Abstract
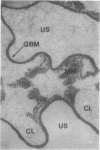
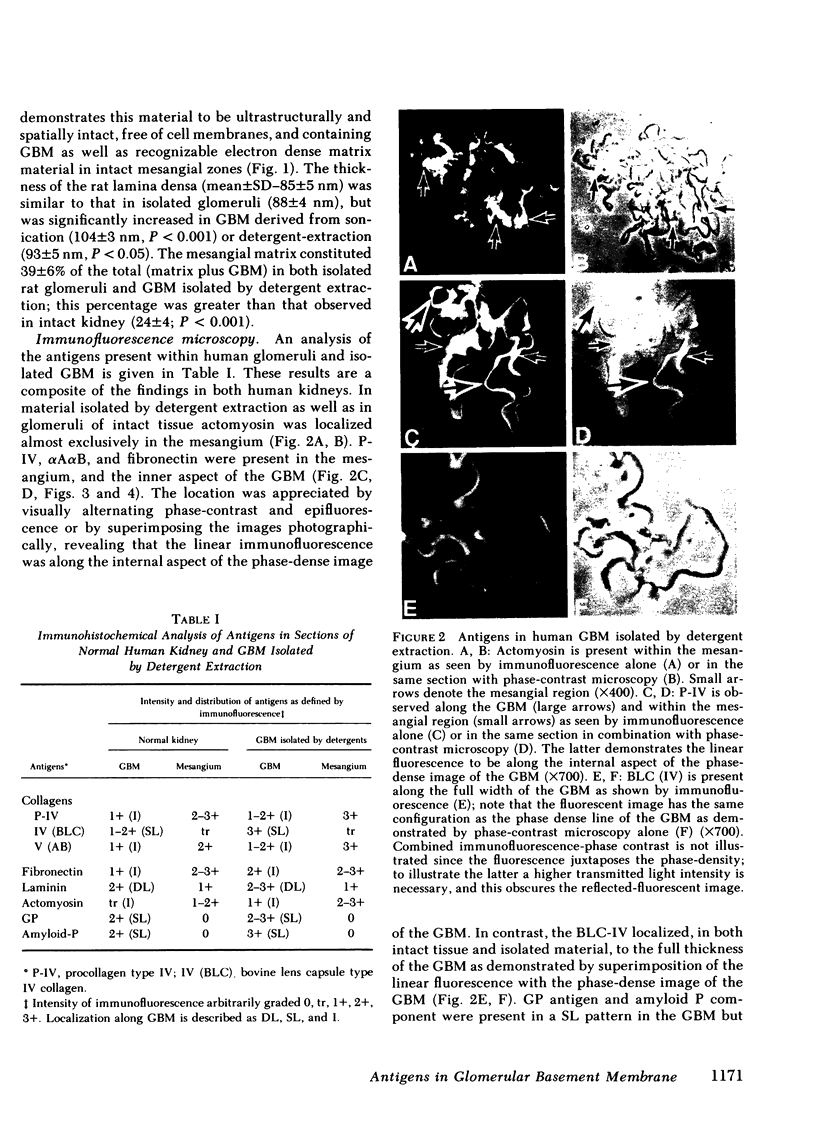
To define the characteristics of isolated glomerular basement membrane (GBM), immunohistochemical and morphometric analyses have been carried out on rat and human tissues. Site-specific arrays of antigens were identified in detergent-isolated GBM in a distribution similar to that observed in intact kidney. In the human, fibronectin, procollagen IV, and collagen V were observed along the internal aspect of GBM continuous with antigenic sites in the mesangium. Another array of antigens was identified in the GBM but not within the mesangium--Goodpasture's antigen, bovine lens capsule type IV collagen, and amyloid P component. In addition, sites reactive with rabbit antiserum to laminin were present on both sides of the lamina densa as well as within the mesangial region. Actomyosin, a presumed mesangial cell antigen persisted in the mesangium of isolated GBM. Mesangial matrix was identified in detergent-isolated GBM in an amount equivalent to that present in intact glomeruli. Sonicated GBM contained the same antigens but it was not possible to quantitate the amount of mesangial material by immunofluorescence or morphometric analysis. The thickness of the lamina densa was greater in sonicated and detergent-treated rat GBM preparations than in native rat kidney. These studies demonstrated that isolated GBM is heterogeneous with respect to its antigenic constituents and in addition contains mesangial matrix, which is morphologically and immunohistochemically distinct from peripheral GBM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisswenger P. G., Spiro R. G. Human glomerular basement membrane: chemical alteration in diabetes mellitus. Science. 1970 May 1;168(3931):596–598. doi: 10.1126/science.168.3931.596. [DOI] [PubMed] [Google Scholar]
- Benson M. D., Skinner M. Amino terminal sequence of amyloid P-component isolated from serum. J Lab Clin Med. 1978 Jul;92(1):78–83. [PubMed] [Google Scholar]
- Blau E., Michael A. F. Rat glomerular basement membrane composition and metabolism in aminonucleoside nephrosis. J Lab Clin Med. 1971 Jan;77(1):97–109. [PubMed] [Google Scholar]
- Cohen M. P., Surma M. Renal glomerular basement membrane. In vivo biosynthesis and turnover in normal rats. J Biol Chem. 1980 Mar 10;255(5):1767–1770. [PubMed] [Google Scholar]
- Courtoy P. J., Kanwar Y. S., Hynes R. O., Farquhar M. G. Fibronectin localization in the rat glomerulus. J Cell Biol. 1980 Dec;87(3 Pt 1):691–696. doi: 10.1083/jcb.87.3.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouch E., Sage H., Bornstein P. Structural basis for apparent heterogeneity of collagens in human basement membranes: type IV procollagen contains two distinct chains. Proc Natl Acad Sci U S A. 1980 Feb;77(2):745–749. doi: 10.1073/pnas.77.2.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck R. F., Lockwood C. M., Kershaw M., McHugh N., Duance V. C., Baltz M. L., Pepys M. B. Amyloid P-component is a constituent of normal human glomerular basement membrane. J Exp Med. 1980 Nov 1;152(5):1162–1174. doi: 10.1084/jem.152.5.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish A. J., Carmody K. M., Michael A. F. Spatial orientation and distribution of antigens within human glomerular basement membrane. J Lab Clin Med. 1979 Sep;94(3):447–457. [PubMed] [Google Scholar]
