Abstract
BACKGROUND
Emerging evidence has suggested that the capability to sustain tumor formation, growth, and chemotherapy resistance in ovarian as well as other human malignancies exclusively resides in a small proportion of tumor cells termed cancer stem cells. During the characterization of CD44+ ovarian cancer stem cells, we found a high expression of the genes encoding for claudin-4. Because this tight junction protein is the natural high-affinity receptor for Clostridium perfringens enterotoxin (CPE), we have extensively investigated the sensitivity of ovarian cancer stem cells to CPE treatment in vitro and in vivo.
METHODS
Real-time polymerase chain reaction and flow cytometry were used to evaluate claudin-3/-4 expression in ovarian cancer stem cells. Small interfering RNA knockdown experiments and MTS assays were used to evaluate CPE-induced cytotoxicity against ovarian cancer stem cell lines in vitro. C.B-17/SCID mice harboring ovarian cancer stem cell xenografts were used to evaluate CPE therapeutic activity in vivo.
RESULTS
CD44+ ovarian cancer stem cells expressed claudin-4 gene at significantly higher levels than matched autologous CD44− ovarian cancer cells, and regardless of their higher resistance to chemotherapeutic agents died within 1 hour after exposure to 1.0 μg/mL of CPE in vitro. Conversely, small-interfering RNA-mediated knockdown of claudin-3/-4 expression in CD44+ cancer stem cells significantly protected cancer stem cells from CPE-induced cytotoxicity. Importantly, multiple intraperitoneal administrations of sublethal doses of CPE in mice harboring xenografts of chemotherapy-resistant CD44+ ovarian cancer stem cells had a significant inhibitory effect on tumor progression leading to the cure and/or long-term survival of all treated animals (ie, 100% reduction in tumor burden in 50% of treated mice; P < .0001).
CONCLUSIONS
CPE may represent an unconventional, potentially highly effective strategy to eradicate chemotherapy-resistant cancer stem cells.
Keywords: ovarian neoplasms, cancer stem cells, Clostridium perfringens, claudin-3, claudin-4
Epithelial ovarian carcinoma (EOC) remains the cancer with the highest mortality rate among gynecological tumors.1 At the time of diagnosis, ⅔ of patients have advanced disease, and unfortunately, after their initial response to the combination of surgery and platinum-based chemotherapy, the majority of ovarian cancer patients develop recurrence and inevitably die from the development of chemotherapy-resistant disease.2 Thus, the identification of novel therapeutic strategies against chemotherapy-resistant/recurrent ovarian cancer remains a high priority.
Emerging evidence has recently been provided to suggest that the capability to sustain tumor formation, growth, and chemotherapy-resistance in ovarian as well as other human malignancies exclusively resides in a small proportion of tumor cells termed cancer stem cells or tumor-initiating cells.3–6 The stem cell-like phenotype of tumor-initiating cells and their limited number within the bulk of the tumor may lead to disease relapse, although the primary lesion is eradicated by conventional therapies; hence, the identification and characterization of tumor stem cells remains of paramount importance for the development of therapeutic strategies capable of affecting the survival of tumor-initiating as well as nontu-morigenic cells.
In an attempt to unravel the mechanisms for repair and chemotherapy resistance of these highly lethal gynecologic tumors, several research groups have recently described the identification and characterization of the molecular phenotype of human ovarian cancer stem cells.7–10 In a large number of studies, CD44 has been extensively reported as a marker that can enrich for cancer stem cells in multiple solid tumors, and its specific correlation with ovarian cancer stem cells has been consistently established.7–10 Accordingly, in this study, cancer stem cells were isolated from ascites and solid tumors of multiple ovarian cancer patients and characterized as CD44+/MyD88+ cells showing constitutive nuclear factor κ B (NF-KB) activity, cytokine and chemokine production, high capacity for repair, resistance to conventional chemotherapies, resistance to tumor necrosis factor α-mediated apoptosis, capacity to form spheroids in suspension, and more importantly, ability to recapitulate in vivo the original tumor.
Of great interest, we found CD44+ ovarian cancer stem cells to express high levels of the gene encoding the tight junction protein claudin-4. Because this protein has been shown to represent the high-affinity natural receptor for Clostridium perfringens enterotoxin (CPE), and is capable of mediating CPE binding and cytolysis,11,12 in this study, we have: 1) carefully analyzed and quantified by quantitative real-time polymerase chain reaction (qRT-PCR) and flow cytometry the expression levels of claudin-3/-4 receptors in CD44+ cancer stem cell populations derived from multiple freshly explanted ovarian tumors, 2) tested the ability of CPE to kill chemotherapy-resistant ovarian CD44+ cancer stem cells overexpressing claudin-4 in vitro, 3) investigated in functional assays the effects of claudin-3/-4 receptors knockdown on CPE-induced CD44+ cancer stem cell toxicity in vitro, and 4) studied the in vivo efficacy and toxicity of intraperitoneal (i.p.) CPE therapy in SCID mice harboring xenografts of highly relevant clinical models of chemotherapy-resistant freshly explanted ovarian CD44+ cancer stem cells. We report for the first time that ovarian CD44+ cancer stem cells overexpress the high-affinity claudin-4 CPE receptor and that these chemotherapy-resistant cells are highly sensitive to CPE treatment in vitro. More importantly, we report that in vivo therapy with repeated i.p. injections of well-tolerated doses of CPE induces the cure or long-term survival of animals harboring established chemotherapy-resistant ovarian CD44+ cancer stem cell-initiated tumors. CPE-mediated therapy may thus represent a novel, potentially highly effective strategy for the eradication of ovarian CD44+ cancer stem cells resistant to chemotherapy.
