Abstract
Gene therapy products for the treatment of genetic diseases are currently in clinical trials, and one of these, an adeno-associated viral (AAV) product, has recently been licensed. AAV vectors have achieved positive results in a number of clinical and preclinical settings, including hematologic disorders such as the hemophilias, Gaucher disease, hemochromatosis, and the porphyrias. Because AAV vectors are administered directly to the patient, the likelihood of a host immune response is high, as shown by human studies. Preexisting and/or recall responses to the wild-type virus from which the vector is engineered, or to the transgene product itself, can interfere with therapeutic efficacy if not identified and managed optimally. Small-scale clinical studies have enabled investigators to dissect the immune responses to the AAV vector capsid and to the transgene product, and to develop strategies to manage these responses to achieve long-term expression of the therapeutic gene. However, a comprehensive understanding of the determinants of immunogenicity of AAV vectors, and of potential associated toxicities, is still lacking. Careful immunosurveillance conducted as part of ongoing clinical studies will provide the basis for understanding the intricacies of the immune response in AAV-mediated gene transfer, facilitating safe and effective therapies for genetic diseases.
Introduction
Recent studies document therapeutic successes for several genetic diseases using gene transfer vectors,1-7 and an adeno-associated viral (AAV) vector product for one of these, lipoprotein lipase deficiency, has now been licensed in Europe.8 Thus, vector-mediated gene therapies are progressing from investigational agents to approved products. A number of these are being developed for hematologic disorders, so that it is critical for hematologists to understand the risks and benefits of this new class of therapeutics. The recent success of a single infusion of an AAV vector leading to >2 years of therapeutic levels of factor IX (F.IX) in men with severe hemophilia B4 illustrates clearly that gene therapy has the potential to improve outcomes and simplify management of serious hematologic disorders.
At a basic level, all gene transfer strategies are characterized by 3 critical elements, the gene to be transferred, the target tissue into which the gene will be introduced, and the vector (gene delivery vehicle) used to facilitate entry of the gene to the target tissue (Figure 1A).9 The gene is the active agent of the therapeutic, but the vector, in most cases derived from a virus, is also a critical determinant of therapeutic success and of the toxicity profile of the product. In order to achieve long-term expression of the donated gene, 1 of 2 strategies can be employed: either using an integrating vector (typically retroviral or lentiviral) to introduce the gene ex vivo into a stem cell, which allows the donated gene to be passed to every daughter cell, or introducing the gene into a long-lived postmitotic cell in vivo, in which case, as long as the donated DNA can be stabilized in the cell (integrated or episomal), long-term expression will result. This review article will focus on the in vivo administration of AAV vectors, which has been successful in several settings.10,11
Figure 1.
Structure and tropism of wild-type AAV and of recombinant AAV vectors. (A) Gene therapy vectors are complex therapeutics requiring proper assembly of both DNA and protein components to generate the final product. Wild-type AAVs are small nonenveloped viruses, 20 to 25 nm in diameter, with a single-stranded DNA genome of ∼4.7 kb encoding 2 sets of genes, the rep genes required for replication and virion assembly and the cap genes that encode the 3 proteins that assemble to form the 60-mer viral capsid (upper bar). AAV vectors are composed of an outer protein shell, an exact or close replica of the AAV viral capsid, carrying a therapeutic gene of interest under the control of an appropriate promoter (lower bar). The vector is 74% protein by molecular weight. Maximum packaging capacity is ∼5 kb DNA, a limitation of AAV as a gene delivery vehicle. (B) Dozens of different naturally occurring AAV capsids, as well as genetically engineered ones, have been isolated for study, from humans and from other species. The capsid sequences are highly conserved, from 60% to >99%, but studies with naturally occurring serotypes56 and purpose-engineered capsids57-59 have shown that even small differences in capsid sequence may affect tissue tropism of a vector and can be exploited to improve therapeutic outcomes. Figure 1A reprinted from Xie et al9 with permission. Copyright 2000 National Academy of Sciences, USA. Figure 1B reprinted from Arrunda and Xiao60 with permission. Copyright 2006 John Wiley and Sons.
As one would predict, the in vivo administration of a viral vector necessitates grappling at close quarters with the human immune response to foreign antigens. Immune responses may be directed against the AAV vector component or the transgene product, or both. Animal models predicted many aspects of the human immune response to the transgene product but largely failed to predict responses to AAV capsid. Delineation of these responses, and crafting of strategies to circumvent or manage them, is critical to achieving clinical success with AAV vectors. What follows is a review of data from both clinical and preclinical studies, which, viewed together, allow the assembly of a mosaic that has led to working strategies for identifying and managing host immune responses in AAV-mediated gene transfer. There are clearly areas where the image is blurred, however, and issues that require further investigation in order to extend clinical applications will be highlighted.
AAV vectors have been used to transduce cells in the retina,5-7,12 the central nervous system,13,14 the liver,4,15 and skeletal16-18 and cardiac muscle.19 The review leans more heavily on data from studies of greatest relevance to hematologists, but other studies are also included to allow the current state of understanding to emerge.
AAV viruses and recombinant AAV virions
AAV vectors are engineered from a naturally occurring parvovirus, adeno-associated virus, which was first isolated in 1966 in studies of pathogens in the respiratory tract.20 AAV is not autonomously replicating but is rather dependent on a helper virus such as adenovirus or herpes simplex for replication. This initial exposure during a coinfection may account for the generation of a memory T-cell response to AAV, with the autonomous helper virus promoting the immune response. AAV itself is not associated with any known illnesses in the human population. Serological studies document that most people are exposed to wild-type AAV during childhood and adolescence; prevalence rates for antibody titers exceed 60% among adults.21
Recombinant AAV vectors are fully deleted of viral coding sequences, retaining only the viral inverted terminal repeats, which serve as signals for packaging of the genome into the viral capsid.22 In 1996, 2 groups reported that recombinant AAV vectors could direct long-term expression of a transgene after introduction into skeletal muscle in immunocompetent mice.23,24 The first trials of AAV vectors in human subjects were in the setting of cystic fibrosis25; the first trial in hemophilia B, in which vector was injected into skeletal muscle, began in 1999.26
A key concept in the study of immune responses triggered by in vivo gene transfer with AAV is that the viral vector, a recombinant molecule consisting of a eukaryotic transgene and a viral capsid, is not a virus and is in fact incapable of directing the synthesis of any viral proteins. However, the recombinant capsid is a close mimic, or in some cases an exact replica, of a true viral capsid, and thus the immune responses to the vector will be influenced by the prior exposure of the human immune system to the wild-type virus from which the vector was engineered. Concepts related to the kinetics of the immune response to viruses are likely to translate poorly to viral vectors, which consist of preformed antigen administered as a single bolus, compared with wild-type viruses, for which the continual synthesis and presentation of viral antigens are critical to the tempo of the host immune response. Thus, it seems logical that viral vectors may initiate memory responses at the time of infusion, but that these responses may not play out in the accustomed time frame given the absence of any ongoing viral replication. Despite the differences from a true virus, several organizing principles that define the current understanding of the human immune response to viruses are critical for optimizing clinical outcomes with viral vectors; these include tissue-specific aspects of the immune response, innate immunity, and the distinct roles of B cells and T cells in viral response.
Innate immunity to AAV vectors
Innate immune responses constitute the frontline defense against viral infections and the main toxicity feature encountered in the development of gene transfer strategies using adenoviral vectors.27,28 AAV vectors, conversely, have gained wide acceptance as in vivo gene transfer vectors due to their very mild proinflammatory profile.29 However, a growing body of work indicates that the interactions of AAV vector components with the innate immune system are potentially important determinants of the outcome of gene transfer. In murine and human plasmacytoid dendritic cells (DCs) in vitro, the AAV vector single-stranded DNA genome interacts with the innate immune system through the Toll-like receptor (TLR) 9/MyD88 and type I interferon cascade.30,31 AAV vectors also trigger nuclear factor κB–dependent production of cytokine and chemokine release in mouse liver,32 and MyD88 signaling in B cells also seems to control the production of capsid-specific T helper 1 antibody responses.33 TLR9-dependent release of inflammatory cytokines may also result in enhanced transgene immunogenicity, as shown by Martino et al34 for self-complementary, but not for single-stranded, AAV vectors in the context of liver gene transfer in mice. Aside from the viral genome, the capsid of AAV serotype 2 (AAV2) may also interact with the innate immune system via TLR2, recently shown to occur in nonparenchymal cells (Kupffer and liver sinusoidal endothelial cells) of human liver.35 Although these studies provide strong evidence that innate immune recognition of AAV occurs in animals, little is known about the consequences of these interactions in the clinical setting, particularly how innate immunity to AAV impacts adaptive responses to the recombinant vector. Clinical observation of more than 300 subjects injected with AAV has shown no evidence of acute clinical responses such as changes in vital signs or nausea and vomiting36; administration to a wide range of tissues has proceeded uneventfully.
Adaptive immunity to AAV vectors
B-cell responses to AAV
Humoral immunity to wild-type AAV represents the first-encountered and one of the most effective barriers to successful systemic gene delivery with AAV vectors. In the first clinical trial in which AAV vectors were delivered through the bloodstream (in this case for hemophilia B), neutralizing antibody (NAb) titers were measured but were not used to exclude subjects from the trial because it was unclear what level of titers would actually block transduction. The study showed that relatively low NAb titers completely neutralized large doses of vector.15 Of 2 subjects enrolled in the high-vector-dose cohort, a dose of 2 × 1012 vector genomes per kg body weight (vg/kg), 1 subject with a pretreatment NAb titer of 1:17 did not express any detectable circulating F.IX, whereas the other with a low titer of 1:2 developed circulating levels of ∼10% of normal. Studies in mice37,38 and nonhuman primates (NHPs),39 have shown that NAb titers as low as 1:5 can block AAV vector transduction of liver completely, and that vector remains susceptible to antibody-mediated neutralization for several hours after intravascular delivery.38 Similarly, antibodies against AAV vectors are likely to present an obstacle to efficient transduction for vector delivery within the joint space to target synoviocytes,40,41 into the limb vasculature to target muscle,42,43 into the ventricles to target the central nervous system,44 or into the coronary arteries to target cardiac muscle.19 Conversely, the presence of anti-AAV antibodies does not seem to affect transduction efficiency when the vector is injected intraparenchymally or into the eye.5-7,13,17,18,26,45-52
Prevalence of anti-AAV antibodies in humans
Studies in healthy humans show that a significant proportion of individuals develop humoral immunity against the capsid early in life, starting at ∼2 years of age, as a consequence of natural exposure to wild-type AAV.53-55 Additionally, maternal anti-AAV antibodies can be found in newborns and decrease a few months after birth before increasing again.53,55 Thus, the window of time in which a majority of humans lack AAV antibodies is narrow.
