Abstract
Context
There has been rapid adoption of newer radiation treatments such as intensitymodulated radiation therapy (IMRT) and proton therapy despite greater cost and limited demonstrated benefit compared with previous technologies.
Objective
To determine the comparative morbidity and disease control of IMRT, proton therapy, and conformal radiation therapy for primary prostate cancer treatment.
Design, Setting, and Patients
Population-based study using Surveillance, Epidemiology, and End Results–Medicare-linked data from 2000 through 2009 for patients with nonmetastatic prostate cancer.
Main Outcome Measures
Rates of gastrointestinal and urinary morbidity, erectile dysfunction, hip fractures, and additional cancer therapy.
Results
Use of IMRT vs conformal radiation therapy increased from 0.15% in 2000 to 95.9% in 2008. In propensity score–adjusted analyses (N=12 976), men who received IMRT vs conformal radiation therapy were less likely to receive a diagnosis of gastrointestinal morbidities (absolute risk, 13.4 vs 14.7 per 100 person-years; relative risk [RR], 0.91; 95% CI, 0.86–0.96) and hip fractures (absolute risk, 0.8 vs 1.0 per 100 person-years; RR, 0.78; 95% CI, 0.65–0.93) but more likely to receive a diagnosis of erectile dysfunction (absolute risk, 5.9 vs 5.3 per 100 person-years; RR, 1.12; 95% CI, 1.03–1.20). Intensitymodulated radiation therapy patients were less likely to receive additional cancer therapy (absolute risk, 2.5 vs 3.1 per 100 person-years; RR, 0.81; 95% CI, 0.73–0.89). In a propensity score–matched comparison between IMRT and proton therapy (n=1368), IMRT patients had a lower rate of gastrointestinal morbidity (absolute risk, 12.2 vs 17.8 per 100 person-years; RR, 0.66; 95% CI, 0.55–0.79). There were no significant differences in rates of other morbidities or additional therapies between IMRT and proton therapy.
Conclusions
Among patients with nonmetastatic prostate cancer, the use of IMRT compared with conformal radiation therapy was associated with less gastrointestinal morbidity and fewer hip fractures but more erectile dysfunction; IMRT compared with proton therapy was associated with less gastrointestinal morbidity.
Prostate cancer is the most common malignancy in men, with more than 200 000 diagnoses and 30 000 deaths per year.1 Recent advances in technology have led to costlier treatments such as minimally invasive radical prostatectomy, intensity-modulated radiation therapy (IMRT), and proton therapy. The adoption of these technologies resulted in a $350 million increase in health care expenditures in 2005 alone.2 The Institute of Medicine, Agency for Healthcare Research and Quality, Secretary of the Department of Health and Human Services, and others have called for comparative effectiveness research of localized prostate cancer treatments,3–5 which is especially relevant for radiation therapy, for which IMRT has gradually replaced the older technique of con-formal radiation therapy during the past 10 years. More recently, there has been a substantial increase in the number of proton facilities built, and direct-to-consumer advertising is likely to lead to an increase in its use.6–8 The clinical benefit from these newer treatments is un-proven, and comparative effectiveness research examining different radiation techniques is lacking. Given these trends in use, multiple recent reports have specifically called for research on proton therapy.9
The objective of this study was to examine the comparative morbidity and disease control outcomes after different radiation techniques in a recent cohort of prostate cancer patients with the Surveillance, Epidemiology, and End Results (SEER)–Medicare-linked database. Specifically, we compared IMRT, which has been rapidly adopted and is currently the most commonly used technique, with the older conformal radiation therapy. We further compared proton therapy, whose use is increasing,6 with IMRT.
METHODS
Data Source
Surveillance, Epidemiology, and End Results data are composed of 16 population-based cancer registries representing approximately 26% of the US population. SEER-Medicare links the registry data to Medicare administrative and health care claims data, which include 97% of US residents aged 65 years and older and has been documented extensively.10 University of North Carolina institutional review board approval was waived.
Study Cohorts
According to SEER-Medicare data, 251 787 patients received a diagnosis of prostate cancer between 2000 and 2007; had no additional cancers, meta-static disease, or disease diagnosis at autopsy; and had month and year of diagnosis in the database. To allow assessment of baseline comorbidity, the patient population was further restricted to men with at least 1 year of claims data before diagnosis.11 To ensure complete capture of health services, we excluded men who were enrolled in a health maintenance organization within 1 year of diagnosis or not enrolled in both Medicare Part A and Part B for the study duration, which resulted in a cohort of 108 756 patients. A sensitivity analysis excluded patients who had any health maintenance organization enrollment for the study duration and demonstrated similar results (eAppendix, available at http://www.jama.com).
Using Current Procedural Terminology/Healthcare Common Procedure Coding System procedure codes, we identified 15 963 men who received radiation as primary treatment within 1 year of diagnosis (eAppendix). Patients who received radiation in combination with brachytherapy or prostatectomy were excluded. For the IMRT vs conformal radiation therapy comparison, we observed a large shift in use of these techniques during the study period (eFigure). To enable propensity score weighting in the 2 treatment groups, analysis was restricted to 12 976 men who received treatment between 2002 and 2006 to maximize the overlap in baseline characteristics: 6666 treated with IMRT and 6310 with conformal radiation therapy. Median follow-up for this comparison was 44 months for IMRT (range, 0.1–91.5 months) and 64 months for conformal radiation therapy (range, 0–91.7 months).
For the proton therapy vs IMRT comparison, we identified 684 men treated with proton therapy from 2002 to 2007. Because few institutions offered proton therapy, there was lack of overlap in baseline characteristics between proton therapy and IMRT patients largely because of 2 higher-level variables: SEER region and institutional affiliation with the Radiation Therapy Oncology Group. Therefore, we used propensity score matching to compare proton therapy with IMRT patients. Median follow-up for this comparison was 46 months for IMRT (range, 0.4–88.3 months) and 50 months for proton therapy (range, 0.3–90.2 months).
Outcomes
Morbidity outcomes included conditions associated with radiation therapy for prostate cancer: gastrointestinal morbidity, urinary incontinence, non-incontinence urinary morbidity, sexual dysfunction, and hip fractures.12–16 Diagnoses and procedures in each morbidity category were counted as separate outcomes. Because the goal of this study was to examine long-term morbidity, we excluded person-time and diagnoses and procedures that occurred within 1 year of radiation therapy; acute radiation therapy–related morbidity commonly resolves and does not become long-term morbidity.12
Consistent with previous studies, we identified men requiring additional cancer therapy after radiation therapy as an indicator of disease recurrence.17–19 Because radiation therapy is commonly used in combination with brachytherapy20 and sometimes as neoadjuvant treatment for planned prostatectomy,21 we defined additional cancer treatment as that occurring 9 months or more after initiation of radiation therapy. Furthermore, for patients who received radiation therapy concurrently with androgen deprivation therapy, additional treatment was defined as cessation of all treatment for 9 months or more, followed by reinitiation of androgen deprivation therapy or another salvage treatment.
Survival was not examined because death caused by prostate cancer is minimal within 5 years of diagnosis and not expected to be significantly different by radiation therapy technique within this period.1
Control Variables
The SEER registry provided patient-level demographic variables, including race, age at diagnosis, and marital status; census tract measures of income and education; SEER region; and population density (urban vs rural). Medicare claims data provided information on treatment dates, enrollment, and institutional affiliation with the Radiation Therapy Oncology Group, a radiation-specific clinical trials cooperative group. Radiation Therapy Oncology Group affiliation was used in propensity score weighting for the IMRT vs conformal radiation therapy comparison.