MATERIALS AND METHODS
CPE Production
CPE production and purification was performed as previously described.13 Purified, endotoxin-free CPE protein was sterilized by 0.2 μm filtration and frozen in aliquots at −70°C before its use in in vitro and in vivo studies.
Ovarian Cancer Stem Cell Lines and Culture Conditions
Primary cultures of human ovarian cancer cells were obtained from ascites or tumor tissue at the time of primary or secondary (ie, recurrence) tumor debulking and used to isolate and culture CD44+ cancer stem cells as previously described.9,10,14 Characteristics of patients from whom CD44+ cancer stem cells were obtained are described in Table 1. Briefly, CD44+ cancer stem cells were isolated using a 2-step process: 1) staining with anti-CD44 fluorescein isothiocyanate (FITC) (eBioscience, San Diego, Calif) and 2) isolation using anti-FITC microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to manufacturers’ instructions. For the in vitro and in vivo studies described below, we used a panel of freshly isolated CD44+ cancer stem cell lines derived from ovarian cancer patients harboring advanced stage tumors (ie, stage III-IV) with serous papillary and clear cell histology (Table 1), as well as clones of previously characterized CD44+/NF-KB-high/MyD88+ ovarian cancer stem cells.9,10 The use of patients’ samples was approved by Yale University’s Human Investigations Committee. Control cell lines evaluated in our experiments included matched autologous CD44− ovarian cancer cells (ie, obtained from the same tumor from which CD44+ cancer stem lines were isolated), Vero cells, normal ovarian epithelium, normal cervical keratinocytes, Epstein-Barr–transformed B lymphocytes, and human fibroblasts. Only primary cancer stem cell cultures that had at least 90% viability and contained >95% CD44+ cells after sorting were used for the in vitro and in vivo experiments.
Table 1.
Patient Characteristics
| Patient | Age, y | Race | Grade | Stagea | Histology | Source | CD44%b |
|---|---|---|---|---|---|---|---|
| OVA 1 | 33 | C | G3 | IIC | OSPC | Ovary | 3.13 |
| OVA 2 | 79 | C | G3 | IIC | OSPC | Omentum | 5.46 |
| OVA 3 | 37 | C | G3 | IIIC | OSPC | Ovary | 2.46 |
| OVA 4 | 32 | C | G3 | IC | Clear cell | Ovary | 28.47 |
| OVA 5 | 64 | C | G3 | IIIC | OSPC | Ascites | 6.62 |
| OVA 1R | 33 | C | G3 | Recurrence | OSPC | Ascites | 22.66 |
| OVA 2R | 55 | C | G3 | Recurrence | OSPC | Ascites | 14.09 |
| OVA 3R | 78 | C | G3 | Recurrence | OSPC | Ascites | 15.14 |
| OVA 4R | 69 | C | G3 | Recurrence | OSPC | Omentum | 21.94 |
| OVA 5R | 53 | C | G3 | Recurrence | OSPC | Ascites | 6.43 |
Abbreviations: C, Caucasian; OSPC, ovarian serous papillary carcinoma.
International Federation of Gynecology and Obstetrics operative staging system.
Mean percentage of CD44+ cancer stem cells ± standard error = 12.6% ± 2.9%.
RNA Extraction and qRT-PCR
RNA isolation from primary freshly isolated CD44+ cancer stem cell lines, cancer stem cell clones, and control cells was performed using TRIzol Reagent (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions as previously described.13 qRT-PCR was performed with a 7500 Real-Time PCR System (Applied Biosystems, Foster City, Calif), using the manufacturer’s recommended protocol, to evaluate expression of claudin-3/-4 in all samples. The primers for claudin-3/-4 were obtained from Applied Biosystems as Assay-on-Demand products. Assays ID were Hs00265816_s1 (claudin-3) and Hs00433616_s1 (claudin-4). The comparative threshold cycle method (PE Applied Biosystems) was used to determine gene expression in each sample relative to the value observed in the nonmalignant ovarian epithelial cells, using glyceraldehyde-3-phosphate dehydrogenase (Assay-on-Demand Hs99999905_m1) mRNA as internal control.
Flow Cytometry Experiments
We used fluorescence-activated cell sorting analysis to confirm high surface expression of the CPE receptors on primary freshly isolated CD44+ cancer stem cell lines and control cells using a FITC-labeled Clostridium perfringens carboxy-terminal fragment peptide (CPE290-319). The FITC-CPE290-319 peptide was synthesized by Invitrogen, with the sequence FITC-SLDAGQYVLVMKANSSYSG-NYPYSILFQKF and a purity level >95%. Control cells included CD44− ovarian cancer cells obtained from the same patients from whom CD44+ cancer stem cell lines were isolated and normal cells (ie, peripheral blood lymphocytes, Epstein-Barr–transformed B lymphocytes, and human fibroblasts), which show low and/or negligible claudin-3/-4 receptor expression. Vero cells, expressing high levels of claudin-3/-4 receptors, were used as positive controls in all assays. Briefly, approximately 2.5 × 105 cells were resuspended with 50 μL phosphate-buffered saline (PBS) 1% bovine serum albumin that contained 2 μg of FITC-labeled CPE peptide. Cells were then incubated for 1 hour at room temperature. Cells stained with FITC-labeled CPE peptide were then washed with PBS, resuspended in 500 μL PBS, and immediately analyzed by FACSCalibur (BD Biosciences, San Diego, Calif).
