Abstract
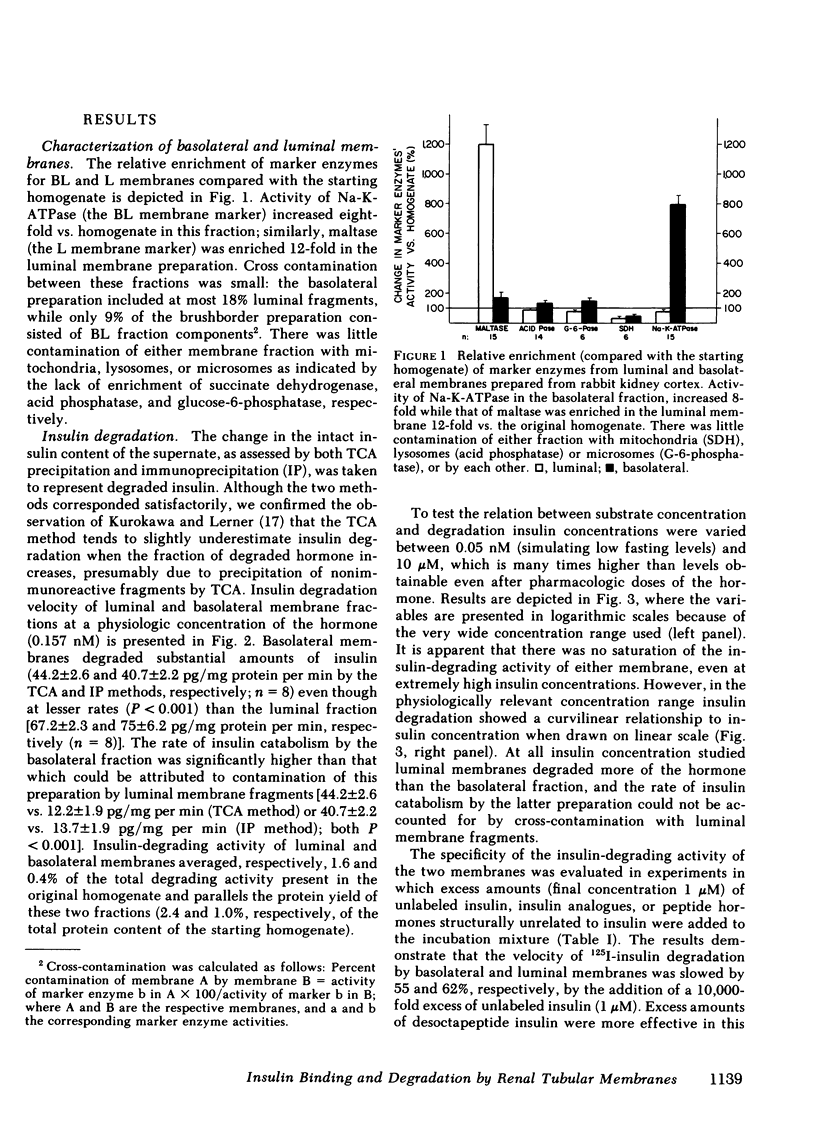
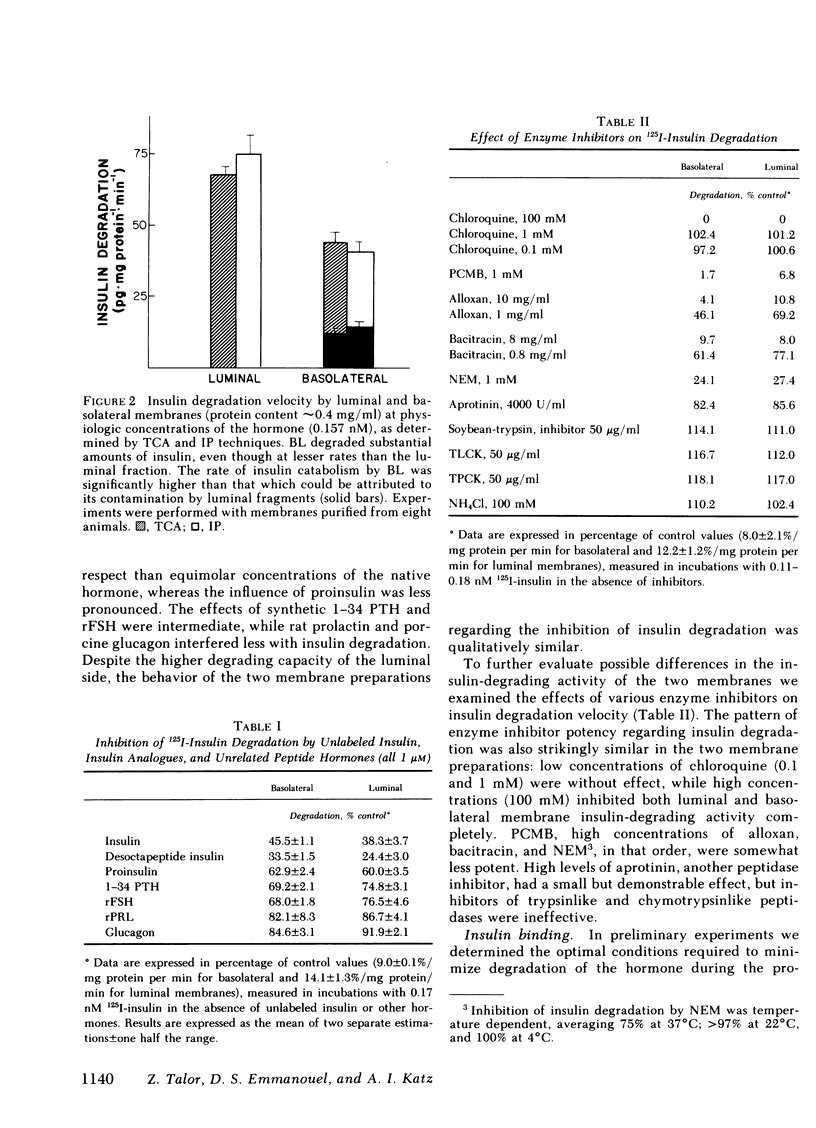
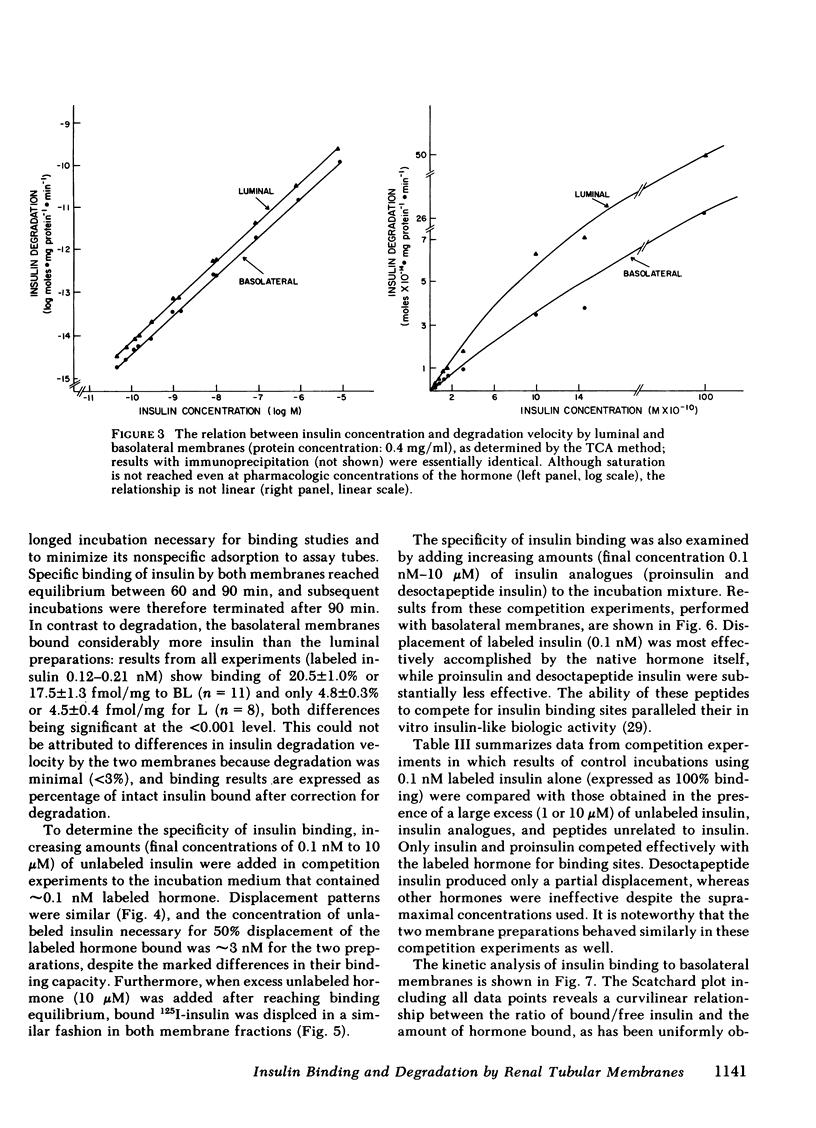
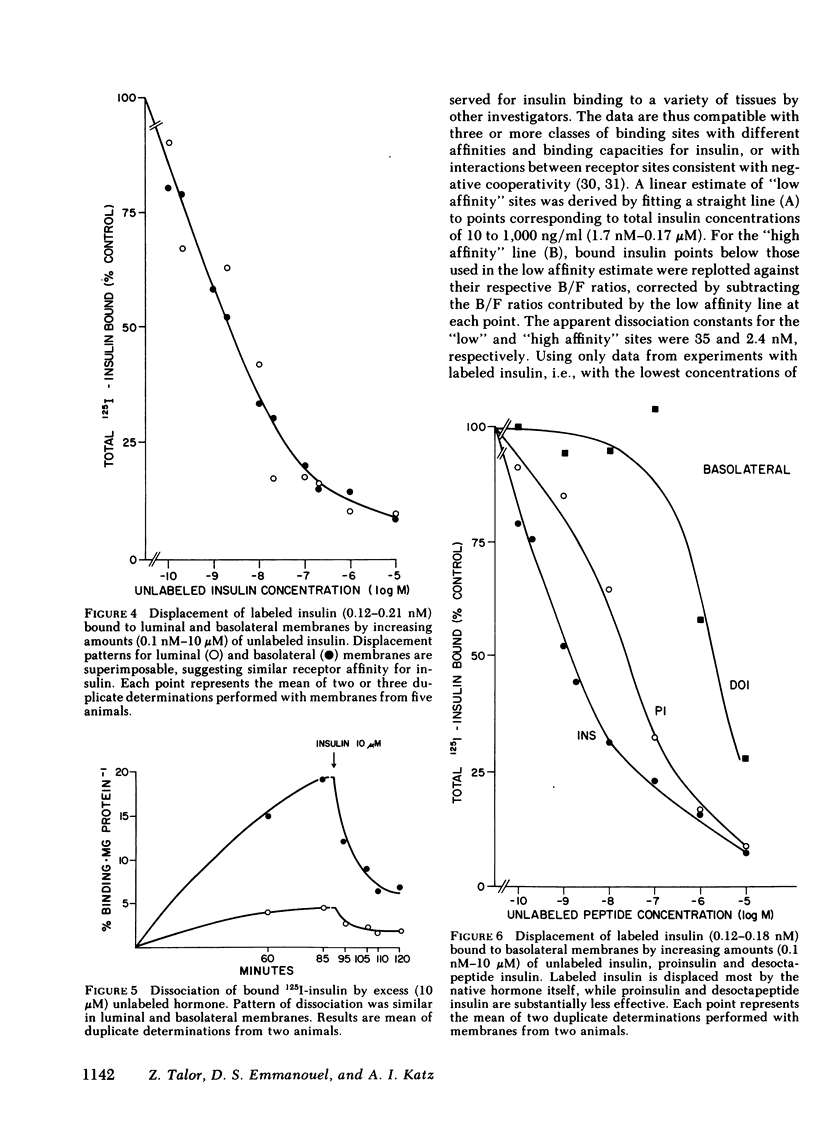
Insulin influences certain metabolic and transport renal functions and is avidly degraded by the kidney, but the relative contribution of the luminal and basolateral tubular membranes to these events remains controversial. We studied 125I-insulin degradation [TCA and immunoprecipitation (IP) methods] and the specific binding of the hormone by purified luminal (L) and basolateral (BL) tubular membranes. These were prepared from rabbit kidney cortical homogenates by differential and gradient centrifugation and ionic precipitation steps in sequence, which resulted in enrichment vs. homogenate of marker enzymes' activities (sodium-potassium-activated adenosine triphosphatase for BL and maltase for L) of 8- and 12-fold, respectively. Both fractions degraded insulin avidly and bound the hormone specifically without saturation even at pharmacologic concentrations (10 μM). At physiologic insulin concentrations (0.157 nM) BL membranes degraded substantial amounts of insulin (44.2±2.6 and 40.7±2.2 pg/mg protein per min by the TCA and IP methods, respectively), even though at lesser rates (P < 0.001) than the luminal fraction (67.2±2.3 and 75±6.2 pg/mg protein per min, respectively); the rate of insulin catabolism by BL membranes was significantly higher (P < 0.001) than