Abstract
The central nervous system has been considered off-limits to antibody therapeutics. However, recent advances in preclinical and clinical drug development suggest that antibodies can cross the blood–brain barrier in limited quantities and act centrally to mediate their effects. In particular, immunotherapy for Alzheimer’s disease has shown that targeting beta amyloid with antibodies can reduce pathology in both mouse models and the human brain, with strong evidence supporting a central mechanism of action. These findings have fueled substantial efforts to raise antibodies against other central nervous system targets, particularly neurodegenerative targets, such as tau, beta-secretase, and alpha-synuclein. Nevertheless, it is also apparent that antibody penetration across the blood–brain barrier is limited, with an estimated 0.1–0.2 % of circulating antibodies found in brain at steady-state concentrations. Thus, technologies designed to improve antibody uptake in brain are receiving increased attention and are likely going to represent the future of antibody therapy for neurologic diseases, if proven safe and effective. Herein we review briefly the progress and limitations of traditional antibody drug development for neurodegenerative diseases, with a focus on passive immunotherapy. We also take a more in-depth look at new technologies for improved delivery of antibodies to the brain.
Electronic supplementary material
The online version of this article (doi:10.1007/s13311-013-0187-4) contains supplementary material, which is available to authorized users.
Keywords: Alzheimer’s disease, Passive immunotherapy, Blood-brain barrier, Beta amyloid, Tau, α-synuclein, Transferrin receptor
Introduction
Developing effective therapies for disorders of the central nervous system (CNS) is one of the greatest unmet medical challenges facing our society. As a result of the growing aging population worldwide, incidences of neurodegenerative diseases, in particular, are projected to increase considerably in the coming decades [1]. At present, there is a dearth of effective therapeutics for neurodegenerative diseases owing, in part, to the inherent difficulty of developing safe and efficacious drugs that will cross the blood–brain barrier (BBB).
Because of their larger size, development of antibody therapeutics for CNS diseases has been particularly challenging. However, target specificity, reduced off-target side effects, and better pharmacokinetics make antibody and protein therapeutics an attractive and promising approach for targeting CNS diseases [2, 3]. Furthermore, progress in the field of Alzheimer’s passive immunotherapy—particularly results showing that peripherally administered beta amyloid (Aβ) antibodies can cross the BBB and reduce amyloid plaque—have spurred efforts to raise antibodies to other CNS targets [4]. We review the recent advances in antibody drug development for neurodegenerative disease, focusing almost exclusively on passive antibody therapy with only a brief comment on active immunization approaches that provided initial proof-of-concept for immunotherapy. A focus is also placed on antibodies designed to treat or prevent Alzheimer’s disease (AD), paying particular attention to Aβ, BACE1, and tau, with an emphasis on our current understanding of the associated mechanisms of action supported by the most recent findings in the field. We also explore the limitations of traditional antibody development for CNS diseases, namely limited antibody exposure in brain.
The inherent biological limitation of antibody uptake in brain with roughly 0.1–0.2 % of peripherally administered antibody crossing the BBB has resulted in efforts to engineer antibodies to cross the BBB by utilizing endogenous transport mechanisms, such as receptor-mediated transcytosis of large molecules. We end our review by summarizing the recent progress in utilizing endogenous transport mechanism to boost antibody uptake in brain, and propose that this approach may lead to the next generation of CNS antibody therapeutics designed to treat a wide range of CNS diseases.
The BBB
A major obstacle in the development of antibody therapeutics for CNS diseases is the tightly regulated BBB that is localized to the brain vasculature. The BBB is one of many distinct barriers that limit the transport of peripheral substances into the CNS; the blood–cerebrospinal fluid (CSF)-barrier, blood–retinal barrier, and blood–spinal cord barriers all contribute to the limited movement of compounds from the systemic circulation to the CNS [5]. The primary function of these barriers is to maintain homeostasis in the CNS. Specificity of brain uptake of various endogenous ligands, including amino acids, glucose, iron, and other nutrients, is controlled by transporters and receptors expressed at the barrier. For exogenous drugs, generally only lipophilic compounds with a molecular weight less than ~400 Da are able to diffuse through the barrier to any appreciable degree. Thus, by restricting the movement of compounds between the blood and the brain, the BBB has severely limited the success of therapeutics for CNS disease.
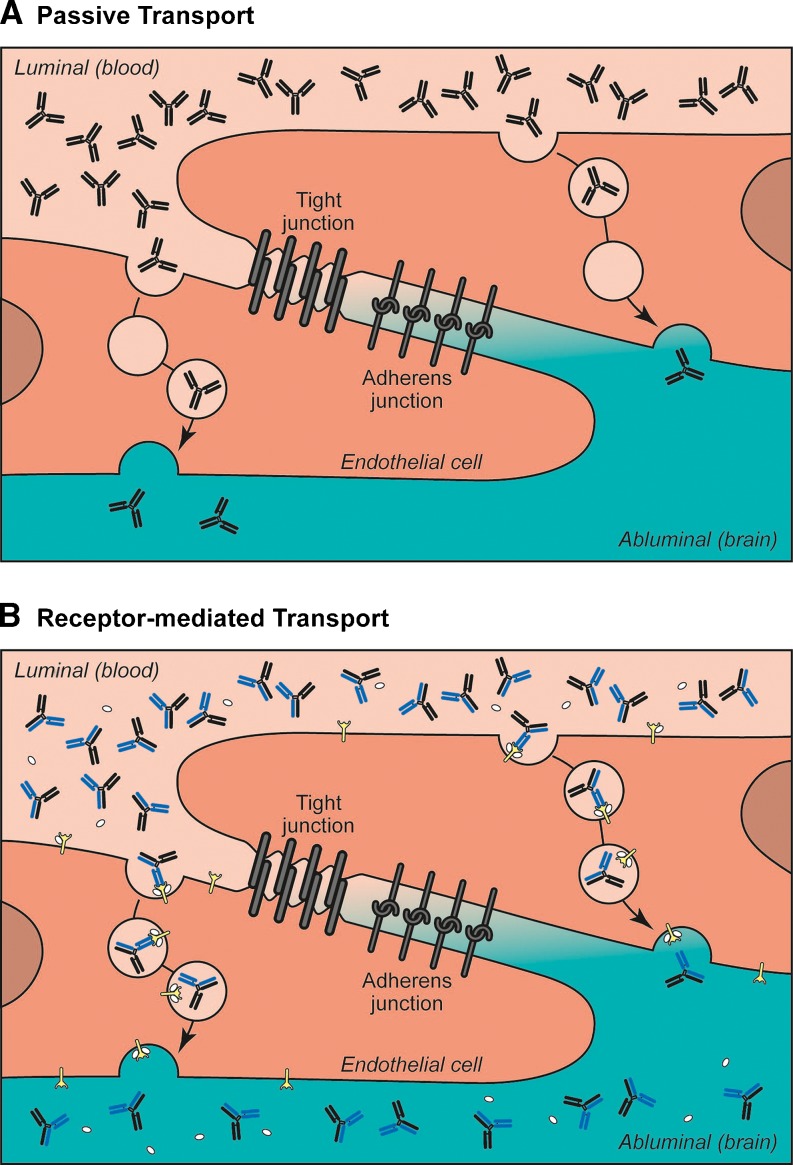
The BBB is comprised of a continuous monolayer of brain endothelial cells that constitute the brain microvasculature (Fig. 1a; reviewed in [6, 7]). A network of transmembrane tight junction proteins (e.g., claudin-1, claudin-5, occludin) between adjacent endothelial cells serves to create a physical barrier, which results in a low paracellular permeability of hydrophilic molecules from the blood to the brain [8, 9]. Besides these structural elements, transporters, and enzymes present on both the luminal (blood) and abluminal (brain) side of endothelial cells regulate the influx, efflux, and metabolism of substances between the periphery and the CNS [10–12]. Beyond the endothelial cells, the astrocytes and pericytes that surround the endothelial cell layer also play a role in the development and maintenance of BBB integrity and constitute the highly regulated neurovascular unit [13–17].
Fig. 1.
Endogenous transport systems of the blood–brain barrier. a Passive fluid phase uptake of peripheral antibodies diffuse nonspecifically across endothelial cells of the blood–brain barrier to reach the brain. Chemical modifications, such as cationization, can enhance this absorptive-mediated transport by enhancing the binding of antibodies to negatively charged endothelial cell membranes. b Receptor-mediated transport relies on substrate-specific binding of antibodies to endocytic receptors expressed on the luminal surface of endothelial cells. Bispecific antibodies that bind to both a receptor-mediated transport and therapeutic target can enhance brain uptake of the therapeutic agent
Ultimately, this tightly regulated neurovascular unit restricts movement of substances from circulation into the CNS, thus posing a challenge for systemically administered drugs. However, given that every CNS cell is no more than ~40 μm away from the ~400 miles of capillary network in the human brain, a number of approaches have been made to take advantage of this extensive vascular barrier in order to deliver drugs from the periphery to the brain [18–20]. These approaches most often rely on the existing biological machinery found in the brain vasculature, either via passive or receptor-mediated transport.
Limited Quantities of Therapeutic Antibodies Cross the BBB
In general, only a mere 0.1–0.2 % of circulating antibodies cross the BBB and enter the brain or CSF [21, 22]. This uptake is similar in magnitude to other endogenous circulating proteins that are taken up into brain in a nonspecific manner, such as serum albumin. With a limited amount of antibody uptake in brain, there is some uncertainty as to whether antibodies do cross the BBB or if the small amount of antibody measured in brain is a result of blood contamination. At least two major lines of evidence support the conclusion that antibodies do cross the BBB and act centrally to mediate their effects. First, taking a lesson from nature, there are rare autoimmune diseases in which antibodies are raised against CNS targets [23, 24]. Indeed, there are a number of examples where autoantibodies are generated against extracellular targets, such as N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, with associated syndromes consistent with blocking receptor function. The second line of evidence is recent success with preclinical and clinical drug development in AD, particularly the fact that peripherally administered anti-Aβ and anti-BACE1 antibodies can reduce plaque load and amyloid production, respectively. Results from these efforts and associated data supporting a direct mechanism of action will be reviewed in the next section.