Baseline diagnoses of diabetes and conditions associated with the use of anticoagulation (atrial fibrillation and valvular disease) were determined by using claims within the year before radiation therapy (eAppendix); both have been shown to increase the morbidity risk from radiation therapy.22–24 Use of androgen deprivation therapy in conjunction with radiation therapy was included as a covariate because of its potential effects on both disease control and erectile dysfunction.25
Statistical Analysis
For the IMRT vs conformal radiation therapy comparisons, we used propensity score weighting to adjust for potentially important baseline characteristics. We used logistic regression to estimate the probability of receiving IMRT vs conformal radiation therapy (the propensity score) as a function of relevant covariates (Table 1 and 2). We evaluated the distributions of propensity scores by treatment group to check for sizeable overlap demonstrating that the groups are comparable. A propensity score weight was calculated as the inverse of the propensity for the treatment received and then stabilized, reflecting the sample size for each treatment group.
Table 1.
Baseline Demographic Characteristics for the IMRT vs CRT Comparison
| No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Before Propensity Weighting | After Propensity Weighting | |||||||
| Characteristics | IMRT (n = 6666) |
CRT (n = 6310) |
P Value |
IMRT (n = 6438) |
CRT (n = 6478) |
P Value |
||
| Year of radiation | ||||||||
| 2002 | 448 (6.7) | 2402 (38.1) | <.001 | 1203 (18.7) | 1380 (21.3) | <.001 | ||
| 2003 | 917 (13.8) | 1846 (29.3) | 1431 (22.2) | 1340 (20.7) | ||||
| 2004 | 1334 (20.0) | 1149 (18.2) | 1287 (20.0) | 1193 (18.4) | ||||
| 2005 | 1841 (27.6) | 601 (9.5) | 1261 (19.6) | 1214 (18.7) | ||||
| 2006 | 2126 (31.9) | 312 (4.9) | 1256 (19.5) | 1351 (20.9) | ||||
| Age at diagnosis, y | ||||||||
| 66–69 | 1338 (20.1) | 1265 (20.1) | .50 | 1341 (20.8) | 1306 (20.2) | .59 | ||
| 70–74 | 2415 (36.2) | 2345 (37.2) | 2353 (36.6) | 2368 (36.5) | ||||
| ≥75 | 2913 (43.7) | 2700 (42.8) | 2743 (42.6) | 2804 (43.3) | ||||
| Race | ||||||||
| White | 5694 (85.4) | 5325 (84.4) | <.001 | 5436 (84.5) | 5443 (84.0) | .45 | ||
| Black | 521 (7.8) | 657 (10.4) | 586 (9.1) | 630 (9.7) | ||||
| Other/unknown | 451 (6.8) | 328 (5.2) | 415 (6.4) | 405 (6.3) | ||||
| SEER region | ||||||||
| Atlanta and rural Georgia | 121 (1.8) | 126 (2.0) | <.001 | 129 (2.0) | 130 (2.0) | .06 | ||
| California | 2195 (32.9) | 1546 (24.5) | 2038 (31.7) | 2024 (31.2) | ||||
| Connecticut | 661 (9.9) | 608 (9.6) | 592 (9.2) | 562 (8.7) | ||||
| Detroit | 437 (6.6) | 849 (13.5) | 541 (8.4) | 602 (9.3) | ||||
| Hawaii | 143 (2.2) | 71 (1.1) | 105 (1.6) | 80 (1.2) | ||||
| Iowa | 345 (5.2) | 646 (10.2) | 501 (7.8) | 488 (7.5) | ||||
| Kentucky | 322 (4.8) | 682 (10.8) | 405 (6.3) | 462 (7.1) | ||||
| Louisiana | 489 (7.3) | 340 (5.4) | 452 (7.0) | 464 (7.2) | ||||
| New Jersey | 1415 (21.2) | 788 (12.5) | 1122 (17.4) | 1099 (17.0) | ||||
| New Mexico | 287 (4.3) | 178 (2.8) | 228 (3.5) | 226 (3.5) | ||||
| Seattle | 205 (3.1) | 368 (5.8) | 222 (3.4) | 265 (4.1) | ||||
| Utah | 46 (0.7) | 108 (1.7) | 103 (1.6) | 78 (1.2) | ||||
| Marital status | ||||||||
| Married | 4733 (71.0) | 4465 (70.8) | .02 | 4513 (70.1) | 4570 (70.6) | .68 | ||
| Not married | 1308 (19.6) | 1323 (21.0) | 1341 (20.8) | 1347 (20.8) | ||||
| Missing/unknown | 625 (9.4) | 522 (8.3) | 584 (9.1) | 560 (8.6) | ||||
| Census income, % | ||||||||
| Low income (0–25) | 1545 (23.2) | 1840 (29.2) | <.001 | 1718 (26.7) | 1716 (26.5) | .95 | ||
| Low-medium income (26–50) | 1648 (24.7) | 1696 (26.9) | 1652 (25.7) | 1662 (25.7) | ||||
| Medium-high income (51–75) | 1698 (25.5) | 1481 (23.5) | 1582 (24.6) | 1579 (24.4) | ||||
| High income (>75) | 1775 (26.6) | 1293 (20.5) | 1485 (23.1) | 1521 (23.5) | ||||
| Census proportion with at least high school education, % |
||||||||
| Low education (0–25) | 1461 (21.9) | 1862 (29.5) | <.001 | 1690 (26.3) | 1680 (25.9) | .90 | ||
| Low-medium education (26–50) | 1579 (23.7) | 1606 (25.5) | 1576 (24.5) | 1589 (24.5) | ||||
| Medium-high education (51–75) | 1799 (27.0) | 1547 (24.5) | 1637 (25.4) | 1682 (26.0) | ||||
| High education (>75) | 1827 (27.4) | 1295 (20.5) | 1535 (23.8) | 1526 (23.6) | ||||
| Population density | ||||||||
| Metropolitan | 5750 (86.3) | 5024 (79.6) | <.001 | 5339 (82.9) | 5380 (83.0) | .86 | ||
| Nonmetropolitan | 916 (13.7) | 1286 (20.4) | 1099 (17.1) | 1098 (17.0) | ||||
| RTOG affiliation | 917 (13.8) | 740 (11.7) | <.001 | 852 (13.2) | 825 (12.7) | .40 | ||
Abbreviations: CRT, conformal radiation therapy; IMRT, intensity-modulated radiation therapy; RTOG, Radiation Therapy Oncology Group; SEER, Surveillance, Epidemiology, and End Results.
Table 2.
