Abstract
Background
Subsyndromal symptoms of depression (SSD) in patients with schizophrenia are common and clinically important. While treatment of depression in major depressive disorder may partially ameliorate cognitive deficits, the cognitive effects of antidepressant medications in patients with schizophrenia or schizoaffective disorder and SSD are unknown.
Methods
The goal of this study was to assess the impact of SSD and their treatment on cognition in participants with schizophrenia or schizoaffective disorder aged ≥40 years. Participants were randomly assigned to a flexible dose treatment with citalopram or placebo augmentation of their current medication for 12 weeks. An ANCOVA compared improvement in the cognitive composite scores, and a linear model determined the moderation of cognition on treatment effects based on the Hamilton Depression Rating Scale and the Calgary Depression Rating Scale scores between treatment groups.
Results
There were no differences between the citalopram and placebo groups in changes in cognition. Baseline cognitive status did not moderate antidepressant treatment response.
Conclusions
Although there are other cogent reasons why SSD in schizophrenia warrant direct intervention, treatment does not substantially affect the level of cognitive functioning. Given the effects of cognitive deficits associated with schizophrenia on functional disability, there remains an ongoing need to identify effective means of directly ameliorating them.
Key Words: Schizophrenia, Citalopram, Cognition, Subsyndromal depression
Introduction
Cognitive impairment is a core symptom of schizophrenia and a prime determinant of the functional disability associated with this disorder [1]. The severity of cognitive deficits in individual patients with schizophrenia tends to be very stable and antipsychotic medications have minimal positive or deleterious influences on cognition. In contrast, the cognitive deficits in patients with mood disorders appears to be more state-related as they tend to be at least partially responsive to antidepressant medication [2,3,4].
As previously reported [5] we found augmentation of antipsychotic treatment with an antidepressant (citalopram) results in a significant reduction of the severity of subsyndromal depressive symptoms (SSD) among middle-aged and older patients with schizophrenia or schizoaffective disorder. We found no significant effects of citalopram on the Folstien Mini-Mental Status Examinations (MMSE) [6], but the MMSE is a course measure designed to stage the severity of dementia; it may not be sensitive to the less severe cognitive deficits that tend to characterize schizophrenia [1]. In this report, we examine the hypothesis that treatment augmentation with citalopram among patients with schizophrenia/schizoaffective disorder and SSD will improve cognitive functioning as measured by a more comprehensive and sensitive battery of neuropsychological tests than provided by the MMSE alone.
Methods
Participants
The 198 participants had DSM-IV-TR schizophrenia or schizoaffective disorder and SSD (defined by having 2–4 of the 9 DSM-IV-TR symptoms of a major depressive episode present most of the time for at least 2 weeks as well as a Hamilton Depression Rating Scale (HDRS 17-item score >8) [7]. Data were collected as part of an NIMH-funded 12-week double-blind randomized placebo-controlled trial of augmentation of a primary antipsychotic medication with citalopram to decrease depressive symptoms in schizophrenia/schizoaffective disorder and SSD [5]. Participants were recruited through the University of California, San Diego (UCSD) Advanced Center for Innovation in Services and Interventions Research on Late-Life Psychoses and the VA Cincinnati Health Care System, Cincinnati, Ohio. The institutional review boards for these facilities reviewed and approved the study protocol, and all participants provided written informed consent prior to enrollment. Between February 2001 and January 2006, 106 participants were recruited from the San Diego site and 92 from the Cincinnati site. For full inclusion and exclusion criteria please refer to Zisook et al. [5]. Briefly, the study included people with schizophrenia or schizoaffective disorder and SSD who were aged ≥40 years and who did not have any other Axis I disorder within 2 months of enrollment. All participants were outpatients, competent to consent and diagnoses were established via the Structured Clinical Interview for DSM-IV-TR (SCID) [8] administered by the clinical research coordinator.
Study Treatments
Participants were randomly assigned in this double-blind trial to either citalopram (20 mg/day) or placebo augmentation of their current stable antipsychotic treatment. The study dose of citalopram was allowed to be increased or decreased according to clinical response and/or side effects, within the range of 10– 40 mg/day at the discretion of the blinded study physician. Potential participants currently on any medication to treat depression who consented were tapered and discontinued from this medication for at least 4 weeks before entering the trial. Some participants (n = 8, 4%) were allowed to continue on a stable low-dose antidepressant medication, only if prescribed for the treatment of insomnia or chronic pain.