Although there is evidence that peripherally originating or administered antibodies can act centrally [25–28], the mechanism(s) for such uptake is not entirely clear. Nevertheless, current evidence supports a steady-state equilibrium being established through a passive fluid phase mechanism relying on nonspecific uptake by endocytic vesicles in brain vasculature (Fig. 1a). We have confirmed this proposed mechanism of antibody uptake in brain indirectly by showing that increasing the doses of anti-BACE1 antibodies (discussed later) results in increased CNS exposure in a nonsaturating fashion [29]. Furthermore, we have observed that brain uptake of IgG is comparable in both wild-type and severe combined immunodeficiency mice (unpublished data), suggesting that IgG does not compete for uptake at the BBB. It can therefore be concluded that penetration of traditional antibodies is limited by the amount of circulating antibody, and that a non-saturable steady state is established roughly equaling 1 antibody in brain for every 1000 circulating antibodies in the blood. This limited BBB uptake and associated minimal brain exposure makes it imperative that therapeutic antibodies directed against CNS targets either have extremely high affinities for their target or are administered at large doses to achieve therapeutic effects. Fortunately, antibodies targeting aggregated proteins in neurodegenerative disease, such as Aβ and tau, may take advantage of improved potency based on substantial improvements in perceived affinity as a result of robust avidity towards their target (multiple binding sites in close proximity), as shown for Aβ [30], or even accumulate in brain around their aggregated target [31]. Nevertheless, in order to tackle more traditional antibody targets in brain, such as receptor/ligand interactions or extracellular enzymes (e.g., BACE1), or even go after more novel targets such as G protein-coupled receptors or ion channels, improved brain uptake will substantially increase the probability of success.
Targeting Neurodegenerative Disease With Antibodies
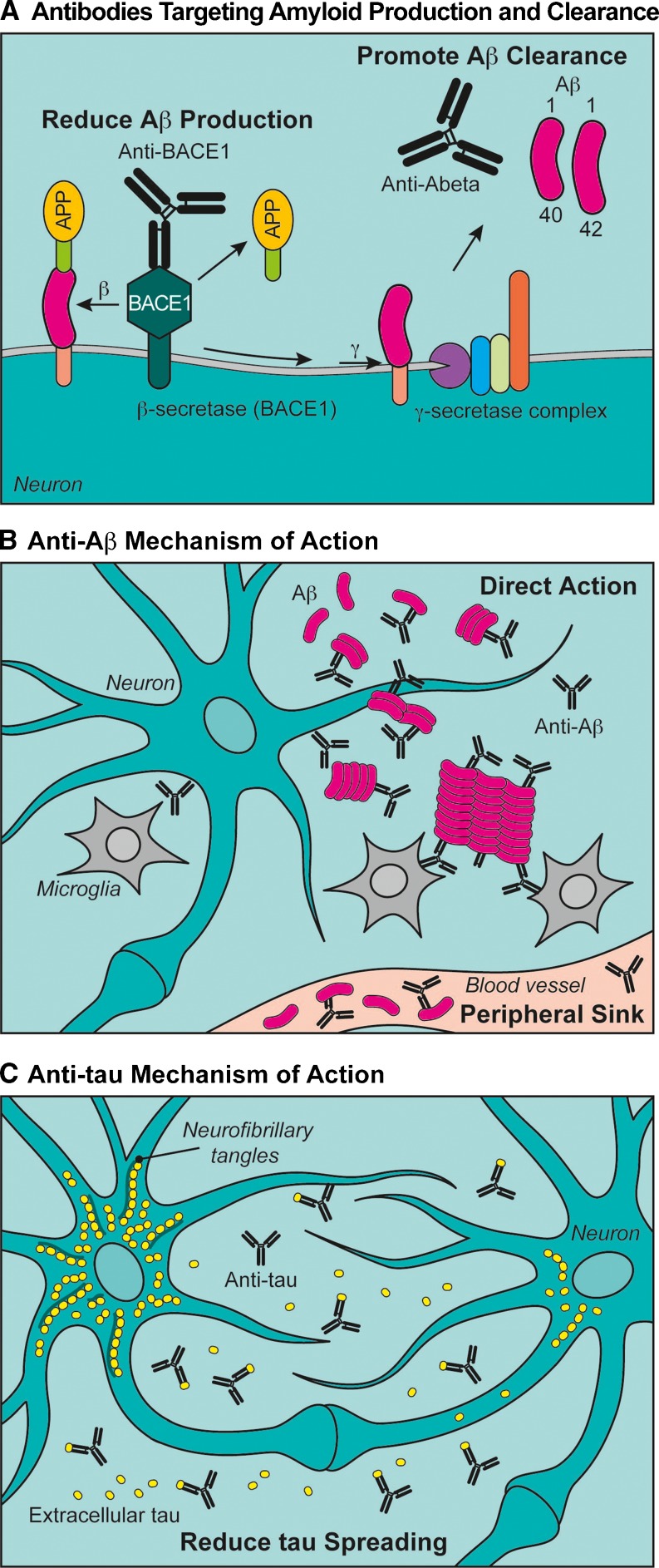
Aβ and the associated enzymes responsible for its production are major targets of Alzheimer’s drug development (Fig. 2a; [32–35]). The reason for such intense efforts is based on compelling human genetic and pathologic findings. In addition to mutations in amyloid precursor protein (APP) causing early onset Alzheimer’s disease as a result of increased Aβ production or a shift in the ratio of toxic Aβ species [36], a recent variant in APP was discovered that reduces BACE1 cleavage of APP and is associated with a significant reduction in the risk of developing AD [37].
Fig. 2.
Alzheimer’s disease antibody approaches and associated mechanisms. a Amyloid precursor protein (APP) is cleaved sequentially by β-site APP cleaving enzyme (BACE1) and the γ-secretase complex to give rise to toxic beta amyloid (Aβ). Two antibody approaches to mitigate the toxic effects of Aβ include reducing the initial cleavage of APP by BACE1 with an antibody. A second, and more common, approach is to clear existing Aβ with antibodies targeting Aβ directly. b Two models for how anti-Aβ clears amyloid from the brain have been proposed. The “direct action” hypothesis postulates that systemically delivered anti-Aβ antibodies cross the blood–brain barrier and promote clearance of amyloid by activating microglia, inhibiting Aβ aggregation, promoting Aβ disaggregation, and/or directly inhibit Aβ oligomer toxicity to neurons. The “peripheral sink” hypothesis proposes that Aβ captured in the periphery (blood vessels) shifts the equilibrium of Aβ and pulls amyloid from the brain. c Recent evidence supports a prion-like spreading of tau from one neuron to neuron. We propose that immunologic approaches to tau may act primarily by reducing tau spreading, as anti-tau antibodies cross the blood–brain barrier and bind extracellular tau blocking its ability to seed pathology in adjacent neurons
Hallmark pathologic findings in AD also include extracellular accumulations of Aβ and intracellular neurofibrillary tangles made of hyperphosphorylated tau [35, 38]. Recent studies evaluating imaging and CSF biomarkers in both early onset and idiopathic AD suggest a long time course of amyloid accumulation, followed by tau pathology and neuronal loss tracking with cognitive decline [39]. Furthermore, the prion-like spreading of tau has been proposed based on numerous examples of pathologic spreading along neuronal networks [40, 41] and studies where tau aggregates are introduced ectopically to tau transgenic mouse brains serve as seeds for tau pathology [42].
Taken together, the overwhelming genetic and pathologic evidence pointing toward Aβ as a viable drug target in AD has resulted in numerous approaches to reduce production (secretase inhibitors), block aggregation and/or promote disaggregation, and promote clearance of Aβ. In this review we focus on passive immunotherapy approaches with therapeutic antibodies, specifically anti-Aβ, anti-BACE1, and anti-tau. We will also briefly discuss other passive immunotherapy approaches for neurodegeneration, focusing on similarities in proposed mechanisms of action, such as slowing the prion-like spreading of aggregating proteins (e.g., tau and α-synuclein).
Using Anti-BACE1 to Reduce Aβ Production
The aspartyl protease β-site APP cleaving enzyme (BACE1) is a highly sought after Alzheimer’s drug target, as it is the first enzyme in the amyloidogenic processing of APP giving rise to toxic Aβ (Fig. 2a) [43–45]. Until recently, it has been considered difficult to develop BACE1 small molecule inhibitors that are selective, potent, and BBB penetrant [46]. Nevertheless, recent advances have been made in the clinic that show promise for small molecule inhibition of BACE1 [47], yet most of these approaches still lack the specificity that an antibody approach will offer. In particular, there are clear risks associated with cathepsin-D inhibition [48–50], and the risks associated with BACE2 inhibition are not yet evident. Recent genetic studies have also raised the concern that complete inhibition of BACE1 itself may be deleterious [51–53].
In an attempt to tackle BACE1 in a novel and selective way, we, and others, have recently developed antibodies that selectively bind and inhibit BACE1 activity [29, 54]. This approach revealed several important aspects about antibody therapeutics directed against CNS targets. First, there was a direct pharmacokinetic/pharmacodynamic relationship between antibody levels in brain and inhibition of BACE1 activity as measured by Aβ levels. Second, with the current level of potency (cellular IC50s ~5nM and in vivo IC50s ~15nM), very high doses of drug were needed to substantially inhibit BACE1 activity. Third, there is a direct steady-state relationship between drug levels in brain versus drug levels in blood at multiple dose levels, equalling, approximately, 1 to 1000 as reported previously [21, 22]. Ultimately, these findings lead to the conclusion that most antibodies targeting CNS targets could potentially benefit from improved CNS uptake and/or extremely high affinities against that selected target in order to advance toward clinical applications.