Baseline Clinical Characteristics for the IMRT vs CRT Comparison
| No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Before Propensity Weighting | After Propensity Weighting | ||||||||
| Characteristics | IMRT (n = 6666) |
CRT (n = 6310) |
P Value |
IMRT (n = 6438) |
CRT (n = 6478) |
P Value |
|||
| Concurrent androgen deprivation therapy | 3786 (56.8) | 3906 (61.9) | <.001 | 3843 (59.7) | 3893 (60.1) | .65 | |||
| Tumor grade | |||||||||
| Well/moderately differentiated | 3390 (50.9) | 3850 (61.0) | <.001 | 3512 (54.5) | 3579 (55.3) | .68 | |||
| Poorly differentiated | 3177 (47.7) | 2334 (37.0) | 2817 (43.8) | 2785 (43.0) | |||||
| Unknown/not assessed | 99 (1.5) | 126 (2.0) | 109 (1.7) | 113 (1.7) | |||||
| Clinical stage | |||||||||
| T1 | 3375 (50.6) | 2502 (39.7) | <.001 | 2933 (45.6) | 2984 (46.1) | .79 | |||
| T2 | 3070 (46.1) | 3556 (56.3) | 3273 (50.8) | 3255 (50.3) | |||||
| T3/T4 | 221 (3.3) | 252 (4.0) | 232 (3.6) | 239 (3.7) | |||||
| Baseline comorbidities | |||||||||
| Diabetes | 1750 (26.2) | 1681 (26.6) | .62 | 1702 (26.4) | 1728 (26.7) | .75 | |||
| Anticoagulation, arrhythmia, or valvular disease | 1685 (25.3) | 1533 (24.3) | .20 | 1597 (24.8) | 1622 (25.0) | .76 | |||
| Gastrointestinal diagnosis/procedure | 1359 (20.4) | 1238 (19.6) | .27 | 1343 (20.9) | 1340 (20.7) | .80 | |||
| Urinary nonincontinence diagnosis/procedure | 1453 (21.8) | 1331 (21.1) | .33 | 1444 (22.4) | 1510 (23.3) | .23 | |||
| Urinary incontinence diagnosis/procedure | 1475 (22.1) | 1032 (16.3) | <.001 | 1326 (20.6) | 1349 (20.8) | .75 | |||
| Erectile dysfunction diagnosis/procedure | 615 (9.2) | 501 (7.9) | .009 | 569 (8.8) | 556 (8.6) | .61 | |||
| Hip fracture | 20 (0.3) | 14 (0.2) | .38 | 20 (0.3) | 27 (0.4) | .32 | |||
Abbreviations: CRT, conformal radiation therapy; IMRT, intensity-modulated radiation therapy.
For each outcome, we calculated number of events per 100 person-years of follow-up to be consistent with existing literature.3 We calculated the probability of additional treatment after propensity score weighting with the Kaplan-Meier method and used log-rank statistics to assess for potential difference in patients treated with IMRT vs conformal radiation therapy. Follow-up time was determined from the start of follow-up (12 months after the start of radiation therapy for morbidity and 9 months for additional therapies) until an event or censoring at the end of the study (December 31, 2009).
For the proton therapy vs IMRT comparisons, we applied propensity score matching to balance the 2 groups and calculated propensity score–matched rates for each outcome. Because of unequal distribution of proton patients across institutional-level variables, we performed sensitivity analysis with the Radiation Therapy Oncology Group affiliation as an instrumental variable to assess potential unmeasured confounding. Of all measured covariates, this affiliation was the strongest predictor of treatment receipt and was considered a priori as a possible instrument. In sensitivity analysis, we applied methods to test for the strength of the affiliation as an instrument among subgroups of the population and found that overall, the Radiation Therapy Oncology Group qualifies as a preference-based instrument.26 To maintain comparability between our other rate models, a modified 2-stage least-squares approach was applied.27
Statistical significance was set at .05; all tests were 2-tailed and no adjustment was made for multiple comparisons. Analyses were performed with SAS version 9.2.
RESULTS
Among the patients undergoing primary radiation therapy for nonmetastatic prostate cancer, use of IMRT vs conformal radiation therapy increased from 0.15% in 2000 to 95.9% in 2008 (eFigure). After propensity score weighting, baseline characteristics among conformal radiation therapy and IMRT patients were balanced, except for small residual differences in year of treatment (Table 1 and Table 2).
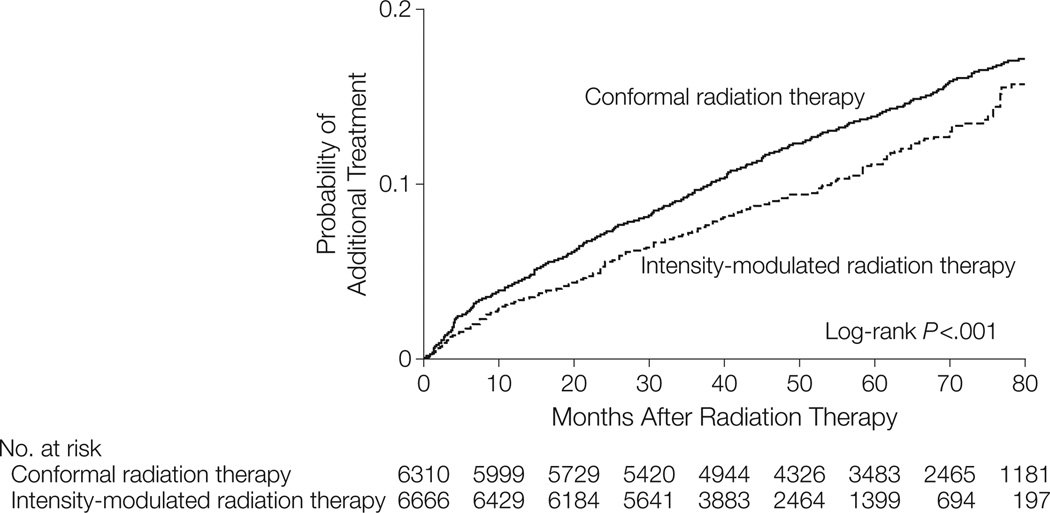
Unadjusted and propensity score–adjusted outcomes for IMRT vs conformal radiation therapy are presented in Table 3. In the adjusted analysis, men treated with IMRT were less likely to receive a diagnosis of gastrointestinal morbidity (13.4 for IMRT vs 14.7 for conformal radiation therapy per 100 person-years; relative risk [RR], 0.91; 95% CI, 0.86–0.96; P<.001) and hip fracture (0.8 for IMRT vs 1.0; RR, 0.78; 95% CI, 0.65–0.93; P = .006) but more likely to receive a diagnosis of erectile dysfunction (5.9 for IMRT vs 5.3; RR, 1.12; 95% CI, 1.03–1.20;P=.006). Furthermore, IMRT patients were less likely to receive additional cancer therapy (2.5 for IMRT vs 3.1; RR, 0.81; 95% CI, 0.73–0.89; P<.001) (Table 3, Figure 1).
Table 3.
Unadjusted and Propensity-Model Adjusted Outcomes for IMRT vs CRT
| Unadjusted | Adjusteda | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CRT (n = 6310) |
IMRT (n = 6666) |
CRT (n = 6478) |
IMRT (n = 6438) |
|||||||||||
| Outcome per 100 Person-Years |
Total Events |
100 Person Years |
Rate | Total Events |
100 Person-Years | Rate | IMRT vs CRT Rate Ratio (95% CI) |
Total Events | 100 Person Years |
Rate | Total Events |
100 Person Years |
Rate | IMRT vs CRT Rate Ratio (95% CI) |
| Gastrointestinal events | ||||||||||||||
| Procedures (including colonoscopy) |
3187 | 195 | 16.3 | 2940 | 167 | 17.6 | 1.08 (1.03–13) | 2989 | 180 | 16.6 | 3011 | 177 | 17.0 | 1.02 (0.97–.07) |
| Diagnoses | 2946 | 212 | 13.9 | 2450 | 182 | 13.5 | 0.97 (0.92–1.02) | 2828 | 192 | 14.7 | 2594 | 194 | 13.4 | 0.91 (0.86–0.96) |
| Urinary nonincontinence events | ||||||||||||||
| Procedures | 564 | 293 | 1.9 | 439 | 234 | 1.9 | 0.98 (0.86–.11) | 493 | 260 | 1.9 | 483 | 257 | 1.9 | 0.99 (0.87–.12) |
| Diagnoses | 2003 | 248 | 8.1 | 1747 | 199 | 8.8 | 1.09 (1.02–1.16) | 1941 | 220 | 8.8 | 1869 | 214 | 8.8 | 0.99 (0.93–1.06) |
| Urinary incontinence events | ||||||||||||||
| Procedures | 1904 | 246 | 7.7 | 1854 | 194 | 9.5 | 1.23 (1.16–.32) | 1867 | 219 | 8.5 | 1888 | 211 | 8.9 | 1.05 (0.98–.12) |
| Diagnoses | 970 | 283 | 3.4 | 785 | 226 | 3.5 | 1.01 (0.92–1.11) | 917 | 251 | 3.7 | 858 | 249 | 3.5 | 0.94 (0.86–1.04) |
| Erectile dysfunction events | ||||||||||||||
| Procedures | 202 | 303 | 0.7 | 200 | 240 | 0.8 | 1.25 (1.03–.52) | 224 | 268 | 0.8 | 200 | 265 | 0.8 | 0.90 (0.75–.09) |
| Diagnoses | 1186 | 265 | 4.5 | 1342 | 208 | 6.5 | 1.44 (1.33–1.56) | 1239 | 235 | 5.3 | 1342 | 228 | 5.9 | 1.12 (1.03–1.20) |
| Hip fracture | 301 | 302 | 1.0 | 186 | 240 | 0.8 | 0.78 (0.65–0.93) | 272 | 267 | 1.0 | 209 | 265 | 0.8 | 0.78 (0.65–0.93) |
| Additional cancer therapy | 896 | 302 | 3.0 | 575 | 249 | 2.3 | 0.78 (0.70–0.87) | 839 | 270 | 3.1 | 677 | 270 | 2.5 | 0.81 (0.73–0.89) |
Abbreviations: CRT, conformal radiation therapy; IMRT, intensity-modulated radiation therapy.