Measures
Descriptive Information. Demographic and clinical history information was collected via self-report and/or review of available records.
Severity of Psychopathology. Subjects were assessed with the 17-item HDRS [7] and the Calgary Depression Rating Scale (CDRS) [9], as well as the Positive and Negative Syndrome Scale (PANSS) for schizophrenia [10].
Cognitive Functioning. Neurocognitive functioning was evaluated with the following test battery at baseline and at 12 weeks: MMSE [6], Digit Span Distractibility Test [11], letter and category fluency [12], the Trail-Making Test (part A) [13,14], the Continuous Performance Test – identical pairs [15], Digit-Symbol-Coding [16], Symbol Search [16] and Letter-Number Sequencing [16]. This battery was selected to provide a general assessment of cognitive abilities known to be affected by schizophrenia and/or depression. Raw scores were converted to standardized z-scores using the baseline means and SDs for each measure (scaled such that higher scores represent better functioning), and the mean z-score across measures for each subject was used as an index of overall cognitive functioning.
Statistical Analysis
All variables were examined for violation of assumptions required for parametric analyses. No site-by-group interactions were significant, so this term was dropped from any further analyses. Two-way analyses of variance (ANOVAs) were used to evaluate differences in clinical and demographic characteristics. Cochran-Mantel-Haenszel tests compared the characteristics across treatments. Differences between treatment groups on the cognitive composite score were compared using analysis of covariance (ANCOVA). The main dependent variable in the ANCOVA model was the final cognitive composite score at the endpoint of the study, while the independent variables were baseline-symptom severity and treatment group. To determine whether cognition moderated the effects of depression treatment, we used linear modeling that included endpoint CDRS or HDRS as the dependent variables, and treatment, baseline cognition, treatment by baseline cognition, baseline CDRS and site as independent variables [17]. Significance was defined as p < 0.05 (2-tailed).
Results
Ninety-four of the participants were randomized to the placebo, 104 were randomized to citalopram. The average dose of citalopram given throughout the trial was 28.9 mg (SD = 9.8, range 10–40) per day. The demographic and clinical characteristics among the 2 groups have been reported elsewhere [5], but, as briefly summarized in table 1, other than a higher proportion of widowed patients than the placebo group, there were no significant demographic or clinical differences between groups. As reported previously [5], citalopram augmentation resulted in significantly more improvement in depressive symptoms relative to the changes seen in the placebo group over the 12-week course of the study.
