Cudkowicz ME. New considerations in the design of clinical trials for amyotrophic lateral sclerosis. Clin Invest. 2011;1:1375–1389. doi: 10.4155/cli.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kitamoto T, Tateishi J, Tashima T, et al. Amyloid plaques in Creutzfeldt-Jakob disease stain with prion protein antibodies. Ann Neurol. 1986;20:204–208. doi: 10.1002/ana.410200205. [DOI] [PubMed] [Google Scholar]
- 138.Cohen FE, Pan KM, Huang Z, et al. Structural clues to prion replication. Science. 1994;264:530–531. doi: 10.1126/science.7909169. [DOI] [PubMed] [Google Scholar]
- 139.Goldfarb LG, Brown P, Haltia M, et al. Creutzfeldt-Jakob disease cosegregates with the codon 178Asn PRNP mutation in families of European origin. Ann Neurol. 1992;31:274–281. doi: 10.1002/ana.410310308. [DOI] [PubMed] [Google Scholar]
- 140.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 141.Enari M, Flechsig E, Weissmann C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc Natl Acad Sci U S A. 2001;98:9295–9299. doi: 10.1073/pnas.151242598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Feraudet C, Morel N, Simon S, et al. Screening of 145 anti-PrP monoclonal antibodies for their capacity to inhibit PrPSc replication in infected cells. J Biol Chem. 2005;280:11247–11258. doi: 10.1074/jbc.M407006200. [DOI] [PubMed] [Google Scholar]
- 143.Perrier V, Solassol J, Crozet C, et al. Anti-PrP antibodies block PrPSc replication in prion-infected cell cultures by accelerating PrPC degradation. J Neurochem. 2004;89:454–463. doi: 10.1111/j.1471-4159.2004.02356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.White AR, Enever P, Tayebi M, et al. Monoclonal antibodies inhibit prion replication and delay the development of prion disease. Nature. 2003;422:80–83. doi: 10.1038/nature01457. [DOI] [PubMed] [Google Scholar]
- 145.Wuertzer CA, Sullivan MA, Qiu X, et al. CNS delivery of vectored prion-specific single-chain antibodies delays disease onset. Mol Ther. 2008;16:481–486. doi: 10.1038/sj.mt.6300387. [DOI] [PubMed] [Google Scholar]
- 146.Padiolleau-Lefevre S, Alexandrenne C, Dkhissi F, et al. Expression and detection strategies for an scFv fragment retaining the same high affinity than Fab and whole antibody: Implications for therapeutic use in prion diseases. Mol Immunol. 2007;44:1888–1896. doi: 10.1016/j.molimm.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 147.Mallucci GR, Ratte S, Asante EA, et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 2002;21:202–210. doi: 10.1093/emboj/21.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mallucci G, Dickinson A, Linehan J, et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science. 2003;302:871–874. doi: 10.1126/science.1090187. [DOI] [PubMed] [Google Scholar]
- 149.White MD, Farmer M, Mirabile I, et al. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc Natl Acad Sci U S A. 2008;105:10238–10243. doi: 10.1073/pnas.0802759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Verity NC, Mallucci GR. Rescuing neurons in prion disease. Biochem J. 2011;433:19–29. doi: 10.1042/BJ20101323. [DOI] [PubMed] [Google Scholar]
- 151.Stieger K, Belbellaa B, Le Guiner C, et al. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goverdhana S, Puntel M, Xiong W, et al. Regulatable gene expression systems for gene therapy applications: progress and future challenges. Mol Ther. 2006;12:189–211. doi: 10.1016/j.ymthe.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 550 kb)