Chemotherapy Resistance MTS Assay In Vitro
Freshly isolated CD44+ cancer stem cell lines and control cells were seeded in 96-well culture plates at a dose of 2500 cells per well and allowed to attach for 24 hours. Media were removed after 24 hours and replaced with 100 μL of culture medium or the same volume of medium containing various concentrations of carboplatin or paclitaxel (ie, from 1 μM to 1000 μM and from 1 nM to 20,000 nM, respectively). After 72-hour incubation, 20 μL of CellTiter 96 AQueous One Solution Reagent containing a tetrazolium compound (3-[4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H tetrazolium, inner salt; MTS) (Promega Corporation, Madison, Wis) was added, and incubated, according to the manufacturer’s instructions. Absorbance was measured by a 96-well VERSA max plate-reader (Molecular Devices, Sunnyvale, Calif) at a reference wavelength of 490 nm. Each drug concentration was obtained in 3 replicate wells, and each experiment was performed in parallel a minimum of 3× using cell lines established from surgical biopsies.
CPE Treatment of Cancer Stem Cell Clones and Cancer Stem Cell Lines and Trypan Blue Exclusion Test
Freshly isolated CD44+ ovarian cancer stem cell lines and CD44− matched ovarian cancer cells obtained from the same patients from whom cancer stem cell lines were obtained were isolated and seeded at a concentration of 1 × 105 cells/well into 6-well culture plates (Costar, Cambridge, Mass) with the appropriate medium for 24 hours. After washing and renewal of the medium, CPE was added to final concentrations ranging from 0.01 to 1.0 μg/mL. After incubation for 60 minutes to 24 hours at 37°C, 5% CO2, floating cells were removed and stored, and attached cells were trypsinized and pooled with the floating cells. After staining with trypan blue, viability was determined by counting the number of trypan blue-positive cells and the total cell number.
Small Interfering RNA Knockdown Experiments
Claudin-3/-4 specific small interfering (siRNA) oligonucleotides were purchased from Ambion, Inc. (Austin, Tex). Briefly, freshly isolated CD44+ ovarian cancer stem cell lines cultured in 6-well plates were transfected with siRNA duplexes using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer’s instructions. Twelve nanomolars of each specific siRNA were incubated with 5 μL of Lipofectamine RNAiMAX. Mock transfections and nonspecific siRNA duplexes were used as negative controls. CD44+ cancer stem cells were treated for 72 hours (ie, the time we found required for maximal down-regulation of claudin-3/-4, based on qRT-PCR), after which they were either collected for RNA extraction and qRT-PCR experiments, as described above, or exposed to different concentrations of CPE in MTS cytotoxicity assays. Briefly, CD44+ cancer stem cells were detached, washed, and seeded in 96-well culture plates at optimum density and allowed to attach for 3 hours in 80 μL of culture medium. Twenty microliters of medium containing recombinant CPE was then added to reach a final concentration of 0.5 μg/mL or 1 μg/mL of the toxin. After 1 hour of CPE incubation, 20 μL of CellTiter 96 AQueous 1 Solution Reagent MTS was added to the wells, and CD44+ cancer stem cells were incubated according to the manufacturer’s instructions for an additional 1 hour before absorbance was measured by a 96-well VERSA max plate-reader (Molecular Devices) at a reference wavelength of 490 nm. Each CPE concentration was obtained in 3 replicate wells, and each experiment was repeated a minimum of 3×.
In Vivo CPE Treatment of SCID Mouse CD44+ Ovarian Cancer Stem Cell Tumor Xenografts
C.B-17/SCID female mice 5 to 7 weeks old were obtained from Harlan Sprague-Dawley (Indianapolis, Ind) and used to generate ovarian CD44+ cancer stem cell tumor xenografts. The Yale University Institutional Animal Care and Use Committee approved all in vivo studies described. Briefly, the ovarian CD44+ cancer stem cell line was injected i.p. at a dose of 5 × 106 into C.B-17/SCID mice in groups of 5. One week after i.p. tumor injection, mice were injected i.p. with 7.5 μg of CPE dissolved in 1 mL of sterile saline at 72- to 96-hour intervals for a total of 8 injections, whereas control animals were injected with sterile saline. All animals were observed twice daily and weighed weekly, and survival was monitored. In addition, groups of 3 mice injected i.p. at a dose of 5 × 106 ovarian CD44+ cancer stem cells were killed at 1, 2, 3, and 4 weeks for necropsy and pathologic analysis. The remaining animals were killed and examined just before they died of intraperitoneal carcinomatosis and malignant ascites.
Statistics
Statistical differences in siRNA knockdown experiments of MTS cytotoxicity, trypan blue cytotoxicity, and in claudin-3/-4 expression between isolated CD44+ ovarian cancer stem cell lines, cancer stem cell clones, CD44− matched autologous ovarian cancer cells, and normal control cells were tested using Student t test. For the response to chemotherapy, the concentration that inhibits 50% (IC50) for a given drug was determined from the dose-response curve fit to the cell count data using the XLFit program (ID Business Solutions, Parsippany, NJ). The Lavenburg-Marquardt algorithm was used to obtain and calculate the IC50 and IC50 curves for each drug in each cell line using a nonlinear regression curve fit. To facilitate statistical inference on IC50 fold changes, IC50s were transformed to log10 units. Differences in log10(IC50)s between CD44+ ovarian cancer stem cell lines and cancer stem cell clones versus matched CD44− autologous ovarian cancer cells were assessed for significance via unequal-variance t test. For the ovarian CD44+ cancer stem cell animal model, survival was plotted using Kaplan-Meier methods and compared using the log-rank test. A P value < .05 was used for statistical significance.