Because of the high degree of conservation in the amino acid sequence among AAV capsids,56 anti-AAV antibodies show cross-reactivity over a wide range of serotypes (Figure 1B).57-60 Thus, although antibodies to AAV2 are clearly the most prevalent in humans (up to 70%), the natural host for this serotype, antibodies recognizing virtually all AAV serotypes can be found in a large proportion of individuals. Among the most commonly used AAV vectors, antibodies to AAV5, carrying one of the least-conserved capsid sequences,61 and to AAV8,56 are among the least prevalent.21,40,62
Antibody subclass analysis63 shows that anti-AAV IgG1 antibodies are highly prevalent in humans exposed to the wild-type virus, although lower levels of IgG2, 3, and 4 are also found. Following AAV vector delivery to muscle and liver, IgG1 antibodies remain the preponderant subclass,63 with some subjects developing a robust anticapsid IgG3 antibody response.63,64 The significance of these responses in terms of their possible impact on vector transduction or their possible prognostic value for capsid-triggered T-cell immunity is not fully understood.
Strategies to overcome humoral immunity to AAV
Gene transfer to subjects with preexisting immunity to AAV remains a major challenge for all methods of delivery through the circulation. The current approach to NAb to AAV is simply to exclude patients with antibodies to AAV, but because this involves up to 70% of the population, a better solution must be found.
Some of the proposed strategies to overcome the limitation of humoral immunity to AAV, which could be used alone or in combination, are summarized in Table 1.65-71 Switching serotypes seems unlikely to succeed because of the high degree of cross-reactivity of NAb across AAV serotypes. Conversely, some promising results have been obtained with pharmacologic and physical (plasmapheresis) modulation of anti-AAV NAb titers, and the use of catheters followed by saline flushing has been explored in the setting of AAV vector gene transfer with some promising results.72
Table 1.
Possible strategies to overcome humoral immunity to AAV in systemic gene transfer
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Select subjects with low-to-undetectable anti-AAV NAb. | Enrollment of NAb-negative subjects has been effective in allowing successful gene transfer. | Antibody assays are relatively insensitive (risk of false negatives) particularly for AAV serotypes performing poorly in vitro56; |
| More than 50% of humans develop anti-AAV humoral immunity after the age of 3,53,55 and thus exclusion of those with antibodies restricts the patient population. | ||
| Administer high vector doses. | Simple approach that may be effective in the presence of low-titer NAb. | High vector doses may trigger anticapsid CTL responses4,15,95,128; |
| Relatively low-titer antibodies (1:5-1:17) can neutralize large doses of vector.15,39 | ||
| Use empty capsids to adsorb anti-AAV antibodies, thus allowing for vector transduction. | Effectively overcomes humoral immunity to AAV119; | Increase antigen load in target organ, thus potentially triggering anticapsid T-cell immunity.95 |
| Does not require any pharmacologic intervention; | ||
| Empty capsids are an easy-to-manufacture by-product of AAV vector production. | ||
| Administer IS to prevent or eradicate humoral immune responses to AAV.65 | Drugs selectively targeting B cells and plasma cells are approved for use in humans66; | Potential risks associated with systemic IS; Risks associated with blocking the induction of regulatory T cells with IS 67,91; |
| The approach has been shown to be at least partially effective.40 | ||
| Many IS drugs, particularly biologics, are not effective in animal models of gene transfer, making modeling IS strategies in animals difficult; | ||
| Not effective in the complete eradication of preexisting high-titer NAb.40 | ||
| Switch AAV serotype62 or engineer AAV capsids that are less susceptible to NAb.68,69 | This approach has been shown to be effective in some instances.70 | Switching or altering the capsid may modify significantly the AAV vector tissue tropism; |
| Anti-AAV capsid antibodies are highly cross-reactive among serotypes.21 | ||
| Use repeated plasma exchange cycles to adsorb immunoglobulins and therefore reduce the anti-AAV antibody titer. | Does not require IS; | Requires several cycles of plasmapheresis to achieve significant drop in antibody titer; |
| Relatively safe, noninvasive procedure; | ||
| Effective in dropping anti-AAV antibody titer.71 | Does not completely eradicate high-titer anti-AAV antibodies.71 | |
| Use delivery techniques such as balloon catheters followed by saline flushing to isolate the target tissue from the systemic circulation to avoid vector dilution in blood and exposure to NAb.72 | Limits systemic exposure to the vector. | Relatively invasive, safety in diseased target organs/tissues needs to be assessed; |
| Not useful if systemic gene transfer is required for therapeutic efficacy, not feasible for all target tissues. |
CTL, cytotoxic T lymphocyte; IS, immunosuppression.
T-cell responses to the capsid and lessons from the first trial of AAV delivery to the liver
The first clear indication that human CD8+ T-cell responses to AAV capsid had not been accurately predicted by animal models and could limit efficacy of gene transfer was uncovered in the initial trial of AAV gene transfer to liver for hemophilia.15 An AAV2 vector expressing human F.IX under the control of a liver-specific promoter was introduced into the livers of men with severe hemophilia B; the first subject infused at the high dose, 2 × 1012 vg/kg, initially expressed F.IX at levels in the range of 10% to 12%, establishing that the therapeutic dose had been accurately predicted by studies in hemophilic dogs.73 However, after a period of several weeks, F.IX expression disappeared, accompanied by a self-limited and asymptomatic transaminase elevation (Figure 2A). This series of events had never been seen in experimental animal models.
Figure 2.
Clinical results following hepatic artery infusion of AAV2-F.IX. (A) Time course of F.IX levels and of transaminases in first subject to receive high-dose AAV2-F.IX (2 × 1012 vg/kg) in hemophilia B trial. An F.IX level (red) of ∼10% persists for 4 weeks and then slowly declines, concomitant with a self-limited rise in liver transaminases, which peaked at levels close to 10-fold over the baseline and then gradually fell, in tandem with a falling F.IX level, so that all laboratory parameters had returned to the patient’s baseline by ∼12 weeks after vector infusion (ALT, blue; AST, green). The patient remained asymptomatic throughout these events and, when tested subsequently, responded normally to infused recombinant F.IX. (B) A subsequent subject received a fivefold lower dose, 4 × 1011 vg/kg. F.IX levels (red) did not rise above 1%, but liver enzymes (ALT, blue; AST, green) rose and fell in a time course similar to that seen in the previous subject. Note difference in scales of liver enzymes. ALT, alanine aminotransferase; AST, aspartate aminotransferase. Reprinted from Manno et al.15
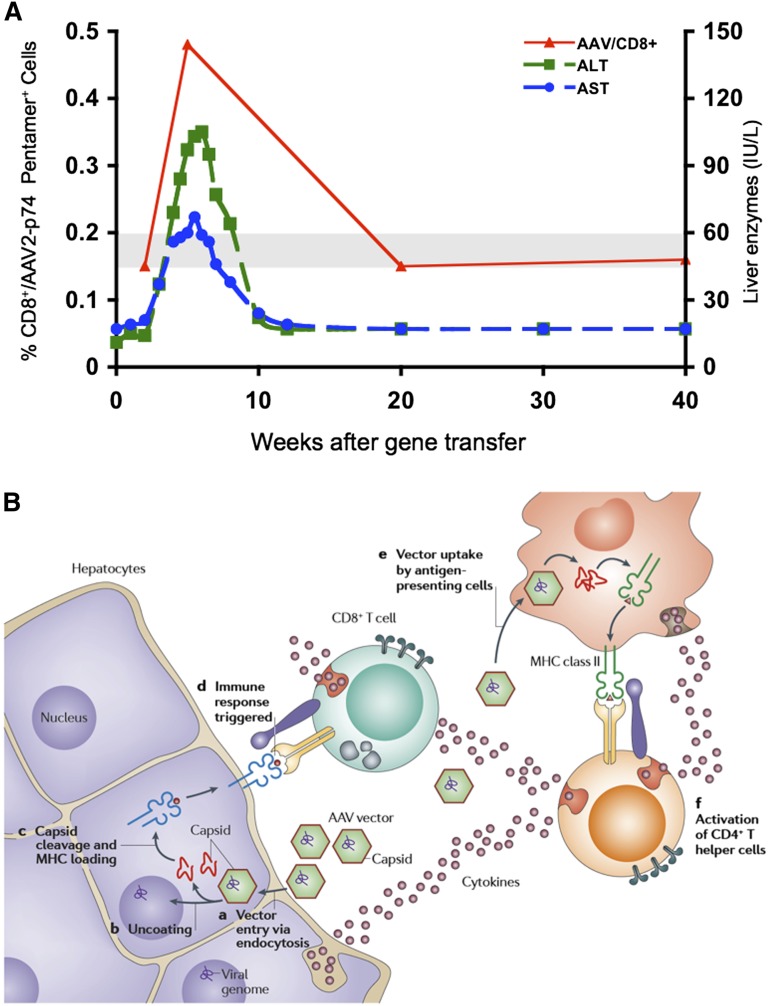
Attention focused on factors that differed between experimental animals and human subjects including previous exposure to wild-type AAV in most human subjects, and previous exposure to hepatitis C virus (HCV), hepatitis B virus, or both in most hemophilic trial subjects, which conceivably altered the hepatic microenvironment. Evaluation for known causes of elevated transaminases was negative, and direct toxicity from the vector infusion procedure seemed unlikely because the liver function tests were normal for the first several weeks after vector administration. The time course was consistent with some sort of immune response, and the trial was thus amended to allow the collection of peripheral blood mononuclear cells (PBMCs) at baseline and after vector infusion, with the goal of screening for immune responses to both vector (AAV capsid) and transgene product (F.IX). The next subject to enroll was negative for serological evidence of exposure to HCV or hepatitis B virus, eliminating this as a confounding variable, but he also developed transaminase elevation in a similar time course (Figure 2B). Analysis of PBMCs by interferon-γ enzyme-linked immunospot (IFN-γ ELISPOT) showed a response to AAV capsid but not to F.IX (Figure 3A). These findings led to the working hypothesis that preformed capsid that had entered the hepatocyte at the time of transduction, but was not being actively synthesized by the cell, had nonetheless gained access to major histocompatibility complex class I (MHC I) presentation pathways. This resulted in the expansion of a preexisting pool of capsid-specific CD8+ memory T cells, generated in the context of a prior infection with wild-type AAV together with a helper virus such as adenovirus. The requirements for reactivation of memory T cells are less stringent than those for induction of primary responses. Memory CD8+ T cells can commence effector function or undergo expansion upon recognition of antigen presented by any type of cell in the context of MHC I molecules. The immune response then effected a clean excision of the vector-transduced hepatocytes, resulting in transaminase elevation and loss of circulating F.IX (Figure 3B). Thus human subjects, undergoing reexposure, have an outcome different from experimental animals, undergoing what amounts to a primary exposure to AAV.