Rates shown are adjusted for the variables presented in Tables 1 and 2, using propensity scores implemented by inverse probability of treatment weighting.
Figure 1.
Propensity Score–Adjusted Rates of Additional Cancer Treatment for Patients Treated With Intensity-Modulated Radiation Therapy vs Conformal Radiation Therapy
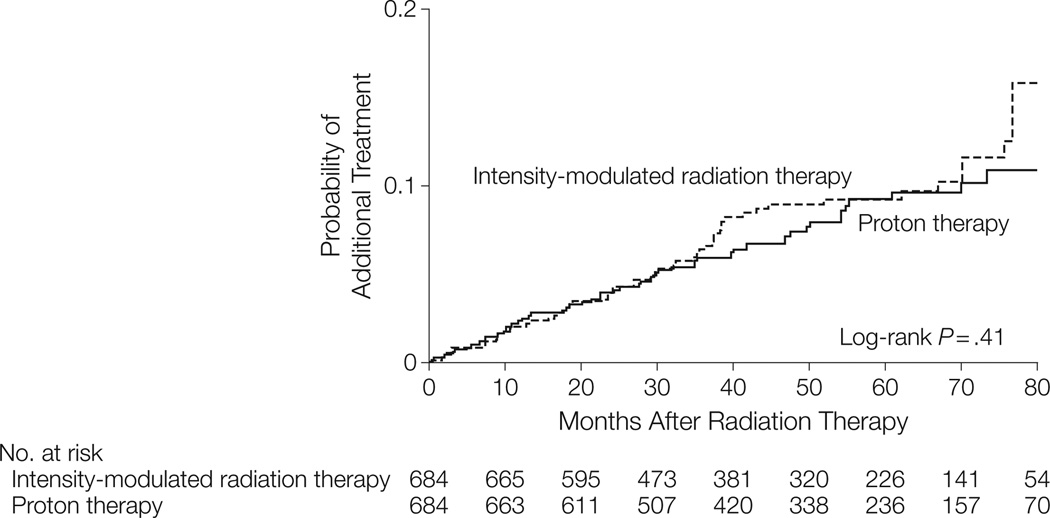
For the proton therapy vs IMRT comparison, propensity-matched baseline characteristics are presented in Table 4 and 5. Proton therapy patients had slightly less frequent baseline anticoagulation use. For morbidity and additional therapy outcomes, results from propensity score– matched and instrumental variable analyses were consistent (Table 4 and Table 5). There was no significant difference in proton therapy– vs IMRT-treated patients in urinary nonincontinence or incontinence diagnoses or procedures, erectile dysfunction, or hip fractures. The low hip fracture incidence precluded calculation of rate ratios in the propensity score–matched model. In both models, proton therapy– treated patients were more likely to receive a diagnosis of gastrointestinal morbidity and undergo gastrointestinal procedures. Rates of additional cancer therapy were no different between the 2 groups (Table 6, Figure 2).
Table 4.
Baseline Demographic Characteristics for the IMRT vs Proton Therapy Comparison
| No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Before Propensity Matching | After Propensity Matching | |||||||
| Characteristics | IMRT (n = 9437) |
Proton (n = 685) |
P Value |
IMRT (n = 684) |
Proton (n = 684) |
P Value |
||
| Year of radiation | ||||||||
| 2002 | 448 (4.8) | 119 (17.4) | <.001 | 110 (16.1) | 118 (17.3) | .34 | ||
| 2003 | 917 (9.7) | 127 (18.5) | 131 (19.2) | 127 (18.6) | ||||
| 2004 | 1334 (14.1) | 122 (17.8) | 122 (17.8) | 122 (17.8) | ||||
| 2005 | 1841 (19.5) | 102 (14.9) | 79 (11.6) | 102 (14.9) | ||||
| 2006 | 2226 (23.6) | 97 (14.2) | 99 (14.5) | 97 (14.2) | ||||
| 2007 | 2671 (28.3) | 118 (17.2) | 143 (20.9) | 118 (17.3) | ||||
| Age at diagnosis, y | ||||||||
| 66–69 | 1924 (20.4) | 248 (36.2) | <.001 | 244 (35.7) | 247 (36.1) | .61 | ||
| 70–74 | 3366 (35.7) | 233 (34.0) | 249 (36.4) | 233 (34.1) | ||||
| ≥75 | 4147 (43.9) | 204 (29.8) | 191 (27.9) | 204 (29.8) | ||||
| Race | ||||||||
| White | 7991 (84.7) | 634 (92.6) | <.001 | 635 (92.8) | 633 (92.5) | .77 | ||
| Black | 772 (8.2) | 20 (2.9) | 16 (2.3) | 20 (2.9) | ||||
| Other/unknown | 674 (7.1) | 31 (4.5) | 33 (4.8) | 31 (4.5) | ||||
| SEER region | ||||||||
| Atlanta and rural Georgia | 161 (1.7) | <11 (<2.0) | <.001 | <11 (<2.0) | <11 (<2.0) | .83 | ||
| California | 2928 (31.0) | 546 (79.7) | 560 (81.9) | 546 (79.8) | ||||
| Connecticut | 921 (9.8) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| Detroit | 619 (6.6) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| Hawaii | 230 (2.4) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| Iowa | 526 (5.6) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| Kentucky | 510 (5.4) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| Louisiana | 662 (7.0) | <11 (<2.0) | 13 (1.9) | <11 (<2.0) | ||||
| New Jersey | 2134 (22.6) | <11 (<2.0) | <11 (<2.0) | <11 (<2.0) | ||||
| New Mexico | 381 (4.0) | 26 (3.8) | 19 (2.8) | 26 (3.8) | ||||
| Seattle | 306 (3.2) | 42 (6.1) | 32 (4.7) | 42 (6.1) | ||||
| Utah | 59 (0.6) | 22 (3.2) | 22 (3.2) | 21 (3.1) | ||||
| Marital status | ||||||||
| Married | 6657 (70.5) | 530 (77.4) | <.001 | 539 (78.8) | 529 (77.3) | .70 | ||
| Not married | 1866 (19.8) | 109 (15.9) | 106 (15.5) | 109 (15.9) | ||||
| Missing/unknown | 914 (9.7) | 46 (6.7) | 39 (5.7) | 46 (6.7) | ||||
| Census income | ||||||||
| (%) Low income (0–25) | 2213 (23.5) | 175 (25.5) | .004 | 128 (18.7) | 131 (19.2) | .84 | ||
| Low-medium income (26–50) | 2324 (24.6) | 177 (25.8) | 211 (30.9) | 211 (30.9) | ||||
| Medium-high income (51–75) | 2399 (25.4) | 195 (28.5) | 180 (26.3) | 190 (27.8) | ||||
| High income (>75) | 2501 (26.5) | 138 (20.2) | 165 (24.1) | 152 (22.2) | ||||
| Census proportion with at least high school education (%) |
||||||||
| Low education (0–25) | 2074 (22.0) | 131 (19.1) | <.001 | 163 (23.8) | 175 (25.6) | .50 | ||
| Low-medium education (26–50) | 2240 (23.7) | 211 (30.8) | 189 (27.6) | 177 (25.9) | ||||
| Medium-high education (51–75) | 2525 (26.8) | 191 (27.9) | 179 (26.2) | 195 (28.5) | ||||
| High education (>75) | 2598 (27.5) | 152 (22.2) | 153 (22.4) | 137 (20.0) | ||||
| Population density | ||||||||
| Metropolitan | 8091 (85.7) | 617 (90.1) | .002 | 72 (10.5) | 68 (9.9) | .72 | ||
| Nonmetropolitan | 1346 (14.3) | 68 (9.9) | 612 (89.5) | 616 (90.1) | ||||
Abbreviations: IMRT, intensity-modulated radiation therapy; SEER, Surveillance, Epidemiology, and End Results.