Removing Amyloid Via Anti-Aβ Treatment
Numerous reviews have addressed the immunotherapeutic approaches to target Aβ [4, 34, 55–57]; thus, we will focus on the debate around the mechanism(s) of anti-Aβ action (Fig. 2b) and the most recent clinical advances. From the earliest observations that active immunization against Aβ in APP transgenic mice could reduce plaque load [58] to the most current clinical data showing that peripheral levels of Aβ increase after dosing and that plaque can be reduced in patients treated with Aβ immunotherapy [59–62], the mechanism by which anti-Aβ antibodies exert their effects have remained somewhat controversial.
Based on early publications in the anti-Aβ field, two opposing, but not mutually exclusive mechanisms have been proposed, namely “direct action” and “peripheral sink” (Fig. 2b) [30, 63]. In the strictest sense of the definition, the “peripheral sink” hypothesis stipulates that Aβ captured by anti-Aβ antibodies in the periphery (blood) would shift the equilibrium and “pull” Aβ from the brain into the blood in an attempt to re-establish Aβ equilibrium. This hypothesis relies on the assumption that antibodies do not cross the BBB and that Aβ exists in a passive equilibrium between the brain and blood. Addressing the latter point: if Aβ were to exist in peripheral/central equilibrium, any approach that lowers Aβ in the periphery should subsequently reduce brain Aβ levels. Unfortunately, the BBB is not a generally permeable barrier and many examples abound where peripheral decreases in Aβ do not result in brain levels being decreased, particularly associated with efforts to develop secretase inhibitors [46]. Indeed, as just reviewed, anti-BACE1 antibodies that inhibit BACE1 activity in the periphery do not show the same reduction of Aβ in brain. Rather, the reduction in brain is related to the amount of anti-BACE1 that crosses the BBB [29]. Finally, preclinical experiments have been conducted to directly test the idea that peripherally administered anti-Aβ antibodies pull Aβ from brain [28]. Results from these studies suggested the opposite: anti-Aβ/Aβ complexes actually are cleared more slowly from the brain and provide additional proof that anti-Aβ antibodies may be exerting their effects centrally in the CNS.
Support is mounting for a “direct action” hypothesis by which anti-Aβ reduce plaque load, inhibit aggregation, promote disaggregation, and possibly directly block the toxicity of oligomeric Aβ (Fig. 2b). This hypothesis stipulates that anti-Aβ antibodies cross the BBB and directly block the toxic effects of Aβ while mediating its clearance by promoting microglial engulfment or clearance as a complex via CSF/interstitial fluid (ISF) bulk flow. The latter may explain the peripheral rise in Aβ observed after dosing [28, 63]. Consistent with this hypothesis, antibodies that fully engage microglia while binding plaque may cause reduction of amyloid plaque, but also disrupt the BBB causing vasogenic edema [62], a phenomenon renamed amyloid-related imaging abnormalities-edema [64]. These adverse effects have been dose-limiting for bapineuzumab [62]. Mechanistically, it appears as though anti-Aβ antibodies that recognize aggregated Aβ and are on a human IgG1 backbone show a dose-dependent increase in amyloid-related imaging abnormalities-edema, which is likely related to BBB penetration of antibody and activation of microglia. Indeed, human IgG1 antibodies have the greatest affinity to Fc-gamma receptors, thus potentially activating microglia maximally. It may also be the case that unique epitopes must be targeted to limit toxicity while still engaging plaques, such as proposed by a recent study in which antibodies were raised to pyroglutamate n-terminally truncated Aβ(p3-42) [65].
In contrast to these approaches, we have engineered an anti-Aβ antibody (crenezumab) on an IgG4 backbone that shows reduced activation of microglia, while retaining the ability to promote Aβ engulfment [59]. This decreased immune response allows for higher doses of crenezumab, thus increasing the exposure in brain and allowing for maximizing the “direct action” of blocking Aβ toxicity.
The ultimate proof of Aβ immunotherapy is showing a clinical benefit for patients. Unfortunately, a number of recent phase III clinical trials in mild-to-moderate AD with two anti-Aβ antibodies, bapineuzumab and solanezumab, did not meet their primary endpoints. However, there were some signs of efficacy on cognitive endpoints in a prespecified secondary analysis of mild AD patients treated with solanezumab. These data support the growing consensus that therapies designed to target Aβ may need to be given early, even in a preventative setting [66–69]. Indeed, two recent publications assessing dominantly inherited early onset cases of Alzheimer’s show that amyloid may accumulate as early as 25 years before the first signs of cognitive decline [70, 71]. Thus, the future of anti-amyloid approaches may, indeed, be in a preventative setting, which will require the development of additional biomarkers and clinical endpoints to assess benefits to patients.
Slowing the Spreading of Tau Pathology With Anti-tau Antibodies
Unlike the extended lead-time of pathologic accumulation of Aβ prior to cognitive decline, tau intracellular pathology spreads as cognitive impairment increases. Braak staging in AD, which is based on presence of neurofibrillary tangles formed by hyperphosphorylated tau, shows a direct relationship between pathologic spreading and worsening of cognitive scores [72]. These data, combined with evidence that tau pathology can spread in mice [40, 41], and the observation that tau can be found extracellularly in interstitial fluid [73], suggests that tau may be spread in a prion-like fashion and may thus be a valid antibody target to treat AD.
Similar to the amyloid immunotherapy field, tau active immunization provided the first evidence that pathologic spreading could be reduced in long-term efficacy studies [74–77]. These studies have more recently been followed by passive immunotherapy studies also showing success in reducing pathology and even rescuing motor deficits in tau transgenic mice [78, 79]. The model we propose for anti-tau mechanism of action relies on anti-tau antibodies crossing the BBB, and binding to phosphorylated or aggregated tau and reducing the prion-like behavior of seeding tau aggregates in adjacent cells (Fig. 2c). Consistent with this hypothesis, powerful assays have been developed recently that show that tau can be secreted and taken up by adjacent cells to seed pathology, a process that can be blocked with anti-tau antibodies [80]. The evidence for cellular spreading has also extended to in vivo studies in mice using genetic means to express human tau in a small subpopulation of neurons, and then follow the spread of human tau and associated aggregation of murine tau [40, 41].
Although still in its early days, anti-tau therapy may be a more attractive approach than anti-Aβ therapy to treat individuals with early signs of cognitive decline. This is especially promising when considering the fact that patients with prodromal AD are just beginning to develop tau pathology. Nevertheless, much remains to be understood about the mechanisms of tau spreading and the ultimate contribution of this prion-like hypothesis to pathology in humans, as opposed to a cell intrinsic mechanism for tau accumulation downstream of Aβ. Also, similar to Aβ, it is not entirely clear what toxic form of tau should be targeted to maximize efficacy while limiting safety liabilities. Although many years of drug development will be necessary before we can realize the hope from such an approach, the coming years of preclinical and clinical drug development for anti-tau will be insightful.
Targeting Other Toxic Proteins in Neurodegeneration With Antibodies
This review has focused largely on antibody approaches to treat AD; however, similar principles may apply to other aggregating proteins. One such example is α-synuclein. Similar to tau, α-synuclein intracellular aggregates form Lewy bodies and spread pathologically as Parkinson’s disease progresses; indeed, Braak also developed a staging system for Parkinson’s disease, in this case based on α-synculein Lewy body pathology [81]. Although anti-α-synuclein immunotherapy is also in its early days, there are examples of preclinical success with both active and passive immunotherapy against α-synuclein in transgenic mouse models [82, 83]. Recent in vitro cellular data also support the idea of α-synuclein spreading from cell to cell [84, 85], a conclusion supported by in vivo data in both mice [86] and humans [87, 88].
Other targets, such as huntingtin, TAR-DNA binding protein 43 kDa, superoxide dismutase 1, and prion protein are less validated as potential antibody targets; however, there is accumulating evidence supporting the possibility that these pathologic molecules can be modulated with immunotherapy [89–93]. However, many of these suffer the same uncertainty as tau and α-synuclein, as they are considered primarily to be intracellular molecules (with the exception of prion protein). It is not known how much of the pathologic and toxic action of these proteins are mediated extracellularly, and thus what portion of toxicity can be alleviated with antibody therapy. In addition to this uncertainty, antibody therapeutics against CNS targets face an equally large challenge of crossing the BBB. Many targets may be difficult to validate based on the limited uptake of antibody in brain, and thus strategies to increase uptake of antibodies will have a broad impact on CNS drug development and may open the window for targets beyond the currently pursued list of aggregating proteins in neurodegenerative diseases.
Implication of Extracellular Versus Intracellular Targets
We have proposed that “classical” intracellular aggregating proteins, such as tau and α-synuclein, are antibody targets based on a prion spreading hypothesis (Fig. 2c), as it is generally believed that antibodies cannot penetrate cells effectively and access the cytoplasm directly. In contrast, Aβ is largely extracellular and thus readily accessible to antibodies (Fig. 2b). Although it is interesting to note that both extracellular and intracellular targeting of aggregating proteins showed initial proof-of-concept via active immunization, Aβ immunotherapy is substantially advanced relative to tau and α-synuclein immunotherapy. As such, studies aimed at elucidating the mechanism by which antibodies slow the spreading of intracellular pathology are needed. These studies may include assessing the role of effector function, epitope specificity, and dose relationships to efficacy. Furthermore, there may be mechanisms that allow for antibodies to access the cytoplasm of neurons, but without any clear molecular and cellular pathways currently conceivable, such a proposal remains implausible.