Figure 3.
Expansion of a population of capsid-specific CD8+ T cells after vector infusion, and working model of capsid processing and presentation in hepatocyte. (A) Time course of serum transaminases and frequency of AAV2 capsid peptide–specific CD8+ T cells in PBMCs, in subject infused at 4 × 1011 vg/kg dose. (B) Working hypothesis of CD8+ T-cell–mediated destruction of AAV-transduced hepatocytes. On transduction, vector enters hepatocytes, and following escape from the endosome and uncoating, vector DNA directs synthesis of F.IX. Some capsid remains behind in the cytosol and undergoes proteasomal processing. Capsid-derived peptides are then transported to the endoplasmic reticulum and loaded onto MHC I molecules, making the transduced hepatocyte a target for circulating capsid-specific CD8+ T cells. Note that activation of CD8+ T cells and presentation of capsid-derived peptides must occur in a similar time course to result in a clinically detectable event. Figure 3A reprinted from Mingozzi et al,67 and Figure 3B reprinted from Mingozzi and High.10
Efforts were subsequently directed toward developing an animal model that could recapitulate the human immune response to AAV capsid. Multiple attempts to generate a mouse model failed.74-76 More recent attempts that have made use of systems that increase the frequency of epitope-specific T cells (incorporation of SIINFEKL peptide epitopes into the AAV vector capsid and use of OT-1 mice77), or that further promote the adaptive immune response through the use of adjuvants,78 have allowed recapitulation of some features of the human response to AAV-mediated liver-directed gene transfer, but their utility in devising solutions in humans remains to be demonstrated. Because NHPs are also natural hosts for some serotypes of AAV, it seemed logical that one could model the human immune response in these animals, but multiple attempts to do so have also failed.39,79 Recent studies suggest differences in frequencies and subset distributions of AAV capsid-specific CD4+ and CD8+ T cells in humans and NHPs,80 perhaps related to differences in the AAV life cycle in the 2 species. In addition, Varki and colleagues have previously shown that differences in proliferative responses of T cells between humans and NHPs may be the result of the loss of sialic acid–binding Ig-like lectins on human T cells. Loss of these inhibitory signaling molecules could explain why humans mount more robust responses to AAV than other mammalian species.81
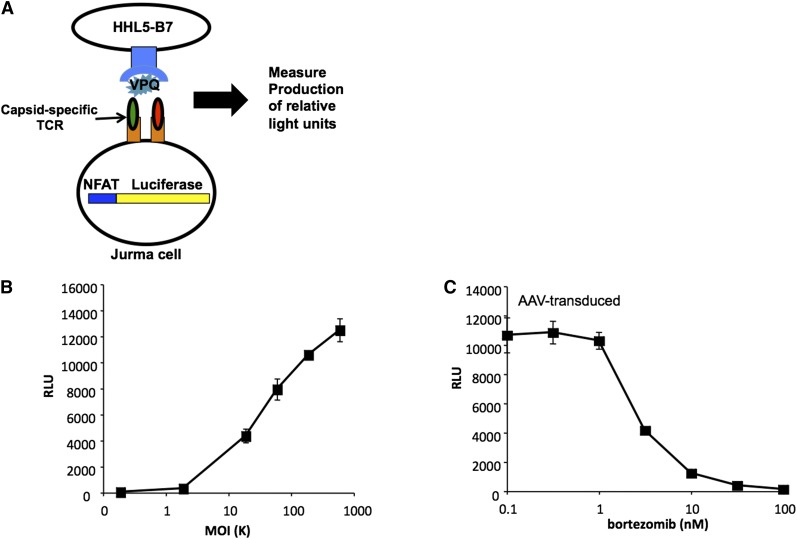
The inability to recapitulate the human findings in an animal model led to ongoing controversy about the pathophysiological basis of the transaminase elevation and loss of F.IX expression,82 and multiple alternative hypotheses were advanced (Table 2).83-88 However, if the observed toxicity was indeed related to the catabolism of the capsid, then this aspect of drug metabolism needed to be understood in greater detail. Early studies by Engelhardt and colleagues89,90 showed that AAV vector particles are indeed ubiquitinated once internalized in the cytosol, a step that is fundamental for targeting intracellular proteins for degradation through proteasomes. These findings are supported by the fact that proteasome inhibitors increase AAV transduction efficiency in vitro89-91 and in vivo,91-93 and that mutating surface-exposed tyrosines, which are targets for phosporylation and ubiquitination, results in enhanced AAV vector transduction.94 Evidence continued to emerge to support the hypothesis that presentation via MHC I pathways of the preformed capsid antigen had led to immune-mediated destruction of transduced hepatocytes. For example Pien et al95 generated a soluble T-cell receptor (TCR) reagent that recognized the B*0702 molecule complexed to a peptide from the AAV capsid and used this reagent to demonstrate peptide-MHC complexes on the surface of transduced hepatocytes by confocal microscopy. The soluble TCR also blocked lysis of transduced target cells in a dose-dependent fashion when added to a cytotoxic T-cell assay in which human PBMCs expanded in vitro against capsid were used as effector cells. In other studies, the development of a modified T-cell line that expresses the capsid-specific TCR and that also expresses luciferase as a function of engagement of the TCR (Figure 4A) demonstrated dose-dependent luciferase expression following exposure to hepatocytes transduced with AAV at progressively higher multiplicities of infection (Figure 4B). Moreover, luciferase expression could be completely blocked by the addition of proteasome inhibitors (Figure 4C), suggesting that the proteasomal processing of input capsid was required for peptide presentation.91
Table 2.
Competing hypotheses explaining AAV vector immune responses in humans
| Hypothesis | Supporting observations | Observations not supporting the model |
|---|---|---|
| Capsid antigen presentation and memory T-cell activation leads to clearance of AAV-transduced cells.67 | Human studies show expansion of capsid T cells following vector delivery4,15,67; | Most humans are exposed to wild-type AAV, but not all react against the AAV capsid. |
| Healthy subjects carry capsid-reactive T cells67,85; | ||
| Capsid antigen is processed and presented on MHC I.91,95 | ||
| Expression of rep/cap from vector impurities triggers CTLs against transduced cells.82 | AAV capsid packaging DNA impurities can be found in vector preparations.84,85 | Studies in mice failed to detect expression of Rep and Cap in animals injected with high vector doses.86 |
| Translation of ARFs within expression cassettes results in CTLs against AAV-transduced cells.82 | Animal studies show that it is possible to express epitopes from ARFs.87 | Screening of subjects for anti-ARF T-cell reactivity did not support the model.4 |
| Preferential uptake of AAV2 by APCs via heparin-binding domain results in higher immunogenicity. | Animal studies showed that AAV2 binds more efficiently to APCs than AAV8.88 | Subjects dosed with AAV8 vectors (not binding to heparin) developed T-cell responses against the capsid.4 |
APC, antigen-presenting cell; ARF, alternate open reading frame.
Figure 4.