Table 5.
Baseline Clinical Characteristics for the IMRT vs Proton Therapy Comparison
| No. (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Before Propensity Matching | After Propensity Matching | |||||||
| Characteristics | IMRT (n = 9437) |
Proton (n = 685) |
P Value |
IMRT (n = 684) |
Proton (n = 684) |
P Value |
||
| Concurrent androgen deprivation therapy | 5293 (56.1) | 212 (31.0) | <.001 | 200 (29.2) | 212 (31.0) | .48 | ||
| Tumor grade | ||||||||
| Well/moderately differentiated | 4528 (48.0) | 413 (60.3) | <.001 | 426 (62.3) | 412 (60.2) | .74 | ||
| Poorly differentiated | 4786 (50.7) | 268 (39.1) | 254 (37.1) | 268 (39.2) | ||||
| Unknown/not assessed | 123 (1.3) | 4 (0.6) | 4 (0.6) | 4 (0.6) | ||||
| Clinical stage | ||||||||
| T1 | 4989 (52.9) | 348 (50.8) | .57 | 346 (50.6) | 347 (50.7) | .81 | ||
| T2 | 4131 (43.8) | 314 (45.8) | 319 (46.6) | 314 (45.9) | ||||
| T3/T4 | 317 (3.4) | 23 (3.4) | 19 (2.8) | 23 (3.4) | ||||
| Baseline comorbidities | ||||||||
| Diabetes | 2583 (27.4) | 130 (19.0) | <.001 | 119 (17.4) | 130 (19.0) | .44 | ||
| Anticoagulation, arrhythmia, or valvular disease |
2384 (25.3) | 144 (21.0) | .01 | 176 (25.7) | 144 (21.1) | .04 | ||
| Gastrointestinal diagnosis/procedure |
1905 (20.2) | 148 (21.6) | .37 | 123 (18.0) | 148 (21.6) | .09 | ||
| Urinary nonincontinence diagnosis/procedure |
2084 (22.1) | 104 (15.2) | <.001 | 111 (16.2) | 104 (15.2) | .60 | ||
| Urinary incontinence diagnosis/procedure |
2233 (23.7) | 109 (15.9) | <.001 | 119 (17.4) | 109 (15.9) | .47 | ||
| Erectile dysfunction diagnosis/procedure |
901 (9.5) | 83 (12.1) | .03 | 72 (10.5) | 83 (12.1) | .35 | ||
| Hip fracture | 31 (0.3) | 0 | .27 | 1 (0.2) | 0 | >.99 | ||
Abbreviation: IMRT, intensity-modulated radiation therapy.
Table 6.
Outcomes for IMRT vs Proton Therapy With Propensity Score Matching and Instrumental Variable Analyses
| Propensity Score Matcheda | Instrumental Variable Analysis | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMRT (n = 684) |
Proton (n = 684) |
IMRT (n = 8144)b |
Proton (n = 1978)b |
|||||||||||
| Outcome per 100 Person-Years |
Total Events |
100 Person Years |
Rate | Total Events |
100 Person Years |
Rate | IMRT vs PT Rate Ratio (95% CI) |
Total Event s |
100 Person Years |
Rate | Total Events |
100 Person Years |
Rate | IMRT vs PT Rate Ratio (95% CI) |
| Gastrointestinal events | ||||||||||||||
| Procedures (including colonoscopy) |
302 | 17 | 17.7 | 347 | 16.2 | 21.4 | 0.82 (0.70–.97) | 3074 | 169 | 18.2 | 883 | 41 | 21.6 | 0.60 (0.46–.78) |
| Diagnoses | 235 | 19 | 12.2 | 301 | 16.9 | 17.8 | 0.66 (0.55–.79) | 2620 | 182 | 14.4 | 714 | 45 | 16.0 | 0.66 (0.49–.88) |
| Urinary nonincontinence events | ||||||||||||||
| Proceduresc | 44 | 25 | 1.8 | 42 | 25.8 | 1.6 | 1.06 (0.69–.63) | 466 | 233 | 2.0 | 113 | 62 | 1.8 | 1.71 (0.87–.36) |
| Diagnoses | 161 | 22 | 7.5 | 144 | 22.9 | 6.3 | 1.25 (0.99–.58) | 1864 | 198 | 9.4 | 454 | 53 | 8.6 | 1.10 (0.78–.58) |
| Urinary incontinence events | ||||||||||||||
| Procedures | 161 | 21 | 7.6 | 173 | 22.1 | 7.8 | 0.97 (0.77–.20) | 2029 | 194 | 10.5 | 511 | 51 | 10.0 | 1.06 (0.76–.50) |
| Diagnosesc | 75 | 24 | 3.1 | 82 | 24.8 | 3.3 | 0.96 (0.70–.32) | 816 | 226 | 3.6 | 200 | 59 | 3.4 | 1.03 (0.63–.71) |
| Erectile dysfunction events | ||||||||||||||
| Proceduresc | 21 | 25 | 0.8 | 36 | 26.2 | 1.4 | 0.61 (0.35–.06) | 206 | 239 | 0.9 | 70 | 63 | 1.1 | 0.58 (0.24–.41) |
| Diagnoses | 145 | 22 | 6.6 | 164 | 22.2 | 7.4 | 0.89 (0.70–.12) | 1454 | 208 | 7.0 | 436 | 53 | 8.3 | 0.78 (0.54–.13) |
| Hip fractured | 21 | 26 | 0.8 | 18 | 26.6 | 0.7 | 192 | 239 | 0.8 | 40 | 63 | 0.6 | 1.42 (0.50–.02) | |
| Additional cancer therapy | 58 | 26 | 2.2 | 52 | 27.5 | 1.9 | 1.26 (0.86–.84) | 588 | 252 | 2.3 | 124 | 67 | 1.9 | 1.60 (0.85–.00) |
Abbreviation: IMRT, intensity-modulated radiation therapy.