- Gunson D. E., Kefalides N. A. The use of the radioimmunoassay in the characterization of antibodies to basement membrane collagen. Immunology. 1976 Oct;31(4):563–569. [PMC free article] [PubMed] [Google Scholar]
- Jensen E. B., Gundersen H. J., Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979 Jan;115(1):19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Biochemical properties of human glomerular basement membrane in normal and diabetic kidneys. J Clin Invest. 1974 Feb;53(2):403–407. doi: 10.1172/JCI107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Isolation of a collagen from basement membranes containing three identical - chains. Biochem Biophys Res Commun. 1971 Oct 1;45(1):226–234. doi: 10.1016/0006-291x(71)90073-8. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Roll F. J., Furthmayr H., Foidart J. M. Ultrastructural localization of fibronectin and laminin in the basement membranes of the murine kidney. J Cell Biol. 1980 Aug;86(2):682–687. doi: 10.1083/jcb.86.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meezan E., Hjelle J. T., Brendel K., Carlson E. C. A simple, versatile, nondisruptive method for the isolation of morphologically and chemically pure basement membranes from several tissues. Life Sci. 1975 Dec 1;17(11):1721–1732. doi: 10.1016/0024-3205(75)90119-8. [DOI] [PubMed] [Google Scholar]
- Michael A. F., Brown D. M. Increased concentration of albumin in kidney basement membranes in diabetes mellitus. Diabetes. 1981 Oct;30(10):843–846. doi: 10.2337/diab.30.10.843. [DOI] [PubMed] [Google Scholar]
- Miller K., Michael A. F. Immunopathology of renal extracellular membranes in diabetes mellitus. Specificity of tubular basement-membrane immunofluorescence. Diabetes. 1976 Aug;25(8):701–708. doi: 10.2337/diab.25.8.701. [DOI] [PubMed] [Google Scholar]
- Pettersson E. E., Colvin R. B. Cold-insoluble globulin (fibronectin, LETS protein) in normal and diseased human glomeruli: papain-sensitive attachment to normal glomeruli and deposition in crescents. Clin Immunol Immunopathol. 1978 Dec;11(4):425–436. doi: 10.1016/0090-1229(78)90170-8. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Berg R., Martin G. R., Foidart J. M., Robey P. G. Enzyme-linked immunoassay (ELISA) for connective tissue components. Anal Biochem. 1980 May 1;104(1):205–214. doi: 10.1016/0003-2697(80)90300-0. [DOI] [PubMed] [Google Scholar]