Capsid antigen presentation is dose-dependent, and can be blocked with a protosomal inhibitor. (A) Generation of a genetically modified T-cell line used as a sensitive detector of peptide-MHC complexes on the cell surface. The T-cell line is stably transfected with a luciferase gene under the control of a promoter containing the NFAT sequence. The cell also carries a TCR cloned from a human subject that recognizes a specific peptide from the AAV capsid complexed with HLA B*0702. Upon engagement of the TCR luciferase is produced. (B) Human hepatocyte cell line HHL5-B7 is transduced with AAV at progressively higher MOIs (x-axis). When the TCR recognizes its cognate peptide-MHC complex, luciferase expression increases (y-axis) in proportion to the number of peptide-MHC complexes engaged. (C) Treatment of cells with the proteasome inhibitor bortezomib reduces AAV capsid antigen presentation in a dose-dependent manner. MOI, multiplicity of infection; NFAT, nuclear factor of activated T cells; RLU, relative light units. Reprinted from Finn et al.91
The AAV8-F.IX trial
The AAV8 trial proposed by Nathwani and colleagues (sponsored by St. Jude Children’s Research Hospital and University College London) synthesized multiple developments in the field to generate a novel vector and a modified trial design to elude or overcome immune obstacles identified in the previous trial. First, the trial excluded individuals with neutralizing antibodies to AAV (titers >1:5) (vide supra). Second, the vector was reengineered with 3 modifications, including a switch to a capsid serotype (AAV8) with strong tropism for liver, allowing intravenous delivery, a change in the DNA conformation from single stranded to double stranded, which had been proposed to improve transduction efficiency,96 and codon optimization of the F.IX sequence to enhance translational efficiency.97
Additionally, in an attempt to thwart an immune response should it occur, the trial design incorporated a provision for administering high-dose steroids should any subject develop transaminase elevation and/or loss of F.IX expression. This necessitated excluding subjects who were positive for HCV RNA viral load because steroids are contraindicated in this setting. The first 2 dose cohorts proceeded uneventfully, and all of the subjects showed an increase in circulating levels of F.IX, in the range of 1% to 3%. These data, although modest, were nonetheless exciting, as the increases were persistent and, in some cases, allowed subjects to dramatically reduce the amount of clotting factor concentrates they used to manage their disease.4
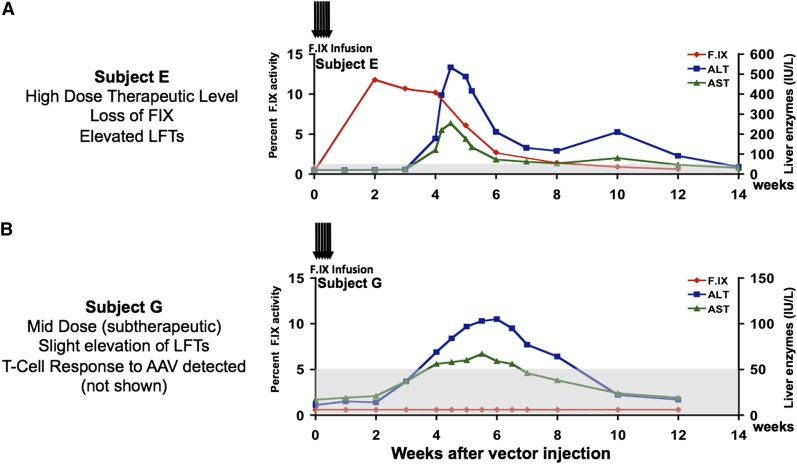
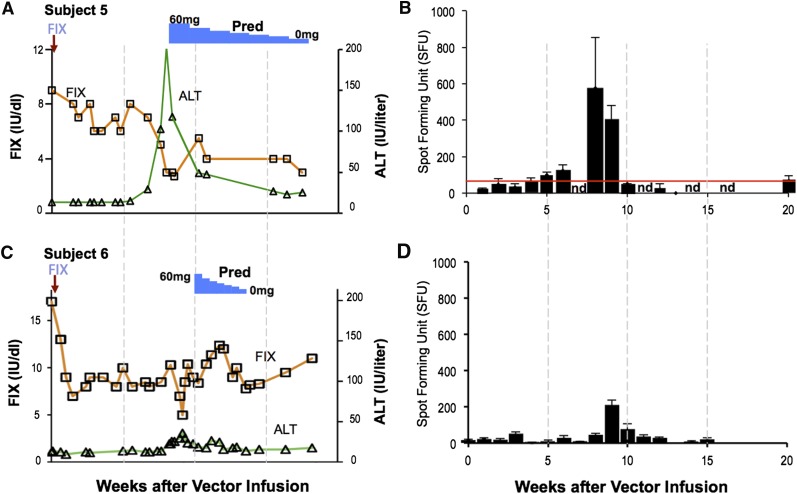
The first subject to receive a dose of 2 × 1012 vg/kg of AAV8-F.IX had circulating F.IX levels of 8% to 10% for a period of 8 weeks (Figure 5A). However, at that point, the serum transaminases rose rapidly, and F.IX levels began to fall. The subject was begun on high-dose prednisolone, 60 mg/d, which was tapered over a period of 8 weeks. This maneuver resulted in partial “rescue” of F.IX levels, which stabilized eventually at ∼2%. Liver function tests also rapidly returned to normal. Subsequent studies of PBMCs from the subject documented a marked rise in circulating capsid-specific T cells at ∼8 weeks after vector infusion and disappearance of these with administration of high-dose prednisolone (Figure 5B). These data seemed to confirm the findings from the AAV2 trial, that is, that a dose-dependent immune response directed against epitopes in the AAV capsid arises weeks after vector administration, and that it has the capacity to destroy the F.IX-expressing hepatocytes. A subsequent subject, who also developed a modest rise in transaminase levels at 8 weeks, was begun on a short course of steroids at the first sign of increase in ALT (Figure 5C-D). This subject has now maintained a circulating level of F.IX in the range of 4% to 6% for more than 24 months, with observation ongoing. His hemophilia has been changed from severe disease to mild.
Figure 5.
T-cell–mediated immunity to the capsid in the AAV8-F.IX trial. (A-B) Subject 5 received a vector dose of 2 × 1012 vg/kg. At week 8 postinfusion, his F.IX levels (red line) began to decline and his liver enzymes increased (ALT, green line). Concomitantly, capsid-specific T cells became detectable in peripheral blood. A course of prednisolone was associated with resolution of the transaminitis and partial rescue of F.IX transgene expression levels. (C-D) Subject 6, dosed at 2 × 1012 vg/kg, experienced an increase in liver enzymes and was promptly treated with steroids. In this subject, increase in liver enzymes and decrease in F.IX transgene expression levels was also associated with detection of capsid-reactive T cells in PBMCs. Reprinted from Nathwani et al.4
It should be noted that subjects in the intermediate dose cohort in this trial also exhibited detectable numbers of circulating capsid-specific T cells on IFN-γ ELISPOT, without any ensuing transaminase elevation or decline in circulating levels of F.IX. However, the development of clinically detectable transaminase elevation requires at least 2 conditions: first, the proliferation of capsid-specific T cells; and second, the display on the surface of the transduced hepatocytes of sufficient numbers of peptide-MHC complexes (peptides derived from AAV capsid) to flag the cell for destruction by circulating CD8+ T cells. The first condition is measureable by IFN-γ ELISPOT, but the second cannot be measured clinically. It is likely, however, that this second condition is dose dependent (Figure 4), and that the levels of capsid-derived antigen presentation in the lower-dose cohorts may be insufficient to trigger immune-mediated destruction of transduced hepatocytes.
The key advance in this trial with regard to the immune response was the demonstration that it could be controlled or at least ameliorated by a short course of high-dose steroids. This finding raises a number of follow-up questions, including what proportion of subjects will require a course of high-dose steroids, and whether this maneuver will reliably control the immune response in those who develop it, particularly when vector doses higher than the ones tested so far are administered. Ongoing and planned clinical trials will help to gather data on these questions, but progress would be facilitated by the availability of an animal model that recapitulated the human immune response.
Capsid T-cell responses in muscle gene transfer
Extensive T-cell monitoring performed in muscle gene transfer trials with AAV vectors resulted in the generation of one of the largest data sets on capsid T-cell responses in humans.17,19,64,98-102 As for liver gene transfer, the cumulative results suggest that the magnitude of T-cell responses directed against the AAV capsid roughly correlates with the dose of vector administered, with an increasing number of subjects showing T-cell reactivity to the AAV capsid in PBMCs at higher vector doses and, in some cases, a faster kinetics of detection of T cells in peripheral blood at higher vector doses.64 However, because of the fact that most of the therapeutic transgenes tested in muscle trials were nonsecreted, and that efficacy end points were more difficult to determine than with a circulating protein such as F.IX, correlation between results of immune response analysis and clinical outcome was more difficult to establish. Furthermore, several factors appear to influence the outcome of gene transfer in muscle, including upregulation of MHC I expression in some settings,100,101 apoptosis of reactive T cells,101,103 immunomodulatory properties of the transgene product17,98,104 as well as transgene immunogenicity (vide infra), and baseline levels of inflammation within the target tissue.105 Additional studies are needed to identify determinants that may lead to a destructive immune response following AAV vector administration to muscle and to establish whether IS is needed (eg, when large doses of AAV vectors are administered). Recently, long (>10 years) duration of expression of an AAV-administered transgene has been reported in a human subject106; the generally low level of MHC I expression in healthy skeletal muscle may reduce the likelihood of cytotoxic T lymphocyte (CTL)–mediated events in this setting.
Data accumulated so far support the safety of muscle gene transfer and indicate that in skeletal muscle, as in liver and presumably other tissues with high regenerative capacity, T-cell responses against the capsid, if they occur, are mild, mostly resulting in loss of therapeutic efficacy with no other sequelae.
Gene transfer to immune-privileged body compartments
The eye and central nervous system are known to be immune-privileged compartments of the body due to adaptations that limit immune responses, likely in order to preserve these tissues with limited regenerative capacities from destructive inflammatory reactions. Delivery of small amounts of AAV vectors directly into the brain has been tested in a number of trials for Parkinson disease,13,51,52,107 Canavan disease,14,108 and late infantile neuronal ceroid lipofuscinosis.50 Similarly, subretinal vector delivery has been performed in a number of clinical studies of gene transfer for RPE65 deficiency,5-7,47 and in the immunologically stringent setting of vector administration to the contralateral eye of previously injected subjects.46 In all these studies, AAV vector administration was generally associated with little to no detectable immune response to the capsid or the transgene in serum and PBMCs. Future studies will help define the upper limit of vector dose that can be safely administered to humans without breaking the immunologic unresponsiveness of brain and eye, for example, in the proposed setting of global brain transduction through intra–cerebrospinal fluid AAV vector delivery.44
Trade-offs around the immune response and problems to be solved
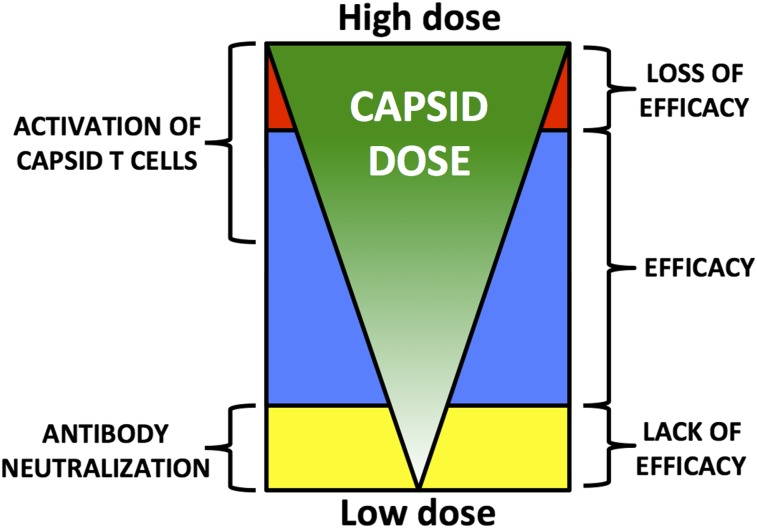
The limitations imposed by the human immune response to AAV capsid are well illustrated in the hemophilia B trials. To avoid the neutralizing antibodies, one can simply exclude these patients from the trials, but this prevents as many as 70% of subjects from enrolling (vide supra). A potential remedy to the issue is the use of higher doses, but this risks increasing the capsid load to the target cell (Figure 6). At the maximum doses used thus far in the hemophilia trials, 2 × 1012 vg/kg, the CD8+ T-cell response to capsid can be reduced or controlled using a short course of high-dose steroids, but it is unclear that this same strategy will be successful at higher doses of vector. Thus, it may be critical to develop alternative strategies (Table 3) such as stronger IS regimens if higher doses of vector are needed. Alternatively, high specific-activity transgenes, such as F.IX Padua,109-111 may allow a reduction in dose to a level that can be tolerated without a need for steroids (vide infra), although the risks of immune responses to variant clotting factor molecules inherent in this approach must be carefully considered (http://www.novonordisk.com/investors/sea/sea.asp?sShowNewsItemGuID=a64ed4f3-6393-4ce7-bb74-4814aa5ec29a&sShowLanguageCode=en-GB&sSearchText=rFVIII+OR+Turoctocog+alfa+OR+NN7008). Finally, the elimination of excess empty capsids from the vector preparations may reduce the total capsid load to a level that can be tolerated without coadministration of an IS regimen (vide infra).