Rates shown are adjusted for the variables presented in Tables 4 and 5, using propensity scores implemented by matching.
Rates for IMRT and proton were adjusted with a 2-stage least-squares instrumental variable approach in which Radiation Therapy Oncology Group affiliation predicts proton use: this predicted value was subsequently used as exposure in an adjusted outcome model to estimate the effect of IMRT vs proton on the outcome.
Because of zero cell counts, Surveillance, Epidemiology, and End Results region was not included in propensity score–matched models.
Because of the small number of events and zero cell counts in some covariates in the propensity score–matched model, rate ratio could not be calculated.
Figure 2.
Propensity Score–Matched Rates of Additional Cancer Treatment for Patients Treated With Intensity-Modulated Radiation Therapy vs Proton Therapy
COMMENT
Comparative effectiveness research in localized prostate cancer treatments is needed because of the large number of men with this disease and the continued trend of a rapid increase in use of newer and costlier treatments with un-proven clinical benefit. The Institute of Medicine included localized prostate cancer as a “first quartile” priority topic in its top 100 topics for comparative effectiveness research.3 Although the theoretic rationale for the new treatments such as minimally invasive prostatectomy and IMRT is convincing, studies directly comparing the outcomes of newer vs older treatments are lacking. A recent SEER-Medicare study that reported mixed results when comparing minimally invasive prostatectomy vs the older open prostatectomy technique demonstrated that theoretic advantages for newer treatments may not necessarily translate into a clinical benefit.18
Radiation treatment can cause damage to organs surrounding the prostate, leading to long-term gastrointestinal and urinary morbidity, erectile dysfunction, and hip fractures. In the past 10 years, data have demonstrated that the risk of long-term morbidity is directly associated with the radiation dose received by each organ.28–31 These data led to the development of dose guidelines for the bowel and rectum, femoral heads, and bladder, which were widely adopted and are now a standard part of radiation treatment planning. Dose guidelines to minimize erectile dysfunction are not widely used because no organ or structure has been identified that consistently demonstrates an association between dose of radiation to the structure and erectile dysfunction.30
Delivering a high radiation dose to the prostate while limiting doses to surrounding organs to minimize long- term morbidity presents a significant challenge. In addition, between 2002 and 2006, 3 randomized trials were published that consistently demonstrated that higher radiation doses (78–79 Gy) resulted in improved freedom from recurrence compared with lower doses (68–70 Gy).32–34 However, higher doses also resulted in increased morbidity in these patients treated with conformal radiation techniques. Because these results changed the standard of care to “dose-escalated” radiation therapy for prostate cancer, simultaneously minimizing doses received by surrounding organs, especially the bowel and femoral heads, became even more difficult.
The potential advantage of IMRT compared with conformal radiation therapy is its ability to deliver high radiation doses to the prostate while minimizing doses to surrounding organs.35 However, the clinical benefit of this approach is largely untested. In a retrospective, single-institution study, Zelefsky et al12 reported that the 10-year rate of gastrointestinal morbidity was lower for IMRT-treated patients (5%) compared with those who received conformal radiation therapy (13%), despite a higher dose to the prostate prescribed among the IMRT patients. To our knowledge, no study has compared the nongastrointestinal morbidity and disease control outcomes between conformal radiation therapy and IMRT. Yet, as we have demonstrated, there has been an almost complete adoption of IMRT for prostate cancer.
From 2000 to 2008, IMRT use increased from 0.15% to 95.9%, and the timing is consistent with a surge in the use of IMRT to deliver dose-escalated treatment after publication of the 3 aforementioned trials.32,36,37 Despite a higher dose administered, patients who received IMRT compared with conformal radiation therapy were less likely to receive a diagnosis of gastrointestinal morbidity and hip fracture. Because bowel and femoral head dose limitations are routinely considered in radiation planning, the improved ability of IMRT to minimize doses to these organs is a likely explanation. Another possibility is an improvement in physician understanding of and attention to organ dose guidelines, which may have paralleled the adoption of IMRT, resulting in better treatment plans that may have also been achievable with conformal radiation therapy, if such constraints were addressed. No difference was observed in the rates of urinary morbidity. Erectile dysfunction was diagnosed more frequently in IMRT patients. Because the relevant anatomic structures associated with radiation-induced erectile dysfunction have not yet been identified, it is possible that increased erectile dysfunction from IMRT is due to delivery of dose-escalated treatment, resulting in a higher unintentional dose administered to these structures. These results are similar to those reported from a study of comparable design, which used an older patient cohort and had shorter follow-up.15
Patients receiving IMRT were less likely than those receiving conformal radiation therapy to undergo additional cancer treatments, which is consistent with the use of IMRT to deliver dose-escalated treatment, resulting in improved cancer control, as demonstrated by the randomized trials. Taken together, these results suggest that IMRT facilitated radiation dose escalation without compromising acceptable long-term morbidity.
Proton therapy is a high-profile, high-cost prostate cancer treatment. Since 2007, multiple proton facilities have been built, and direct-to-consumer advertising is likely to lead to a substantial increase in use.6–8 The potential advantage of proton therapy compared with IMRT is unclear. Radiation planning studies demonstrated that proton therapy relative to IMRT may reduce the proportion of each surrounding organ that receives low doses of radiation, which has unclear clinical significance but may be more prone to errors related to daily patient setup and positioning, as well as organ movement during treatment.38–40
To our knowledge, this study includes the largest series of proton therapy patients. We found no significant differences among patients treated with proton therapy vs IMRT in morbidity or receipt of additional cancer therapy, except an association with increased gastrointestinal morbidity in proton therapy patients. Another recent study also found higher gastrointestinal morbidity rates in proton therapy patients relative to IMRT patients.41 One possible explanation is the higher vulnerability of proton therapy to organ movement, which may lead to an unintentional higher dose to the rectum compared with IMRT. Whether the use of better image guidance reduces gastrointestinal morbidity is unknown. Overall, our results do not clearly demonstrate a clinical benefit to support the recent increase in proton therapy use for prostate cancer.
The strengths of our study include the use of a large, population-based cohort that reflects treatment outcomes in the community setting. The follow-up duration is longer than that of previous studies15,18 and may there- fore provide more stable estimates of long-term outcomes. We adjusted for baseline morbidity and included co-variates that could influence treatment outcomes, such as diabetes and a proxy for anticoagulation. We applied several analytic approaches to test the stability of our effect estimates, including propensity score weighting and matching, instrumental variable analyses, and Poisson and negative binomial models. Effect estimates and conclusions were consistent regardless of model choice. Our data on proton therapy are unique, and, to our knowledge, this is the first study to examine morbidity and disease control outcomes among the 3 most commonly used radiation techniques for prostate cancer. Because IMRT and proton therapy are relatively new technologies, several more years of use and patient follow-up are needed to examine comparative survival outcomes.
There are limitations to the use of SEER-Medicare data for the assessment of clinical outcomes. Claims files are not designed to provide detailed clinical information, so the data may be subject to misclassification. For example, morbidity diagnoses may be attributable to reasons other than the type of radiation therapy received, and certain outcomes (such as erectile dysfunction) may be underreported.42 However, these potential limitations should be balanced in the different patient cohorts to allow a comparison of relative rates of morbidity. There may also be bias in patient and physician reporting of morbidity, as well as use of additional cancer therapies; whether this bias affects patients treated with one form of radiation therapy more than another is unknown. An examination of our data, along with those reported by Hu et al,18 who used SEER-Medicare data to examine the morbidity diagnoses after prostatectomy, indicates relatively higher rates of urinary incontinence and erectile dysfunction in prostatectomy patients and noncontinence urinary and bowel morbidity in radiation patients. These findings are consistent with those of previous studies reporting physician-assessed morbidity and patient-reported outcomes.43–45 Furthermore, prostate cancer risk group was not available and can affect radiation dose and extent of treatment, with consequent effects on morbidity. However, cohorts were balanced for tumor grade and clinical stage. In addition, although SEER was designed to provide data representative of the US population,10 whether our results are generalizable with respect to choice of treatments, disease severity, and rates of outcomes requires further study.