RESULTS
Claudin-3 and Claudin-4 Transcript Levels in Isolated CD44+ Cancer Stem Cells and in Matched CD44− EOC Cell Lines
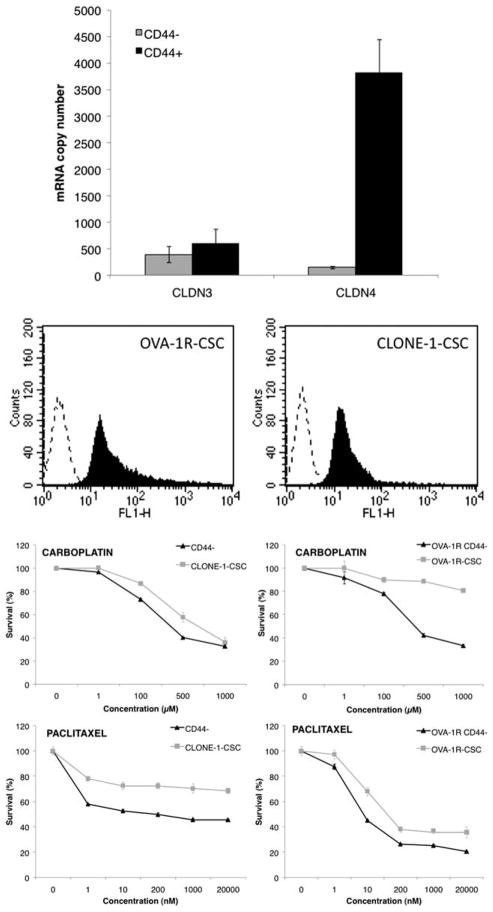
We used qRT-PCR assays to get highly sensitive measurements of claudin-3/-4 expression in normal ovarian epithelium, CD44+ cancer stem cells, and CD44− matched autologous tumor cells isolated from fresh ovarian tumor tissues and/or ascites. Table 1 describes patient characteristics and the different percentages of CD44+ cancer stem cells found in the ovarian cancer specimens tested. Both claudin-3 and claudin-4 genes were found to be significantly overexpressed in primary ovarian carcinomas when compared with normal ovarian epithelium control cells. Claudin-3 had a mean ± standard error (SE) copy number of 1094 ± 371 in the tumor samples, compared with 3 ± 1 in the normal ovarian epithelium (P = .002). Claudin-4 had a mean ± SE copy number of 143 ± 29 in the tumor samples, compared with 6 ± 3 in the normal ovarian epithelium (P = .0001). Similarly, claudin-3/-4 expression was low in all other control tissues examined, including normal endometrial epithelium, normal cervical keratinocytes, and normal human fibroblasts (data not shown). Of great interest, as shown in Figure 1 (Top), we found CD44+ cancer stem cell populations and CD44+ cancer stem cell clones to express significantly higher levels of the claudin-4 receptor when compared with the matched CD44− ovarian tumor specimens (P = .001). In contrast, claudin-3 was not found to be significantly overexpressed in the CD44+ cancer stem cell populations and CD44+ clones when compared with matched CD44− autologous tumor cells (P = .5, Fig. 1, Top).
Figure 1.
(Top) Quantitative real-time polymerase chain reaction analysis of claudin-3 and claudin-4 expression is shown. The Y axis represents the fold induction relative to normal ovary expression. The X axis represents CD44+ cancer stem cells and autologous matched CD44− control sample tested for claudin-3 and claudin-4. Claudin-4 expression was significantly higher in CD44+ cancer stem cell lines and cancer stem cell clones when compared with matched autologous CD44− control samples (P < .004). (Middle) Representative flow cytometry histograms are shown of claudin-3 and claudin-4 expression in a representative CD44+ cancer stem cell population (OVA-1R-CSC, left panel) and 1 CD44+ cancer stem cell clone (CLONE-1-CSC, right panel) after labeling with fluorescein isothiocyanate (FITC)-conjugated-C-CPE290-319 peptide. Dashed lines indicate isotype; solid black peaks indicate FITC-conjugated-C-CPE290-319 peptide. (Bottom) Representative dose-response curves after exposure to carboplatin and paclitaxel of OVA-1R-CSC line and matched autologous CD44− control tumor cells (right panel) and of 1 representative cancer stem cell clone (CLONE-1-CSC) versus matched autologous CD44− control tumor cells (left panel) are shown. Survival was assessed by MTS assay as described in the Materials and Methods section. Each point on the cell line graph represents the mean of 3 estimations ± standard error. A significant difference was observed in the IC50 of the CD44+ cancer stem cell populations versus autologous CD44− matched tumor cells when treated with carboplatin or paclitaxel (P < .01).
CPE Surface Receptor Expression by Flow Cytometry in Isolated CD44+ Cancer Stem Cells
To determine whether the high expression of the claudin-4 gene detected by qRT-PCR in primary ovarian CD44+ cancer stem cells also resulted in high expression of the protein on the surface of cancer stem cells, we evaluated the ability of the FITC-conjugated CPE peptide to label freshly isolated CD44+ ovarian cancer stem cell cultures in flow cytometry experiments. As representatively demonstrated in Figure 1 (Middle) for a primary ovarian CD44+ cancer stem cell and 1 cancer stem cell clone, FITC-conjugated CPE peptide was found to be highly reactive against all primary CD44+ ovarian cancer stem cell lines tested, with positivity in 100% of tumor cells and a mean fluorescence intensity (MFI ± standard deviation [SD]) of 60 ± 15. These values of MFI were not significantly different from those found by testing CPE receptor expression on Vero cells (ie, positive controls, data not shown). Consistent with qRT-PCR results, negligible or no binding was detected by flow-cytometry against normal ovarian cell controls expressing low levels of claudin-3 and/or claudin-4 (ie, normal ovarian epithelium, Epstein-Barr–transformed B lymphocytes, and fibroblasts, data not shown).