Table 1.
Demographic and baseline clinical characteristics of participants with SSD receiving antipsychotic augmentation with citalo-pram or placebo (n = 198)
| Characteristic | Placebo (n = 94) mean ± SD | Citalopram (n = 104) mean ± SD | F (d.f.) | p |
|---|---|---|---|---|
| Age, years | 51.7±6.3 | 53.1±7.7 | 2.032 (1,192) | 0.156 |
| Age at onset of first psychotic episode, years | 27.3±10.3 | 28.4±10.7 | 0.484 (1,167) | 0.488 |
| Education level, years | 11.8±2.3 | 12.1±2.1 | 0.938 (1,194) | 0.334 |
| HDRS | 13.4±4.1 | 13.6±4.4 | 0.057 (1,194) | 0.812 |
| CDRS | 7.0±3.1 | 6.5±3.2 | 1.957 (1,194) | 0.163 |
| PANSS | ||||
| Positive | 16.43±4.60 | 15.26±6.41 | 2.492 (1,194) | 0.116 |
| Negative | 16.17±5.45 | 15.40±4.44 | 3.870 (1,193) | 0.510 |
| n (%) | n (%) | χ2 (d.f) | p | |
| Female gender | 20 (21.3) | 23 (22.1) | 0.0149 (1) | 0.903 |
| Race | 3.029 (3) | 0.387 | ||
| White | 54 (57.4) | 54 (51.9) | ||
| Black | 26 (27.7) | 40 (38.5) | ||
| Hispanic | 9 (9.6) | 5 (4.8) | ||
| Other | 5 (5.3) | 5 (4.8) | ||
| Marital status | 15.169 (3) | 0.002 | ||
| Never (single) | 48 (32) | 32 (30.8) | ||
| Married or cohabitating | 10 (18) | 18 (17.3) | ||
| Divorced or separated | 35 (41) | 41 (39.4) | ||
| Widowed | 1 (13) | 13 (12.5) | ||
| Age <18 years at onset of first psychotic episode | 12 (9) | 9 (10.1) | 0.409 (1) | 0.522 |
| Ever attempted suicide | 41 (48) | 48 (47.5) | 1.891 (2) | 0.389 |
| Diagnosed schizoaffective | 33 (35.1) | 48 (46.2) | 1.521 (1) | 0.218 |
| Medications | 0.036 (2) | 0.982 | ||
| First-generation antipsychotics | 9 (9.9) | 10 (10.2) | ||
| Second-generation antipsychotics | 65 (71.4) | 69 (70.4) | ||
| Both first- and second-generation antipsychotics | 17 (18.7) | 19 (19.4) | ||
| Anticholinergics/antihistamines | 26 (28.6) | 30 (30.6) | 0.035 (1) | 0.853 |
| Antidepressant tapered off from ≥4 weeks prior to study | 15 (16.0) | 19 (18.3) | 0.386 (1) | 0.534 |
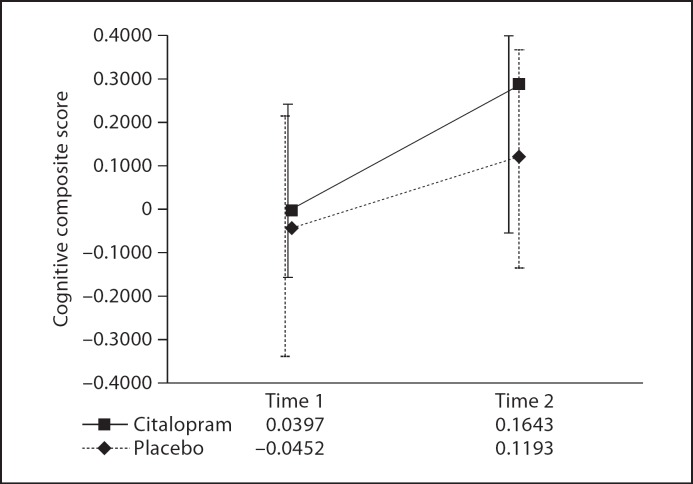
Means and SDs for the cognitive composite z-scores as well as for the 10 component neurocognitive measures are shown in table 2. The cognitive composite score showed good internal consistency (Cronbach alpha = 0.843). As illustrated in figure 1, from the baseline observation to the last observation at 12 weeks, there were no differences between the 2 treatments in terms of cognitive functioning – as measured by the cognitive composite [F(1,133) = 0.072, p = 0.789] for the whole sample, or when the participants with schizoaffective disorder were excluded from the analysis [F(1,85) = 0.058, p = 0.811].
Table 2.