Given the near-complete adoption of IMRT, it is unlikely that a randomized trial of IMRT vs conformal radiation therapy could ever be performed. An IMRT vs proton therapy trial would require many years to provide results. With the recent rapid increase in the number of proton facilities,7 comparative data are needed. Despite limitations in SEER-Medicare data, they represent an important data source with an established methodology for comparative effectiveness research.18 Furthermore, SEER-Medicare data are drawn from a large population-based sample and are therefore more likely to reflect results from treatments widely available in the community, broadening the generalizability of results in comparison to single-institutional studies or even clinical trials, which often have stringent patient selection criteria.
CONCLUSIONS
Among patients with nonmetastatic prostate cancer, use of IMRT vs conformal radiation therapy increased substantially from 2000 to 2008. Compared with conformal radiation therapy, IMRT was associated with fewer diagnoses of gastrointestinal morbidity, hip fractures, and additional cancer therapy but more erectile dysfunction. Proton therapy was associated with more gastrointestinal morbidity than IMRT. This population-based study suggests that IMRT may be associated with improved disease control without compromising morbidity compared with conformal radiation therapy, although proton therapy does not appear to provide additional benefit.
Supplementary Material
Acknowledgments
Funding/Support: This research was funded by the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the DEcIDE program, contract HHSA290-2005-0040-I-TO6. This publication was made possible by grant 2T32NR008856 from the National Institute of Nursing Research (NINR) at the NIH.
Role of the Sponsor: The sponsor was involved in the review and approval of the manuscript; however, the sponsor did not participate in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation of the manuscript.
Disclaimer: This study used the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. This article's contents are solely the responsibility of the authors and do not necessarily represent the official views of NINR.
Footnotes
Author Contributions: Dr Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Sheets, Goldin, and Meyer contributed equally to this article.
Study concept and design: Sheets, Goldin, Meyer, Holmes, Godley, Carpenter, Chen.
Acquisition of data: Goldin, Carpenter, Chen.
Analysis and interpretation of data: Sheets, Goldin, Meyer, Wu, Chang, Stürmer, Reeve, Godley, Carpenter, Chen.
Drafting of the manuscript: Sheets, Goldin, Meyer, Carpenter, Chen.
Critical revision of the manuscript for important intellectual content: Sheets, Goldin, Wu, Chang, Stu¨rmer, Holmes, Reeve, Godley, Carpenter, Chen.
Statistical analysis: Meyer, Wu, Chang, Holmes, Reeve, Chen.
Obtained funding: Meyer, Godley, Carpenter, Chen. Administrative, technical, or material support: Stu¨ rmer, Carpenter, Chen.
Study supervision: Godley, Chen.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Stürmer reports receiving investigator-initiated research funding and support as principal investigator (RO1 AG023178) and coinvestigator (RO1 AG018833) from the National Institute on Aging at the National Institutes of Health (NIH), funding as principal investigator of the University of North Carolina (UNC) Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) center from the Agency for Healthcare Research and Quality, salary support from the UNC Center of Excellence in Pharmacoepidemiology and Public Health, consultancy on the Genentech CER advisory board, and from unrestricted research grants from Merck and sanofi-aventis to the UNC. Dr Godley reports receiving compensation for participating in the data and safety monitoring committee for Ferring Pharmaceuticals, which manufactures Degarelix, a hormonal treatment for prostate cancer that is not directly related to the subject of this study, and receiving compensation for consultancy with GlaxoSmithKline. No other disclosures were reported.
Previous Presentations: Presented at the American Society for Radiation Oncology Annual Meeting, Miami, Florida, October 4, 2011; and the Genitourinary Cancers Symposium, San Francisco, California, February 2, 2012.
Online-Only Material: The eFigure and 3 eTables are available at http://www.jama.com.
Additional Contributions: We thank Tzy-Mey Kuo, MPH, PhD (University of North Carolina at Chapel Hill), Jane Darter, BS (University of North Carolina at Chapel Hill), Seth Tyree, MS (University of North Carolina at Chapel Hill), and Laura Hendrix, MS (University of North Carolina at Chapel Hill), for technical assistance; William Lawrence, MD, MS (Agency for Healthcare Research and Quality), for assistance with the study and manuscript; Centers for Medicare & Medicaid Services and the SEER program tumor registries in the creation of the SEER-Medicare database; and M. Alan Brookhart, PhD (University of North Carolina at Chapel Hill) for his assistance with the instrumental variable analysis. Work on this study was supported by the Integrated Cancer Information and Surveillance System, UNC Lineberger Comprehensive Cancer Center, with funding provided by the University Cancer Research Fund via the state of North Carolina. No one received any additional compensation beyond usual salary for his or her contributions.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen PL, GU X, Lipsitz SR, et al. Cost implications of the rapid adoption of newer technologies for treating prostate cancer. J Clin Oncol. 2011;29(12):1517–1524. doi: 10.1200/JCO.2010.31.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academies Press; 2009. [Google Scholar]
- 4.Institute for Clinical and Economic Review. Management Options for Low-Risk Prostate Cancer: A Report on Comparative Effectiveness and Value. Boston, MA: Institute for Clinical & Economic Review; 2009. [Google Scholar]
- 5.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148(6):435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 6.Goozner M. The proton beam debate: are facilities outstripping the evidence? J Natl Cancer Inst. 2010;102(7):450–453. doi: 10.1093/jnci/djq112. [DOI] [PubMed] [Google Scholar]
- 7.StatBite. StatBite: particle beam therapy facilities worldwide. J Natl Cancer Inst. 2010;102(7):450–453. doi: 10.1093/jnci/djq108. [DOI] [PubMed] [Google Scholar]
- 8.Zietman AL. The Titanic and the iceberg: prostate proton therapy and health care economics. J Clin Oncol. 2007;25(24):3565–3566. doi: 10.1200/JCO.2007.11.9768. [DOI] [PubMed] [Google Scholar]
- 9.Terasawa T, Dvorak T, Ip S, Raman G, Lau J, Trikalinos TA. Systematic review: charged-particle radiation therapy for cancer. Ann Intern Med. 2009;151(8):556–565. doi: 10.7326/0003-4819-151-8-200910200-00145. [DOI] [PubMed] [Google Scholar]
- 10.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40(8) suppl:IV-3–IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17(8):584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Zelefsky MJ, Levin EJ, Hunt M, et al. Incidence of late rectal and urinary toxicities after three-dimensional conformal radiotherapy and intensitymodulated radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2008;70(4):1124–1129. doi: 10.1016/j.ijrobp.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 13.Al-Mamgani A, Heemsbergen WD, Peeters ST, Lebesque JV. Role of intensity-modulated radiotherapy in reducing toxicity in dose escalation for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2009;73(3):685–691. doi: 10.1016/j.ijrobp.2008.04.063. [DOI] [PubMed] [Google Scholar]