- Roll F. J., Madri J. A., Albert J., Furthmayr H. Codistribution of collagen types IV and AB2 in basement membranes and mesangium of the kidney. an immunoferritin study of ultrathin frozen sections. J Cell Biol. 1980 Jun;85(3):597–616. doi: 10.1083/jcb.85.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T., Spiro R. G. Studies on the subunit composition of the renal glomerular basement membrane. J Biol Chem. 1976 Jul 10;251(13):4062–4070. [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J., Matas A. J., Michael A. F. The immunohistopathology of glomerular antigens. II. The glomerular basement membrane, actomyosin, and fibroblast surface antigens in normal, diseased, and transplanted human kidneys. Am J Pathol. 1978 Jan;90(1):71–88. [PMC free article] [PubMed] [Google Scholar]
- Scheinman J. I., Fish A. J., Michael A. F. The immunohistopathology of glomerular antigens. The glomerular basement membrane, collagen, and actomyosin antigens in normal and diseased kidneys. J Clin Invest. 1974 Nov;54(5):1144–1154. doi: 10.1172/JCI107858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinman J. I., Foidart J. M., Gehron-Robey P., Fish A. J., Michael A. F. The immunohistology of glomerular antigens. IV. Laminin, a defined noncollagen basement membrane glycoprotein. Clin Immunol Immunopathol. 1980 Feb;15(2):175–189. doi: 10.1016/0090-1229(80)90029-x. [DOI] [PubMed] [Google Scholar]
- Scheinman J. I., Foidart J. M., Michael A. F. The immunohistology of glomerular antigens. V. The collagenous antigens of the glomerulus. Lab Invest. 1980 Oct;43(4):373–381. [PubMed] [Google Scholar]
- Scheinman J. I., Steffes M. W., Brown D. M., Mauer S. M. The immunohistopathology of glomerular antigens. III. Increased mesangial actomyosin in experimental diabetes in the rat. Diabetes. 1978 Jun;27(6):632–637. doi: 10.2337/diab.27.6.632. [DOI] [PubMed] [Google Scholar]
- Skinner M., Vaitukaitis J. L., Cohen A. S., Benson M. D. Serum amyloid P-component levels in amyloidosis, connective tissue diseases, infection, and malignancy as compared to normal serum. J Lab Clin Med. 1979 Oct;94(4):633–638. [PubMed] [Google Scholar]
- Steffes M. W., Brown D. M., Basgen J. M., Matas A. J., Mauer S. M. Glomerular basement membrane thickness following islet transplantation in the diabetic rat. Lab Invest. 1979 Aug;41(2):116–118. [PubMed] [Google Scholar]
- Steffes M. W., Brown D. M., Basgen J. M., Mauer S. M. Amelioration of mesangial volume and surface alterations following islet transplantation in diabetic rats. Diabetes. 1980 Jul;29(7):509–515. doi: 10.2337/diab.29.7.509. [DOI] [PubMed] [Google Scholar]
- Stenman S., Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978 Apr 1;147(4):1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West T. W., Fox J. W., Jodlowski M., Freytag J. W., Hudson B. G. Bovine glomerular basement membrane. Properties of the collagenous domain. J Biol Chem. 1980 Nov 10;255(21):10451–10459. [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane. Preparation and composition. Biochemistry. 1970 Sep 15;9(19):3837–3846. doi: 10.1021/bi00821a025. [DOI] [PubMed] [Google Scholar]
- Westberg N. G., Michael A. F. Human glomerular basement membrane: chemical composition in diabetes mellitus. Acta Med Scand. 1973 Jul-Aug;1-2(1):39–47. doi: 10.1111/j.0954-6820.1973.tb19411.x. [DOI] [PubMed] [Google Scholar]