Neurocognitive performance of participants with SSD receiving antipsychotic augmentation with either citalopram (n = 104) or placebo (n = 94)
| Characteristic | Placebo, mean (SD) |
Citalopram, mean (SD) |
||
|---|---|---|---|---|
| time 1 | time 2 | time 1 | time 2 | |
| Animals (total correct) | 14.6 (5.0) | 15.1 (4.5) | 14.7 (4.7) | 14.8 (4.7) |
| Continuous Performance Task (d’) | 1.0 (0.9) | 0.9 (0.9) | 1.0 (0.7) | 0.9 (0.7) |
| MMSE (total score) | 26.7 (2.8) | 27.0 (2.9) | 27.2 (2.3) | 27.7 (2.1) |
| Digit Span nondistracted (% correct) | 67.7 (26.0) | 73.8 (22.7) | 69.8 (20.3) | 69.0 (20.8) |
| Digit Span distracted (% correct) | 66.2 (24.2) | 71.9 (20.6) | 71.2 (21.0) | 73.1 (20.1) |
| Controlled Oral Word Association Test – FAS (total correct) | 27.2 (11.6) | 26.8 (11.3) | 28.2 (10.5) | 28.3 (15.4) |
| Letter-number sequencing (scaled score) | 6.8 (3.0) | 7.0 (3.1) | 7.5 (2.6) | 7.4 (2.7) |
| Symbol search (scaled score) | 6.2 (3.0) | 6.3 (3.0) | 6.5 (2.7) | 6.8 (2.6) |
| Digit-symbol (scaled score) | 5.5 (2.1) | 5.7 (2.2) | 5.9 (2.0) | 6.0 (1.8) |
| Trail-Making Test – part A (t-score) | 37.9 (10.9) | 40.2 (10.6) | 40.3 (9.5) | 40.6 (10.3) |
| Cognitive composite score (z-score)* | −0.05 (1.11) | 0.12 (1.05) | 0.04 (0.92) | 0.16 (0.96) |
| Cognitive composite score (z-score)** | −0.07 (1.20) | 0.36 (1.13) | 0.04 (0.94) | 0.13 (0.04) |
* Total sample [F(1,133) = 0.072, p = 0.789].
** Schizophrenia participants only [F(1,85) = 0.058, p = 0.811].
Fig. 1.
Mean cognitive composite z-scores for the placebo (n = 94) and citalopram (n = 104) groups from time 1 to time 2.
There was no relationship between the scores on the HDRS or the CDRS and the cognitive composite at baseline (r = −0.01, p > 0.05 and r = 0.12, p > 0.05, respectively). Furthermore, the linear model evaluating moderation indicated that the interaction between cognition and treatment group did not detect differences between scores on the HDRS [F(1,145) = 0.456, p = 0.501] or the CDRS [F(1,145) = 0.127, p = 0.722] from the baseline to the 12-week assessments. Thus, we did not detect any significant moderating effect of cognition on change in depression severity. In addition, no improvements in cognition were found in those who responded to treatment versus those who did not, based on either the HDRS [F(1,134) = 0.497, p = 0.609] or the CDRS [F(1,134) = 0.633, p = 0.532].
Discussion
Contrary to our hypothesis, citalopram augmentation to treat SSD among patients with schizophrenia did not yield significant improvements in cognitive function, even though, as described elsewhere [5], citalopram augmentation did result in a significant improvement in depressive and negative symptoms relative to the placebo. The lack of observed cognitive effects of treating SSD in schizophrenia may speak to the general tenacity of cognitive deficits in schizophrenia [1], but may also reflect the relatively moderate-to-mild severity of depressive symptoms in this sample. As this was a study of SSD in schizophrenia, we did not include patients with major depression; it is possible that cognitive deficits among patients with more severe depressive symptoms would be more responsive to antidepressant treatment.
Another potential limitation of the study is the 12-week timeframe. Although 12 weeks is longer than most studies of antidepressant treatment, it is possible that cognitive improvements lag behind improvements in affective symptoms. Other limitations included the restricted age range, the absence of first-break or inpatient participants, and the fact that participants were reported to be on several different antipsychotics (see Zisook et al. [5]). It is possible that schizophrenia and schizoaffective disorder are more related to bipolar than to unipolar depression [18,19,20]. Thus, in future studies it would be useful to consider whether mood stabilizers, which have been found to be effective augmentation agents for patients with schizophrenia [21], might provide cognitive improvement in schizophrenic patients with SSD.
Although citalopram did not positively impact cognition, it is important to note that we did not find any detrimental effects either. Given the positive effects of citalopram augmentation on SSD, and the importance of the latter in terms of patient-suffering and quality of life, it remains important for clinicians to be alert to depressive symptoms in schizophrenia patients and to consider direct treatment of these symptoms as well as addressing the psychotic symptoms of the disorder [5]. In addition, as cognitive impairment has been consistently shown to be the most robust predictor of functional disability in schizophrenia [22], there is a continued need to find a direct means of effectively treating attendant cognitive deficits [23,24]. To resolve the symptoms of psychosis or depressive symptoms that occur simultaneously are only the initial steps in trying to improve the overall quality of life and functional ability of schizophrenia patients. The future of treatment must prioritize enhancing cognition in order to fully realize the potential recovery of people with schizophrenia.