Figure 6.
Model of the relationship between capsid dose and outcome of gene transfer following systemic vector delivery. Low capsid doses are more likely to be neutralized by anti-AAV antibodies, even low-titer NAb. This results in lack of efficacy. Higher capsid doses overcome this limitation, leading to therapeutic efficacy. Capsid-specific T-cell activation is detected as the total capsid dose administered increases. This does not affect efficacy until a critical threshold is reached, above which immune-mediated clearance of transduced target cells results in loss of efficacy.
Table 3.
Possible strategies to overcome capsid-specific T-cell responses
| Strategy | Advantages | Disadvantages |
|---|---|---|
| Select subjects naive to AAV. | Avoid memory responses to AAV. | Data suggest that capsid-reactive T cells fail to circulate in peripheral blood, and thus the most reliable way to screen subjects for T-cell reactivity to AAV is to use splenocytes,85 an approach not feasible clinically. |
| Decrease the therapeutic vector dose using highly efficient AAV vectors4 of hyperactive variants of the therapeutic transgenes.110,111 | Available data suggest that the approach is feasible and may be effective in preventing T-cell–mediated clearance of transduced cells. | This strategy may not be applicable for those gene transfer protocols in which the therapeutic dose is expected to be considerably higher than the doses tested so far in humans. |
| Hyperactive variants are not available for every transgene. | ||
| Administer IS to prevent or block T-cell responses to AAV. | Steroids effectively block T-cell responses directed against the AAV capsid at doses tested thus far; | Potential risks associated with systemic IS; |
| Risks associated with blocking the induction of regulatory T cells with IS67,91; | ||
| Extensive experience with IS comes from transplant medicine, and several IS drugs can be safely administered for prolonged periods. | Many IS drugs, particularly biologics, are not effective in animal models, precluding preclinical testing; | |
| Timing of capsid presentation on MHC I in vivo is unknown, and thus timing of IS administration may be difficult to define except through clinical experience. | ||
| Switch AAV serotypes56 or engineer AAV capsids to remove MHC I epitopes. | In theory, the approach could work. | Because of AAV capsid sequence conservation, a high degree of cross-recognition by capsid-specific T cells is expected67; |
| Switching or altering the capsid can significantly modify the AAV capsid tissue tropism; | ||
| Complexity of MHC I recognition in humans and presence of subdominant T-cell epitopes may hinder efforts in this direction. | ||
| Use proteasome inhibitors91 or mutant capsids that are not efficiently ubiquitinated.94 | Both measures have shown efficacy in reducing AAV capsid antigen presentation on MHC I. | The effect may be only partial or may require prolonged pharmacotherapy. |
Vector-manufacturing considerations
AAV vector production and purification methodologies affect to a substantial degree the nature and amount of impurities in the final product. Impurities commonly found in AAV vector preparations include host cell proteins, mammalian DNA, and other contaminants, which may impact the immunogenicity of the final product itself. Thus far, the only AAV vector preparations administered to humans intravascularly and at high doses have been those prepared by triple transfection of HEK293 cells112 in the context of the 2 hemophilia B trials (vide supra).4,15 Vectors produced with other technologies, such as baculovirus expression systems113 or with the use of live helper virus,114 have also been used in human trials but were administered intramuscularly,16 or subretinally,5 or at lower doses than in the hemophilia studies.19,115
Aside from the host cell platform used to produce AAV vectors, one of the major determinants of the content of vector preparations is the downstream purification process. Typically, column purification methods cannot be used to separate empty from full capsids, with some exceptions,116 whereas addition of a gradient centrifugation step to the vector purification procedure can effectively isolate empty from full capsids.117,118
Excess capsid antigen may favor vector transduction in vivo by overcoming vector neutralization by anti-AAV antibodies.119 However, both full and empty capsids can enter a target cell120 and contribute to the amount of antigen being presented on MHC I molecules95 with consequent recognition of transduced cells by capsid-specific T cells. Manufacturers may thus need to balance competing demands for higher purity and better scalability of the process. Modifications that reduce empty capsids while preserving scalability are needed.
Immune responses against the transgene product
Because immune responses to the transgene product can be affected by the innate immune response to the vector, the relatively low proinflammatory profile and the inefficiency in transducing DCs of AAV constitute advantages for this vector in terms of risk of transgene-triggered immune responses compared with, for example, adenoviral or lentiviral vectors. Several groups showed that delivery of AAV vectors to the liver induces transgene-specific tolerance121-125; recent studies demonstrate that this effect is mediated by regulatory T cells.126-128 AAV-mediated gene transfer has also been used in hemophilic mice and dogs with neutralizing antibodies to clotting factors (both VIII and IX) to eradicate these antibodies127,129 (clinically termed inhibitors), further underscoring the protolerogenic properties of liver gene transfer with these vectors and providing proof of concept for an additional application of gene transfer (ie, eradication of inhibitors in hemophilia).
Thus far, clinical studies of liver gene transfer have been conducted only in the context of hemophilia B.4,15 As predicted by preclinical studies, none of the subjects enrolled in these trials developed immune responses against the donated therapeutic transgene, despite the fact that some of them had null mutations in the F.IX gene. However, all subjects who had a history of inhibitor following protein replacement therapy were excluded from these studies; thus additional studies will be required to address the risk of mounting an immune response against the transgene product in previously untreated patients. Finally, studies conducted so far in dogs with AAV vectors expressing the F.IX Padua hyperactive variant109 would argue that the approach is safe in the context of gene transfer.110,111 This approach will need to be carefully tested, particularly when nonnative transgene variants are expressed with vectors that may interact with the innate immune system (vide supra and Martino et al34 and Wu et al130).
The development of transgene-specific immune responses is highly dependent on the route of AAV vector administration. Preclinical studies of AAV gene transfer to muscle suggest that IS may be needed to maintain gene expression following intramuscular vector delivery in nontolerant animals.131-134
In the clinic, direct intramuscular gene transfer using AAV vectors has been explored for a number of indications, including hemophilia B,18,26 lipoprotein lipase deficiency,16,48 α1-antitrypsin deficiency,17,98,135 and muscular dystrophies.99-102 In addition, cardiac muscle has also been targeted through an intravascular approach, for cardiac failure.19 Immune responses against the therapeutic transgene were not detectable in most of these studies. However, in a recent study of AAV gene transfer in children with Duchenne muscular dystrophy in which an AAV vector encoding a functional truncated version of the dystrophin protein was delivered intramuscularly, analysis of muscle biopsies showed no transgene expression in any of the subjects, and analysis of PBMCs showed the presence of transgene-specific CD4+ and CD8+ T cells in 4 of 6 subjects. Of interest, the dystrophin-specific T cells were present prior to vector injection in 2 of 4 subjects, having arisen most likely in response to spontaneous revertant fibers.99 In another study of intramuscular gene transfer for α1-antitrypsin deficiency,98 vector administration was associated with detection of capsid-specific T-cell reactivity and increase in serum creatine kinase in some subjects at approximately day 30 post vector administration. Some subjects also experienced a drop in circulating levels of transgene product associated with detection of CD4+ and CD8+ T-cell infiltrates in muscle biopsies. T-cell reactivity against the α1-antitrypsin protein was confirmed in 1 subject, but the clinical relevance of this finding is unclear.
The role of tissue-specific promoters in reducing or abrogating immune responses to the transgene product has been demonstrated in the setting of animal models of muscular dystrophy.136,137 The use of tissue-specific micro RNA elements to detarget transgene expression in DCs, first used in the context of lentiviral vectors138 and more recently extended to AAV vectors introduced into skeletal muscle,139 prevented cellular immune responses to a human sarcoglycan transgene following intramuscular injection in normal mice but not in sarcoglycan knockout mice, where the presence of ongoing inflammation may alter or amplify immune responses. Long-term expression of the dystrophin gene in canine muscular dystrophy, achieved following short-term IS at the time of AAV vector administration, may provide another strategy for avoiding immune responses in this challenging setting.140
Despite the potential risk of transgene immune responses, muscle is an important target tissue not only for diseases like muscular dystrophies, but also for protein deficiencies in which liver (the main site of synthesis of several of these proteins) is compromised and cannot be used as the target organ. Recent developments141 suggest that it is possible to target the skeletal muscle through the intravascular route, resulting in high transduction efficiency and lower transgene immunogenicity.42,132,142
Concluding remarks
Over the past several years, studies in humans have demonstrated the therapeutic potential of in vivo gene transfer with AAV vectors. Initially, investigators underestimated the extent of the interactions of recombinant viral vectors with the human immune system because these had not been predicted by studies in animals. AAV vectors are complex biological therapeutics consisting of a viral capsid, a DNA genome, and the therapeutic transgene product that the DNA encodes. Each of these components can interact at multiple levels with the innate and adaptive immune system. Consistent with current concepts in immunology, the human immune response to vector may vary substantially depending on the tissue in which the vector is encountered, with outcomes ranging from unresponsiveness (eg, gene transfer in the eye), to tolerance (eg, to the transgene product following expression in the liver), to clearance of transduced cells (eg, capsid T-cell responses in the liver). Small-scale clinical studies have yielded a tremendous amount of information about these interactions, how to measure them, and ultimately, how to modulate them, which is key to attaining optimal outcomes for clinical application.