CD44+ Cancer Stem Cells Are More Resistant to Chemotherapy When Compared With CD44− Matched Autologous Tumor Cells by MTS Chemoresponse Assay
We next compared the effects of carboplatin and paclitaxel single-agent chemotherapy (ie, the gold standard chemotherapy agents used in the clinic in ovarian cancer patients) against multiple primary CD44+ ovarian cancer stem cell lines, cancer stem cell clones, and matched CD44− ovarian cancer control cells by MTS assays. Consistent with previously reported results,9,10 we found primary CD44+ ovarian cancer stem cells to be consistently and significantly more resistant to carboplatin and paclitaxel when compared with CD44− matched cancer cells in multiple experiments (P = .01). Results are displayed as the average ± SD from 3 independent experiments. As representatively shown in Figure 1 (Bottom) for a CD44+ ovarian cancer stem cell population and a CD44+ ovarian cancer stem cell clone, a higher IC50 (ie, higher resistance) to carboplatin and paclitaxel was consistently detected in the CD44+ cancer stem cell populations when compared with their matched CD44− ovarian cancer control cell cultures (Fig. 1, Bottom; P = .01).
Effects of CPE on Primary CD44+ Ovarian Cancer Stem Cells
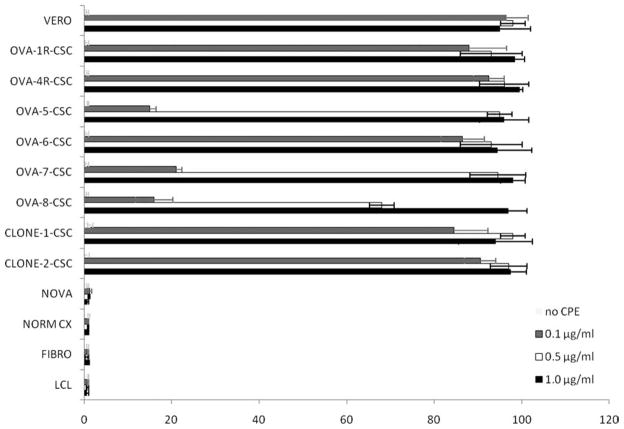
On the basis of the high expression of claudin-4 found at mRNA and protein level on primary CD44+ ovarian cancer stem cell lines, it was expected that ovarian CD44+ cancer stem cells expressing claudin-4 would be sensitive to CPE-mediated lysis. However, it was important to demonstrate this directly on freshly isolated human CD44+ ovarian cancer stem cells, and cancer stem cell clones were showed to be highly resistant to chemotherapy. Accordingly, the sensitivity of primary ovarian CD44+ cancer stem cell cultures to CPE-mediated cytolysis was tested along with an appropriate claudin-3 and claudin-4–expressing positive control (ie, Vero cells) and negative controls that do not express detectable levels of either claudin-3 or claudin-4. As shown in Figure 2, regardless of their high resistance to chemotherapy, all ovarian CD44+ cancer stem cell populations tested (ie, a total of 8 different ovarian CD44+ cancer stem cell lines) were found to be highly sensitive to CPE-mediated cytolysis (P = .001). The cytotoxic effect was dose dependent and was positively correlated to the levels of CPE-receptor expression as tested by qRT-PCR in tumor samples. Importantly, no ovarian CD44+ cancer stem cell population was found viable after 24 hours of exposure to CPE at the concentration of 1.0 μg/mL (Fig. 2). In contrast, all normal controls tested, including ovarian epithelium, cervical keratinocytes, human fibroblasts, and human lymphocytes, lacking or expressing negligible levels of claudin-3/-4, were not affected by CPE exposure (Fig. 2).
Figure 2.
Representative dose-dependent Clostridium perfringens enterotoxin (CPE)-mediated cytotoxicity of multiple CD44+ cancer stem cell (CSC) populations and CSC clones, positive control cells (ie, VERO cells), negative controls including normal ovarian epithelium (NOVA), normal cervical keratinocytes (NORM CX), normal human fibroblasts (FIBRO), and lymphoblastoid B cells (LCL) after 24 hours incubation with CPE is shown. With no exception, all 8 CD44+ CSC populations tested were found highly sensitive to CPE exposure in vitro (P = .001). Viability was determined by counting the number of trypan blue-positive cells and the total cell number and comparing such numbers to those of the untreated control cells (ie, 100% viable cells).