Acknowledgements
This work was supported by RO-1 MH 063931 Citalopram Augmentation of Older Patients with Schizophrenia (S.Z.), NIMH grants MH66248, MH19934, P30 MH080002 and the Department of Veterans Affairs. J.K. was supported by R01 MH6398, the VISN 4 MIRECC and a VISN 4 CPPF award.
References
- 1.Palmer BW, Dawes SE, Heaton RK. What do we know about neuropsychological aspects of schizophrenia? Neuropsychol Rev. 2009;19:365–384. doi: 10.1007/s11065-009-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bench CJ, Frackowiak RS, Dolan RJ. Changes in regional cerebral blood flow on recovery from depression. Psychol Med. 1995;25:247–261. doi: 10.1017/s0033291700036151. [DOI] [PubMed] [Google Scholar]
- 3.Mayberg HS, Brannan SK, Tekell JL, Silva JA, Mahurin RK, McGinnis S, Jerabek PA. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry. 2000;48:830–843. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- 4.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, Steffens DC, Doraiswamy PM. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60:58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Zisook S, Kasckow JW, Golshan S, Fellows I, Solorzano E, Lehman D, Mohamed S, Jeste DV. Citalopram augmentation for subsyndromal symptoms of depression in middle-aged and older outpatients with schizophrenia and schizoaffective disorder: a randomized controlled trial. J Clin Psychiatry. 2009;70:562–571. doi: 10.4088/jcp.08m04261. [DOI] [PubMed] [Google Scholar]
- 6.Folstein MF, Folstein SE, McHugh PR. ‘Mini-Mental State’: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 7.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 8.First MB, Spitzer RL, Gibbon M. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition with psychotic screen (scid-i/p w/psy screen) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 9.Addington D, Addington J, Maticka-Tyndale E. Assessing depression in schizophrenia: the Calgary Depression Scale. Br J Psychiatry suppl. 1993;39–44 [PubMed] [Google Scholar]
- 10.Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 11.Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- 12.Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: demographic corrections for age, education, and ethnicity. Assessment. 1999;6:147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- 13.Partington JE, Leiter RG. Partington's pathway test. Psychol Serv Cent J. 1949;1:9–20. [Google Scholar]
- 14.Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. ed 2. Tucson: Neuropsychology Press; 1993. [Google Scholar]
- 15.Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler D. Wechsler Adult Intelligence Scale – ed 3 (WAIS-III). Administration and scoring manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 17.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 18.Carroll LS, Owen MJ. Genetic overlap between autism, schizophrenia and bipolar disorder. Genome Med. 2009;1:102. doi: 10.1186/gm102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potash JB, Bienvenu OJ. Neuropsychiatric disorders: shared genetics of bipolar disorder and schizophrenia. Nat Rev Neurol. 2009;5:299–300. doi: 10.1038/nrneurol.2009.71. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein P, Yip BH, Bjork C, Pawitan Y, Cannon TD, Sullivan PF, Hultman CM. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. Lancet. 2009;373:234–239. doi: 10.1016/S0140-6736(09)60072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tiihonen J, Wahlbeck K, Kiviniemi V. The efficacy of lamotrigine in clozapine-resistant schizophrenia: a systematic review and meta-analysis. Schizophr Res. 2009;109:10–14. doi: 10.1016/j.schres.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 22.Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, Kumar A. Executive dysfunction and memory in older patients with major and minor depression. Arch Clin Neuropsychol. 2006;21:669–676. doi: 10.1016/j.acn.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Harvey PD. Pharmacological cognitive enhancement in schizophrenia. Neuropsychol Rev. 2009;19:324–335. doi: 10.1007/s11065-009-9103-4. [DOI] [PubMed] [Google Scholar]
- 24.Carpenter WT, Koenig JI. The evolution of drug development in schizophrenia: past issues and future opportunities. Neuropsychopharmacology. 2008;33:2061–2079. doi: 10.1038/sj.npp.1301639. [DOI] [PMC free article] [PubMed] [Google Scholar]