that which could be attributed to their contamination by luminal components [12.2±1.9 pg/mg per min (TCA method), or 13.7±1.9 pg/mg per min (IP method)]. Competition experiments suggested that insulin-degrading activity in both fractions includes both specific and nonspecific components. In contrast to degradation, insulin binding by both membranes was highly specific for native insulin and was severalfold higher in BL than L membranes [17.5±1.3 vs. 4.5±0.4 fmol/mg protein (P < 0.001) at physiologic insulin concentrations]. Despite the marked difference in the binding capacity for insulin by the two membranes, the patterns of labeled insulin displacement by increasing amounts of unlabeled hormone were superimposable (50% displacement required ∼3 nM), suggesting that their receptors' affinity for insulin was similar. These observations provide direct evidence that interaction of insulin with the kidney involves binding and degradation of the hormone at the peritubular cell membrane.
Full text
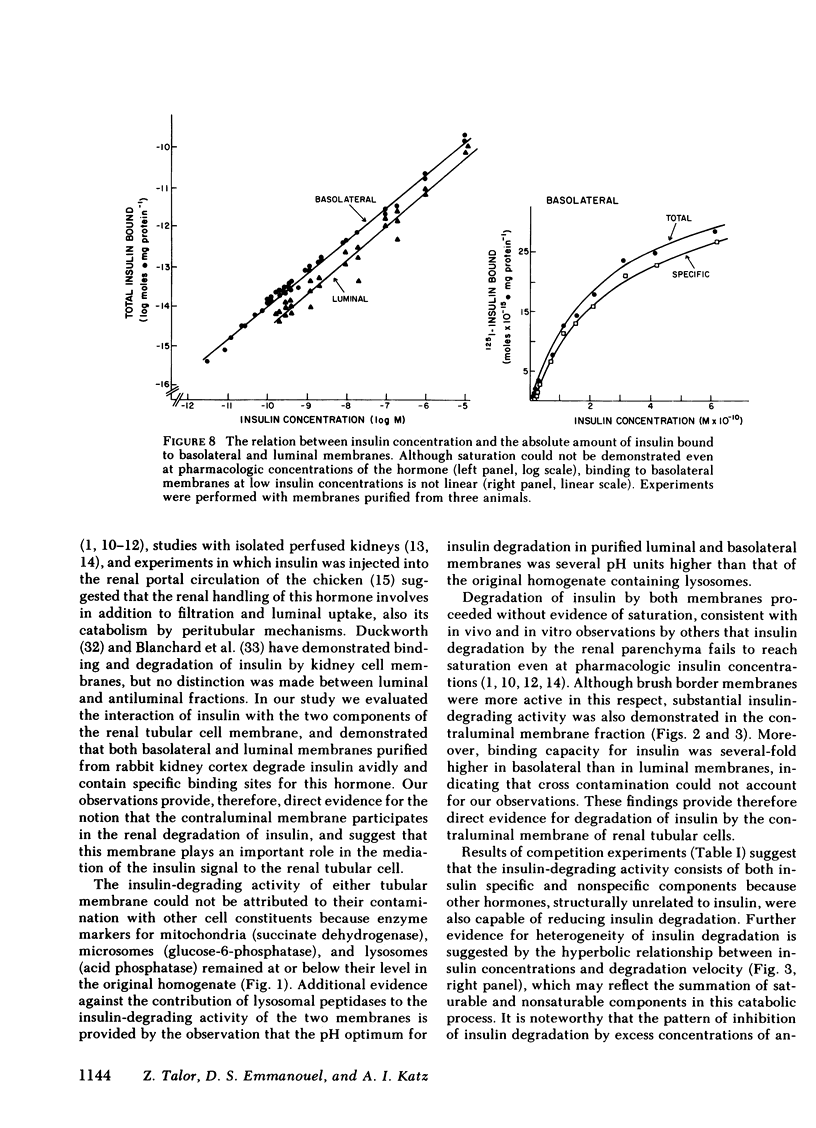
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blanchard R. F., Davis P. J., Blas S. D. Physical characteristics of insulin receptors on renal cell membranes. Diabetes. 1978 Feb;27(2):88–95. doi: 10.2337/diab.27.2.88. [DOI] [PubMed] [Google Scholar]
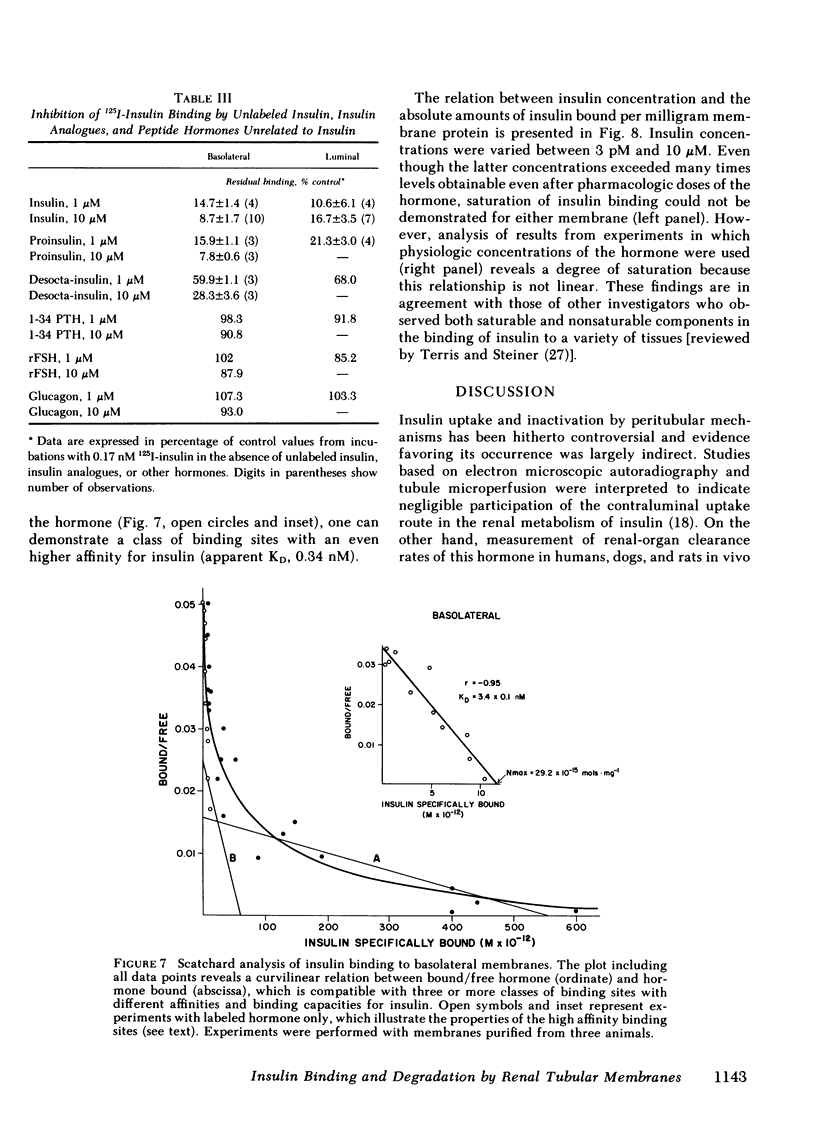
- Bourdeau J. E., Chen E. R., Carone F. A. Insulin uptake in the renal proximal tubule. Am J Physiol. 1973 Dec;225(6):1399–1404. doi: 10.1152/ajplegacy.1973.225.6.1399. [DOI] [PubMed] [Google Scholar]