Knockdown of Claudin-3 and Claudin-4 Expression in CD44+ Ovarian Cancer Stem Cells Confers Resistance to CPE-Mediated Cytotoxicity
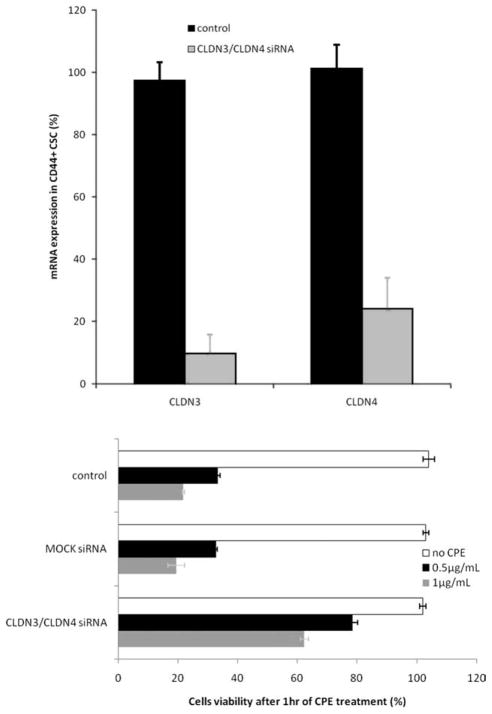
To experimentally demonstrate that expression of claudin-3/-4 is required for the biological effects of CPE against ovarian CD44+ cancer stem cells in functional assays, we used siRNA to knock down claudin-3/-4 expression in multiple ovarian CD44+ cancer stem cell populations before their exposure to lethal doses of CPE. Transient siRNA transfections were carried using claudin-3 and claudin-4–specific siRNA duplexes, and silencing efficiency was evaluated by qRT-PCR at different time points. As representatively shown in Figure 3 (Top) for the ovarian CD44+ cancer stem cell line, transfection with claudin-specific siRNA duplexes efficiently repressed the expression of claudin-3 and claudin-4 by 90.4% ± 6.1% (mean ± SD) and 72.5% ± 9.1% (mean ± SD), respectively, at 72 hours, whereas expression remained unchanged in mock or control siRNA-treated cells (data not shown). Importantly, as shown in Figure 3 (Bottom) for a representative ovarian CD44+ cancer stem cell population, down-regulation of claudin-3/-4 expression was able to significantly increase CD44+ cancer stem cell resistance to CPE exposure when compared with untreated or mock transfected control cells (P < .004).
Figure 3.
(Top) Representative knockdown of the Clostridium perfringens enterotoxin (CPE) receptors by anticlaudin-3 and anticlaudin-4 small interfering RNA (siRNA) in ovarian cancer stem cells (CSCs) is shown. The levels of claudin-3 and claudin-4 mRNA in OVA-1R-CSC CD44+ cells transfected with claudin-3 and claudin-4 siRNA duplexes (gray bars) were analyzed using quantitative real-time polymerase chain reaction and expressed as percentage of control cells (black bars). (Bottom) Representative dose-dependent CPE-mediated cytotoxicity of OVA-1R-CSCs transfected with claudin-3/-4 siRNA duplexes after 1 hour exposure to 0.5 μg/mL (black bars) and 1 μg/mL (gray bars) of CPE (P = .004) is shown. Mock-transfected cells and untransfected cancer stem cells were used as controls. Results are presented as percentage of viable cells when compared with number of untreated control cells (ie, 100% viable cells).
Eradication of CD44+ Ovarian Cancer Stem Cell Xenografts in SCID Mice by Administration of CPE In Vivo
For in vivo confirmation of our in vitro data, we developed xenograft tumors in SCID mice by i.p. injection with ovarian CD44+ cancer stem cells. This ovarian tumor was selected as a source of ovarian CD44+ cancer stem cells because of its high resistance to multiple chemotherapeutic agents in vivo and in vitro (as tested in chemosensitivity MTS assay, Fig. 1 (Bottom), and extreme drug resistance assay by Oncotech Inc., Irvine, Calif; data not shown) as well as its high content of CD44+/NF-KB-high/MyD88+ cancer stem cells (ie, 22.6%, Table 1). Primary ovarian CD44+ cancer stem cells grew progressively as numerous serosal nodules adherent to virtually all intra-abdominal organs and exhibited the capacity for local tissue invasion and formation of malignant ascites 2 to 3 weeks from injection. Necropsies revealed massive hemorrhagic ascites and numerous tumor nodules, measuring 1 to 8 mm in diameter, studding the entire peritoneal surface and implanting the serosa of virtually all intra-abdominal organs. Our previous toxicology studies in mice have reported 0.5 μg/g of CPE administered i.p. to be a well-tolerated and safe dose in 100% of the animals (ie, 16.5 ± 1.0 g female mice).13 Accordingly, in the in vivo experiments, groups of mice harboring established ovarian tumor burden xenografts (ie, 1 week after ovarian CD44+ cancer stem cell injection) were treated with repeated i.p. CPE injections (ie, 7.5 μg of CPE in 500 μL of sterile saline) every 72 to 96 hours for a total of 8 administrations. Control mice harboring ovarian CD44+ cancer stem cells received saline alone. CPE injections were well tolerated, and no adverse events were observed throughout the complete treatment protocol either in control mice receiving CPE alone or in CPE-treated mice harboring tumor burden. Mice harboring ovarian CD44+ cancer stem cells treated with saline all died within 10 weeks from tumor injection, with a mean survival of 54 days (Fig. 4). In contrast, animals treated with multiple i.p. CPE injections survived significantly longer than control animals (P < .0001, Fig. 4). Importantly, 4 of 8 (50%) of the mice treated with i.p. injections of CPE remained alive and free of detectable tumor for the entire duration of the study period (ie, over 150 days, P < .0001).
Figure 4.
Survival of C.B-17/SCID mice after intraperitoneal (i.p.) injection of 5 × 106 viable ovarian CD44+ cancer stem cells is shown. Animals harboring ovarian CD44+ cancer stem cell tumors established for 1 week were injected i.p. with 7.5 μg of Clostridium perfringens enterotoxin (CPE) as described in the Material and Methods section for a total of 8× 72 to 96 hours apart. Mice were evaluated on a daily basis and sacrificed when showing significant sign of disease and/or disease-induced distress as per Yale Institutional Animal Care and Use Committee regulations. The log-rank test yielded P < .0001 for the differences in survival.