Much more remains to be done to gain a more nuanced understanding of immune responses to AAV vectors and to better understand the safety implications of administering high vector doses to humans. For example, little is known about what drives these responses aside from the total vector dose administered. In the case of immune responses to capsid in liver, basic principles suggest that the simultaneous occurrence of T-cell activation and capsid antigen presentation are required to trigger a transaminase elevation and loss of transduced hepatocytes (Figure 3B); however, although it is straightforward to measure the former (through IFN-γ ELISPOT on PBMCs), there is no clinical method for measuring the latter. Also unknown is the pharmacogenetics of gene transfer and whether, for example, certain human HLA alleles may be associated with higher vector immunogenicity. Clearly, the availability of predictive animal models would constitute an important advance if the model could recapitulate effects of capsid structure, DNA conformation, and adjuvant immunomodulatory regimens on capsid antigen presentation and T-cell responses. Similarly, better solutions are needed to allow for enrollment of AAV seropositive individuals in gene transfer trials for which systemic vector delivery is required. In the case of hemophilia, there is no clinical experience with gene transfer in previously untreated patients, and therefore no knowledge about transgene immunogenicity in this patient population. Finally, innate immunity to AAV and its clinical implications are largely unknown.
Nonetheless, current results are promising and suggest that investigators have now defined a population of patients that can expect long-lasting expression of a donated gene from an AAV vector. These developments raise other questions: how long expression will last, whether readministration will be possible, and whether short-term IS with steroids will be effective at higher vector doses. These questions will only be answered by further clinical investigation.
The idea of treating AAV-naive subjects, thus avoiding almost completely the issue of capsid immunity, is appealing. However, humans are exposed to AAV early in life, and AAV gene transfer in rapidly dividing tissues is not long lasting, unless strategies like genome editing with zinc finger nucleases are used.143
The human immune response to AAV vectors is modest in intensity, although it has the ability to limit efficacy if not appropriately managed. On balance, however, so far it is clearly more an efficacy issue than a safety one. The immune responses highlighted here occur relatively early after gene transfer; they are now well-understood enough to be tractable from a clinical standpoint. This leaves as the major safety issue the risk of insertional mutagenesis.144 Although AAV vectors are regarded as nonintegrating, it is clear that integration does occur to some degree.145,146 In more than 15 years of clinical experience with AAV, there have been no observations of clinical events related to AAV integration. Continued monitoring in clinical trials, and ongoing laboratory studies, should help to define and reduce this long-term risk of AAV vector administration.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute and in part by grants from the National Institutes of Health National Heart, Lung, and Blood Institute (P01 HL64190 and P01 HL078810) (K.A.H.).
Authorship
Contribution: K.A.H. and F.M. worked together to outline, draft, revise and edit the manuscript.
Conflict-of-interest disclosure: K.A.H. holds patents in the area of AAV-F.IX and has waived all financial interest in these; is a coinventor on patents related to AAV manufacture and purification, and lentiviral vector manufacture; holds equity in Bluebird Bio Inc., which has clinical programs in X-linked adrenoleukodystrophy and thalassemia; and has served as a consultant to BioMarin Pharmaceuticals, Genzyme, Novo Nordisk, Nordic Biotech Advisors, and Shire regarding gene therapy approaches for genetic disease. F.M. is a coinventor on patents related to AAV-mediated gene delivery and modulation of immunity to AAV vectors.
The current address for F.M. is Université Pierre and Marie Curie, Paris, France; and Genethon, Evry, France.
Correspondence: Katherine A. High, The Children’s Hospital of Philadelphia, 3501 Civic Center Blvd, 5060 CTRB, Philadelphia, PA 19104; e-mail: high@email.chop.edu.
References
- 1.Aiuti A, Cattaneo F, Galimberti S, et al. Gene therapy for immunodeficiency due to adenosine deaminase deficiency. N Engl J Med. 2009;360(5):447–458. doi: 10.1056/NEJMoa0805817. [DOI] [PubMed] [Google Scholar]
- 2.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. doi: 10.1126/science.1171242. [DOI] [PubMed] [Google Scholar]
- 3.Cavazzana-Calvo M, Payen E, Negre O, et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467(7313):318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathwani AC, Tuddenham EG, Rangarajan S, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 6.Maguire AM, High KA, Auricchio A, et al. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374(9701):1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack A. European agency backs approval of a gene therapy. New York Times. July 20, 2012:B1.
- 9.Xie Q, Bu W, Bhatia S, et al. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(16):10405-10410. [DOI] [PMC free article] [PubMed]
- 10.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12(5):341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 11.High KA. The gene therapy journey for hemophilia: are we there yet? Blood. 2012;120(23):4482–4487. doi: 10.1182/blood-2012-05-423210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson SG, Cideciyan AV, Ratnakaram R, et al. Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years. Arch Ophthalmol. 2012;130(1):9–24. doi: 10.1001/archophthalmol.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 14.Leone P, Shera D, McPhee SW, et al. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4(165):165ra163. [DOI] [PMC free article] [PubMed]
- 15.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response [published correction appears in Nat Med. 2006;12(5):592]. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 16.Carpentier AC, Frisch F, Labbé SM, et al. Effect of alipogene tiparvovec (AAV1-LPL(S447X)) on postprandial chylomicron metabolism in lipoprotein lipase-deficient patients. J Clin Endocrinol Metab. 2012;97(5):1635–1644. doi: 10.1210/jc.2011-3002. [DOI] [PubMed] [Google Scholar]
- 17.Brantly ML, Chulay JD, Wang L, et al. Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci USA. 2009;106(38):16363–16368. doi: 10.1073/pnas.0904514106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 19.Jaski BE, Jessup ML, Mancini DM, et al. Calcium Up-Regulation by Percutaneous Administration of Gene Therapy In Cardiac Disease (CUPID) Trial Investigators. Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase 1/2 clinical trial. J Card Fail. 2009;15(3):171–181. doi: 10.1016/j.cardfail.2009.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoggan MD, Blacklow NR, Rowe WP. Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci USA. 1966;55(6):1467–1474. doi: 10.1073/pnas.55.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boutin S, Monteilhet V, Veron P, et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. 2010;21(6):704–712. doi: 10.1089/hum.2009.182. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Muzyczka N. In vitro packaging of adeno-associated virus DNA. J Virol. 1998;72(4):3241–3247. doi: 10.1128/jvi.72.4.3241-3247.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler PD, Podsakoff GM, Chen X, et al. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc Natl Acad Sci USA. 1996;93(24):14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70(11):8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner JA, Reynolds T, Moran ML, et al. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351(9117):1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 26.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24(3):257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 27.Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80(1-2):148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Marshall E. Gene therapy on trial. Science. 2000;288(5468):951–957. [PubMed] [Google Scholar]
- 29.Vandendriessche T, Thorrez L, Acosta-Sanchez A, et al. Efficacy and safety of adeno-associated viral vectors based on serotype 8 and 9 vs. lentiviral vectors for hemophilia B gene therapy. J Thromb Haemost. 2007;5(1):16–24. doi: 10.1111/j.1538-7836.2006.02220.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhu J, Huang X, Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J Clin Invest. 2009;119(8):2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki M, Bertin TK, Rogers GL, et al. Differential type I interferon-dependent transgene silencing of helper-dependent adenoviral vs. adeno-associated viral vectors in vivo. Mol Ther. 2013;21(4):796–805. doi: 10.1038/mt.2012.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayandharan GR, Aslanidi G, Martino AT, et al. Activation of the NF-kappaB pathway by adeno-associated virus (AAV) vectors and its implications in immune response and gene therapy. Proc Natl Acad Sci USA. 2011;108(9):3743–3748. doi: 10.1073/pnas.1012753108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Sudres M, Ciré S, Vasseur V, et al. MyD88 signaling in B cells regulates the production of Th1-dependent antibodies to AAV. Mol Ther. 2012;20(8):1571–1581. doi: 10.1038/mt.2012.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martino AT, Suzuki M, Markusic DM, et al. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117(24):6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hösel M, Broxtermann M, Janicki H, et al. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55(1):287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 36.High KA, Aubourg P. rAAV human trial experience. Methods Mol Biol. 2011;807:429–457. doi: 10.1007/978-1-61779-370-7_18. [DOI] [PubMed] [Google Scholar]
- 37.Scallan CD, Jiang H, Liu T, et al. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107(5):1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- 38.Murphy SL, Li H, Zhou S, Schlachterman A, High KA. Prolonged susceptibility to antibody-mediated neutralization for adeno-associated vectors targeted to the liver [published correction appears in Mol Ther. 2008;16(3):633]. Mol Ther. 2008;16(1):138–145. doi: 10.1038/sj.mt.6300334. [DOI] [PubMed] [Google Scholar]
- 39.Jiang H, Couto LB, Patarroyo-White S, et al. Effects of transient immunosuppression on adenoassociated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. 2006;108(10):3321–3328. doi: 10.1182/blood-2006-04-017913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mingozzi F, Chen Y, Edmonson SC, et al. Prevalence and pharmacological modulation of humoral immunity to AAV vectors in gene transfer to synovial tissue. Gene Ther. 2013 doi: 10.1038/gt.2012.55. 20(4):417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cottard V, Valvason C, Falgarone G, Lutomski D, Boissier MC, Bessis N. Immune response against gene therapy vectors: influence of synovial fluid on adeno-associated virus mediated gene transfer to chondrocytes. J Clin Immunol. 2004;24(2):162–169. doi: 10.1023/B:JOCI.0000019781.64421.5c. [DOI] [PubMed] [Google Scholar]