- Campbell B. J., Woodward G., Borberg V. Calcium-mediated interactions between the antidiuretic hormone and renal plasma membranes. J Biol Chem. 1972 Oct 10;247(19):6167–6175. [PubMed] [Google Scholar]
- Caro J. F., Amatruda J. M. Evidence for modulation of insulin action and degradation independently of insulin binding. Am J Physiol. 1981 Mar;240(3):E325–E332. doi: 10.1152/ajpendo.1981.240.3.E325. [DOI] [PubMed] [Google Scholar]
- Chamberlain M. J., Stimmler L. The renal handling of insulin. J Clin Invest. 1967 Jun;46(6):911–919. doi: 10.1172/JCI105597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyts P., Van Obberghen E., Roth J. Mapping of the residues responsible for the negative cooperativity of the receptor-binding region of insulin. Nature. 1978 Jun 15;273(5663):504–509. doi: 10.1038/273504a0. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Cooke C. R., Andres R., Faloona G. R., Davis P. J. The effect of insulin on renal handling of sodium, potassium, calcium, and phosphate in man. J Clin Invest. 1975 Apr;55(4):845–855. doi: 10.1172/JCI107996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Goldberg M., Agus Z. S. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest. 1976 Jul;58(1):83–90. doi: 10.1172/JCI108463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bella F. P., Dousa T. P., Miller S. S., Arnaud C. D. Parathyroid hormone receptors of renal cortex: specific binding of biologically active, 125I-labeled hormone and relationship to adenylate cyclase activation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):723–726. doi: 10.1073/pnas.71.3.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth W. C. Insulin and glucagon binding and degradation by kidney cell membranes. Endocrinology. 1978 Jun;102(6):1766–1774. doi: 10.1210/endo-102-6-1766. [DOI] [PubMed] [Google Scholar]
- Freychet P., Kahn R., Roth J., Neville D. M., Jr Insulin interactions with liver plasma membranes. Independence of binding of the hormone and its degradation. J Biol Chem. 1972 Jun 25;247(12):3953–3961. [PubMed] [Google Scholar]
- Freychet P., Roth J., Neville D. M., Jr Monoiodoinsulin: demonstration of its biological activity and binding to fat cells and liver membranes. Biochem Biophys Res Commun. 1971 Apr 16;43(2):400–408. doi: 10.1016/0006-291x(71)90767-4. [DOI] [PubMed] [Google Scholar]
- HUEBSCHER G., WEST G. R. SPECIFIC ASSAYS OF SOME PHOSPHATASES IN SUBCELLULAR FRACTIONS OF SMALL INTESTINAL MUCOSA. Nature. 1965 Feb 20;205:799–800. doi: 10.1038/205799a0. [DOI] [PubMed] [Google Scholar]
- Joseph P. K., Subrahmanyam K. Effect of growth hormone, insulin, thyroxine and cortisone on renal gluconeogenesis. Arch Biochem Biophys. 1968 Sep 20;127(1):288–291. doi: 10.1016/0003-9861(68)90228-2. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Katz A. I., Rubenstein A. H. Metabolism of proinsulin, insulin, and C-peptide in the rat. J Clin Invest. 1973 May;52(5):1113–1121. doi: 10.1172/JCI107277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinsella J. L., Holohan P. D., Pessah N. I., Ross C. R. Isolation of luminal and antiluminal membranes from dog kidney cortex. Biochim Biophys Acta. 1979 Apr 19;552(3):468–477. doi: 10.1016/0005-2736(79)90191-3. [DOI] [PubMed] [Google Scholar]
- Kurokawa K., Lerner R. L. Binding and degradation of insulin by isolated renal cortical tubules. Endocrinology. 1980 Mar;106(3):655–662. doi: 10.1210/endo-106-3-655. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lloyd J. B., Whelan W. J. An improved method for enzymic determination of glucose in the presence of maltose. Anal Biochem. 1969 Sep;30(3):467–470. doi: 10.1016/0003-2697(69)90143-2. [DOI] [PubMed] [Google Scholar]
- Lupiáez J. A., Dileepan K. N., Wagle S. R. Interrelationship of somatostatin, insulin, and calcium in the control of gluconeogenesis in kidney cortex slices. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1153–1158. doi: 10.1016/0006-291x(79)91157-4. [DOI] [PubMed] [Google Scholar]
- Mahler R. J., Szabo O. Metabolic effects of insulin on rat kidney after inhibiting degradation of the hormone. Endocrinology. 1968 Dec;83(6):1166–1172. doi: 10.1210/endo-83-6-1166. [DOI] [PubMed] [Google Scholar]