DISCUSSION
Multiple human cancers including, but not limited to, leukemia, brain, breast, ovarian, prostate, and pancreatic tumors have recently been reported to contain stem-like cancer cells called cancer stem cells.4–6 Although it is still open to question whether tumors derive from organ stem cells that retain self-renewal properties but acquire epigenetic and genetic changes required for tumorigenicity or whether tumor stem cells are proliferative progenitors that acquire self-renewal capacity, the identification of molecules differentially or uniquely expressed in human cancer stem cells may provide novel diagnostic and/or therapeutic markers critical for the elimination of this fraction of cancer cells, which is highly resistant to chemotherapy and therefore likely responsible for the recurrence and death of the majority of patients diagnosed with cancer.
Consistent with this hypothesis, several groups have reported the identification and molecular characterization of ovarian cancer stem cells.7–10,15–18 Multiple putative ovarian cancer stem cell markers including but not limited to CD133 and ALDH1 have recently been identified15–18; in the present study, ovarian cancer stem cells were identified and enriched as CD44+ cells present in primary, meta-static, and recurrent ovarian tumors, as well as in malignant ascites.7–10,19 Ovarian cancer stem cells are characterized by a unique genetic profile8–10 and microRNA signatures19 that confer the distinctive characteristics of cancer stem cells in terms of their tumorigenicity, resistance to chemotherapy, differentiation potential both in vitro and in vivo, and capacity to promote a proinflammatory microenvironment.7–10,19
During the cloning and further characterization of the molecular phenotype of CD44+/NF-KB-high/MyD88+ ovarian cancer stem cells, we found a high expression of the gene encoding for claudin-4. This tight junction protein is the natural high affinity human receptor for the cytotoxic effect of CPE, a single polypeptide of 35 kd comprised of 319 amino acids that is associated with C. perfringens type A food poisoning.11,12 Because CPE is able to trigger the lysis of claudin-4–positive epithelial cells by initiating massive permeability changes, osmotic cell ballooning, and lysis within several minutes,11,12,20–22 whereas mammalian cells that do not express the high-affinity CPE receptor (ie, the majority of normal tissues in the body) fail to bind CPE and are not susceptible to CPE cytotoxicity,11–13,20,21 to target ovarian CD44+ cancer stem cells based on their high levels of claudin-4 is potentially a novel, unconventional, and highly effective therapeutic approach to eradicate chemotherapy-resistant/residual ovarian disease.
In this study, we have extensively investigated the expression of claudin-3/-4 in CD44+ ovarian cancer stem cells and tested their sensitivity to CPE treatment in vitro and in vivo. Our results demonstrate for the first time a high expression of the high-affinity CPE receptor (claudin-4) at both RNA and protein levels in multiple primary CD44+/NF-KB-high/MyD88+ ovarian cancer stem cell populations. These results are consistent with previous reports from our own group demonstrating a high expression of the CPE receptors in multiple primary ovarian cancer cell lines characterized by a high resistance to chemotherapy,13 as well as the study evaluating the proteomes of cisplatin-resistant ovarian cancer cells by Stewart et al, who also found claudin-4 as 1 of the top differentially expressed proteins in cisplatin-resistant ovarian tumors.23 Importantly, all CD44+/NF-KB-high/MyD88+ ovarian cancer stem cell populations evaluated, regardless of their higher resistance to chemotherapy when compared with autologous CD44− matched ovarian cancer cells, were found to be sensitive to CPE-mediated killing in vitro. This was in strong contrast with the lack of sensitivity of normal ovarian epithelium as well as other normal control cells to CPE-mediated cytolysis. These findings are likely explained by a limited expression of claudin-4 in normal epithelia compared with chemotherapy-resistant CD44+ cancer stem cells. Accordingly, an increased resistance to CPE was consistently noted in all CD44+ cancer stem cell populations tested after transfection with siRNA to knock down claudin-3/-4 expression. These data suggest that claudin-3/-4 are indeed required for mediating the biological effects of CPE on CD44+ ovarian cancer stem cells.
Importantly, pharmacologic studies in ovarian cancer patients have demonstrated a marked therapeutic advantage to the intraperitoneal delivery of drugs and biological agents combined with a significant reduction in systemic toxicity resulting from i.p. drug administration when compared with an identical dose of the drug given intravenously.24 These clinical observations, combined with the finding that ovarian cancer remains confined to the peritoneal cavity for much of its natural history, suggest that i.p. administration of CPE in human patients harboring chemotherapy-resistant/residual disease (ie, a population of tumor cells potentially enriched in CD44+ cancer stem cells) may result in reduced toxicity and better therapeutic responses compared with an identical dose of CPE given intravenously. Accordingly, we have also conducted a careful evaluation of the efficacy and toxicity of i.p. injections of CPE in vivo in a clinically relevant animal model of CD44+ ovarian cancer stem cells derived from the biopsy of a patient harboring recurrent chemotherapy-resistant ovarian carcinoma and confirmed to be highly resistant in vitro by multiple chemoassays. We found that multiple i.p. injections of sublethal doses of CPE in vivo were well tolerated, and no adverse events were observed throughout the complete treatment protocol either in control mice receiving CPE alone or CPE-treated mice harboring CD44+ ovarian cancer stem cell xenografts. Thus, these data demonstrate that CPE doses found effective in vitro to kill ovarian CD44+ cancer stem cells may be safely administered i.p. in mice harboring ovarian cancer disease. More importantly, we found that survival of mice harboring an established burden of chemotherapy-resistant ovarian CD44+ cancer stem cells was significantly prolonged in all treated animals, with 50% of mice remaining alive and free of detectable tumor for the entire duration of the study (ie, >150 days). Collectively, these results provide strong experimental evidence to suggest that CPE-based therapy may have great potential for the targeted treatment of ovarian cancer patients harboring chemotherapy-resistant CD44+ cancer stem cells.