Enhancing Antibody Uptake in Brain
Endogenous Transport Systems as a Means to Enhance Antibody Uptake in Brain
Among the non-invasive strategies of delivering antibodies to the CNS, the most explored strategy has been to take advantage of a number of endogenous transport systems that are present at the BBB (Fig. 1b;). Most nutrients and small metabolites do not diffuse across the BBB through passive paracellular diffusion in sufficient quantities to serve the needs of the highly metabolically active CNS. Instead, delivery of these important molecules is regulated by active endogenous transport systems present on brain endothelial cells. There are three main classes of transport systems at the BBB: 1) nonspecific absorptive-mediated endocytosis (including transmembrane diffusion and charged-based interactions which do not require receptor interaction); 2) substrate-selective carrier-mediated transport (e.g., glucose, amino acids); and 3) receptor-mediated transcytosis (RMT) for larger molecules (e.g., transferrin [94], insulin [95], leptin [96]).
Drug targeting using absorptive-mediated endocytosis utilizes chemical modifications, such as cationization of carboxyl groups, to nonspecifically increase cellular uptake through binding of the positively charged modified antibody to the negatively charged plasma membrane [97–99]. This type of drug targeting is nonselective in that it does not require binding of the drug to receptors; rather, endocytosis of cationic-bound membrane results in the internalization of compounds into intracellular endosomes. Polyamination and glycation of antibodies and proteins have shown increased BBB penetration [21, 98]. However, the nonspecific nature of absorptive-mediated transport greatly limits its therapeutic potential, as indiscriminate cellular uptake is not only a major disadvantage in terms of off-target effects, but also for their reduced pharmacokinetic properties. Additionally, cationic compounds have been shown to cause acute toxicity and disruption of BBB permeability in vivo, which further brings into question the utility of altering surfaces charges as a means of increasing brain uptake [100].
Unlike absorptive-mediated transport, both carrier-mediated transport (CMT) and RMT mechanisms are substrate-selective. CMT is responsible for the delivery of small molecule nutrients that include glucose, amino acids, monocarboxylic acids, hormones, ions, and vitamins [101]. Binding and transport of these nutrients are stereospecific, bidirectional, and independent of endocytic trafficking. Drug targeting using CMT requires either mimicking the natural small molecule ligand or conjugation to the endogenous substrate of the carrier protein. This can be successful for small molecule CNS drugs, such as gabapentin and L-dopa, both of which are delivered primarily through the cerebrovascular large neutral amino acid transporter (LAT1) carrier system [102, 103]. However, neither approach is conducive to large molecule transport required for brain uptake of antibody therapeutics.
Is Receptor-Mediated Transcytosis the Answer to Enhance Antibody Uptake in Brain?
Of the three transport routes, perhaps the most widely studied and most promising approach for targeting antibody therapeutics to the brain is RMT (Fig. 1b). The specificity of this mechanism of drug delivery takes advantage of endogenous receptors expressed on the luminal side of the BBB that function to deliver macromolecule nutrients to the brain. Large molecule nutrients, such as iron-bound transferrin, insulin, and leptin, are delivered into the brain via vesicular trafficking of the ligand-receptor complex [94–96]. This process involves binding of the ligand onto the extracellular domain of the receptor, endocytosis into the cytoplasm of the ligand-bound receptor, and release of the ligand either inside the endosomal compartment or exocytosis on the abluminal (brain) side of the capillary endothelium into the interstitial space (Fig. 1b).
Transferrin Receptor
One of the most widely explored RMT systems for drug delivery is the transferrin (Tf) /transferrin receptor (TfR) pathway. Highly expressed on brain capillary endothelium, TfR is a transmembrane homodimer of two 90-kDa glycoprotein subunits that functions to mediate the delivery of iron into the brain [94]. Linked by a disulfide bridge, each TfR subunit can bind one iron-carrying Tf protein [104, 105]. Within the CNS, TfR is expressed at very high levels on capillary endothelial cells, but is also expressed to a lesser extent on neurons [94, 106–108].
TfR is a constitutively recycling receptor, and thus its internalization into clathrin-coated pits is independent of Tf binding [109]. Together with the fact that TfR recycling is rapid and occurs within minutes [110], this suggests that trafficking of TfRs from the plasma membrane into internal compartments is a highly dynamic process. Many groups have thus attempted to take advantage of this active transport pathway by either targeting the Tf ligand or by engineering antibodies to the receptor. Competition with high concentrations of endogenous Tf may be a significant obstacle for targeting the ligand itself, though a few groups have been able to demonstrate some modest success with this method [111, 112].
A much more promising approach for antibody delivery to the CNS has been to target TfR. Proof-of-concept studies using a mouse monoclonal antibody against the rat TfR, OX-26, were among the first to show brain delivery of a TfR antibody [113–115]. OX-26 binds to an extracellular epitope of TfR that does not interfere with Tf binding, and thus iron transport is unaffected. Conjugation of a therapeutic cargo to TfR antibodies has also been explored, where fusion with compounds such as brain-derived neurotrophic factor or nerve growth factor correlated with indirect improvements in neuroprotection [116–118]. Because OX-26 recognizes only the rat TfR, murine cross-reactive 8D3 and R17-217 are additional anti-TfRs that have been generated and explored for their ability to delivery large molecules to the brain [115, 119].
Although clearly pioneering in nature, several practical caveats exist for these early studies as it relates to translatability to a clinical setting. The first is that that nearly all use either radiolabeled anti-TfR at trace doses or doses lower than 2 mg/kg. Although successful therapeutic results could, theoretically, be achieved with high-affinity binding to the therapeutic target, the feasibility of such low doses of the antibody–drug conjugates providing a sustained effect in brain is unlikely translatable for CNS disease therapeutics in chronic dosing paradigms. Second, many of the studies do not measure directly the pharmacokinetic or pharmacodynamic effects of the anti-TfR conjugates in therapeutic dosing paradigms, but, instead, use a more downstream effect as the readout of target engagement, such as plaque reduction for anti-Aβ [120]. This method restricts the interpretations of the engineered anti-TfR drug’s effects, relying on correlative, rather than causative proximal, endpoints, and thus creates limitations on fine-tuning the therapeutic antibody’s dose response. Third, it remains unclear from these studies whether brain uptake of TfR antibody resulted in broad distribution of antibody throughout the brain parenchyma. Indeed, one of the major criticisms of using TfR as a RMT target is that the majority of antibodies mentioned above accumulate primarily in the brain capillary endothelium, instead of distributing throughout the brain [121]. This is obviously less than ideal, as target engagement required to produce a therapeutic response in the CNS would occur beyond the brain vasculature.
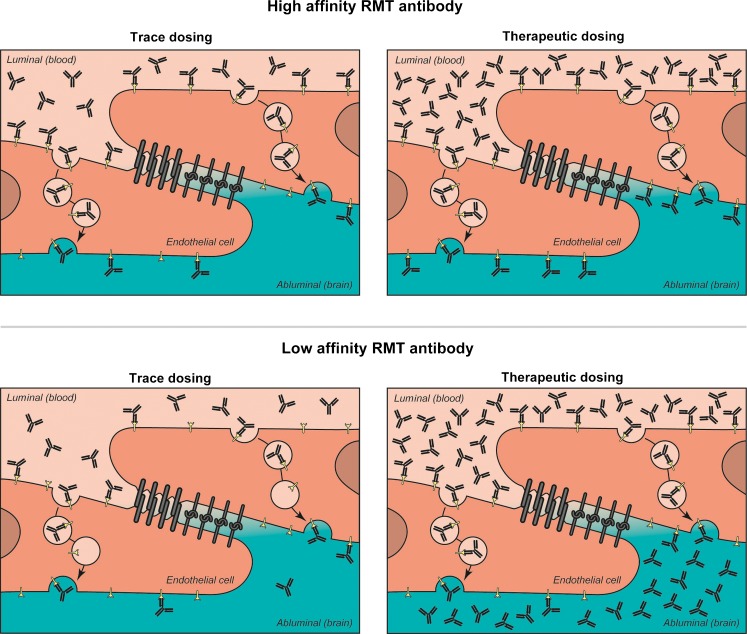
What has caused the lack of broad brain distribution of systemically administered anti-TfR in these studies? One contributing factor is likely the low amount of antibody that was systemically dosed in the majority of these studies. Because TfR is expressed not only in the brain, but also in many other tissues [122], target-mediated clearance of the antibody in peripheral organs likely contributes to an even more reduced amount of antibody available for brain delivery. Another major factor for the retention of antibodies in the brain capillaries is that these antibodies are selected to have high binding affinity for TfR. To directly test this latter explanation for vascular accumulation of anti-TfR antibodies, we recently described that systemic administration of lower-affinity TfR antibodies results in improved brain accumulation and distribution compared to higher-affinity antibodies [123]. In fact, brain concentration of systemically administered antibody in mice increased from 2-fold with a high-affinity anti-TfR to almost 6-fold with a low-affinity anti-TfR 24 hours postdose compared with control IgG. This inverse relationship is observed when therapeutically relevant doses of anti-TfR are given (high dose), in which case the luminal TfRs are saturated by both low- and high-affinity anti-TfR antibodies (Fig. 3). These quantitiave data are consistent with the conclusion that lower affinity TfR antibody would have a greater likelihood of dissociating after binding, resulting in improved brain uptake and broader brain distribution. Importantly, when different anti-TfR affinity variants are administered at radiolabeled trace doses (low dose), similar to what is done in most BBB studies, the exact opposite relationship is observed; that is, higher-affinity anti-TfRs showed higher brain “uptake” than lower-affinity antibodies. However, it is important to note that, as reported previously [113–115, 121], trace doses of antibody are accumulating in brain endothelial cells [123], thus what is measured as “uptake” is actually brain endothelial accumulation of high-affinity anti-TfR antibodies. This suggests that to appropriately test an antibody’s ability to cross the BBB and achieve therapeutic concentrations in the brain, the affinity of the RMT targeting antibody, such as anti-TfR, should be in the lower range to maximize brain uptake and distribution.