- Maude D. L., Handelsman D. G., Babu M., Gordon E. E. Handling of insulin by the isolated perfused rat kidney. Am J Physiol. 1981 Apr;240(4):F288–F294. doi: 10.1152/ajprenal.1981.240.4.F288. [DOI] [PubMed] [Google Scholar]
- Meezan E., Freychet P. Rat renal glomeruli and tubules have specific insulin receptors of differing affinity. Mol Pharmacol. 1979 Nov;16(3):1095–1100. [PubMed] [Google Scholar]
- Michell A. R., Lindheimer M. D., Katz A. I. The optimum pH of renal adenosine triphosphatase and its variation with the type of ATP. Enzyme. 1977;22(5):341–347. doi: 10.1159/000458815. [DOI] [PubMed] [Google Scholar]
- Mohr H., Hesch R. D. Different handling of parathyrin by basal-lateral and brush-border membranes of the bovine kidney cortex. Biochem J. 1980 Jun 15;188(3):649–656. doi: 10.1042/bj1880649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris B. J. Specific radioactivity of radioimmunoassay tracer determined by self-displacement: a re-evaluation. Clin Chim Acta. 1976 Nov 15;73(1):213–216. doi: 10.1016/0009-8981(76)90328-4. [DOI] [PubMed] [Google Scholar]
- Nizet A., Lefebvre P., Crabbé J. Control by insulin of sodium potassium and water excretion by the isolated dog kidney. Pflugers Arch. 1971;323(1):11–20. doi: 10.1007/BF00586561. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Jones J., Kitabchi A. E. Insulin extraction from the renal peritubular circulation in the chicken. Endocrinology. 1977 Dec;101(6):1828–1833. doi: 10.1210/endo-101-6-1828. [DOI] [PubMed] [Google Scholar]