In conclusion, we have shown that CD44+/NF-KB-high/MyD88+ ovarian cancer stem cells, which are inherently resistant to chemotherapeutic drugs, overexpress claudin-4 and may be highly susceptible to killing by CPE-mediated therapy in vitro as well as in vivo. Taken altogether, our findings suggest for the first time that intraperitoneal CPE administration may represent an unconventional, potentially highly effective strategy to eradicate ovarian CD44+ cancer stem cells after the elimination of the bulk of the disease using conventional cytoreductive surgery followed by adjuvant chemotherapy.
Acknowledgments
FUNDING SOURCES
Supported in part by grants from the Nocivelli, Berlucchi, and Golgi Foundations, Brescia, Italy, National Institutes of Health (NIH) R01 CA122728-01A2 to A.D.S., and grants 501/A3/3 and 0027557 from the Italian Institute of Health to A.D.S. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
We thank Dr. Gil Mor of Yale University School of Medicine for the kind gift of CD44+/NF-KB-high/MyD88+ ovarian cancer stem cells.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Copeland L. Epithelial ovarian cancer. In: DiSaia PJ, Creasman WT, editors. Clinical Gynecologic Oncology. St Louis: Mosby Year Book; 1997. pp. 313–367. [Google Scholar]
- 3.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 4.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 5.Hermann PC, Huber SL, Heeschen C. Metastatic cancer stem cells: a new target for anti-cancer therapy? Cell Cycle. 2008;7:188–193. doi: 10.4161/cc.7.2.5326. [DOI] [PubMed] [Google Scholar]
- 6.Mueller MT, Hermann PC, Heeschen C. Cancer stem cells as new therapeutic target to prevent tumour progression and metastasis. Front Biosci. 2010;2:602–613. doi: 10.2741/e117. [DOI] [PubMed] [Google Scholar]
- 7.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–3029. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Balch C, Chan MW, et al. Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvero AB, Fu HH, Holmberg J, et al. Stem-like ovarian cancer cells can serve as tumor vascular progenitors. Stem Cells. 2009;27:2405–2413. doi: 10.1002/stem.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alvero AB, Chen R, Fu HH, et al. Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle. 2009;8:158–166. doi: 10.4161/cc.8.1.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katahira J, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Molecular cloning and functional characterization of the receptor for Clostridium perfringens enterotoxin. J Cell Biol. 1977;136:1239–1247. doi: 10.1083/jcb.136.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes 2 structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 13.Santin AD, Cane S, Bellone S, et al. Treatment of chemotherapy-resistant human ovarian cancer xenografts in C.B-17/SCID mice by intraperitoneal administration of Clostridium perfringens enterotoxin. Cancer Res. 2005;65:4334–4342. doi: 10.1158/0008-5472.CAN-04-3472. [DOI] [PubMed] [Google Scholar]
- 14.Kamsteeg M, Rutherford T, Sapi E, et al. Phenoxodiol—an isoflavone analog—induces apoptosis in chemoresistant ovarian cancer cells. Oncogene. 2003;22:2611–2620. doi: 10.1038/sj.onc.1206422. [DOI] [PubMed] [Google Scholar]
- 15.Landen CN, Jr, Goodman B, Katre AA, et al. Targeting aldehyde dehydrogenase cancer stem cells in ovarian cancer. Mol Cancer Ther. 2010;9:3186–3199. doi: 10.1158/1535-7163.MCT-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baba T, Convery PA, Matsumura N, et al. Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene. 2009;28:209–218. doi: 10.1038/onc.2008.374. [DOI] [PubMed] [Google Scholar]
- 17.Deng S, Yang X, Lassus H, et al. Distinct expression levels and patterns of stem cell marker, aldehyde dehydrogenase isoform 1 (ALDH1), in human epithelial cancers. PLoS One. 2010;5:e10277. doi: 10.1371/journal.pone.0010277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curley MD, Therrien VA, Cummings CL, et al. CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells. 2009;27:2875–2883. doi: 10.1002/stem.236. [DOI] [PubMed] [Google Scholar]
- 19.Yin G, Chen R, Alvero AB, et al. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene. 2010;29:3545–3553. doi: 10.1038/onc.2010.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McClane BA. An overview of Clostridium perfringens enterotoxin. Toxicon. 1996;34:1335–1343. doi: 10.1016/s0041-0101(96)00101-8. [DOI] [PubMed] [Google Scholar]
- 21.Long H, Crean CD, Lee WH, Cummings OW, Gabig TG. Expression of Clostridium perfringens enterotoxin receptors claudin-3 and claudin-4 in prostate cancer epithelium. Cancer Res. 2001;61:7878–7881. [PubMed] [Google Scholar]
- 22.Michl P, Buchholz M, Rolke M, et al. Claudin-4: a new target for pancreatic cancer treatment using Clostridium perfringens enterotoxin. Gastroenterology. 2001;121:678–684. doi: 10.1053/gast.2001.27124. [DOI] [PubMed] [Google Scholar]
- 23.Stewart JJ, White JT, Yan X, et al. Proteins associated with cisplatin resistance in ovarian cancer cells identified by quantitative proteomic technology and integrated with mRNA expression levels. Mol Cell Proteomics. 2006;5:433–443. doi: 10.1074/mcp.M500140-MCP200. [DOI] [PubMed] [Google Scholar]
- 24.Alberts DS, Markman M, Armstrong D, Rothenberg ML, Muggia F, Howell SB. Intraperitoneal therapy for stage III ovarian cancer: a therapy whose time has come. J Clin Oncol. 2002;20:3944–3946. doi: 10.1200/JCO.2002.20.19.3944. [DOI] [PubMed] [Google Scholar]