- 42.Arruda VR, Stedman HH, Haurigot V, et al. Peripheral transvenular delivery of adeno-associated viral vectors to skeletal muscle as a novel therapy for hemophilia B. Blood. 2010;115(23):4678–4688. doi: 10.1182/blood-2009-12-261156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodino-Klapac LR, Montgomery CL, Bremer WG, et al. Persistent expression of FLAG-tagged micro dystrophin in nonhuman primates following intramuscular and vascular delivery. Mol Ther. 2010;18(1):109–117. doi: 10.1038/mt.2009.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samaranch L, Salegio EA, San Sebastian W, et al. Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther. 2012;23(4):382–389. doi: 10.1089/hum.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amado D, Mingozzi F, Hui D, et al. Safety and efficacy of subretinal readministration of a viral vector in large animals to treat congenital blindness. Sci Transl Med. 2010;2(21):21ra16. doi: 10.1126/scitranslmed.3000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett J, Ashtari M, Wellman J, et al. AAV2 gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4(120):120ra15. [DOI] [PMC free article] [PubMed]
- 47.Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105(39):15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stroes ES, Nierman MC, Meulenberg JJ, et al. Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol. 2008;28(12):2303–2304. doi: 10.1161/ATVBAHA.108.175620. [DOI] [PubMed] [Google Scholar]
- 49.Eberling JL, Jagust WJ, Christine CW, et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 50.Worgall S, Sondhi D, Hackett NR, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19(5):463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- 51.Marks WJ, Jr, Bartus RT, Siffert J, et al. Gene delivery of AAV2-neurturin for Parkinson’s disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(12):1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 52.Marks WJ, Jr, Ostrem JL, Verhagen L, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 53.Calcedo R, Morizono H, Wang L, et al. Adeno-associated virus antibody profiles in newborns, children, and adolescents. Clin Vaccine Immunol. 2011;18(9):1586–1588. doi: 10.1128/CVI.05107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erles K, Sebökovà P, Schlehofer JR. Update on the prevalence of serum antibodies (IgG and IgM) to adeno-associated virus (AAV). J Med Virol. 1999;59(3):406–411. doi: 10.1002/(sici)1096-9071(199911)59:3<406::aid-jmv22>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 55.Li C, Narkbunnam N, Samulski RJ, et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 2012 doi: 10.1038/gt.2011.90. 19(3):288-294. [DOI] [PubMed] [Google Scholar]
- 56.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grimm D, Lee JS, Wang L, et al. In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses. J Virol. 2008;82(12):5887–5911. doi: 10.1128/JVI.00254-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Asokan A, Conway JC, Phillips JL, et al. Reengineering a receptor footprint of adeno-associated virus enables selective and systemic gene transfer to muscle. Nat Biotechnol. 2010;28(1):79–82. doi: 10.1038/nbt.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maheshri N, Koerber JT, Kaspar BK, Schaffer DV. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat Biotechnol. 2006;24(2):198–204. doi: 10.1038/nbt1182. [DOI] [PubMed] [Google Scholar]
- 60.Arruda VR, Xiao W. It’s all about the clothing: capsid domination in the adeno-associated viral vector world. J Thromb Haemost. 2007;5(1):12-15. [DOI] [PubMed]
- 61.Chiorini JA, Kim F, Yang L, Kotin RM. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73(2):1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calcedo R, Vandenberghe LH, Gao G, Lin J, Wilson JM. Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis. 2009;199(3):381–390. doi: 10.1086/595830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy SL, Li H, Mingozzi F, et al. Diverse IgG subclass responses to adeno-associated virus infection and vector administration. J Med Virol. 2009;81(1):65–74. doi: 10.1002/jmv.21360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mingozzi F, Meulenberg JJ, Hui DJ, et al. AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells. Blood. 2009;114(10):2077–2086. doi: 10.1182/blood-2008-07-167510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miao CH. Advances in overcoming immune responses following hemophilia gene therapy. J Genet Syndr Gene Ther. 2011;S1:007. [PMC free article] [PubMed]
- 66.Townsend MJ, Monroe JG, Chan AC. B-cell targeted therapies in human autoimmune diseases: an updated perspective. Immunol Rev. 2010;237(1):264–283. doi: 10.1111/j.1600-065X.2010.00945.x. [DOI] [PubMed] [Google Scholar]
- 67.Mingozzi F, Maus MV, Hui DJ, et al. CD8(+) T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 68.Asuri P, Bartel MA, Vazin T, Jang JH, Wong TB, Schaffer DV. Directed evolution of adeno-associated virus for enhanced gene delivery and gene targeting in human pluripotent stem cells. Mol Ther. 2012;20(2):329–338. doi: 10.1038/mt.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19(6):694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- 70.Stone D, Koerber JT, Mingozzi F, Podsakoff GM, High KA, Schaffer DV. Directed evolution of adeno-associated virus variants that evade human neutralizing antibodies [abstract]. Mol Ther. 2010;18(suppl 1):S3. Abstract 8. [Google Scholar]
- 71.Monteilhet V, Saheb S, Boutin S, et al. A 10 patient case report on the impact of plasmapheresis upon neutralizing factors against adeno-associated virus (AAV) types 1, 2, 6, and 8. Mol Ther. 2011;19(11):2084–2091. doi: 10.1038/mt.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mimuro J, Mizukami H, Hishikawa S, et al. Minimizing the inhibitory effect of neutralizing antibody for efficient gene expression in the liver with adeno-associated virus 8 vectors. Mol Ther. 2013;21(2):318–323. doi: 10.1038/mt.2012.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mount JD, Herzog RW, Tillson DM, et al. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99(8):2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 74.Li C, Hirsch M, Asokan A, et al. Adeno-associated virus type 2 (AAV2) capsid-specific cytotoxic T lymphocytes eliminate only vector-transduced cells coexpressing the AAV2 capsid in vivo. J Virol. 2007;81(14):7540–7547. doi: 10.1128/JVI.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li H, Murphy SL, Giles-Davis W, et al. Pre-existing AAV capsid-specific CD8+ T cells are unable to eliminate AAV-transduced hepatocytes. Mol Ther. 2007;15(4):792–800. doi: 10.1038/sj.mt.6300090. [DOI] [PubMed] [Google Scholar]
- 76.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18(3):185–194. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 77.Li H, Tuyishime S, Wu TL, et al. Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice. Mol Ther. 2011;19(3):536–546. doi: 10.1038/mt.2010.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martino AT, Basner-Tschakarjan E, Markusic DM, et al. Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells. Blood. 2013;121(12):2224–2233. doi: 10.1182/blood-2012-10-460733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao G, Wang Q, Calcedo R, et al. Adeno-associated virus-mediated gene transfer to nonhuman primate liver can elicit destructive transgene-specific T cell responses. Hum Gene Ther. 2009;20(9):930–942. doi: 10.1089/hum.2009.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H, Lasaro MO, Jia B, et al. Capsid-specific T-cell responses to natural infections with adeno-associated viruses in humans differ from those of nonhuman primates. Mol Ther. 2011;19(11):2021–2030. doi: 10.1038/mt.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nguyen DH, Hurtado-Ziola N, Gagneux P, Varki A. Loss of Siglec expression on T lymphocytes during human evolution. Proc Natl Acad Sci USA. 2006;103(20):7765–7770. doi: 10.1073/pnas.0510484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Report on immune responses to adeno-associated virus (AAV) vectors. Office of Biotechnology Activities, NIH Recombinant DNA Advisory Committee Meeting, June 19, 2007. http://oba.od.nih.gov/rdna_rac/rac_meetings.html. Accessed May 20, 2012.
- 83.Hui DJ, Mingozzi F, Basner-Tschakarjan E, et al. Characterization of AAV capsid responses in humans using normal donor splenocytes. Mol Ther. 2007;15:S99. [Google Scholar]
- 84.Chadeuf G, Ciron C, Moullier P, Salvetti A. Evidence for encapsidation of prokaryotic sequences during recombinant adeno-associated virus production and their in vivo persistence after vector delivery. Mol Ther. 2005;12(4):744–753. doi: 10.1016/j.ymthe.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 85.Hui DJ, Basner-Tschakarjan E, Chen Y, et al. Characterization of AAV T cells epitopes presented by splenocytes from normal human donors [abstract]. Mol Ther. 2012;20(suppl 1):S215. Abstract 557. [Google Scholar]
- 86.Hauck B, Murphy SL, Smith PH, et al. Undetectable transcription of cap in a clinical AAV vector: implications for preformed capsid in immune responses. Mol Ther. 2009;17(1):144–152. doi: 10.1038/mt.2008.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li C, Goudy K, Hirsch M, et al. Cellular immune response to cryptic epitopes during therapeutic gene transfer. Proc Natl Acad Sci USA. 2009;106(26):10770–10774. doi: 10.1073/pnas.0902269106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vandenberghe LH, Wang L, Somanathan S, et al. Heparin binding directs activation of T cells against adeno-associated virus serotype 2 capsid. Nat Med. 2006;12(8):967–971. doi: 10.1038/nm1445. [DOI] [PubMed] [Google Scholar]
- 89.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105(11):1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yan Z, Zak R, Luxton GW, Ritchie TC, Bantel-Schaal U, Engelhardt JF. Ubiquitination of both adeno-associated virus type 2 and 5 capsid proteins affects the transduction efficiency of recombinant vectors. J Virol. 2002;76(5):2043–2053. doi: 10.1128/jvi.76.5.2043-2053.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Finn JD, Hui D, Downey HD, et al. Proteasome inhibitors decrease AAV2 capsid derived peptide epitope presentation on MHC class I following transduction. Mol Ther. 2010;18(1):135–142. doi: 10.1038/mt.2009.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Monahan PE, Lothrop CD, Sun J, et al. Proteasome inhibitors enhance gene delivery by AAV virus vectors expressing large genomes in hemophilia mouse and dog models: a strategy for broad clinical application. Mol Ther. 2010;18(11):1907–1916. doi: 10.1038/mt.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nathwani AC, Cochrane M, McIntosh J, et al. Enhancing transduction of the liver by adeno-associated viral vectors. Gene Ther. 2009;16(1):60–69. doi: 10.1038/gt.2008.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci USA. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 97.Nathwani AC, Gray JT, Ng CY, et al. Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver. Blood. 2006;107(7):2653–2661. doi: 10.1182/blood-2005-10-4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Flotte TR, Trapnell BC, Humphries M, et al. Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing α1-antitrypsin: interim results. Hum Gene Ther. 2011;22(10):1239–1247. doi: 10.1089/hum.2011.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mendell JR, Campbell K, Rodino-Klapac L, et al. Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med. 2010;363(15):1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mendell JR, Rodino-Klapac LR, Rosales XQ, et al. Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol. 2010;68(5):629–638. doi: 10.1002/ana.22251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, et al. Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol. 2009;66(3):290–297. doi: 10.1002/ana.21732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herson S, Hentati F, Rigolet A, et al. A phase I trial of adeno-associated virus serotype 1-γ-sarcoglycan gene therapy for limb girdle muscular dystrophy type 2C. Brain. 2012;135(pt 2):483–492. doi: 10.1093/brain/awr342. [DOI] [PubMed] [Google Scholar]