- 14.Elliott SP, Jarosek SL, Alanee SR, Konety BR, Dusenbery KE, Virnig BA. Three-dimensional external beam radiotherapy for prostate cancer increases the risk of hip fracture. Cancer. 2011;117(19):4557–4565. doi: 10.1002/cncr.25994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekelman JE, Mitra N, Efstathiou J, et al. Outcomes after intensity-modulated versus conformal radiotherapy in older men with nonmetastatic prostate cancer. Int J Radiat Oncol Biol Phys. 2011;81(4):e325–e334. doi: 10.1016/j.ijrobp.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lips I, Dehnad H, Kruger AB, et al. Health-related quality of life in patients with locally advanced prostate cancer after 76 Gy intensity-modulated radiotherapy vs 70 Gy conformal radiotherapy in a prospective and longitudinal study. Int J Radiat Oncol Biol Phys. 2007;69(3):656–661. doi: 10.1016/j.ijrobp.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Lu-Yao GL, Potosky AL, Albertsen PC, Wasson JH, Barry MJ, Wennberg JE. Follow-up prostate cancer treatments after radical prostatectomy: a population-based study. J Natl Cancer Inst. 1996;88(3–4):166–173. doi: 10.1093/jnci/88.3-4.166. [DOI] [PubMed] [Google Scholar]
- 18.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302(14):1557–1564. doi: 10.1001/jama.2009.1451. [DOI] [PubMed] [Google Scholar]
- 19.Lowrance WT, Elkin EB, Jacks LM, et al. Comparative effectiveness of prostate cancer surgical treatments: a population based analysis of postoperative outcomes. J Urol. 2010;183(4):1366–1372. doi: 10.1016/j.juro.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hurwitz MD. Technology insight: combined external-beam radiation therapy and brachytherapy in the management of prostate cancer. Nat Clin Pract Oncol. 2008;5(11):668–676. doi: 10.1038/ncponc1224. [DOI] [PubMed] [Google Scholar]
- 21.Pontes JE, Montie J, Klein E, Huben R. Salvage surgery for radiation failure in prostate cancer. Cancer. 1993;71(3) suppl:976–980. doi: 10.1002/1097-0142(19930201)71:3+<976::aid-cncr2820711413>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Choe KS, Jani AB, Liauw SL. External beam radiotherapy for prostate cancer patients on anticoagulation therapy: how significant is the bleeding toxicity? Int J Radiat Oncol Biol Phys. 2010;76(3):755–760. doi: 10.1016/j.ijrobp.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 23.Valdagni R, Rancati T, Fiorino C, et al. Development of a set of nomograms to predict acute lower gastrointestinal toxicity for prostate cancer 3D–CRT. Int J Radiat Oncol Biol Phys. 2008;71(4):1065–1073. doi: 10.1016/j.ijrobp.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 24.Giordano SH, Lee A, Kuo YF, Freeman J, Goodwin JS. Late gastrointestinal toxicity after radiation for prostate cancer. Cancer. 2006;107(2):423–432. doi: 10.1002/cncr.21999. [DOI] [PubMed] [Google Scholar]
- 25.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 26.Brookhart MA, Schneeweiss S. Preference-based instrumental variable methods for the estimation of treatment effects: assessing validity and interpreting results. Int J Biostat. 2007;3(1):14. doi: 10.2202/1557-4679.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brookhart MA, Rassen JA, Schneeweiss S. Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19(6):537–554. doi: 10.1002/pds.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michalski JM, Gay H, Jackson A, Tucker SL, Deasy JO. Radiation dose-volume effects in radiation-induced rectal injury. Int J Radiat Oncol Biol Phys. 2010;76(3) suppl:S123–S129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Viswanathan AN, Yorke ED, Marks LB, Eifel PJ, Shipley WU. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76(3) suppl:S116–S122. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roach M, III, Nam J, Gagliardi G, El Naqa I, Deasy JO, Marks LB. Radiation dose-volume effects and the penile bulb. Int J Radiat Oncol Biol Phys. 2010;76(3) suppl:S130–S134. doi: 10.1016/j.ijrobp.2009.04.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorino C, Valdagni R, Rancati T, Sanguineti G. Dose-volume effects for normal tissues in external radiotherapy: pelvis. Radioth Oncol. 2009;93(2):153–167. doi: 10.1016/j.radonc.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Dearnaley DP, Sydes MR, Graham JD. RT01 Collaborators. Escalated-dose versus standard-dose conformal radiotherapy in prostate cancer: first results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2007;8(6):475–487. doi: 10.1016/S1470-2045(07)70143-2. [DOI] [PubMed] [Google Scholar]
- 33.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pollack A, Zagars GK, Starkschall G, et al. Prostate cancer radiation dose response: results of the M. D. Anderson phase III randomized trial. Int J Radiat Oncol Biol Phys. 2002;53(5):1097–1105. doi: 10.1016/s0360-3016(02)02829-8. [DOI] [PubMed] [Google Scholar]
- 35.Nutting CM, Convery DJ, Cosgrove VP, et al. Reduction of small and large bowel irradiation using an optimized intensity-modulated pelvic radiotherapy technique in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2000;48(3):649–656. doi: 10.1016/s0360-3016(00)00653-2. [DOI] [PubMed] [Google Scholar]
- 36.Zietman AL, DeSilvio ML, Slater JD, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA. 2005;294(10):1233–1239. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 37.Pollack A, Zagars GK, Smith LG, et al. Preliminary results of a randomized radiotherapy dose-escalation study comparing 70 Gy with 78 Gy for prostate cancer. J Clin Oncol. 2000;18(23):3904–3911. doi: 10.1200/JCO.2000.18.23.3904. [DOI] [PubMed] [Google Scholar]
- 38.Trofimov A, Nguyen PL, Coen JJ, et al. Radiotherapy treatment of early-stage prostate cancer with IMRT and protons: a treatment planning comparison. Int J Radiat Oncol Biol Phys. 2007;69(2):444–453. doi: 10.1016/j.ijrobp.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newhauser WD, Giebeler A, Langen KM, Mirkovic D, Mohan R. Can megavoltage computed tomography reduce proton range uncertainties in treatment plans for patients with large metal implants? Phys Med Biol. 2008;53(9):2327–2344. doi: 10.1088/0031-9155/53/9/009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tepper JE. Protons and parachutes. J Clin Oncol. 2008;26(15):2436–2437. doi: 10.1200/JCO.2008.17.1165. [DOI] [PubMed] [Google Scholar]
- 41.Kim S, Shen S, Moore DF, et al. Late gastrointestinal toxicities following radiation therapy for prostate cancer. Eur Urol. 2011;60(5):908–916. doi: 10.1016/j.eururo.2011.05.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Potosky AL, Warren JL, Riedel ER, Klabunde CN, Earle CC, Begg CB. Measuring complications of cancer treatment using the SEER-Medicare data. Med Care. 2002;40(8) suppl:IV-62–IV-68. doi: 10.1097/00005650-200208001-00009. [DOI] [PubMed] [Google Scholar]
- 43.Potosky AL, Davis WW, Hoffman RM, et al. Five-year outcomes after prostatectomy or radiotherapy for prostate cancer: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2004;96(18):1358–1367. doi: 10.1093/jnci/djh259. [DOI] [PubMed] [Google Scholar]
- 44.Sanda MG, Dunn RL, Michalski J, et al. Quality of life and satisfaction with outcome among prostate-cancer survivors. N Engl J Med. 2008;358(12):1250–1261. doi: 10.1056/NEJMoa074311. [DOI] [PubMed] [Google Scholar]
- 45.Chen RC, Clark JA, Talcott JA. Individualizing quality-of-life outcomes reporting: how localized prostate cancer treatments affect patients with different levels of baseline urinary, bowel, and sexual function. J Clin Oncol. 2009;27(24):3916–3922. doi: 10.1200/JCO.2008.18.6486. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.