Fig. 3.
Inverse relationship between an antibody’s affinity for a receptor-mediated transport (RMT) target and its brain uptake. When administered at low trace doses a high-affinity RMT antibody will bind more receptors on the luminal side of the brain endothelium (top left panel) than a low-affinity RMT antibody (bottom left panel), with minimal dissociation of antibody on the abluminal side during and after transport. At therapeutic doses (right panels, top and bottom), binding to the RMT receptors on the luminal side is saturated and occurs regardless of affinity. However, lower affinity antibodies will have a great likelihood of dissociation from the receptor during its transcytosis route, resulting in increased brain uptake and broad parenchymal distribution
A direct CNS pharmacodynamic effect has generally been lacking in the functional assessment of BBB-crossing antibodies. To address this, we engineered a bispecific antibody that binds both TfR and BACE1 [123]. As discussed earlier, function-blocking antibodies to BACE1 have been shown to reduce Aβ levels in vivo by inhibiting APP cleavage [29, 54]. Unfortunately, in order to have a modest reduction of Aβ in brain, very high and frequent dosing was required. In the bispecific format, however, anti-TfR/BACE1 significantly reduced both brain and peripheral Aβ levels even after a single intravenous injection [123]. The improved brain distribution and accumulation of a lower affinity anti-TfR arm, combined with the ability to directly measure antibody activity via anti-BACE1 reduction of Aβ in both the periphery and brain, provides evidence that targeting CNS diseases with antibodies in this manner may be a promising approach. Although this TfR antibody does not interfere with Tf binding and iron transport, a more thorough analysis of the safety implications of targeting TfR (or any endogenous transport system) will need to be addressed. Furthermore, these studies were all conducted in rodents, thus application to primates is still unknown.
Insulin Receptor
Another widely studied receptor system for drug targeting via RMT is the insulin receptor (InsR). Expressed on brain endothelial cells, the InsR is a tyrosine kinase receptor comprised of two alpha and two beta subunits. Binding of insulin induces both kinase activation and endocytosis [124]. Like TfR, antibodies to InsR have also been generated for large molecule delivery across the BBB. Among the most studied is the mouse monoclonal 83–14 antibody against the human InsR, where trace doses of radiolabeled antibody showed an increase in brain uptake, but also a fast clearance, suggesting that this antibody has high affinity for InsR [125, 126]. Nevertheless, 83–14 has been characterized in vivo using a number of fusion proteins, including Aβ40 for amyloid imaging [127], single-chain anti-Aβ [128, 129], tissue-specific gene targeting, and glial cell-derived neurotrophic factor [130]. However, as with most TfR antibody studies in the past, these were performed using subtherapeutic doses, where the high affinity 83–14 may potentially be demonstrating a brain uptake largely restricted to brain endothelial cells.
Other RMT Targets
In addition to TfR and InsR, several other endogenous transport systems have been studied for their drug delivery potential to the brain. The low-density lipoprotein receptor-related proteins 1 and 2 (Lrp1 and Lrp2) are two such targets. Lrp1 and Lrp2 are transmembrane receptors belonging to the low-density lipoprotein receptor gene family. Among their many interacting ligands are receptor-associated protein, apolipoprotein E, and melanotransferrin/p97 [131–133]. Drug targeting using p97 is perhaps the most studied of these ligands, where injections of the p97-conjugated chemotherapeutics paclitaxel or adriamycin were reported to result in increased brain uptake, with the latter also improving survival in a rat model of intracranial glioma [134]. More recently, Angiochem Inc. has developed a 19-amino-acid peptide against Lrp1, called Angiopep-2, to which paclitaxel was conjugated [135]. Administered systemically, this conjugate was found to localize to gliomas in mice, and its uptake was dependent on Lrp1 expression. Although these data suggest that targeting Lrp1 as an RMT target may be possible, to date there has yet to be successful attempts at delivering antibody therapeutics using Lrp receptors. Effective RMT delivery of antibodies across the BBB would most likely require high expression of the target receptor (e.g., TfR) on capillary endothelial cells for sufficient transport capacity. However, both electron microscopy and immunohistochemical evidence suggest that Lrp1 is expressed on neurons and perciytes, but not brain endothelial cells [136, 137]. Thus, Lrp1 is unlikely an ideal RMT candidate for CNS antibody therapy.
Along with high transport capacity (i.e., expression), high brain specificity is also an important criterion for an ideal RMT transport system. In order to address this, subtractive panning approaches have been explored to identify brain-specific RMT candidates. One such approach involves utilizing a naïve camelid phage display library of single-domain antibody (sdAb) to identify receptors enriched in human cerebrovascular endothelial cells compared with lung endothelial cells [138, 139]. sdAb consist of only the variable heavy chain and lack both the light chain and the Fc domain of a full antibody, and are thus much smaller in size (~15 kDa). Two novel sdAbs, FC5 and FC44, were identified using subtractive panning of the sdAb phage library that were both enriched in human cerebrovascular endothelial cells and showed evidence of brain accumulation after systemic injection in mice [138]. The receptor for FC5 was identified as Cdc50A (also known as TMEM30), which is expressed on the luminal side of brain endothelial cells and undergoes clathrin-dependent endocytosis [140]. However, their small size results in a considerably short plasma half-life (~10 min), and, thus, improving the pharmacokinetic properties of these sdAbs will be critical for their therapeutic utility.
The Future of CNS Antibody Drug Development
In this brief review we have highlighted some of the exciting progress that has been made in developing antibody therapeutics for neurodegenerative disease, with a focus on AD and novel technologies that will further open the CNS to large-molecule therapeutics. Regardless of the success of this first generation of traditional antibodies targeting aggregating proteins, such as Aβ, tau, and α-synuclein, the next generation of engineered antibodies taking advantage of new technologies to boost antibody uptake in brain, such as bispecific antibody platforms, will provide further incentive to target molecules in the CNS previously thought to be off-limits to antibodies.
Much work remains to engineer safe and effective CNS antibody platforms. Essential to the success of exploiting BBB transport routes for antibody therapeutics will be validating the safety of targeting these specific pathways. This is especially critical when the transport protein target is expressed on peripheral tissues in addition to brain endothelial cells (i.e., TfR and InsR). One major consideration is to ensure that the antibodies do not affect the normal function of these receptors. For instance, the TfR antibodies utilized for in vivo validation of the anti-TfR/BACE1 bispecific does not compete with transferrin binding, and thus are not expected to alter iron transport and normal TfR function [123]. In the case of InsR a recent publication has shown severe safety observations in monkeys that may be related to InsR [141]. In general, it will be necessary to validate each new BBB target not only for its brain uptake potential, but also for the safety liabilities they may bring, including the need for extensive testing in higher species. Nevertheless, the future for antibody therapeutics for neurodegenerative diseases is bright, including the pursuit of novel approaches to access the brain with therapeutic antibodies.