- 103.Velazquez VM, Bowen DG, Walker CM. Silencing of T lymphocytes by antigen-driven programmed death in recombinant adeno-associated virus vector-mediated gene therapy. Blood. 2009;113(3):538–545. doi: 10.1182/blood-2008-01-131375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hudig D, Haverty T, Fulcher C, Redelman D, Mendelsohn J. Inhibition of human natural cytotoxicity by macromolecular antiproteases. J Immunol. 1981;126(4):1569–1574. [PubMed] [Google Scholar]
- 105.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296(5566):301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 106.Buchlis G, Podsakoff GM, Radu A, et al. Factor IX expression in skeletal muscle of a severe hemophilia B patient 10 years after AAV-mediated gene transfer. Blood. 2012;119(13):3038–3041. doi: 10.1182/blood-2011-09-382317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Christine CW, Starr PA, Larson PS, et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McPhee SW, Janson CG, Li C, et al. Immune responses to AAV in a phase I study for Canavan disease. J Gene Med. 2006;8(5):577–588. doi: 10.1002/jgm.885. [DOI] [PubMed] [Google Scholar]
- 109.Simioni P, Tormene D, Tognin G, et al. X-linked thrombophilia with a mutant factor IX (factor IX Padua). N Engl J Med. 2009;361(17):1671–1675. doi: 10.1056/NEJMoa0904377. [DOI] [PubMed] [Google Scholar]
- 110.Cantore A, Nair N, Della Valle P, et al. Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice. Blood. 2012;120(23):4517–4520. doi: 10.1182/blood-2012-05-432591. [DOI] [PubMed] [Google Scholar]
- 111.Finn JD, Nichols TC, Svoronos N, et al. The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy. Blood. 2012;120(23):4521–4523. doi: 10.1182/blood-2012-06-440123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matsushita T, Elliger S, Elliger C, et al. Adeno-associated virus vectors can be efficiently produced without helper virus. Gene Ther. 1998;5(7):938–945. doi: 10.1038/sj.gt.3300680. [DOI] [PubMed] [Google Scholar]
- 113.Kotin RM. Large-scale recombinant adeno-associated virus production. Hum Mol Genet. 2011;20(R1):R2–R6. doi: 10.1093/hmg/ddr141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clark KR, Voulgaropoulou F, Fraley DM, Johnson PR. Cell lines for the production of recombinant adeno-associated virus. Hum Gene Ther. 1995;6(10):1329–1341. doi: 10.1089/hum.1995.6.10-1329. [DOI] [PubMed] [Google Scholar]
- 115.Mease PJ. Improving the routine management of rheumatoid arthritis: the value of tight control. J Rheumatol. 2010;37(8):1570–1578. doi: 10.3899/jrheum.091064. [DOI] [PubMed] [Google Scholar]
- 116.Zhou J, Yang X, Wright JF, High KA, Couto L, Qu G. PEG-modulated column chromatography for purification of recombinant adeno-associated virus serotype 9. J Virol Methods. 2011;173(1):99–107. doi: 10.1016/j.jviromet.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 117.Ayuso E, Mingozzi F, Montane J, et al. High AAV vector purity results in serotype- and tissue-independent enhancement of transduction efficiency. Gene Ther. 2010;17(4):503–510. doi: 10.1038/gt.2009.157. [DOI] [PubMed] [Google Scholar]
- 118.Wright JF. Manufacturing and characterizing AAV-based vectors for use in clinical studies. Gene Ther. 2008;15(11):840–848. doi: 10.1038/gt.2008.65. [DOI] [PubMed] [Google Scholar]
- 119.Mingozzi F, Anguela XM, Pavani G, et al. A novel strategy to circumvent pre-existing humoral immunity to AAV. In: Overcoming pre-existing humoral immunity to AAV using capsid decoys. Sci Transl Med. In press: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Johnson JS, Li C, DiPrimio N, Weinberg MS, McCown TJ, Samulski RJ. Mutagenesis of adeno-associated virus type 2 capsid protein VP1 uncovers new roles for basic amino acids in trafficking and cell-specific transduction. J Virol. 2010;84(17):8888–8902. doi: 10.1128/JVI.00687-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mingozzi F, Liu YL, Dobrzynski E, et al. Induction of immune tolerance to coagulation factor IX antigen by in vivo hepatic gene transfer. J Clin Invest. 2003;111(9):1347–1356. doi: 10.1172/JCI16887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dobrzynski E, Mingozzi F, Liu YL, et al. Induction of antigen-specific CD4+ T-cell anergy and deletion by in vivo viral gene transfer. Blood. 2004;104(4):969–977. doi: 10.1182/blood-2004-03-0847. [DOI] [PubMed] [Google Scholar]
- 123.Franco LM, Sun B, Yang X, et al. Evasion of immune responses to introduced human acid alpha-glucosidase by liver-restricted expression in glycogen storage disease type II. Mol Ther. 2005;12(5):876–884. doi: 10.1016/j.ymthe.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 124.Ziegler RJ, Lonning SM, Armentano D, et al. AAV2 vector harboring a liver-restricted promoter facilitates sustained expression of therapeutic levels of alpha-galactosidase A and the induction of immune tolerance in Fabry mice. Mol Ther. 2004;9(2):231–240. doi: 10.1016/j.ymthe.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 125.Martino AT, Nayak S, Hoffman BE, et al. Tolerance induction to cytoplasmic beta-galactosidase by hepatic AAV gene transfer: implications for antigen presentation and immunotoxicity. PLoS ONE. 2009;4(8):e6376. doi: 10.1371/journal.pone.0006376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cao O, Dobrzynski E, Wang L, et al. Induction and role of regulatory CD4+CD25+ T cells in tolerance to the transgene product following hepatic in vivo gene transfer. Blood. 2007;110(4):1132–1140. doi: 10.1182/blood-2007-02-073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Markusic DM, Herzog RW. Hepatic gene transfer of factor IX reverses inhibitors and protects from anaphylaxis in a murine hemophilia B model [abstract]. Blood. 2011;118(21) Abstract 669. [Google Scholar]
- 128.Mingozzi F, Hasbrouck NC, Basner-Tschakarjan E, et al. Modulation of tolerance to the transgene product in a nonhuman primate model of AAV-mediated gene transfer to liver. Blood. 2007;110(7):2334–2341. doi: 10.1182/blood-2007-03-080093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Finn JD, Ozelo MC, Sabatino DE, et al. Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy. Blood. 2010;116(26):5842–5848. doi: 10.1182/blood-2010-06-288001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu T, Töpfer K, Lin SW, et al. Self-complementary AAVs induce more potent transgene product-specific immune responses compared to a single-stranded genome. Mol Ther. 2012;20(3):572–579. doi: 10.1038/mt.2011.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gao G, Lebherz C, Weiner DJ, et al. Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood. 2004;103(9):3300–3302. doi: 10.1182/blood-2003-11-3852. [DOI] [PubMed] [Google Scholar]
- 132.Haurigot V, Mingozzi F, Buchlis G, et al. Safety of AAV factor IX peripheral transvenular gene delivery to muscle in hemophilia B dogs. Mol Ther. 2010;18(7):1318–1329. doi: 10.1038/mt.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Herzog RW, Mount JD, Arruda VR, High KA, Lothrop CD., Jr Muscle-directed gene transfer and transient immune suppression result in sustained partial correction of canine hemophilia B caused by a null mutation. Mol Ther. 2001;4(3):192–200. doi: 10.1006/mthe.2001.0442. [DOI] [PubMed] [Google Scholar]
- 134.Wang Z, Kuhr CS, Allen JM, et al. Sustained AAV-mediated dystrophin expression in a canine model of Duchenne muscular dystrophy with a brief course of immunosuppression. Mol Ther. 2007;15(6):1160–1166. doi: 10.1038/sj.mt.6300161. [DOI] [PubMed] [Google Scholar]
- 135.Brantly ML, Spencer LT, Humphries M, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther. 2006;17(12):1177–1186. doi: 10.1089/hum.2006.17.1177. [DOI] [PubMed] [Google Scholar]
- 136.Cordier L, Gao GP, Hack AA, et al. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum Gene Ther. 2001;12(2):205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 137.Koo T, Okada T, Athanasopoulos T, Foster H, Takeda S, Dickson G. Long-term functional adeno-associated virus-microdystrophin expression in the dystrophic CXMDj dog. J Gene Med. 2011;13(9):497–506. doi: 10.1002/jgm.1602. [DOI] [PubMed] [Google Scholar]
- 138.Brown BD, Venneri MA, Zingale A, Sergi Sergi L, Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12(5):585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 139.Boisgérault F, Gross DA, Ferrand M, et al. Prolonged gene expression in muscle is achieved without active immune tolerance using microRNA 142.3p-regulated rAAV gene transfer. Hum Gene Ther. 2013 doi: 10.1089/hum.2012.208. 24(4):393-405. [DOI] [PubMed] [Google Scholar]
- 140.Wang Z, Storb R, Halbert CL, et al. Successful regional delivery and long-term expression of a dystrophin gene in canine muscular dystrophy: a preclinical model for human therapies. Mol Ther. 2012;20(8):1501–1507. doi: 10.1038/mt.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Su LT, Gopal K, Wang Z, et al. Uniform scale-independent gene transfer to striated muscle after transvenular extravasation of vector. Circulation. 2005;112(12):1780–1788. doi: 10.1161/CIRCULATIONAHA.105.534008. [DOI] [PubMed] [Google Scholar]
- 142.Toromanoff A, Adjali O, Larcher T, et al. Lack of immunotoxicity after regional intravenous (RI) delivery of rAAV to nonhuman primate skeletal muscle. Mol Ther. 2010;18(1):151–160. doi: 10.1038/mt.2009.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li H, Haurigot V, Doyon Y, et al. In vivo genome editing restores haemostasis in a mouse model of haemophilia. Nature. 2011;475(7355):217–221. doi: 10.1038/nature10177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Russell DW. AAV vectors, insertional mutagenesis, and cancer. Mol Ther. 2007;15(10):1740–1743. doi: 10.1038/sj.mt.6300299. [DOI] [PubMed] [Google Scholar]
- 145.Donsante A, Miller DG, Li Y, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317(5837):477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 146.Li H, Malani N, Hamilton SR, et al. Assessing the potential for AAV vector genotoxicity in a murine model. Blood. 2011;117(12):3311–3319. doi: 10.1182/blood-2010-08-302729. [DOI] [PMC free article] [PubMed] [Google Scholar]