- Rabkin R., Kitabchi A. E. Factors influencing the handling of insulin by the isolated rat kidney. J Clin Invest. 1978 Jul;62(1):169–175. doi: 10.1172/JCI109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin R., Share L., Payne P. A., Young J., Crofton J. The handling of immunoreactive vasopressin by the isolated perfused rat kidney. J Clin Invest. 1979 Jan;63(1):6–13. doi: 10.1172/JCI109279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin R., Simon N. M., Steiner S., Colwell J. A. Effect of renal disease on renal uptake and excretion of insulin in man. N Engl J Med. 1970 Jan 22;282(4):182–187. doi: 10.1056/NEJM197001222820402. [DOI] [PubMed] [Google Scholar]
- Selhub J., Rosenberg I. H. Demonstration of high-affinity folate binding activity associated with the brush border membranes of rat kidney. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3090–3093. doi: 10.1073/pnas.75.7.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Zaharko D. S., Beck L. V., Blankenbaker R. Role of the kidney in the disposal of radioiodinated and nonradioiodinated insulin in dogs. Diabetes. 1966 Sep;15(9):680–685. doi: 10.2337/diab.15.9.680. [DOI] [PubMed] [Google Scholar]
- de Meyts P., Roth J., Neville D. M., Jr, Gavin J. R., 3rd, Lesniak M. A. Insulin interactions with its receptors: experimental evidence for negative cooperativity. Biochem Biophys Res Commun. 1973 Nov 1;55(1):154–161. doi: 10.1016/s0006-291x(73)80072-5. [DOI] [PubMed] [Google Scholar]