Electronic Supplementary Material
(PDF 510 kb)
Acknowledgments
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
References
- 1.Pangalos MN, Schechter LE, Hurko O. Drug development for CNS disorders: strategies for balancing risk and reducing attrition. Nat Rev Drug Discov. 2007;6:521–32. doi: 10.1038/nrd2094. [DOI] [PubMed] [Google Scholar]
- 2.Buss NA, Henderson SJ, McFarlane M, Shenton JM, de Haan L. Monoclonal antibody therapeutics: history and future. Curr Opin Pharmacol. 2012;12:615–22. doi: 10.1016/j.coph.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 3.Carter PJ. Introduction to current and future protein therapeutics: a protein engineering perspective. Exp Cell Res. 2011;317:1261. doi: 10.1016/j.yexcr.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Brody DL, Holtzman DM. Active and passive immunotherapy for neurodegenerative disorders. Annu Rev Neurosci. 2008;31:175–93. doi: 10.1146/annurev.neuro.31.060407.125529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neuwelt EA, Bauer B, Fahlke C, Fricker G, Iadecola C, Janigro D, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–82. doi: 10.1038/nrn2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rubin LL, Staddon JM. The cell biology of the blood–brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 7.Tam SJ, Watts RJ. Connecting vascular and nervous system development: angiogenesis and the blood–brain barrier. Annu Rev Neurosci. 2010;33:379–408. doi: 10.1146/annurev-neuro-060909-152829. [DOI] [PubMed] [Google Scholar]
- 8.Brightman MW, Reese TS. Junctions between intimately apposed cell membranes in the vertebrate brain. J Cell Biol. 1969;40:648–77. doi: 10.1083/jcb.40.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reese TS, Karnovsky MJ. Fine structural localization of a blood–brain barrier to exogenous peroxidase. J Cell Biol. 1967;34:207–17. doi: 10.1083/jcb.34.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee G, Dallas S, Hong M, Bendayan R. Drug transporters in the central nervous system: brain barriers and brain parenchyma considerations. Pharmacol Rev. 2001;53:569–96. [PubMed] [Google Scholar]
- 11.el-Bacha RS, Minn A. Drug metabolizing enzymes in cerebrovascular endothelial cells afford a metabolic protection to the brain. Cell Mol Biol (Noisy-le-grand) 1999;45:15–23. [PubMed] [Google Scholar]
- 12.de Boer AG, van der Sandt IC, Gaillard PJ. The role of drug transporters at the blood–brain barrier. Annu Rev Pharmacol Toxicol. 2003;43:629–56. doi: 10.1146/annurev.pharmtox.43.100901.140204. [DOI] [PubMed] [Google Scholar]
- 13.Ek CJ, Dziegielewska KM, Stolp H, Saunders NR. Functional effectiveness of the blood–brain barrier to small water-soluble molecules in developing and adult opossum (Monodelphis domestica) J Comp Neurol. 2006;496:13–26. doi: 10.1002/cne.20885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janzer RC, Raff MC. Astrocytes induce blood–brain barrier properties in endothelial cells. Nature. 1987;325:253–7. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 15.Stewart PA, Wiley MJ. Developing nervous tissue induces formation of blood–brain barrier characteristics in invading endothelial cells: a study using quail–chick transplantation chimeras. Dev Biol. 1981;84:183–92. doi: 10.1016/0012-1606(81)90382-1. [DOI] [PubMed] [Google Scholar]
- 16.Lai CH, Kuo KH. The critical component to establish in vitro BBB model: pericyte. Brain Res Brain Res Rev. 2005;50:258–65. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Gaillard PJ, van der Sandt IC, Voorwinden LH, Vu D, Nielsen JL, de Boer AG, et al. Astrocytes increase the functional expression of P-glycoprotein in an in vitro model of the blood–brain barrier. Pharm Res. 2000;17:1198–205. doi: 10.1023/A:1026406528530. [DOI] [PubMed] [Google Scholar]
- 18.Banks WA. Developing drugs that can cross the blood–brain barrier: applications to Alzheimer's disease. BMC Neurosci. 2008;9(Suppl 3):S2. doi: 10.1186/1471-2202-9-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pardridge WM. Drug and gene targeting to the brain with molecular Trojan horses. Nat Rev Drug Discov. 2002;1:131–9. doi: 10.1038/nrd725. [DOI] [PubMed] [Google Scholar]
- 20.Gabathuler R. Approaches to transport therapeutic drugs across the blood–brain barrier to treat brain diseases. Neurobiol Dis. 2010;37:48–57. doi: 10.1016/j.nbd.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 21.Poduslo JF, Curran GL, Berg CT. Macromolecular permeability across the blood-nerve and blood–brain barriers. Proc Natl Acad Sci U S A. 1994;91:5705–9. doi: 10.1073/pnas.91.12.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felgenhauer K. Protein size and cerebrospinal fluid composition. Klin Wochenschr. 1974;52:1158–64. doi: 10.1007/BF01466734. [DOI] [PubMed] [Google Scholar]
- 23.Vincent A, Bien CG, Irani SR, Waters P. Autoantibodies associated with diseases of the CNS: new developments and future challenges. Lancet Neurol. 2011;10:759–72. doi: 10.1016/S1474-4422(11)70096-5. [DOI] [PubMed] [Google Scholar]
- 24.Lancaster E, Dalmau J. Neuronal autoantigens–pathogenesis, associated disorders and antibody testing. Nat Rev Neurol. 2012;8:380–90. doi: 10.1038/nrneurol.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks WA, Terrell B, Farr SA, Robinson SM, Nonaka N, Morley JE. Passage of amyloid beta protein antibody across the blood–brain barrier in a mouse model of Alzheimer's disease. Peptides. 2002;23:2223–6. doi: 10.1016/S0196-9781(02)00261-9. [DOI] [PubMed] [Google Scholar]
- 26.Banks WA, Farr SA, Morley JE, Wolf KM, Geylis V, Steinitz M. Anti-amyloid beta protein antibody passage across the blood–brain barrier in the SAMP8 mouse model of Alzheimer's disease: an age-related selective uptake with reversal of learning impairment. Exp Neurol. 2007;206:248–56. doi: 10.1016/j.expneurol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang A, Das P, Switzer RC, 3rd, Golde TE, Jankowsky JL. Robust amyloid clearance in a mouse model of Alzheimer's disease provides novel insights into the mechanism of amyloid-beta immunotherapy. J Neurosci Off J Soc Neurosci. 2011;31:4124–36. doi: 10.1523/JNEUROSCI.5077-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada K, Yabuki C, Seubert P, Schenk D, Hori Y, Ohtsuki S, et al. Abeta immunotherapy: intracerebral sequestration of Abeta by an anti-Abeta monoclonal antibody 266 with high affinity to soluble Abeta. J Neurosci Off J Soc Neurosci. 2009;29:11393–8. doi: 10.1523/JNEUROSCI.2021-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atwal JK, Chen Y, Chiu C, Mortensen DL, Meilandt WJ, Liu Y, et al. A therapeutic antibody targeting BACE1 inhibits amyloid-beta production in vivo. Sci Transl Med. 2011;3:84ra43. doi: 10.1126/scitranslmed.3002254. [DOI] [PubMed] [Google Scholar]
- 30.Bard F, Cannon C, Barbour R, Burke RL, Games D, Grajeda H, et al. Peripherally administered antibodies against amyloid beta-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med. 2000;6:916–9. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 31.Bard F, Fox M, Friedrich S, Seubert P, Schenk D, Kinney GG, et al. Sustained levels of antibodies against Abeta in amyloid-rich regions of the CNS following intravenous dosing in human APP transgenic mice. Exp Neurol. 2012;238:38–43. doi: 10.1016/j.expneurol.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 32.Vassar R, Kovacs DM, Yan R, Wong PC. The beta-secretase enzyme BACE in health and Alzheimer's disease: regulation, cell biology, function, and therapeutic potential. J Neurosci Off J Soc Neurosci. 2009;29:12787–94. doi: 10.1523/JNEUROSCI.3657-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karran E, Mercken M, De Strooper B. The amyloid cascade hypothesis for Alzheimer's disease: an appraisal for the development of therapeutics. Nat Rev Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 34.Nitsch RM, Hock C. Targeting beta-amyloid pathology in Alzheimer's disease with Abeta immunotherapy. Neurother J Am Soc Exp NeuroTher. 2008;5:415–20. doi: 10.1016/j.nurt.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3:77sr1. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertram L, Lill CM, Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron. 2010;68:270–81. doi: 10.1016/j.neuron.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 37.Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer's disease and age-related cognitive decline. Nature. 2012;488:96–9. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- 38.Huang Y, Mucke L. Alzheimer mechanisms and therapeutic strategies. Cell. 2012;148:1204–22. doi: 10.1016/j.cell.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer's disease. Nature. 2009;461:916–22. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu L, Drouet V, Wu JW, Witter MP, Small SA, Clelland C, et al. Trans-synaptic spread of tau pathology in vivo. PLoS One. 2012;7:e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Calignon A, Polydoro M, Suarez-Calvet M, William C, Adamowicz DH, Kopeikina KJ, et al. Propagation of tau pathology in a model of early Alzheimer's disease. Neuron. 2012;73:685–97. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol. 2009;11:909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, et al. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–41. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 44.Roberds SL, Anderson J, Basi G, Bienkowski MJ, Branstetter DG, Chen KS, et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum Mol Genet. 2001;10:1317–24. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh AK, Gemma S, Tang J. beta-Secretase as a therapeutic target for Alzheimer's disease. Neurother J Am Soc Exp NeuroTher. 2008;5:399–408. doi: 10.1016/j.nurt.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ghosh AK, Brindisi M, Tang J. Developing beta-secretase inhibitors for treatment of Alzheimer's disease. J Neurochem. 2012;120(Suppl 1):71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.May PC, Dean RA, Lowe SL, Martenyi F, Sheehan SM, Boggs LN, et al. Robust central reduction of amyloid-beta in humans with an orally available, non-peptidic beta-secretase inhibitor. J Neurosci Off J Soc Neurosci. 2011;31:16507–16. doi: 10.1523/JNEUROSCI.3647-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koike M, Shibata M, Ohsawa Y, Nakanishi H, Koga T, Kametaka S, et al. Involvement of two different cell death pathways in retinal atrophy of cathepsin D-deficient mice. Mol Cell Neurosci. 2003;22:146–61. doi: 10.1016/S1044-7431(03)00035-6. [DOI] [PubMed] [Google Scholar]
- 49.Koike M, Nakanishi H, Saftig P, Ezaki J, Isahara K, Ohsawa Y, et al. Cathepsin D deficiency induces lysosomal storage with ceroid lipofuscin in mouse CNS neurons. J Neurosci Off J Soc Neurosci. 2000;20:6898–906. doi: 10.1523/JNEUROSCI.20-18-06898.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Follo C, Ozzano M, Mugoni V, Castino R, Santoro M, Isidoro C. Knock-down of cathepsin D affects the retinal pigment epithelium, impairs swim-bladder ontogenesis and causes premature death in zebrafish. PLoS One. 2011;6:e21908. doi: 10.1371/journal.pone.0021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cai J, Qi X, Kociok N, Skosyrski S, Emilio A, Ruan Q, et al. beta-Secretase (BACE1) inhibition causes retinal pathology by vascular dysregulation and accumulation of age pigment. EMBO Mol Med. 2012;4:980–91. doi: 10.1002/emmm.201101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hitt B, Riordan SM, Kukreja L, Eimer WA, Rajapaksha TW, Vassar R. Beta-site amyloid precursor protein (APP)-cleaving enzyme 1 (BACE1)-deficient mice exhibit a close homolog of L1 (CHL1) loss-of-function phenotype involving axon guidance defects. J Biol Chem. 2012;287:38408–25. doi: 10.1074/jbc.M112.415505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rajapaksha TW, Eimer WA, Bozza TC, Vassar R. The Alzheimer's beta-secretase enzyme BACE1 is required for accurate axon guidance of olfactory sensory neurons and normal glomerulus formation in the olfactory bulb. Mol Neurodegener. 2011;6:88. doi: 10.1186/1750-1326-6-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou L, Chavez-Gutierrez L, Bockstael K, Sannerud R, Annaert W, May PC, et al. Inhibition of beta-secretase in vivo via antibody binding to unique loops (D and F) of BACE1. J Biol Chem. 2011;286:8677–87. doi: 10.1074/jbc.M110.194860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delrieu J, Ousset PJ, Caillaud C, Vellas B. 'Clinical trials in Alzheimer's disease': immunotherapy approaches. J Neurochem. 2012;120(Suppl 1):186–93. doi: 10.1111/j.1471-4159.2011.07458.x. [DOI] [PubMed] [Google Scholar]
- 56.Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-beta immunotherapy? Nat Rev Neurol. 2010;6:108–19. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Valera E, Masliah E. Immunotherapy for neurodegenerative diseases: focus on alpha-synucleinopathies. Pharmacol Ther 2013. doi:10.1016/j.pharmthera.2013.01.013. [DOI] [PMC free article] [PubMed]
- 58.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–7. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 59.Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An effector-reduced anti-beta-amyloid (Abeta) antibody with unique abeta binding properties promotes neuroprotection and glial engulfment of Abeta. J Neurosci Off J Soc Neurosci. 2012;32:9677–89. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farlow M, Arnold SE, van Dyck CH, Aisen PS, Snider BJ, Porsteinsson AP, et al. Safety and biomarker effects of solanezumab in patients with Alzheimer's disease. Alzheimers Dement J Alzheimers Assoc. 2012;8:261–71. doi: 10.1016/j.jalz.2011.09.224. [DOI] [PubMed] [Google Scholar]
- 61.Ostrowitzki S, Deptula D, Thurfjell L, Barkhof F, Bohrmann B, Brooks DJ, et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 62.Rinne JO, Brooks DJ, Rossor MN, Fox NC, Bullock R, Klunk WE, et al. 11C-PiB PET assessment of change in fibrillar amyloid-beta load in patients with Alzheimer's disease treated with bapineuzumab: a phase 2, double-blind, placebo-controlled, ascending-dose study. Lancet Neurol. 2010;9:363–72. doi: 10.1016/S1474-4422(10)70043-0. [DOI] [PubMed] [Google Scholar]
- 63.DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer's disease. Science. 2002;295:2264–7. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- 64.Sperling RA, Jack CR, Jr, Black SE, Frosch MP, Greenberg SM, Hyman BT, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer's Association Research Roundtable Workgroup. Alzheimers Dement J Alzheimers Assoc. 2011;7:367–85. doi: 10.1016/j.jalz.2011.05.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, et al. A plaque-specific antibody clears existing beta-amyloid plaques in Alzheimer's disease mice. Neuron. 2012;76:908–20. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- 66.Golde TE, Schneider LS, Koo EH. Anti-abeta therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sperling RA, Jack CR, Jr, Aisen PS. Testing the right target and right drug at the right stage. Sci Transl Med. 2011;3:111cm33. doi: 10.1126/scitranslmed.3002609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, et al. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Selkoe DJ. Preventing Alzheimer's disease. Science. 2012;337:1488–92. doi: 10.1126/science.1228541. [DOI] [PubMed] [Google Scholar]
- 70.Bateman RJ, Xiong C, Benzinger TL, Fagan AM, Goate A, Fox NC, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med. 2012;367:795–804. doi: 10.1056/NEJMoa1202753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fleisher AS, Chen K, Quiroz YT, Jakimovich LJ, Gomez MG, Langois CM, et al. Florbetapir PET analysis of amyloid-β deposition in the presenilin 1 E280A autosomal dominant Alzheimer's disease kindred: a cross-sectional study. Lancet Neurol. 2012;11:1057–65. doi: 10.1016/S1474-4422(12)70227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 73.Yamada K, Cirrito JR, Stewart FR, Jiang H, Finn MB, Holmes BB, et al. In vivo microdialysis reveals age-dependent decrease of brain interstitial fluid tau levels in P301S human tau transgenic mice. J Neurosci Off J Soc Neurosci. 2011;31:13110–7. doi: 10.1523/JNEUROSCI.2569-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci Off J Soc Neurosci. 2007;27:9115–29. doi: 10.1523/JNEUROSCI.2361-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau prevents cognitive decline in a new tangle mouse model. J Neurosci Off J Soc Neurosci. 2010;30:16559–66. doi: 10.1523/JNEUROSCI.4363-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Boimel M, Grigoriadis N, Lourbopoulos A, Haber E, Abramsky O, Rosenmann H. Efficacy and safety of immunization with phosphorylated tau against neurofibrillary tangles in mice. Exp Neurol. 2010;224:472–85. doi: 10.1016/j.expneurol.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 77.Bi M, Ittner A, Ke YD, Gotz J, Ittner LM. Tau-targeted immunization impedes progression of neurofibrillary histopathology in aged P301L tau transgenic mice. PLoS One. 2011;6:e26860. doi: 10.1371/journal.pone.0026860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem. 2011;286:34457–67. doi: 10.1074/jbc.M111.229633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J Neurochem. 2011;118:658–67. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287:19440–51. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nat Rev Neurol 2012. doi:10.1038/nrneurol.2012.242 [DOI] [PubMed]
- 82.Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, et al. Effects of alpha-synuclein immunization in a mouse model of Parkinson's disease. Neuron. 2005;46:857–68. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 83.Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS One. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci Off J Soc Neurosci. 2005;25:6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, et al. Antibody-aided clearance of extracellular alpha-synuclein prevents cell-to-cell aggregate transmission. J Neurosci Off J Soc Neurosci. 2012;32:13454–69. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 88.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 89.Miller T. DNA vaccination against mutant huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol Ther. 2003;7:572–9. doi: 10.1016/S1525-0016(03)00063-7. [DOI] [PubMed] [Google Scholar]
- 90.Takeuchi S, Fujiwara N, Ido A, Oono M, Takeuchi Y, Tateno M, et al. Induction of protective immunity by vaccination with wild-type apo superoxide dismutase 1 in mutant SOD1 transgenic mice. J Neuropathol Exp Neurol. 2010;69:1044–56. doi: 10.1097/NEN.0b013e3181f4a90a. [DOI] [PubMed] [Google Scholar]
- 91.Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci U S A. 2007;104:2495–500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexandrenne C, Hanoux V, Dkhissi F, Boquet D, Couraud JY, Wijkhuisen A. Curative properties of antibodies against prion protein: a comparative in vitro study of monovalent fragments and divalent antibodies. J Neuroimmunol. 2009;209:50–6. doi: 10.1016/j.jneuroim.2009.01.025. [DOI] [PubMed] [Google Scholar]
- 93.Sigurdsson EM, Brown DR, Daniels M, Kascsak RJ, Kascsak R, Carp R, et al. Immunization delays the onset of prion disease in mice. Am J Pathol. 2002;161:13–7. doi: 10.1016/S0002-9440(10)64151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jefferies WA, Brandon MR, Hunt SV, Williams AF, Gatter KC, Mason DY. Transferrin receptor on endothelium of brain capillaries. Nature. 1984;312:162–3. doi: 10.1038/312162a0. [DOI] [PubMed] [Google Scholar]
- 95.Duffy KR, Pardridge WM. Blood–brain barrier transcytosis of insulin in developing rabbits. Brain Res. 1987;420:32–8. doi: 10.1016/0006-8993(87)90236-8. [DOI] [PubMed] [Google Scholar]
- 96.Golden PL, Maccagnan TJ, Pardridge WM. Human blood–brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J Clin Investig. 1997;99:14–8. doi: 10.1172/JCI119125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Triguero D, Buciak JB, Yang J, Pardridge WM. Blood–brain barrier transport of cationized immunoglobulin G: enhanced delivery compared to native protein. Proc Natl Acad Sci U S A. 1989;86:4761–5. doi: 10.1073/pnas.86.12.4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Poduslo JF, Curran GL. Polyamine modification increases the permeability of proteins at the blood-nerve and blood–brain barriers. J Neurochem. 1996;66:1599–609. doi: 10.1046/j.1471-4159.1996.66041599.x. [DOI] [PubMed] [Google Scholar]
- 99.Herve F, Ghinea N, Scherrmann JM. CNS delivery via adsorptive transcytosis. AAPS J. 2008;10:455–72. doi: 10.1208/s12248-008-9055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lockman PR, Koziara JM, Mumper RJ, Allen DD. Nanoparticle surface charges alter blood–brain barrier integrity and permeability. J Drug Target. 2004;12:635–41. doi: 10.1080/10611860400015936. [DOI] [PubMed] [Google Scholar]
- 101.Ohtsuki S, Terasaki T. Contribution of carrier-mediated transport systems to the blood–brain barrier as a supporting and protecting interface for the brain; importance for CNS drug discovery and development. Pharm Res. 2007;24:1745–58. doi: 10.1007/s11095-007-9374-5. [DOI] [PubMed] [Google Scholar]
- 102.Cundy KC, Branch R, Chernov-Rogan T, Dias T, Estrada T, Hold K, et al. XP13512 [(+/−)-1-([(alpha-isobutanoyloxyethoxy)carbonyl] aminomethyl)-1-cyclohexane acetic acid], a novel gabapentin prodrug: I. Design, synthesis, enzymatic conversion to gabapentin, and transport by intestinal solute transporters. J Pharmacol Exp Ther. 2004;311:315–23. doi: 10.1124/jpet.104.067934. [DOI] [PubMed] [Google Scholar]
- 103.Gomes P, Soares-da-Silva P. L-DOPA transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res. 1999;829:143–50. doi: 10.1016/S0006-8993(99)01387-6. [DOI] [PubMed] [Google Scholar]
- 104.Pardridge WM, Eisenberg J, Yang J. Human blood–brain barrier transferrin receptor. Metab Clin Exp. 1987;36:892–5. doi: 10.1016/0026-0495(87)90099-0. [DOI] [PubMed] [Google Scholar]
- 105.Moos T, Morgan EH. Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 2000;20:77–95. doi: 10.1023/A:1006948027674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Risau W, Hallmann R, Albrecht U. Differentiation-dependent expression of proteins in brain endothelium during development of the blood–brain barrier. Dev Biol. 1986;117:537–45. doi: 10.1016/0012-1606(86)90321-0. [DOI] [PubMed] [Google Scholar]
- 107.Giometto B, Bozza F, Argentiero V, Gallo P, Pagni S, Piccinno MG, et al. Transferrin receptors in rat central nervous system. An immunocytochemical study. J Neurol Sci. 1990;98:81–90. doi: 10.1016/0022-510X(90)90183-N. [DOI] [PubMed] [Google Scholar]
- 108.Oh TH, Markelonis GJ, Royal GM, Bregman BS. Immunocytochemical distribution of transferrin and its receptor in the developing chicken nervous system. Brain Res. 1986;395:207–20. doi: 10.1016/0165-3806(86)90111-2. [DOI] [PubMed] [Google Scholar]
- 109.Ajioka RS, Kaplan J. Intracellular pools of transferrin receptors result from constitutive internalization of unoccupied receptors. Proc Natl Acad Sci U S A. 1986;83:6445–9. doi: 10.1073/pnas.83.17.6445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5:121–32. doi: 10.1038/nrm1315. [DOI] [PubMed] [Google Scholar]
- 111.Shin SU, Friden P, Moran M, Olson T, Kang YS, Pardridge WM, et al. Transferrin-antibody fusion proteins are effective in brain targeting. Proc Natl Acad Sci U S A. 1995;92:2820–4. doi: 10.1073/pnas.92.7.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mishra V, Mahor S, Rawat A, Gupta PN, Dubey P, Khatri K, et al. Targeted brain delivery of AZT via transferrin anchored pegylated albumin nanoparticles. J Drug Target. 2006;14:45–53. doi: 10.1080/10611860600612953. [DOI] [PubMed] [Google Scholar]
- 113.Friden PM, Walus LR, Musso GF, Taylor MA, Malfroy B, Starzyk RM. Anti-transferrin receptor antibody and antibody-drug conjugates cross the blood–brain barrier. Proc Natl Acad Sci U S A. 1991;88:4771–5. doi: 10.1073/pnas.88.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pardridge WM, Buciak JL, Friden PM. Selective transport of an anti-transferrin receptor antibody through the blood–brain barrier in vivo. J Pharmacol Exp Ther. 1991;259:66–70. [PubMed] [Google Scholar]
- 115.Lee HJ, Engelhardt B, Lesley J, Bickel U, Pardridge WM. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood–brain barrier in mouse. J Pharmacol Exp Ther. 2000;292:1048–52. [PubMed] [Google Scholar]
- 116.Zhang Y, Pardridge WM. Conjugation of brain-derived neurotrophic factor to a blood–brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 2001;889:49–56. doi: 10.1016/S0006-8993(00)03108-5. [DOI] [PubMed] [Google Scholar]
- 117.Friden PM, Walus LR, Watson P, Doctrow SR, Kozarich JW, Backman C, et al. Blood–brain barrier penetration and in vivo activity of an NGF conjugate. Science. 1993;259:373–7. doi: 10.1126/science.8420006. [DOI] [PubMed] [Google Scholar]
- 118.Kordower JH, Charles V, Bayer R, Bartus RT, Putney S, Walus LR, et al. Intravenous administration of a transferrin receptor antibody-nerve growth factor conjugate prevents the degeneration of cholinergic striatal neurons in a model of Huntington disease. Proc Natl Acad Sci U S A. 1994;91:9077–80. doi: 10.1073/pnas.91.19.9077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kissel K, Hamm S, Schulz M, Vecchi A, Garlanda C, Engelhardt B. Immunohistochemical localization of the murine transferrin receptor (TfR) on blood-tissue barriers using a novel anti-TfR monoclonal antibody. Histochem Cell Biol. 1998;110:63–72. doi: 10.1007/s004180050266. [DOI] [PubMed] [Google Scholar]
- 120.Zhou QH, Fu A, Boado RJ, Hui EK, Lu JZ, Pardridge WM. Receptor-mediated abeta amyloid antibody targeting to Alzheimer's disease mouse brain. Mol Pharm. 2011;8:280–5. doi: 10.1021/mp1003515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moos T, Morgan EH. Restricted transport of anti-transferrin receptor antibody (OX26) through the blood–brain barrier in the rat. J Neurochem. 2001;79:119–29. doi: 10.1046/j.1471-4159.2001.00541.x. [DOI] [PubMed] [Google Scholar]
- 122.Gatter KC, Brown G, Trowbridge IS, Woolston RE, Mason DY. Transferrin receptors in human tissues: their distribution and possible clinical relevance. J Clin Pathol. 1983;36:539–45. doi: 10.1136/jcp.36.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yu YJ, Zhang Y, Kenrick M, Hoyte K, Luk W, Lu Y, et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci Transl Med. 2011;3:84ra44. doi: 10.1126/scitranslmed.3002230. [DOI] [PubMed] [Google Scholar]
- 124.McClain DA, Maegawa H, Lee J, Dull TJ, Ulrich A, Olefsky JM. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987;262:14663–71. [PubMed] [Google Scholar]
- 125.Pardridge WM, Kang YS, Buciak JL, Yang J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm Res. 1995;12:807–16. doi: 10.1023/A:1016244500596. [DOI] [PubMed] [Google Scholar]
- 126.Boado RJ, Zhang Y, Zhang Y, Pardridge WM. Humanization of anti-human insulin receptor antibody for drug targeting across the human blood–brain barrier. Biotechnol Bioeng. 2007;96:381–91. doi: 10.1002/bit.21120. [DOI] [PubMed] [Google Scholar]
- 127.Wu D, Yang J, Pardridge WM. Drug targeting of a peptide radiopharmaceutical through the primate blood–brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J Clin Investig. 1997;100:1804–12. doi: 10.1172/JCI119708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Boado RJ, Zhang Y, Zhang Y, Xia CF, Pardridge WM. Fusion antibody for Alzheimer's disease with bidirectional transport across the blood–brain barrier and abeta fibril disaggregation. Bioconjugate Chem. 2007;18:447–55. doi: 10.1021/bc060349x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang Y, Schlachetzki F, Li JY, Boado RJ, Pardridge WM. Organ-specific gene expression in the rhesus monkey eye following intravenous non-viral gene transfer. Mol Vis. 2003;9:465–72. [PubMed] [Google Scholar]
- 130.Boado RJ, Zhang Y, Zhang Y, Wang Y, Pardridge WM. GDNF fusion protein for targeted-drug delivery across the human blood–brain barrier. Biotechnol Bioeng. 2008;100:387–96. doi: 10.1002/bit.21764. [DOI] [PubMed] [Google Scholar]
- 131.Bu G, Geuze HJ, Strous GJ, Schwartz AL. 39 kDa receptor-associated protein is an ER resident protein and molecular chaperone for LDL receptor-related protein. EMBO J. 1995;14:2269–80. doi: 10.1002/j.1460-2075.1995.tb07221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, et al. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gaillard PJ, Visser CC, de Boer AG. Targeted delivery across the blood–brain barrier. Expert Opin Drug Deliv. 2005;2:299–309. doi: 10.1517/17425247.2.2.299. [DOI] [PubMed] [Google Scholar]
- 134.Karkan D, Pfeifer C, Vitalis TZ, Arthur G, Ujiie M, Chen Q, et al. A unique carrier for delivery of therapeutic compounds beyond the blood–brain barrier. PLoS One. 2008;3:e2469. doi: 10.1371/journal.pone.0002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bertrand Y, Currie JC, Poirier J, Demeule M, Abulrob A, Fatehi D, et al. Influence of glioma tumour microenvironment on the transport of ANG1005 via low-density lipoprotein receptor-related protein 1. Br J Cancer. 2011;105:1697–707. doi: 10.1038/bjc.2011.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tooyama I, Kawamata T, Akiyama H, Kimura H, Moestrup SK, Gliemann J, et al. Subcellular localization of the low density lipoprotein receptor-related protein (alpha 2-macroglobulin receptor) in human brain. Brain Res. 1995;691:235–8. doi: 10.1016/0006-8993(95)00735-9. [DOI] [PubMed] [Google Scholar]
- 137.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost. 2005;3:1884–93. doi: 10.1111/j.1538-7836.2005.01371.x. [DOI] [PubMed] [Google Scholar]
- 138.Muruganandam A, Tanha J, Narang S, Stanimirovic D. Selection of phage-displayed llama single-domain antibodies that transmigrate across human blood–brain barrier endothelium. FASEB J Off Publ Fed Am Soc Exp Biol. 2002;16:240–2. doi: 10.1096/fj.01-0343fje. [DOI] [PubMed] [Google Scholar]
- 139.Tanha J, Muruganandam A, Stanimirovic D. Phage display technology for identifying specific antigens on brain endothelial cells. Methods Mol Med. 2003;89:435–49. doi: 10.1385/1-59259-419-0:435. [DOI] [PubMed] [Google Scholar]
- 140.Abulrob A, Sprong H, Van Bergen en Henegouwen P, Stanimirovic D. The blood–brain barrier transmigrating single domain antibody: mechanisms of transport and antigenic epitopes in human brain endothelial cells. J Neurochem. 2005;95:1201–14. doi: 10.1111/j.1471-4159.2005.03463.x. [DOI] [PubMed] [Google Scholar]
- 141.Ohshima-Hosoyama S, Simmons HA, Goecks N, Joers V, Swanson CR, Bondarenko V, et al. A monoclonal antibody-GDNF fusion protein is not neuroprotective and is associated with proliferative pancreatic lesions in parkinsonian monkeys. PLoS One. 2012;7:e39036. doi: 10.1371/journal.pone.0039036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 510 kb)